Abstract
Introduction
Optimal atrial tachyarrhythmia management is facilitated by accurate ECG interpretation, yet typical atrial flutter (AFl) may present without sawtooth F-waves or RR regularity, and atrial fibrillation (AF) may be difficult to separate from atypical AFl or rapid focal atrial tachycardia (AT). We analyzed whether improved diagnostic accuracy using a validated analysis tool significantly impacts costs and patient care.
Methods and Results
We performed a prospective, blinded, multicenter study using a novel quantitative computerized algorithm to identify atrial tachyarrhythmia mechanism from the surface ECG in patients referred for electrophysiology study (EPS). In 122 consecutive patients (age 60±12 years) referred for EPS, 91 sustained atrial tachyarrhythmias were studied. ECGs were also interpreted by 9 physicians from 3 specialties for comparison and to allow healthcare system modeling. Diagnostic accuracy was compared to the diagnosis at EPS. A Markov model was used to estimate the impact of improved arrhythmia diagnosis. We found 13% of typical AFl ECGs had neither sawtooth flutter waves nor RR regularity, and were misdiagnosed by the majority of clinicians (0/6 correctly diagnosed by consensus visual interpretation) but correctly by quantitative analysis in 83% (5/6, p=0.03). AF diagnosis was also improved through use of the algorithm (92%) vs visual interpretation (primary care: 76%, p<0.01). Economically, we found that these improvements in diagnostic accuracy resulted in an average cost-savings of $1,303 and 0.007 quality-adjusted-life-years per patient.
Conclusions
Typical AFl and AF are frequently misdiagnosed using visual criteria. Quantitative analysis improves diagnostic accuracy and results in improved healthcare costs and patient outcomes.
Keywords: Atrial fibrillation, atrial flutter, focal atrial tachycardia, economic modeling, electrocardiogram
INTRODUCTION
Atrial tachyarrhythmias, including typical atrial flutter (AFl), atypical AFl, focal atrial tachycardia (AT), and atrial fibrillation (AF) represent the most common class of heart rhythm disorders presenting to medical attention.1 They are responsible for significant morbidity and mortality2 and are costly to treat.3 Accurate interpretation of the 12-lead electrocardiogram (ECG) is central to their diagnosis and management.
However, typical AFl may present with atypical surface ECG findings4 and irregular RR intervals,5 making ECG interpretation challenging. Similarly, AF may present with coarse fibrillation waves6 that may be mistaken for atrial flutter7 or rapid focal AT. Age-related fibrosis,8 pulmonary disease,9 and prior surgery10 can also significantly alter the ECG appearance of atrial tachyarrhythmias. The difficulties in accurate ECG interpretation are illustrated in a study by Knight et al. showing that an ECG of AF was correctly separated from typical AFl by only 37% of general physicians and 26% of cardiologists.7
The inadequacy of current surface ECG criteria is becoming increasingly recognized,5, 11 and the Task Force of the North American Society for Pacing and Electrophysiology and the European Society of Cardiology (NASPE/ESC) expressed that “the current ECG classification is obsolete for this purpose [of assigning appropriate atrial tachycardia mechanism].”11 Moreover, few studies exist assessing the interpretation of all atrial tachyarrhythmias referenced to intracardiac diagnosis at electrophysiology study. Indeed, a limitation of prior work is that expert visual ECG interpretation has been used as the gold standard instead EPS,12, 13 potentially biasing results in favor of visual interpretation.
Of these arrhythmias, typical AFl is difficult to medically control,14 carries a stroke risk similar to AF,15 but can be ablated in the right atrium (RA) using an electrogram or anatomically guided approach in the majority of patients.16 In contrast to the other atrial tachyarrhythmias, current guidelines recommend catheter ablation without a trial of antiarrhythmic therapy for typical AFl.11 As a result, accurately diagnosing typical AFl from the other tachyarrhythmias has important implications for patient care and costs. Similarly, accurately separating AF versus atypical AFl and focal AT is also important to guide subsequent therapy, including ablation. Prior studies have shown a cost benefit for opportunistic screening for atrial fibrillation17 and for referral for ablation of typical AFl,18 but none have examined the potential impact of implementing a quantitative diagnostic algorithm for atrial arrhythmias at the healthcare system level.
We therefore conducted a prospective, blinded investigation to test the hypothesis that a validated computer algorithm19–21 would improve diagnostic accuracy, particularly for typical AFl and AF, compared to the current criteria for surface ECG interpretation, referenced to the gold standard of diagnosis during invasive EPS. We then evaluated whether differences in diagnostic performance would have a significant impact on healthcare costs and patient quality-of-life using a Markov economic model.
METHODS
Study Design
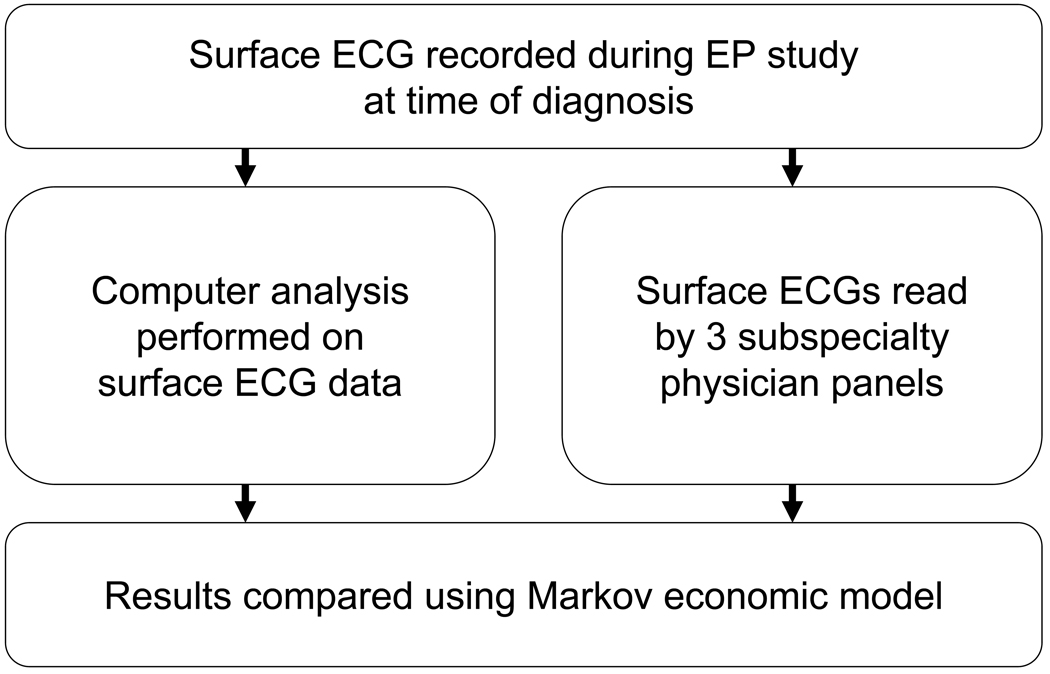
We prospectively enrolled 122 consecutive patients with a documented atrial tachyarrhythmia referred for ablation at the University of California San Diego (UCSD), Veterans Affairs San Diego Healthcare System (VASDHS), and Washington University (WU, St. Louis, Missouri) Medical Centers. Of the study population, 33 patients were excluded due to lack of sustained arrhythmia during EPS (19) or poor technical ECG quality (14). In the remaining 89 patients, we studied 91 sustained arrhythmias. Surface ECG data was analyzed separately by computer algorithm and subspecialty panels of internists, cardiologists, and electrophysiologists (figure 1). Results were compared using a Markov model (figure 2). The study was approved by the Institutional Review Boards of each institution, and all patients provided written informed consent.
