Abstract
Objective
The concept that intraarticular crystals of uric acid of themselves trigger episodes of painful gouty arthritis is inconsistent with the clinical reality. Patients with large deposits of MSU crystals (tophi) do not necessarily suffer from gouty attacks. In fact, it is the excessive consumption of food or alcohol that elicits the inflammation of the acute gout attack. The precise mechanism that initiates flares of gouty arthritis remains unidentified.
Methods
Human PBMC and murine macrophages were stimulated in-vitro with MSU, free fatty acids or the combination. Thereafter, IL-1ß production and caspase-1 activation was determined. Gouty arthritis was induced in caspase-1, ASC, NALP3 and IL-1ß deficient mice and the lack of inflammasome activity on joint swelling or joint pathology was investigated.
Results
In the present study we demonstrate that MSU crystals are without biological effects on PBMC from healthy subjects; rather, in the presence of MSU crystals, FFA (C18.0) induce release of large amounts of IL 1ß following engagement of TLR2. We demonstrated that interaction of FFA, but not alcohol with TLR2 synergizes with MSU crystals to induce a inflammatory reaction. A important event of MSU/FFA-induced acute joint inflammation is the activation of the inflammasome. MSU/fatty acid-induced release of IL 1ß was dependent on caspase-1 and ASC, but surprisingly not on NALP3.
Conclusions
The synergistic effect between free fatty acids and MSU crystals leads to ASC-caspase-1 driven IL-1ß release. This mechanism now explains how constitutionally-derived metabolic events initiates attacks of gout via the induction of IL-1ß mediated joint inflammation.
Gout is a chronic inflammatory disease characterized by recurrent attacks of acute joint inflammation and is regarded as the prototypical crystal-induced arthropathy (1). It is unknown why only a small number of individuals with hyperuricemia develop gout due to the deposition of monosodium urate crystals in joints, and why the attacks of inflammation in gout patients are sporadic, despite continuous deposition of uric acid crystals in the joints. In addition, it is well known that attacks of gout are often related to a copious meal, consumption of alcohol or an infectious process. How food components or their metabolites after an abundant meal precipitate acute joint inflammation in the presence of MSU crystals remains to be elucidated.
Several mechanisms have been proposed for the induction of joint inflammation by the MSU crystals. In the older literature disruption of the phagolysomes and killing of neutrophils, complement activation, production of oxygen radicals, and eicosanoid release have all been implicated (2–4). Induction of pro-inflammatory cytokines and chemokines release by MSU crystals has also been suggested to play an important role (5,6). Among these mediators, interleukin (IL)-1β is a pivotal proinflammatory cytokine strongly associated with the inflammation in gout (7–9). Consequently, treatments that block IL-1 activity, either using recombinant IL-1Ra (anakinra) or anti-IL-1β antibodies, are beneficial in several inflammatory disorders (10). Recently, treatment with IL-1Ra or an IL-1 trap has also been shown to be effective in gout (11,12), which prompted the hypothesis that IL-1β release is a central event in the inflammatory reaction of a gout attack.
IL-1β is one of the very few cytokines that lacks a signal peptide, and its processing and secretion depends on cleavage by proteolytic cysteine proteases such as caspase-1 (13). Caspase-1 in turn is activated within protein platforms called inflammasomes (9). Several inflammasomes are able to activate caspase-1, all comprising proteins of the NOD-like receptor (NLR) family (14). MSU crystals have been reported to activate caspase-1 and to induce IL-1β production via NALP3 inflammasome (8,9,15). Interestingly however, all the in-vitro studies identifying MSU crystals as an inflammasome activator had to use either LPS or PMA to prime cells (7,8,15). Recently, we have shown that purified MSU crystals cannot induce IL-1β by themselves, and a second stimulus is needed (e.g. a bacterial component such as LPS) (16). While this could indeed explain the triggering role of infections on a gout attack (microbial components released during infection synergize with MSU crystals already present in the joint), this cannot explain the induction of an attack by food intake or alcohol consumption.
In the present study we demonstrate that inflammasome activation and IL-1β release takes place after exposure to MSU crystals and free fatty acids (FFA) and this explains the triggering effects of copious meals or alcohol consumption on gout attacks in patients. We demonstrate that C18.0 acting on TLR2 strongly synergize with MSU crystals for the release of IL-1β and induction of inflammation, and this effect can be reversed in mice defective in components of the inflammasome. This demonstrates that the release of FFA after food ingestion or alcohol consumption represents the “missing link” between metabolic changes, inflammasome activation and gout attacks.
Materials and Methods
Animals
Male C57Bl/6 mice were obtained from Charles River Wiga (Sulzfeld, Germany). IL-1β gene deficient mice were kindly provided by J. Mudgett, Merck, NJ, USA. TLR2−/− and TLR4−/− mice were provided by S. Akira, Osaka University, Japan. ASC−/−, NALP3−/−, and caspase-1−/− mice were kindly provided by A. Coyle, J. Bertin, E. Grant (Millennium Pharmaceuticals), G. Nunez (University of Michigan) and R. Flavell (Yale University). Mice were bred at the Central Animal Laboratory, RUNMC, Nijmegen, The Netherlands or St-Jude Children's Research Hospital, Memphis, TN, USA. All mice were housed in filter top cages, water and food were supplied ad libitum and used at 10–12 weeks. All animal experiments were approved by the animal ethic committee of the RUNMC or St-Jude Children's Research Hospital Committee on Use and Care of Animals.
Preparation of MSU crystals
MSU crystals were prepared according to Seegmiller et al. (17). Briefly, a 0.03M solution of MSU at a volume of 200ml was prepared after diluting 1.0g of uric acid (Sigma, St Louis, Missouri, USA) in 200ml of sterile water containing 24g of NaOH. The pH was adjusted to 7.2 after addition of HCl and the solution became pyrogen free after heating for 6h at 120°C. The solution was left to cool at room temperature and stored at 4°C. Crystals produced were 5–25μm long. On each day of experiment, a small amount of the crystals were weighed under sterile conditions for application. LPS contamination was controlled by LAL-assay.
Isolation of murine peritoneal macrophages and stimulation of cytokine production
Isolation of mouse peritoneal macrophages and spleen cells. Groups of five TLR2−/− and TLR4−/− mice and their control littermates were sacrificed, and resident peritoneal macrophages were harvested by injecting 4ml of sterile PBS containing 0.38% sodium citrate. After washing, the cells were resuspended in RPMI 1640 containing 1mM pyruvate, 2mM l-glutamine, 100μg/ml gentamicin and 2% fresh mouse plasma. Cells were cultured in 96-well microtiter plates (Greiner, Alphen, The Netherlands) at 105 cells per well, in a final volume of 200μl. The cells were stimulated with either control medium, Ethanol, MSU, C18.0 (Sigma), MSU/C18.0 or highly purified E. coli LPS (10ng/ml). After 24h of incubation at 37°C, the plates were centrifuged (500g, 10min), and the supernatant were collected and stored at −80 °C until cytokine assays were performed.
Generation of BMDM or BMDC and caspase-1 activation
Bone marrow from mice (8–20 weeks) was flushed out after dissecting mouse legs. Differentiation into macrophages occurred in 5 days at 37°C (5% CO2) in the presence of IMDM medium supplemented with 30% of L929 supernatant containing 10% FCS (Invitrogen), 100U/ml penicillin and 100mg/ml streptomycin. For BMDC, bone marrow cells were cultured in RPMI-1640 medium supplemented with 10% FCS, 100U/ml penicillin, 100μg/ml streptomycin, 50μM 2-mercaptoethanol, 2mM Sodium pyruvate, and 20ng/ml GM-CSF (PeproTech). Fresh medium was given on Day 3. On days 6, 8 and 10 non-adherent cells were harvested, washed, and re-plated in fresh medium. Ultrapure LPS was purchased from Invitrogen and used in a concentration of 10 μg/ml. ATP was from Sigma and used in a final concentration of 3mM. For immunoblotting, cells were washed twice with PBS and lysed in buffer (150mM NaCl, 10mM Tris, pH 7.4, 5mM EDTA, 1mM EGTA, 0.1% Nonidet P-40) which was supplemented with a protease inhibitor cocktail (Roche). After clarification and denaturation with SDS buffer, samples were boiled for 5 minutes. Separation of the proteins was done by using SDS-PAGE and thereafter transferred to a nitrocellulose membrane. These membranes were coated with primary antibodies and active caspase-1 was detected using secondary anti-rabbit antibody conjugated to horseradish peroxidase followed by enhanced chemiluminiscence (18).
Isolation of human peripheral blood mononuclear cells and stimulation of cytokine production
Isolation of peripheral blood mononuclear cells (PBMC) was performed as described elsewhere (19), with minor modifications. 5×105 PBMC in a 100μl volume were added to round-bottom 96-wells plates (Greiner) and incubated with either 100 μl of culture medium, or the various stimuli: highly purified MSU crystals (30–300μg/ml), ultra pure fatty acid 200μM (C18.0), highly purified E. coli LPS (1–10ng/ml) and PAM3cys (10μg/ml). In additional experiments, several TLR or NOD ligands were used as stimulus. In some experimental set, cells were pre-treated with or without double-extracted B. quintana LPS (1μg/ml), a TLR4 antagonist (20), or anti-TLR2 antibody (10μg/ml) 30 minutes before treatment with MSU/C18.0 (300μg/ml-200μM). After 24h of culture, the supernatants were collected, centrifuged at 1200 rpm and stored at −80°C until assayed.
Induction of MSU-induced joint inflammation
Joint inflammation was induced by intraarticular injection (i.a.) of a dose-range highly pure MSU (30–300μg), 200μM C18.0, MSU/C18.0 (300μg/200μM) or 25μg SCW (rhamnose content) in 10 μl of PBS into the right knee joint of naïve mice. 4 hour after i.a. injection, joint swelling was determined, synovial tissue was isolated and knee joints were removed for histology.
Joint swelling measurement
Joint inflammation was measured by either macroscopic scoring or by the 99mTc uptake method. Macroscopic joint swelling was scored on a scale ranging from 0–3. After the skin was removed the knee joint was scored, 0 = no swelling and 3 = severe swelling. 99mTc-uptake method was performed as previously described (21,22). Joint swelling is expressed as the ratio of the 99mTc uptake in the inflamed over the control joint (left knee joint). All values exceeding 1.10 are assigned as joint swelling.