Figure 1.
Study design. Surface ECG data was recorded at the time of diagnosis during electrophysiology study (top) and separately evaluated using a computer algorithm (middle left) versus subspecialty clinician panels (middle right). Diagnostic accuracy of computer versus visual interpretation was then compared using a Markov economic model (bottom).
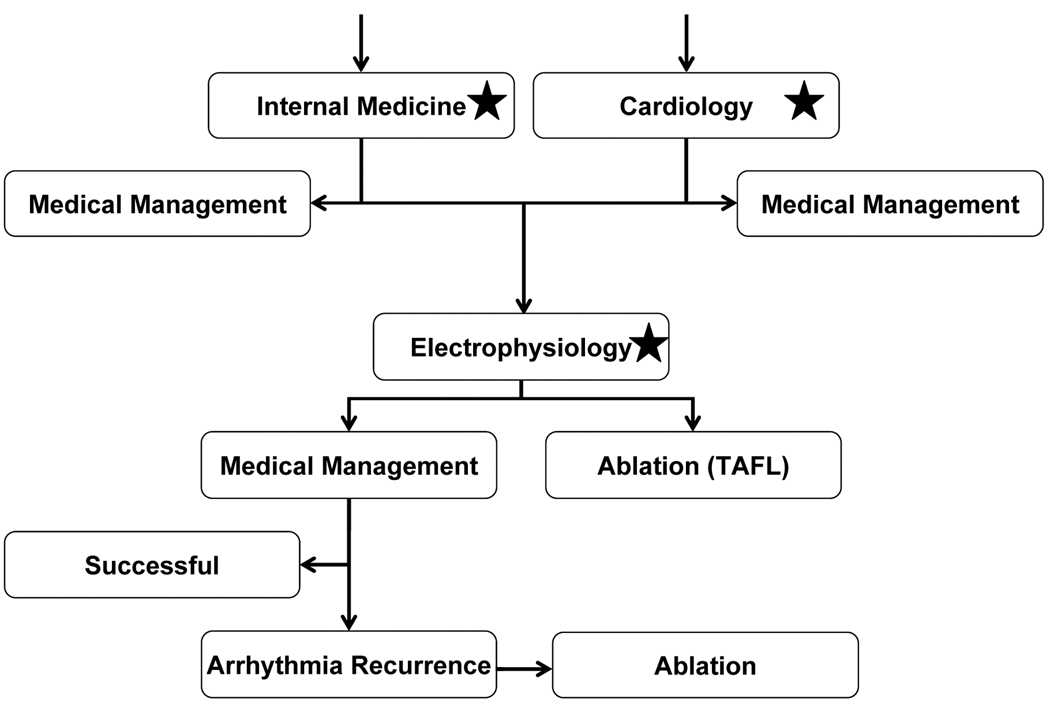
Figure 2.
Outline of Markov model. In this simulation of 1000 patients, economic and patient outcomes were compared between management using quantitative ECG analysis and without. Patients entered this hypothetical healthcare system at in the primary care (internal medicine) and cardiology clinics. Asymptomatic patients diagnosed with atrial fibrillation (AF) were assigned to medical management, while symptomatic patients and those with other tachyarrhythmia diagnoses are referred to electrophysiology (EP). Evaluation in the EP clinic was simulated, and patients diagnosed with typical atrial flutter (TAFL) are referred to ablation, while the remainder was treated with antiarrhythmics. Using published data, a proportion of patients on antiarrhythmic therapy have arrhythmia recurrence, and those patients are referred to ablation. In this simulated system, we incorporated either the specialty panels’ diagnostic accuracy or the computer analysis (locations in model noted by stars), and compared quality of life and cost of care.
Electrophysiologic Study and Ablation
Electrophysiologic study was performed in the fasted state, at least 4 weeks after discontinuing amiodarone (n=2 patients; Table 1) and 5 half-lives after discontinuing other antiarrhythmic medications. Electrophysiology study was performed with clinically indicated catheters and using appropriate diagnostic maneuvers, as determined by the invasive electrophysiologist at each center (GKF, SMN, and MNF). Typically, a decapolar catheter was used to record from the coronary sinus (CS), and quadripolar catheters were placed at the high right atrium, His bundle, and right ventricular apex. In each patient, arrhythmia diagnosis was made using criteria endorsed by the ESC/NASPE guidelines11 including mapping to demonstrate sites of entrainment with concealed fusion, termination with ablation, and lack of reinducibility post-ablation.
Table 1.
Patient Characteristics by EPS Atrial Arrhythmia Diagnosis
| Characteristic | Typical AFl |
Atypical AFl |
Focal AT | AF | p |
|---|---|---|---|---|---|
| Post-EPS diagnosis | 44 | 11 | 5 | 31 | |
| Age, years | 62 ± 10 | 69 ± 13 | 52 ± 8 | 59 ± 14 | 0.07 |
| Male sex, n (%) | 42 (96) | 7 (70) | 2 (40) | 30 (97) | <0.001 |
| Past medical history | |||||
| Congestive heart failure, n (%) | 16 (39) | 3 (30) | 1 (25) | 2 (7) | 0.14 |
| Diabetes mellitus, n (%) | 17 (40) | 2 (20) | 1 (25) | 5 (17) | 0.15 |
| Hypertension, n (%) | 28 (68) | 9 (90) | 1 (25) | 17 (57) | 0.08 |
| Hypercholesterolemia, n (%) | 30 (71) | 5 (50) | 1 (25) | 16 (53) | 0.15 |
| Coronary artery disease, n (%) | 19 (45) | 1 (10) | 0 (0) | 8 (27) | 0.05 |
| Myocardial infarction, n (%) | 7 (17) | 1 (10) | 0 (0) | 1 (3) | 0.28 |
| RCA, n (%) | 1 (3) | 1 (20) | 0 (0) | 3 (13) | 0.41 |
| PCI, n (%) | 7 (17) | 1 (10) | 0 (0) | 4 (13) | 0.79 |
| Coronary artery bypass graft, n (%) | 11 (26) | 1 (10) | 0 (0) | 1 (3) | 0.04 |
| Other cardiovascular surgery, n (%) | 5 (12) | 1 (10) | 0 (0) | 1 (3) | 0.53 |
| Congenital heart disorders, n (%) | 1 (3) | 1 (10) | 0 (0) | 0 (0) | 0.78 |
| COPD, n (%) | 6 (14) | 1 (10) | 1 (25) | 2 (7) | 0.63 |
| Asthma, n (%) | 3 (7) | 1 (10) | 1 (25) | 2 (7) | 0.64 |
| History of AF, n (%) | 13 (32) | 7 (70) | 3 (75) | 27 (90) | <0.001 |
| Prior ablation, n (%) | 1 (2) | 7 (70) | 1 (25) | 5 (17) | <0.001 |
| Valvular disease, n (%) | 5 (13) | 2 (22) | 0 (0) | 2 (7) | 0.54 |
| Echo data | |||||
| Left atrial size, mm | 41 ± 15 | 47 ± 9 | 36 ± 5 | 44 ± 6 | 0.52 |
| Left ventricular ejection fraction, % | 51 ± 17 | 55 ± 7 | 72 ± 7 | 56 ± 8 | 0.08 |
| Left ventricle septal thickness, mm | 12 ± 3 | 11 ± 3 | 11 ± 1 | 12 ± 2 | 0.84 |
| Left ventricle free wall thickness, mm | 11 ± 3 | 11 ± 2 | 11 ± 0 | 12 ± 2 | 0.66 |
| Left ventricular hypertrophy, n (%) | 13 (45) | 1 (25) | 0 (0) | 12 (63) | 0.21 |
| Medications | |||||
| Beta blockers, n (%) | 30 (73) | 5 (56) | 2 (50) | 20 (67) | 0.62 |
| Calcium Channel Blockers, n (%) | 13 (32) | 5 (56) | 1 (25) | 10 (33) | 0.56 |
| Digoxin, n (%) | 10 (24) | 3 (33) | 0 (0) | 8 (27) | 0.63 |
| ACE-I/ARB, n (%) | 25 (61) | 7 (78) | 1 (25) | 13 (45) | 0.16 |
| Flecainide/Propafenone, n (%) | 3 (7) | 1 (11) | 1 (25) | 6 (21) | 0.37 |
| Amiodarone, n (%) | 5 (12) | 1 (11) | 0 (0) | 3 (10) | 0.90 |
| Sotalol/Dofetilide, n (%) | 1 (2) | 2 (22) | 0 (0) | 3 (10) | 0.16 |
| Aspirin/Plavix, n (%) | 19 (46) | 4 (44) | 1 (25) | 4 (14) | 0.03 |
| Coumadin, n (%) | 34 (83) | 8 (89) | 2 (50) | 24 (83) | 0.38 |
Abbreviation Key: EPS = electrophysiology study, AFl = atrial flutter, AT = atrial tachycardia. AF = atrial fibrillation, RCA = right coronary artery, PCI = percutaneous coronary intervention, COPD = chronic obstructive pulmonary disease, ACE-I = angiotensin converting enzyme – inhibitor, ARB – angiotensin receptor blocker.
Specifically, the diagnosis of typical AFl (isthmus dependent AFL, counterclockwise or clockwise) required induction of sustained tachycardia (figure 3B) with concealed entrainment within the cavotricuspid isthmus (CTI). Entrainment pacing was also performed at other sites around the tricuspid valve to verify the reentrant circuit. Finally, we required termination of the tachycardia with ablation within the CTI (figure 3C), and/or noninducibility after demonstrating bidirectional CTI block.
Figure 3.
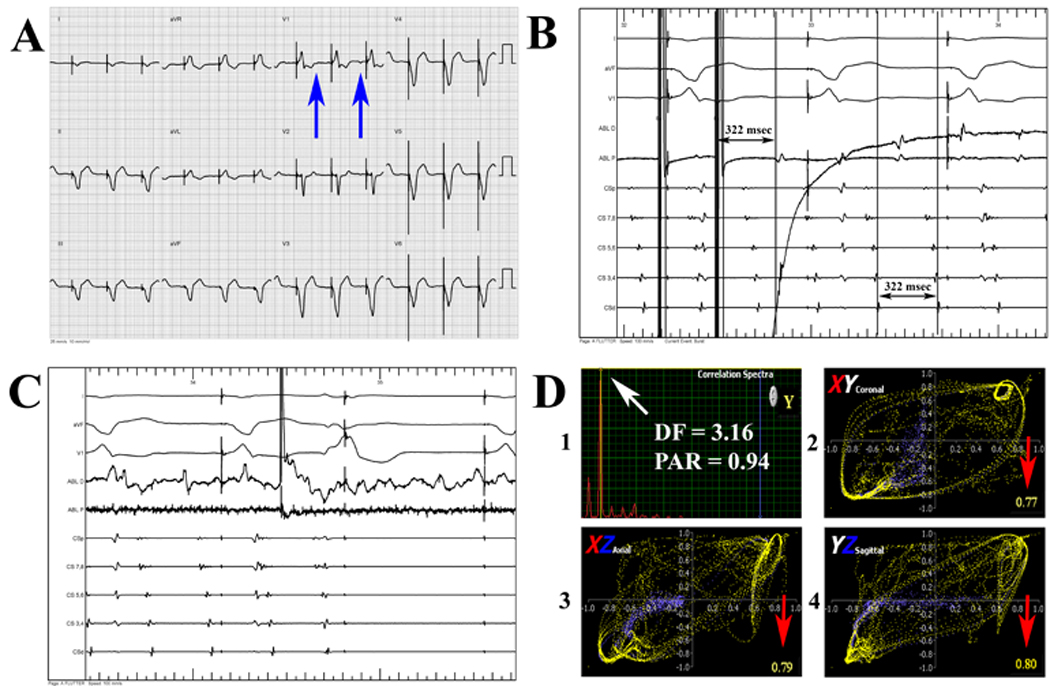
Example surface ECG (A) during tachycardia obtained at electrophysiology study from a patient with coronary artery disease, hypertension, ventricular tachycardia (VT) s/p dual chamber implantable cardioverter-defibrillator (ICD) implantation, and VT ablation for recurrent ICD shocks that had a highly symptomatic supraventricular tachycardia. All clinicians incorrectly diagnosed the surface ECG as atrial fibrillation (AF) or atypical atrial flutter (AFl), based on ICD interrogation showing regular atrial activation). The atypical F-wave appearance on surface ECG (blue arrows) is shown. During electrophysiology study (B), patient was diagnosed with typical AFl with a cycle length of 322 msec by concealed entrainment within the cavo-tricuspid isthmus (CTI) and termination of tachyarrhythmia without reinducibility during ablation within the CTI (C). Correct diagnosis as typical AFl (D) was made independently using quantitative analysis (narrow correlation function spectra [peak area ratio (PAR) = 0.94, D1] reducing probability of AF, and high temporospatial correlation in the XY, YZ, and XZ planes [D1, D2, and D3, correlation values ≥ 0.75 at red arrows] reducing probability of atypical atrial flutter) with a relatively long atrial activation > 45% of tachycardia cycle length [322 msec] reducing probability of atrial tachycardia). The use of quantitative analysis would have saved used of NavX patches (~$1000), and prevented scheduling case in a long EP lab time block. Abbreviation: DF = dominant frequency
For the diagnosis of atypical AFl, we required the presence of concealed entrainment during tachycardia for a reentrant circuit not involving the CTI. We also required termination of atypical AFl during ablation within a critical isthmus and/or noninducibility after ablation.
AF was diagnosed by irregular F-F intervals on intracardiac electrograms (>20 msec), frequent alternation from concentric to eccentric activation of the coronary sinus (figure 4B), irregular ventricular rates when AV node conduction was normal, and the inability to entrain the arrhythmia from one or more sites within the atria. Inducibility was tested using IV isoproterenol and burst pacing. Ablation was continued until AF was noninducible.
Figure 4.