RNA isolation and PCR amplification
Immediately after cervical dislocation synovial tissue was isolated from the inflamed knee joints. The synovium samples were immediately stored in N2 until total RNA isolation. RNA was extracted as described previously (21). To obtain cDNA, standard RT-PCR was performed using oligo dT primers. Subsequently quantitative PCR was performed using ABI/PRISM 7000 Sequence Detection System (Applied Biosystems, Foster City, CA). PCR's of murine GAPDH, IL-1β, IL-6 and KC were performed with Sybr Green PCR Master Mix (Applied Biosystems). Quantification of the PCR signals of each sample was performed by comparing the cycle threshold values (Ct), in duplicate, of the gene of interest with the Ct values of the GAPDH housekeeping gene. We validated all primers according to the protocol and the standard curves were all within the tolerable range (21,22,23).
Cytokine determination
Protein levels of murine IL-1β or KC were measured in patellae washouts. 4h after injection of MSU/C18.0, patellae were isolated from inflamed knee joints and cultured 1 hour at RT in RPMI 1640 medium containing 0.1% bovine serum albumin (200μl/patella). For intracellular IL-1β levels, patellae were frozen directly after isolation. After repeated freeze-thawing IL-1β was determined. Human IL-1β and IL-8 were measured by either specific or commercial ELISA kits (R&D Systems, Abingdon, UK) and Pelikine Compact (Sanquin, Amsterdam, The Netherlands). Sensitivity of both ELISAs are below 5 pg/ml (22,23). Mouse cytokines were determined by Luminex technology, kits for IL-1β and KC were obtained from Bio-Rad (Hercules, CA, USA).
Histological analysis
Mice were sacrificed by cervical dislocation. Whole knee joints were removed and fixed in 4% formaldehyde for 7 days before decalcification in 5% formic acid and processing for paraffin embedding. Tissue sections (7μm) were stained with Haematoxylin/Eosin. Histopathological changes in the knee joints were scored in the patella/femur region on 5 semi-serial sections. Scoring was performed on decoded slides by two separate observers, using the following parameters: the amount of cells infiltrating the synovial lining and the joint cavity was scored from 0–3. (21,22,23).
Statistical analysis
Differences between experimental groups were tested using the Mann-Whitney U-test. Data are expressed as mean±SEM, unless stated elsewhere. *P<0.05, compared to wild control mice.
Results
Highly purified MSU crystals do not induce IL-1β production or joint inflammation
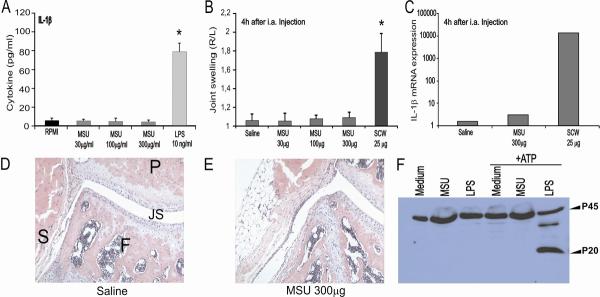
Although it has been reported that exposure of mononuclear cells to MSU crystals leads to the production of active IL-1β (16), many of these studies used LPS- or PHA-primed cells to observe these effects. In the present series of experiments, we confirm that highly pure MSU crystals cannot induce IL-1β production in human PBMCs, even at high concentrations (data not shown). Likewise, murine peritoneal macrophages do not respond to highly pure MSU crystals, but do respond to LPS (Figure 1A). Several reports have indicated that MSU crystals will induce acute inflammation when injected locally, e.g., into the peritoneal cavity of mice (8). Since gout is merely manifested as an acute joint inflammation, we injected highly pure MSU crystals directly into murine knee joints. Figure 1B demonstrates that even at high doses (up to 300μg MSU per joint), MSU did not induce joint swelling when measured with a highly sensitive method (99mTc-uptake). Histology confirmed the lack of joint inflammation after local injections of MSU crystals. Intra-articular injection of 300μg MSU crystals also did not lead to enhanced influx of granulocytes (Figure 1D/E). Even a single dose of 600μg MSU crystals injected intra-articularly did not give rise to signs of joint inflammation (data not shown). Furthermore, MSU crystals were not able to induce a substantial amount of mRNA for IL-1β in synovial tissue (Figure 1C). To explore the lack of effect of highly pure MSU crystals on the induction of joint inflammation further, we analyzed whether MSU could activate caspase-1. Figure 1F shows that this is not the case. Thus, these data convincingly demonstrate that MSU crystals alone do not induce inflammation, and additional stimuli are needed for the activation of the inflammasome, caspase-1 and IL-1β by MSU.
Figure 1. MSU crystals do not induce IL-1β production or joint inflammation.
A. Human PBMC's were exposed to a dose-range of MSU for 24h, and IL-1β was determined in the supernatants using ELISA. LPS (1ng/ml) was used as control. Data are expressed as mean±SEM of 8 healthy donors. B. MSU crystals fails to induce joint inflammation. Joint swelling at 4h after intraarticular injection of a dose-range MSU crystal or 25μg SCW fragments (positive control), measured by radioactive 99mTc-uptake method. The swelling is expressed as a right-left ratio and a ratio > 1.15 is indicated as inflammation. Data are expressed as mean±SEM of 7 mice per group. C. Synovial tissue was isolated 4h after MSU i.a. injection. Subsequently, RNA was isolated and used for determining IL-1β mRNA expression by q-PCR. Values are expressed as fold increase compared to saline injected group (synovial biopsies of 7 mice were pooled). E/F Histology confirmed no joint inflammation after injection of MSU. P= patella, F= femur, JS = joint space and S = synovium. 200× magnification. G. No caspase-1 activation by MSU. BMDM were exposed to medium, MSU or LPS for 24h. Thereafter cells were left untreated of treated with ATP for 30 minutes. Experiment was repeated twice with similar results. *P<0.01, Mann-Whitney U-test.