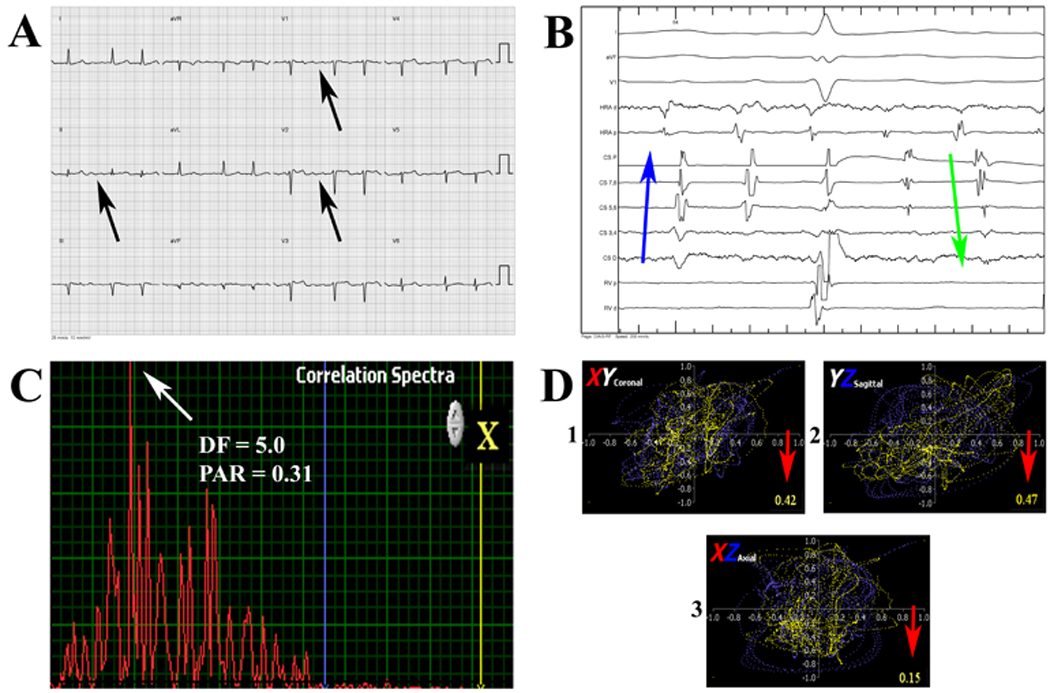
Surface ECG (A) of a 64 year old patient with a history of congestive heart failure, ventricular tachycardia s/p ablation and dual chamber ICD who presented with a symptomatic supraventricular tachycardia. Patient was diagnosed with atypical atrial flutter due to ICD interrogation showed a relatively regular atrial tachycardia cycle length and what appeared to be flutter waves on surface ECG (black arrows). Electrophysiology study showed frequent alternation between concentric and eccentric coronary sinus activation (B), consistent with atrial fibrillation (blue and green arrows). Quantitative analysis showed a broad correlation spectra (peak area ratio (PAR) = 0.31, C), and low temporospatial correlation (red arrows, D), consistent with AF. Abbreviation: DF = dominant frequency
In all cases of focal AT, centrifugal activation (figure 5C) was documented using the EnSite noncontact mapping catheter (St. Jude, St. Paul, MN) or activation mapping using NavX (St. Jude, St. Paul, MN) or Carto (Biosense Webster, Diamond Bar, CA). We required focal AT termination with ablation at the ectopic site (3D) and/or lack of reinducibility. Sinus tachycardia was excluded in all cases by verification that activation did not emanate from the region of the sinoatrial node.
Figure 5.
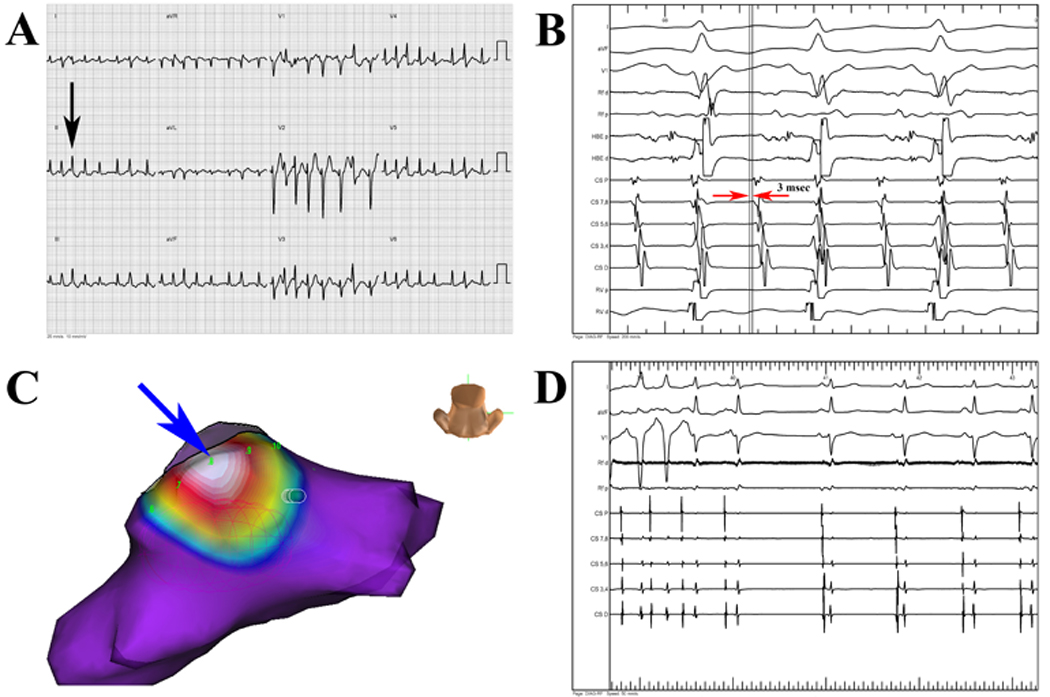
Surface ECG (A) of a patient diagnosed with atrial fibrillation (AF) by all clinicians due to rapid, irregular ventricular activation and lack of regular F-waves (black arrow). Patient was found to have focal atrial tachycardia (AT) at EPS due to regular CS activation (B) with CS 7–8 earlier than CS 9–10 by 3 msec (red arrows). Rapid, focal activation (C) from inferior posterior left atrium (blue arrow) by using noncontact activation mapping (EnSite Array). Ablation at focal source terminated tachycardia (D), which was subsequently non-inducible. Independent quantitative analysis correctly diagnosed arrhythmia as focal AT, potentially helping plan use of noncontact mapping.
The digital 12-lead surface ECG (without intracardiac data) of the index tachyarrhythmia was recorded at the time of diagnosis during EPS (e.g., immediately following entrainment from the CTI [figure 3A and 3B]). Digital data were then downloaded from the electrophysiologic recorder (Bard at UCSD and VASDHS; Prucka Cardiolab at WU) and de-identified for blinded analysis (by DEK, MP, HN, and GH). A panel of electrophysiologists (DEK, MCH, and SMN) then categorized cases of typical AFl by RR regularity and the presence or absence of sawtooth flutter waves on surface ECG.
Blinded Quantitative Analysis
Each de-identified digital surface ECG was imported into computer software we have developed and validated which differentiates atrial tachyarrhythmias using quantitative metrics.19–23 As previously described, the algorithm separates typical AFl, atypical AFl and AF using differences in spatial and temporal organization,24 and identifies focal AT via the duration of atrial activation relative to tachycardia cycle length.22
Briefly, the computer program uses a three step process to assign a diagnosis. First, ECGs were subjected to sliding Pearson correlation and Fourier analysis of the correlation waveform. If the dominant peak in the 3–12 Hz bandwidth is broad (peak area ratio (PAR) ≤ 0.44 of a 1 Hz envelope at the peak relative to a surrounding 2.5 Hz envelope), the ECG was classified as AF (figure 4C).20 Next, if the magnitude of the spatial phase plots in three orthogonal planes (plots of the ECG correlation series in XY, YZ, and XZ planes) is < 0.75 then a diagnosis of atypical AFl is assigned.19, 23 Finally, if P (or F) wave duration was < 45% of ECG-derived cycle length, the arrhythmia is classified as focal AT.21 Remaining cases are diagnosed as typical AFl (figure 3D).
Diagnosis by Clinician Panels
A total of 9 physicians (3 electrophysiologists, 3 cardiologists, and 3 internists) blindly interpreted each ECG. Supplemental clinical information was provided with each ECG, including the patient’s age, gender, past medical history, and symptoms. Panelists were asked to assign one of the four atrial tachyarrhythmia diagnoses (typical AFl, atypical AFl, focal AT, or AF) for each ECG using standard visual criteria. Each physician provided individual diagnoses, and diagnoses were grouped for Markov analysis, described below.
Economic Analysis
We then prospectively developed a discrete-time Markov model to evaluate the economic impact of the application of temporospatial ECG analysis in a typical medical practice versus standard ECG interpretation (figure 2). A hypothetical cohort of 1,000 65-year old patients with atrial tachyarrhythmias was followed for 5 years after initial presentation to an internist or a cardiologist. Management (for instance, referral for ablation, initiation of medical management) was performed according to current treatment guidelines.1, 25 The true diagnosis was revealed if the patient underwent an EPS, and this modified subsequent management.
Accuracy was calculated from the study results, while probability, unit cost and utility data were obtained from peer-reviewed literature26, Medicare tariffs1, 25, and published guidelines.27 Age-adjusted mortality was calculated from the 2003 U.S. Life Tables.28 Direct medical costs were adjusted to 2006 U.S. dollars using the U.S. Consumer Price Index for medical care.29 The effect was measured in terms of quality-adjusted life years (QALYs), calculated by multiplying the life years lived by a preference-weight for the quality of life experienced (with 0 representing death and 1 full health). Future costs and benefits were discounted at 3%.7, 12, 13, 30 The model was created using TreeAge Pro software (Suite 2007, TreeAge Software, Inc., Williamstown, MA). Details of the assumptions and input parameters are provided in the appendix.
Statistical Analysis
Continuous data are represented as mean ± standard deviation (SD). McNemar’s test was used to compare diagnostic accuracy of the algorithm versus the individual and group consensus results. Analysis of variance (ANOVA) and t-test were used to compare continuous variables with normal distributions. Mann-Whitney was used for data with non-normally distributed data. Chi-squared and Fisher’s Exact Test were used to compare nominal data. Statistics were performed using NCSS 2007 (Kaysville, UT, USA). All analyses were two-sided; p values < 5% were considered statistically significant.
RESULTS
Invasive electrophysiology study assigned 45 cases as typical AFl, 10 atypical AFl, 5 focal AT, and 31 as AF. Demographics are summarized in Table 1. As expected, groups showed significant differences in gender, use of sotalol, aspirin and statins, CABG, history of AF, prior ablation, and symptoms of dizziness.
Improved Typical AFl Diagnosis
Of the ECGs diagnosed as typical AFl during EPS, 10 of 45 (22%) did not have sawtooth flutter waves on independent, blinded, consensus assessment by 3 electrophysiologists (MCH, DEK, and SMN). Of the 10 without sawtooth flutter waves, 6 (13% of typical AFl) also had an irregularly irregular (n=5) or paced (n=1) ventricular response. Each ECG within this subgroup of “difficult” typical AFl cases was misdiagnosed by the majority of clinicians (0 of 6 ECGs correctly diagnosed by consensus visual interpretation) but was correctly diagnosed by quantitative analysis in 83% (5 of 6 ECGs, p=0.03).
Overall, the algorithm had a diagnostic accuracy of 89% for typical AFl. This compares favorably against the consensus diagnoses of internists (65%, p=0.001) and electrophysiologists (72%, p=0.03), and is similar to the cardiology panel (73%, p=NS). An example ECG misclassified by visual interpretation but correctly by the algorithm is shown in figure 3.
The Markov model revealed that the observed improvement in typical AFl diagnostic accuracy resulted in significant cost savings and QALY gains versus visual interpretation, resulting from 2 sources: (1) expedited referral for ablation versus trial of medical management and follow-up, and (2) proper procedure planning and resource utilization for patients referred to ablation.
Improved AF Diagnosis
Overall, AF was correctly diagnosed using visual ECG criteria in 82% of cases. The algorithm correctly identified AF in 92% of cases, improving upon the AF accuracy of general internists (73%, p<0.01), and is similar to general cardiologists (86%, p=NS) and electrophysiologists (86%, p=NS). An example of AF incorrectly diagnosed by visual criteria but correctly by the algorithm is shown in figure 4. Markov modeling revealed significant cost savings from the observed improvement in AF diagnosis, due to (1) identification of patients with AF in the primary care clinic who could be managed with rate control and anticoagulation instead of referral to electrophysiology, and (2) proper procedure planning for patient referred to ablation.
Clinical Characteristics of Patients Most Likely to Benefit from Computer Diagnosis
Patients were significantly more likely to benefit from quantitative analysis in the presence of other cardiovascular disease, including hypertension (p=0.05), hyperlipidemia (p=0.03), or coronary artery disease (p=0.02).
Healthcare System Modeling
In a population of 1000 patients with atrial tachyarrhythmias, improved atrial tachyarrhythmia diagnosis using quantitative analysis resulted in a cost savings of $1,302,510, and produced 7 additional QALYs. Sensitivity analyses showed a consistent benefit using the algorithm (figure 6).
Figure 6.