Synergy between MSU and saturated fatty acids
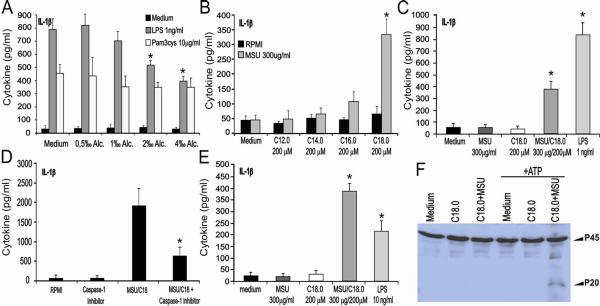
In gout patients, inflammatory attacks are often triggered by ingestion of a copious meal or alcohol consumption. We hypothesized that one or more substances or metabolites released through these processes a stimulatory effect could have on the inflammatory properties of MSU crystals. One obvious candidate is alcohol concentration, which may influence the induction of inflammation by MSU crystals. However, ethanol concentrations up to 4‰ was not able to enhance the effect of MSU crystals on the production of proinflammatory cytokines such as IL-1β (data not shown). Moreover, increasing concentrations of ethanol even inhibited LPS-induced IL-1β production, whereas no influence on TLR2 signaling was found (Figure 2A).
Figure 2. MSU synergizes with FFA for cytokine prodcution.
A. Human PBMC's were exposed to a dose-range of ethanol in combination with TLR2 or TLR4 agonists for 24h. Data are expressed as mean±SEM of 4 healthy donors. B. Several saturated FA (200μM) were tested in combination with MSU using human PBMCs. Data are expressed as mean±SEM of 4 healthy donors. C. Strong synergy between MSU and C18.0 for IL-1β production in human PBMCs. Data are expressed as mean±SEM of 4 healthy donors. D. MSU/C18.0 IL-1β production is reduced by a caspase-1 inhibitor. PBMCs were incubated with YVAD (10mM) and/or MSU/C18.0 (300μg/ml and 200μM concentration, respectively) for 24h. Data are expressed as mean±SEM of 4 healthy donors. E. Murine peritoneal macrophages were exposed to MSU, C18.0 or both for 24h. LPS (10ng/ml) served as positive control. Data are expressed as mean±SEM of 5 mice per group. F. MSU/C18.0 activates caspase-1 in maturated murine BMDCs. BMDCs were exposed to medium, C18.0 MSU/C18.0 for 24h. Thereafter cells were stimulated either with medium (RPMI) or with ATP for 30 minutes. Experiment was repeated twice with similar results. *P<0.01, Mann-Whitney U-test.
A second important candidate is represented by free fatty acids that are released from adipose tissue and liver depots after ingestion of a large meal or alcohol consumption (24). Therefore, we investigated the capacity of several saturated fatty acids, i.e. C12.0, C14.0, C16.0 and C18.0 to synergize with MSU crystals for the induction of IL-1β. Interestingly, the combination of MSU crystals and saturated fatty acid C18.0 resulted in the synergistic production of IL-1β by human PBMCs (Figure 2B/C). Other saturated fatty acids examined appeared to be less potent. Exposure of PBMCs to the combination of MSU and C18.0 FA led to an enhanced production of the chemokine IL-8, which is able to attract neutrophils, an important feature of the gout inflammatory reaction. C18.0 alone was also able to induce IL-8 in PBMCs (data not shown). Exposure to MSU-C18.0 did not result to robust TNFα production in human PBMCs or mouse peritoneal macrophages (2-fold detection limit for TNFα whereas 30-fold more for IL-1β data not shown).
Pharmacological inhibition of caspase-1 with Y-VAD strongly reduced IL-1β production of human PBMCs induced by MSU/C18.0 (Figure 2D), indicating that active caspase-1 is needed for IL-1β production. Murine peritoneal macrophages were also activated by MSU/C18.0 to produce IL-1β (Figure 2E). The role of caspase-1 in MSU-C18.0 induced IL-1β production was confirmed by showing that MSU/C18.0 activates caspase-1 in murine bone-marrow-derived dendritic cells (Figure 2F).
The role of TLR2 and TLR4 for the effects of FFA
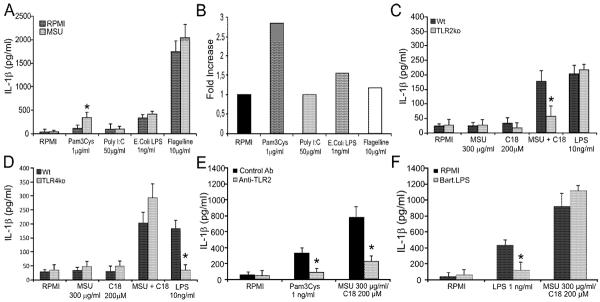
It has been suggested that fatty acids may trigger cell activation through both TLR2 and TLR4 (25). Therefore, we examined whether different known TLR ligands synergized with MSU to induce IL-1β. Figure 3A/B shows that among the TLR ligands tested, only the TLR2 agonist PAM3Cys synergized with MSU to induced IL-1β in human PBMC's. MSU did not synergize with ligands for TLR-3, -4, -5, -7,-8, -9, Nod1 or Nod2 (Figure 3A/data not shown). Combination of low dose LPS (1ng/ml) and MSU results in slightly enhanced IL-1β production (16). To investigate whether TLR2 or TLR4 mediate the synergistic effects of FFA on MSU crystals, peritoneal macrophages from both TLR2- and TLR4-deficient mice were stimulated with MSU/C18.0. Figure 3C/D shows that TLR2, and not TLR4, is involved in the enhanced IL-1β production after exposure to the MSU and C18.0 combinations. To find out whether this is also the case for human cells, PBMCs were stimulated with MSU/C18.0 in combination with an anti-TLR2 antibody or a potent TLR4 antagonist. Figure 3E/F confirms that only TLR2 ligation is needed for IL-1β production induced by the MSU/C18.0 combination.
Figure 3. MSU/C18.0 synergy is TLR2 dependent.
A. Human PBMCs were stimulated with several TLR ligands and MSU (300μg/ml) for 24h. Data are expressed as mean±SEM of 4 healthy donors. B. Ratio of IL-1β production by MSU/TLR ligand versus medium/TLR ligand. C/D. Peritoneal macrophages of wild type, TLR2ko or TLR4ko mice were stimulated with MSU, C18.0 or MSU/C18.0 for 24h. LPS (10ng/ml) served as positive control. Data are expressed as mean±SEM of 5 mice. Experiment was twice repeated with similar results. E/F. Blockade of TLR2 or TLR4 in combination with MSU/C18.0 stimulation. Human PBMCs were pretreated for 30 min with anti-TLR2 (eBioscience, Clone T2.5) or TLR4 antagonist (B.LPS) before exposure to MSU/C18.0. Data are expressed as mean±SEM of 4 healthy donors. *P<0.01, Mann-Whitney U-test.
Induction of gouty arthritis by MSU/C18.0
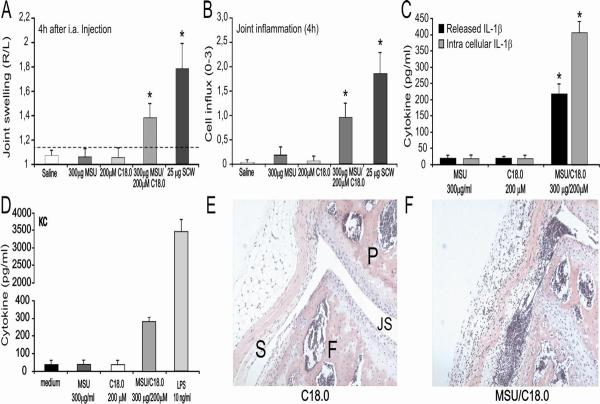
In a follow-up set of experiments, we investigated whether the MSU/C18.0 combination leads to acute arthritis in vivo. Intraarticular injection of MSU and C18.0 alone or in combination was studied in C57/Bl6 mice. Figure 4A/B shows that only the combination of MSU crystals with C18.0 fatty acids induces joint swelling and influx of inflammatory cells in the joint cavity. Analysis of inflamed synovium demonstrated enhanced mRNA expression and protein production for several pro-inflammatory cytokines (data not shown), which was not seen with MSU or C18.0 alone. Both intracellular and released IL-1β was significantly elevated after intraarticular injection of MSU/C18.0 (Figure 4C). Exposure of murine peritoneal macrophages to the MSU/C18.0 combination resulted in elevated concentrations of KC, the murine homolog of IL-8 (Figure 4D). Histology taken 4 hours after local injection of MSU/C18.0 revealed that the MSU/C18.0 combination induces joint inflammation dominated by neutrophils (Figure 4F), which does not occur with MSU or C18.0 alone (Figure 1E/4E).
Figure 4. MSU/C18.0 induced joint inflammation.
A. MSU, C18.0, MSU/C18.0 or SCW fragments were injected i.a. and 4h later joint swelling was determined using 99mTC-uptake method. The swelling is expressed as a right-left ratio and a ratio > 1.15 is indicated as inflammation. Data are expressed as mean±SEM of 7 mice per group. B. Cell influx into the joint cavity at 4h was determined by histology. The amount of cells, predominantly PMNs infiltrating in the synovial lining and the joint cavity was scored from 0–3. C. Both intracellular and released IL-1β was measured in synovial tissue explants at 4h after injection of MSU/C18.0. Data are expressed as mean±SEM of 5 mice per group. D. KC production of peritoneal macrophages after exposure to MSU/C18.0 for 24h. Data are expressed as mean±SEM of 5 mice per group. E/F/Histology 4h after i.a. injection of C18.0 or MSU/C18.0. For details and MSU injection alone see figure 1. Note the cell influx in the joint cavity after MSU/C18.0 injection. *P<0.01, Mann-Whitney U-test.