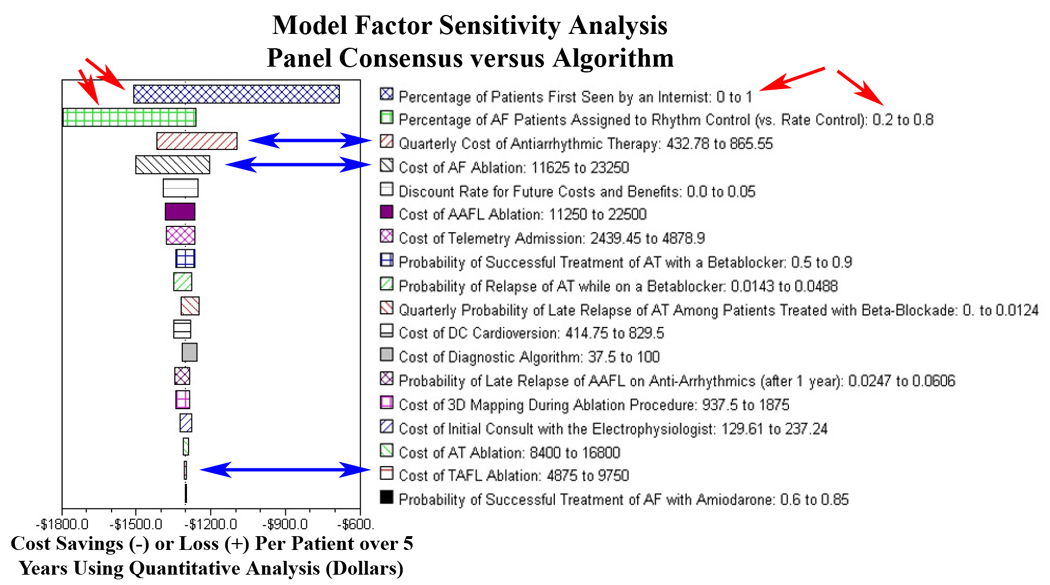
Factor sensitivity analysis for the Markov model reflecting impact of input parameter variation on cost-of-care. Parameter uncertainty in the model was evaluated using a large number of one- and two-way sensitivity analyses, and the results were noted to be robust across the entire range of all tested parameters. This was done separately for cost and QALYs, and for the incremental cost-effectiveness ratio. Important parameters are displayed in descending order of impact on the model for the variations shown. Relatively greater cost differential between primary care diagnosis and algorithm reflected in top 2 parameters (red arrows), highlighting greater diagnostic accuracy using quantitative analysis for all arrhythmias. Relatively lower cost differential in use of algorithm versus without in electrophysiology setting (blue arrows) reflects significant performance improvement in diagnosis of typical atrial flutter only. Note using the algorithm costs less than the not using the algorithm for all variations in input parameters, reflected by negative values for all sensitivity analyses. Abbreviations: QALYs = Quality-Adjusted Life Years, TAFL = typical atrial flutter, AAFL = atypical atrial flutter, AT = focal atrial tachycardia, and AF = atrial fibrillation.
DISCUSSION
There were 3 major findings from this study. First, typical AFl commonly presents without classic ECG findings. Second, quantitative ECG analysis improves upon visual criteria for atrial tachyarrhythmia diagnosis. Third, even modest improvements in diagnostic accuracy of typical AFl and AF result in significant improvements in quality-of-life measures and healthcare cost.
Use of Electrophysiology Study as Gold Standard
The present study is unique in that diagnoses were determined by invasive electrophysiologic study in a broad group referred for management of atrial tachyarrhythmias. Prior studies reporting the accuracy of ECG interpretation have relied upon visual inspection,31 yet expert interpreters often disagree.31, 32 Although the gold standard for atrial tachyarrhythmia diagnosis is invasive electrophysiology study (EPS),7, 30 the diagnostic accuracy of physicians or computerized ECG analysis compared to EPS had previously rarely been evaluated. Recently, interesting work by Weinberg et al.33 used intracardiac diagnosis as the gold standard to develop diagnostic surface ECG criteria for typical AFl using typical flutter waves and RR interval regularity. Of note, however, atypical AFl and focal AT were not included in this study.
Quantitative Analysis Improves Upon Visual Criteria
The difficulty of assigning tachycardia mechanism using visual criteria in this study is in agreement with prior rersearch.12, 34 These findings emphasize that visual analysis alone may be suboptimal for guiding care. Indeed, our results likely overestimate the accuracy of visual analysis, since panelists’ responses were limited to the four diagnoses considered in this study, pertinent clinical information was provided with each ECG, no time limit was placed on ECG interpretation, and only technically adequate, sustained atrial tachyarrhythmia tracings were included.
Current computerized ECG interpretation is valuable,35, 36 but largely applies the same criteria as visual interpretation.10 However, “typical” F-waves may be obscured in patients with left atrial enlargement,5 age-related fibrosis, or pulmonary disease,9 and may appear to be present in atypical AFl37 and AF.38, 39 The visual ECG diagnosis of AF relies heavily upon RR interval irregularity, yet this is nonspecific and also present in other atrial tachyarrhythmias.5, 19 Quantifying subtle variations in F-wave shape and timing among different axes, and detailed measurement of atrial activation time more accurately differentiates AF, typical and atypical AFL, and focal AT.
Improving ECG Diagnostic Accuracy Significantly Impacts Care
In this study, we endeavored to determine if improving diagnostic accuracy results in better care. Anecdotally, we had observed situations in which inaccurate ECG diagnosis lead to suboptimal resource utilization. For instance, misdiagnosis of typical AFl as AF in the primary care clinic can delay ablation and facilitate progression of typical AFl to AF.40 Additionally, misdiagnosis of typical AFl as atypical AFl or AF may waste both expensive medical supplies (electroanatomical mapping equipment) and valuable laboratory time (figure 3). Conversely, misdiagnosis of AF as atrial flutter may cause ablation to be attempted without proper planning for a more complex procedure (figure 4). Finally, failure to identify atrial tachycardia may delay optimal use of activation mapping tools in the EP lab (figure 5).
Our Markov model found that improved diagnostic accuracy, whether from quantitative ECG analysis or any other method, translates into improved healthcare delivery. Our projected cost savings is likely conservative, given inflation, common use of imaging with EP procedures (CT), and availability of newer, more expensive antiarrhythmic medications. This has important implications in physician training and the need to maintain ECG interpretation skills.31
Prior Computerized ECG Analysis Work
Previous studies have shown that computerized ECG analysis can improve the accuracy of family medicine physicians41 and pediatric emergency medicine physicians,42 although visual interpretation by a cardiologist was used as the gold standard. In the study by Woolley et al,43 there was a 67% agreement between family physicians and the electrocardiographer versus an 88% agreement between the computer interpretation and the electrocardiographer in an unselected population of ECGs from family medicine clinics. The study concluded that computer assistance improved family practitioner ECG interpretation, but atrial tachyarrhythmias comprised a small fraction of diagnoses.
In a study by Snyder et al,41 the computer algorithm correctly interpreted 75% of ECGs of moderate clinical importance versus 36% accuracy by pediatric emergency medicine physicians. Importantly, both emergency medicine physician and computer interpretation were inaccurate for ECGs of diagnoses rated as critically important (14% computer accuracy, 28% physician accuracy). The study concluded that computer interpretation, when available to physicians, increased diagnostic accuracy. However, it also concluded that expert interpretation would still be required, particularly for high priority ECG diagnoses such as arrhythmias, due to the low accuracy of both for clinically important diagnoses.
In this study, we looked exclusively at atrial tachyarrhythmias, traditionally an area of difficulty for computer interpretation.1 We showed for the first time a technique that was overall equivalent to expert interpreters (cardiologists and electrophysiologists), and superior to EPs at identifying patients more likely to benefit from a less expensive typical AFl ablation. Supplemental use of the computer algorithm in the primary care clinic is very likely to improve patient management and control costs. As such, future work should confirm these projected benefits during real-time use of these methods at the bedside.
Limitations
First, this study was performed in patients referred for EPS, potentially biasing our study towards patients with more symptomatic arrhythmias. However, the demographics of our patients are similar to those of a general primary care population with atrial tachyarrhythmias.21, 44 Second, male patients were overrepresented, and although gender differences in ECG manifestations of atrial arrhythmias are of unclear significance, additional studies in female patients are required. Third, although the algorithm is relatively rapid (2–3 minutes) to use in current form, integration in current ECG systems is required for the technique to be widely adopted. Fourth, computer analysis of atrial arrhythmias was performed as an independent arm of the current study, and it is unclear how clinicians will incorporate quantitative ECG results into practice. Future work should evaluate the real-world use of quantitative analysis in a large health system to confirm clinical and cost benefits. Fifth, modern healthcare practice is difficult to model. Nevertheless, extensive sensitivity analyses demonstrated that our results were robust to large variations in the input parameters, lending support to the validity of our findings.