Role of the NALP3 inflammasome in MSU crystals- and C18 fatty acids-induced arthritis
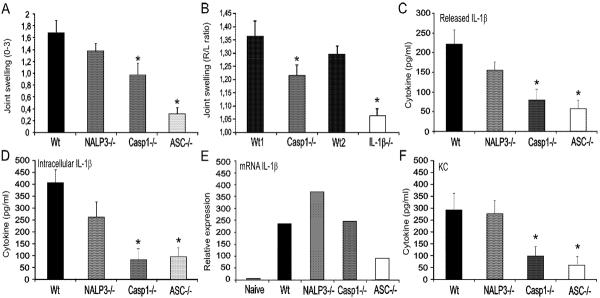
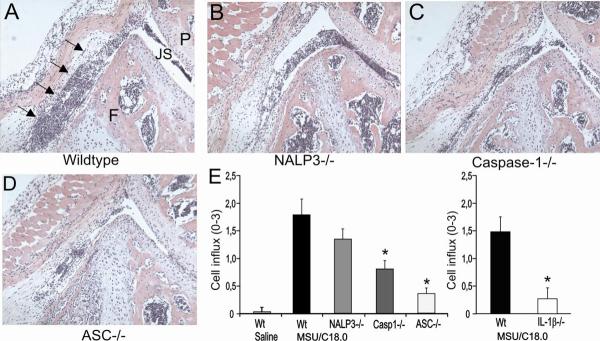
The NALP3 inflammasome has been reported to consist of the components NALP3, ASC and caspase-1, and urid acid crystals recognition by Nalp3 has been suggested to activated caspase-1 (8). Using series of knock-out mice, we examined the contribution of these components for the MSU/C18.0-induced arthritis. Joint swelling, determined 4h after intra-articular injection of MSU/C18.0 was suppressed in caspase-1 and ASC-gene deficient mice. ASC deficient mice showed the strongest reduction (Figure 5A). Surprisingly, NALP3 did not appear to be involved in MSU/C18.0 induced arthritis. As shown in Figure 5B, IL-1β is crucial for joint inflammation induced by MSU/C18.0. Both released and intracellular IL-1β were strongly reduced in synovial tissue from caspase-1 and ASC gene deficient mice (Figure 5C/D), but not in NALP3 gene deficient mice. These results indicate that NALP3 is bypassed for MSU/C18.0-induced IL-1β production. Surprisingly, an increased transcription of mRNA for IL-1β was found in the NALP3−/− mice compared to control mice (Figure 5E). In contrast, ASC deficiency leads to reduced IL-1β mRNA expression in synovial tissue, indicating that ASC also plays a role in transcription and not only for the processing of IL-1β. The chemokine KC was decreased as well in ASC deficient mice (Figure 5F). Not only the protein concentrations of IL-6 and KC showed clear reduction in caspase-1-deficient and ASC-deficient mice, but also at the level of transcription these inflammatory mediators were decreased (data not shown). Finally, histology taken at 4h after injection of MSU/C18.0 showed that ASC deficient mice were almost complete protected against arthritis (Figure 6). Low numbers of inflammatory cells were detected in the joint cavity of the ASC deficient mice, compared to the wild-type (Figure 6D). Caspase-1-deficient mice also exhibited reduced joint inflammation compared to wild-type mice, but not to the same degree as ASC-deficient mice (Figure 6C). Experiments in the NALP3−/− mice showed normal influx of PMN into the joint cavity implying that NALP3 is not crucial for the influx of these cells (Figure 6B). Significantly reduced numbers of PMN were found in the joint of caspase-1-deficient and ASC-deficient mice that received intra-articular MSU/C18.0. Likewise, a strong reduction of PMN influx was seen in the joints of IL-1β deficient mice, underscoring the pivotal role of IL-1β in gout (Figure 6E).
Figure 5. Role of inflammasome components in MSU/C18.0 gouty arthritis.
A. Joint swelling at 4h after injection of MSU/C18.0 in wild type, NALP3ko, caspase-1ko or ASCko mice. Joint swelling was scored macroscopically. B. Joint swelling determined by 99mTC uptake method. Note that MSU/C18.0 induced joint inflammation is highly IL-1β dependent. C/D. Released and intracellular IL-1β concentration 4h after injection of MSU/C18.0 in wild type, NALP3ko, caspase-1ko or ASCko mice. E. Expression of IL-1β mRNA in synovial tissue biopsies at 4h time point. F. Production of KC at 4h after induction of gouty arthritis by MSU/C18.0. For all panels, data are expressed as mean±SEM of 5 mice per group. Experiment was repeated once with similar results.
Figure 6. Joint inflammation after injection of MSU/C18.0 in wild type, NALP3ko, caspase-1ko or ASCko mice.
A. Joint inflammation in wild type mice was induced by intraarticular injection of a combination of MSU and C18.0 FFA. For details see Figure 1. Note cell influx in the joint cavity as indicated by arrows. B. Knee joint of a NALP3ko mouse injected with MSU/C18.0. C. Caspase-1 mouse. D. ASCko mouse. E. Cell influx into the joint cavity at 4h. The amount of cells, predominantly PMNs infiltrating the synovial lining and the joint cavity (scored from 0–3). Note that ASCko and IL-1βko mice showed strong reduced influx of cells. *P<0.01, Mann-Whitney U-test.
Discussion
In the present paper we propose a pathogenetic model to explain the pathways that link metabolic changes with induction of inflammation in gout, mechanisms that involve synergistic effects between C18.0 FFA and MSU crystals. Pure MSU crystals do not activate caspase-1, and they cannot induce the production of active IL-1β. However, costimulatory signals induced by other stimuli can strongly synergize with MSU crystals for the induction of inflammation, and cell stimulation by C18.0 fatty acids through TLR2 can provide such a synergistic impulse. In addition, C18.0 fatty acids in combination with MSU crystals activate caspase-1 and induce IL-1β-driven joint inflammation. The inflammasome components caspase-1 and ASC are also needed for the induction of both IL-1β and acute joint inflammation by MSU/C18.0. Interestingly however, the induction of IL-1β through MSU-C18.0 exposure is NALP3-independent.
Several reports claim that MSU crystals are potent inducers of IL-1β production. However, in most of these studies, the experiments were performed in cell lines that were pre-activated with PMA or LPS. Such pre-activation is necessary for induction of both IL-1β mRNA and pro-IL-1β. Using pure MSU alone, even at very high doses (up to 1000μg/ml, data not shown) we were unable to induce either caspase-1 activation or IL-1β production in human as well as in murine cells. Addition of ATP, which is needed for optimal caspase-1 activation through NALP3 (26, 27), to the stimulation cocktail did not lead to the activation of caspase-1 (Figure 1F). Intraarticular injection of high amounts of MSU crystals in vivo in mice was also unable to induce arthritis.
These data suggest that the induction of inflammation by MSU crystals is not a one-step process that invariably leads to cytokine release. This conclusion fits with the situation in the patients with gout: many of these patients have deposition of tophi in the joints or tendons without experiencing continuous inflammatory reactions. In contrast, the attacks of acute arthritis occur especially in the middle of the night and they are known to be triggered by an abundant meal, alcohol consumption or fasting. A common metabolic consequence of these processes is represented by the release of free fatty acids (FFA) in the circulation (24), which have been recently shown to be recognized by Toll-like receptors and induce an inflammatory reaction (25,28). We hypothesized that FFAs can synergize with MSU crystals in order to induce inflammasome activation and cytokine release. Indeed, while MSU crystals or FFAs alone were very weak inflammatory stimuli, a combination of C18.0 fatty acids and MSU crystals had very strong synergistic effects on cytokine release (Figure 2). Moreover, intraarticular injection of MSU and C18.0 FA induced a severe arthritis, with activation of caspase-1, production of IL-1β and of KC and subsequent neutrophil influx. In the majority of the previous studies of MSU/IL-1β production, LPS was used as first “priming” signal and MSU as a second signal for the induction of IL-1β. However, LPS is not a clinical relevant first signal with respect to gout.
In contrast to FFAs, ethanol did not directly synergize with MSU crystals for IL-1β production (data not shown). Ethanol has been reported to modulate both TLR2 and TLR4 activity (29,30), and this effect is corroborated in our studies in which alcohol inhibited TLR4 signaling (Figure 2A). In addition, increasing alcohol concentrations did not affect TLR2 signaling, and this is in line with previous reports showing that alcohol did not affect TLR2 signaling or recruitment of TLR2 to lipid rafts (31).
The inflammatory effects of MSU crystals have been recently suggested to involve activation of the NALP3 inflammasome (8), followed by caspase-1-dependent processing of pro-IL-1β. The role of IL-1β release for the pathogenesis of inflammation in gout has been strengthened by studies showing that anakinra (recombinant IL-1Ra) has therapeutic effects in gout (11). Induction of a potent IL-1β response depends on the induction of mRNA transcription on the one hand, and the processing of pro-IL-1β into the active cytokine. While we show that IL-1β mRNA transcription by C18.0/MSU crystals is strongly dependent on TLR2 engagement, less clear is the role of the specific inflammasome components necessary for caspase-1 activation, especially NALP3. ASC deficient mice were protected against inflammation induced by the C18.0/MSU combination, suggesting an important role of the inflammasome. Surprisingly however, the MSU/C18.0-induced joint inflammation was NALP3 independent, as NALP3-deficient mice developed gouty arthritis in a similar fashion as wild-type mice. Very recently, it has been shown in model of antigen-induced arthritis that NALP3 was not critical for induction of joint inflammation, although this model was IL-1β dependent (32). These data suggests that a different inflammasome platform is engaged in vivo by MSU crystals/C18.0 fatty acids, and indicates that during certain inflammatory conditions NALP3 is bypassed for IL-1β production.
Both ingestion of an abundant meal or alcohol consumption result in an increase of FFA concentrations in the circulation (33). Fasting is also associated with inflammatory attacks of gout, and it is well-known that during fasting serum concentrations of FFA are elevated (34). As all these well-known risk factors for the induction of a gout attack are accompanied by an increase in circulating concentrations of FFAs, and C18.0 fatty acids are shown here to synergize with MSU crystals for the induction of inflammation, it is tempting to speculate that the engagement of TLR2 by FFAs and the subsequent potentiation of inflammasome activation by MSU crystals, represents the link between the metabolic changes preceding a gout attack and the inflammatory flare in the joints of the patients. In line with this conclusion is the fact that in gout patients elevated levels of FFA were found shortly after exacerbation of joint inflammation (35,36).