CONCLUSION
Quantitative ECG analysis provides significantly increased diagnostic accuracy in both the primary care and subspecialty clinics, resulting in more cost-effective and higher quality medical care. Future work should focus on improving the availability of quantitative bedside ECG tools in the clinical settings, and studying the impact on long-term outcomes.
Supplementary Material
Acknowledgements
We thank Kathleen Mills, BA (University of California and VA San Diego) for coordinating this study.
Supported by an ACC-Merck Fellowship to D. Krummen and by grants from the National Institutes of Health (HL 70529) and the Doris Duke Charitable Foundation to S. Narayan.
S. Narayan reports participation on a research grant sponsored by Biosense-Webster and interest in a patent, relevant to this study, owned by the University of California.
Abbreviations
- AFl
Atrial Flutter
- AF
Atrial Fibrillation
- AT
Atrial Tachycardia
- CL
Cycle Length
- EPS
Electrophysiology Study
Footnotes
Other authors: No disclosures
Supporting Information: Online-only appendix provided in the manuscript zip file.
References
- 1.Blomstrom-Lundqvist C, Scheinman MM, Aliot EM, Alpert JS, Calkins H, Camm AJ, Campbell WB, Haines DE, Kuck KH, Lerman BB, Miller DD, Shaeffer CW, Stevenson WG, Tomaselli GF, Antman EM, Smith SC, Jr, Alpert JS, Faxon DP, Fuster V, Gibbons RJ, Gregoratos G, Hiratzka LF, Hunt SA, Jacobs AK, Russell RO, Jr, Priori SG, Blanc JJ, Budaj A, Burgos EF, Cowie M, Deckers JW, Garcia MA, Klein WW, Lekakis J, Lindahl B, Mazzotta G, Morais JC, Oto A, Smiseth O, Trappe HJ. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias--executive summary. a report of the American college of cardiology/American heart association task force on practice guidelines and the European society of cardiology committee for practice guidelines (writing committee to develop guidelines for the management of patients with supraventricular arrhythmias) developed in collaboration with NASPE-Heart Rhythm Society. J Am Coll Cardiol. 2003;42:1493–1531. doi: 10.1016/j.jacc.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 2.Chugh SS, Blackshear JL, Shen WK, Hammill SC, Gersh BJ. Epidemiology and natural history of atrial fibrillation: clinical implications. J Am Coll Cardiol. 2001;37:371–378. doi: 10.1016/s0735-1097(00)01107-4. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds MR, Essebag V, Zimetbaum P, Cohen DJ. Healthcare resource utilization and costs associated with recurrent episodes of atrial fibrillation: the FRACTAL registry. J Cardiovasc Electrophysiol. 2007;18:628–633. doi: 10.1111/j.1540-8167.2007.00819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bochoeyer A, Yang Y, Cheng J, Lee RJ, Keung EC, Marrouche NF, Natale A, Scheinman MM. Surface electrocardiographic characteristics of right and left atrial flutter. Circulation. 2003;108:60–66. doi: 10.1161/01.CIR.0000079140.35025.1E. [DOI] [PubMed] [Google Scholar]
- 5.Krummen DE, Feld GK, Narayan SM. Diagnostic Accuracy of Irregularly Irregular RR Intervals in Separating Atrial Fibrillation from Atrial Flutter. Am J Cardiol. 2006;98:209–214. doi: 10.1016/j.amjcard.2006.01.088. [DOI] [PubMed] [Google Scholar]
- 6.Yilmaz MB, Guray Y, Guray U, Cay S, Caldir V, Biyikoglu SF, Sasmaz H, Korkmaz S. Fine vs. coarse atrial fibrillation: which one is more risky? Cardiology. 2007;107:193–196. doi: 10.1159/000095416. [DOI] [PubMed] [Google Scholar]
- 7.Knight BP, Michaud GF, Strickberger SA, Morady F. Electrocardiographic differentiation of atrial flutter from atrial fibrillation by physicians. J Electrocardiol. 1999;32:315–319. doi: 10.1016/s0022-0736(99)90002-x. [DOI] [PubMed] [Google Scholar]
- 8.Xi Q, Sahakian AV, Frohlich TG, Ng J, Swiryn S. Relationship between pattern of occurrence of atrial fibrillation and surface electrocardiographic fibrillatory wave characteristics. Heart Rhythm. 2004;1:656–663. doi: 10.1016/j.hrthm.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Rodman DM, Lowenstein SR, Rodman T. The electrocardiogram in chronic obstructive pulmonary disease. J Emerg Med. 1990;8:607–615. doi: 10.1016/0736-4679(90)90458-8. [DOI] [PubMed] [Google Scholar]
- 10.Akar JG, Kok LC, Haines DE, DiMarco JP, Mounsey JP. Coexistence of type I atrial flutter and intra-atrial re-entrant tachycardia in patients with surgically corrected congenital heart disease. J Am Coll Cardiol. 2001;38:377–384. doi: 10.1016/s0735-1097(01)01392-4. [DOI] [PubMed] [Google Scholar]
- 11.Saoudi N, Cosio F, Waldo A, Chen SA, Iesaka Y, Lesh M, Saksena S, Salerno J, Schoels W. Classification of atrial flutter and regular atrial tachycardia according to electrophysiologic mechanism and anatomic bases: a statement from a joint expert group from the Working Group of Arrhythmias of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. J Cardiovasc Electrophysiol. 2001;12:852–866. doi: 10.1046/j.1540-8167.2001.00852.x. [DOI] [PubMed] [Google Scholar]
- 12.Poon K, Okin PM, Kligfield P. Diagnostic performance of a computer-based ECG rhythm algorithm. J Electrocardiol. 2005;38:235–238. doi: 10.1016/j.jelectrocard.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Shah AP, Rubin SA. Errors in the computerized electrocardiogram interpretation of cardiac rhythm. J Electrocardiol. 2007 doi: 10.1016/j.jelectrocard.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Kalman JM, Vohra JK, Jayaprakash S, Sparks PB. Radiofrequency ablation for cure of atrial flutter. Aust N Z J Med. 1997;27:653–657. doi: 10.1111/j.1445-5994.1997.tb00993.x. [DOI] [PubMed] [Google Scholar]
- 15.Singer DE, Albers GW, Dalen JE, Fang MC, Go AS, Halperin JL, Lip GY, Manning WJ. Antithrombotic therapy in atrial fibrillation: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:546S–592S. doi: 10.1378/chest.08-0678. [DOI] [PubMed] [Google Scholar]
- 16.Tada H, Oral H, Sticherling C, Chough SP, Baker RL, Wasmer K, Pelosi F, Jr, Knight BP, Strickberger SA, Morady F. Double potentials along the ablation line as a guide to radiofrequency ablation of typical atrial flutter. J Am Coll Cardiol. 2001;38:750–755. doi: 10.1016/s0735-1097(01)01425-5. [DOI] [PubMed] [Google Scholar]
- 17.Hobbs FD, Fitzmaurice DA, Mant J, Murray E, Jowett S, Bryan S, Raftery J, Davies M, Lip G. A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study. Health Technol Assess. 2005;9(iii–iv, ix–x):1–74. doi: 10.3310/hta9400. [DOI] [PubMed] [Google Scholar]
- 18.Lee BK, Reyes CM, Heranadez J, Pelletier E, Lu Y, Buell H, Feld GK. Catheter ablation is more cost-effective than cardioversion and drug therapy for first-line treatment of atrial flutter. Heart Rhythm. 2005;2:S1. [Google Scholar]
- 19.Narayan SM, Hassankhani A, Feld GK, Bhargava V. Separating non-isthmus- from isthmus-dependent atrial flutter using wavefront variability. J Am Coll Cardiol. 2005;45:1269–1279. doi: 10.1016/j.jacc.2004.12.070. [DOI] [PubMed] [Google Scholar]
- 20.Hoppe BL, Kahn AM, Feld GK, Hassankhani A, Narayan SM. Separating atrial flutter from atrial fibrillation with apparent electrocardiographic organization using dominant and narrow F-wave spectra. J Am Coll Cardiol. 2005;46:2079–2087. doi: 10.1016/j.jacc.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 21.Brown JP, Krummen DE, Feld GK, Narayan SM. Using electrocardiographic activation time and diastolic intervals to separate focal from macro-re-entrant atrial tachycardias. J Am Coll Cardiol. 2007;49:1965–1973. doi: 10.1016/j.jacc.2006.10.080. [DOI] [PubMed] [Google Scholar]
- 22.Narayan SM, Feld GK, Hassankhani A, Bhargava V. Quantifying intracardiac organization of atrial arrhythmias using temporospatial phase of the electrocardiogram. J Cardiovasc Electrophysiol. 2003;14:971–981. doi: 10.1046/j.1540-8167.2003.03213.x. [DOI] [PubMed] [Google Scholar]
- 23.Kahn AM, Krummen DE, Feld GK, Narayan SM. Localizing circuits of atrial macroreentry using electrocardiographic planes of coherent atrial activation. Heart Rhythm. 2007;4:445–451. doi: 10.1016/j.hrthm.2006.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalman JM, Olgin JE, Saxon LA, Lee RJ, Scheinman MM, Lesh MD. Electrocardiographic and electrophysiologic characterization of atypical atrial flutter in man: use of activation and entrainment mapping and implications for catheter ablation. J Cardiovasc Electrophysiol. 1997;8:121–144. doi: 10.1111/j.1540-8167.1997.tb00775.x. [DOI] [PubMed] [Google Scholar]
- 25.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Zamorano JL. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation) J Am Coll Cardiol. 2006;48:854–906. doi: 10.1016/j.jacc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Medicare Fee-for-Service Payment. Center for Medicare and Medicaid Services. pp. http://www.cms.hhs.gov/home/medicare.asp.
- 27.U.S. Life Tables. National Center for Health Statistics. pp. http://www.cdc.gov.mill1.sjlibrary.org/nchs/products/pubs/pubd/lftbls/lftbls.htm.
- 28.U.S. Department of Labor. Bureau of Labor Statistics. Consumer Price Index. pp. http://data.bls.gov/PDQ/servlet/SurveyOutputServlet.
- 29.Lipscomb J, Torrance GW. Time Preference. In: Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996. pp. 214–246. [Google Scholar]
- 30.Bogun F, Anh D, Kalahasty G, Wissner E, Bou Serhal C, Bazzi R, Weaver WD, Schuger C. Misdiagnosis of atrial fibrillation and its clinical consequences. Am J Med. 2004;117:636–642. doi: 10.1016/j.amjmed.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 31.Salerno SM, Alguire PC, Waxman HS. Competency in interpretation of 12-lead electrocardiograms: a summary and appraisal of published evidence. Ann Intern Med. 2003;138:751–760. doi: 10.7326/0003-4819-138-9-200305060-00013. [DOI] [PubMed] [Google Scholar]
- 32.Anh D, Krishnan S, Bogun F. Accuracy of electrocardiogram interpretation by cardiologists in the setting of incorrect computer analysis. J Electrocardiol. 2006;39:343–345. doi: 10.1016/j.jelectrocard.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Weinberg KM, Denes P, Kadish AH, Goldberger JJ. Development and validation of diagnostic criteria for atrial flutter on the surface electrocardiogram. Ann Noninvasive Electrocardiol. 2008;13:145–154. doi: 10.1111/j.1542-474X.2008.00214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinberg KM, Denes P, Kadish AH, Goldberger JJ. Criteria for the electrocardiographic diagnosis of atrial flutter improve diagnostic accuracy. Am J Med. 2007;120:814–818. doi: 10.1016/j.amjmed.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 35.Davidenko JM, Snyder LS. Causes of errors in the electrocardiographic diagnosis of atrial fibrillation by physicians. J Electrocardiol. 2007 doi: 10.1016/j.jelectrocard.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Varriale P, David W, Chryssos BE. Multifocal atrial arrhythmia--a frequent misdiagnosis? A correlative study using the computerized ECG. Clin Cardiol. 1992;15:343–346. doi: 10.1002/clc.4960150507. [DOI] [PubMed] [Google Scholar]
- 37.Horvath G, Goldberger JJ, Kadish AH. Simultaneous occurrence of atrial fibrillation and atrial flutter. J Cardiovasc Electrophysiol. 2000;11:849–858. doi: 10.1111/j.1540-8167.2000.tb00063.x. [DOI] [PubMed] [Google Scholar]
- 38.Marine JE, Korley VJ, Obioha-Ngwu O, Chen J, Zimetbaum P, Papageorgiou P, Milliez P, Josephson ME. Different patterns of interatrial conduction in clockwise and counterclockwise atrial flutter. Circulation. 2001;104:1153–1157. doi: 10.1161/hc3501.095478. [DOI] [PubMed] [Google Scholar]
- 39.Milliez P, Richardson AW, Obioha-Ngwu O, Zimetbaum PJ, Papageorgiou P, Josephson ME. Variable electrocardiographic characteristics of isthmus-dependent atrial flutter. J Am Coll Cardiol. 2002;40:1125–1132. doi: 10.1016/s0735-1097(02)02070-3. [DOI] [PubMed] [Google Scholar]
- 40.Halligan SC, Gersh BJ, Brown RD, Jr, Rosales AG, Munger TM, Shen WK, Hammill SC, Friedman PA. The natural history of lone atrial flutter. Ann Intern Med. 2004;140:265–268. doi: 10.7326/0003-4819-140-4-200402170-00008. [DOI] [PubMed] [Google Scholar]
- 41.Snyder CS, Fenrich AL, Friedman RA, Macias C, O'Reilly K, Kertesz NJ. The emergency department versus the computer: which is the better electrocardiographer? Pediatr Cardiol. 2003;24:364–368. doi: 10.1007/s00246-002-0332-z. [DOI] [PubMed] [Google Scholar]
- 42.Hongo RH, Goldschlager N. Overreliance on computerized algorithms to interpret electrocardiograms. Am J Med. 2004;117:706–708. doi: 10.1016/j.amjmed.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 43.Woolley D, Henck M, Luck J. Comparison of electrocardiogram interpretations by family physicians, a computer, and a cardiology service. J Fam Pract. 1992;34:428–432. [PubMed] [Google Scholar]
- 44.Narayan SM, Bhargava V. Temporal and spatial phase analyses of the electrocardiogram stratify intra-atrial and intra-ventricular organization. IEEE Trans Biomed Eng. 2004;51:1749–1764. doi: 10.1109/TBME.2004.827536. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.