An aspect that should not be omitted is the presence of a residual inflammatory reaction in caspase-1 knock-out mice injected with MSU crystals and C18.0 fatty acids. At least two explanations could account for this: on the one hand, the production of other cytokines/chemokines can also be induced by this inflammatory cocktail (e.g. IL-8/KC). On the other hand, inflammasome-independent mechanisms can also account for partial activation of IL-1β. Indeed, in-vitro studies have previously shown that neutrophil-derived serine proteases such as proteinase-3 or elastase can also process pro-IL-1β into active fragments (37), and we have recently demonstrated the existence inflammasome-independent pathways of IL-1β processing in a model of acute arthritis (21). Inflammasome activation is therefore an important, but by no means the only, pathway through which MSU crystals in combination with FFAs could induce inflammatory reactions in the joints.
In conclusion, in the present study we report strong synergistic effects on the inflammation between MSU crystals and FFAs. As MSU crystals alone are not able to display inflammatory properties to explain the gout attacks, we hypothesized the existence of a metabolic change induced by the risk factors known to predispose for the attacks of the disease, that potentiates the effects of the crystals. Release of FFAs by many of these risk factors, corroborated with their TLR2-dependent synergistic effects on MSU-induced inflammation, place FFAs at the cross-road between the metabolic changes preceding the gout attack and the occurrence of inflammation in the joint. These findings not only contribute to the understanding of the pathogenesis of gout, but they also offer a new therapeutic target in patients with gout, as treatments aimed to impede the interaction between MSU crystals and FFAs could prove of therapeutic value for both prevention and treatment of the gout attacks.
Acknowledgements
M.G.N. was supported by a Vidi Grant of the Netherlands Organization for Scientific Research. This work was also supported by grants from the National Institutes of Health grant number AR056296 and by the American Lebanese and Syrian Associated Charities to T-D.K. Prof. dr. P. Vandenabeele is acknowledges for the kindness to provide the anti-caspase-1 p20 antibody.
References
- 1.Terkeltaub R. Update on gout: new therapeutic strategies and options. Nat Rev Rheumatol. 2010;6:30–8. doi: 10.1038/nrrheum.2009.236. [DOI] [PubMed] [Google Scholar]
- 2.Pope RM, Tschopp J. The role of interleukin-1 and the inflammasome in gout: implications for therapy. Arthritis Rheum. 2007;56:3183–8. doi: 10.1002/art.22938. [DOI] [PubMed] [Google Scholar]
- 3.Tramontini N, Huber C, Liu-Bryan R, Terkeltaub RA, Kilgore KS. Central role of complement membrane attack complex in monosodium urate crystal-induced neutrophilic rabbit knee synovitis. Arthritis Rheum. 2004;50:2633–9. doi: 10.1002/art.20386. [DOI] [PubMed] [Google Scholar]
- 4.Abramson S, Hoffstein ST, Weissmann G. Superoxide anion generation by human neutrophils exposed to monosodium urate. Arthritis Rheum. 1982;25:174–80. doi: 10.1002/art.1780250210. [DOI] [PubMed] [Google Scholar]
- 5.Di Giovine FS, Malawista SE, Thornton E, Duff GW. Urate crystals stimulate production of tumor necrosis factor alpha from human blood monocytes and synovial cells. Cytokine mRNA and protein kinetics, and cellular distribution. J Clin Invest. 1991;87:1375–81. doi: 10.1172/JCI115142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Giovine FS, Malawista SE, Nuki G, Duff GW. Interleukin 1 (IL-1) as a mediator of crystal arthritis. Stimulation of T cell and synovial fibroblast mitogenesis by urate crystal-induced IL-1. J Immunol. 1987;138:3213–8. [PubMed] [Google Scholar]
- 7.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–26. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 8.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–41. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 9.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–65. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 10.Dinarello CA. Blocking IL-1 in systemic inflammation. J Exp Med. 2005;201:1355–9. doi: 10.1084/jem.20050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.So A, De Smedt T, Revaz S, Tschopp J. A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res Ther. 2007;9:R28. doi: 10.1186/ar2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terkeltaub R, Sundy JS, Schumacher HR, Murphy F, Bookbinder S, Biedermann S, et al. The interleukin 1 inhibitor rilonacept in treatment of chronic gouty arthritis: results of a placebo-controlled, monosequence crossover, non-randomised, single-blind pilot study. Ann Rheum Dis. 2009;68:1613–7. doi: 10.1136/ard.2009.108936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–50. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 14.Franchi L, Eigenbrod T, Muñoz-Planillo R, Nuñez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–7. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen CJ, Shi Y, Hearn A, Fitzgerald K, Golenbock D, Reed G, et al. MyD88-dependent IL-1 receptor signaling is essential for gouty inflammation stimulated by monosodium urate crystals. J Clin Invest. 2006;116:2262–71. doi: 10.1172/JCI28075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giamarellos-Bourboulis EJ, Mouktaroudi M, Bodar E, van der Ven J, Kullberg BJ, Netea MG, et al. Crystals of monosodium urate monohydrate enhance lipopolysaccharide-induced release of interleukin 1 beta by mononuclear cells through a caspase 1-mediated process. Ann Rheum Dis. 2009;68:273–8. doi: 10.1136/ard.2007.082222. [DOI] [PubMed] [Google Scholar]
- 17.Seegmiller JE, Howell RR, Malawista SE. The inflammatory reaction to sodium urate. J Am Med Assoc. 1962;180:469–75. [Google Scholar]
- 18.Lamkanfi M, Malireddi RK, Kanneganti TD. Fungal zymosan and mannan activate the cryopyrin inflammasome. J Biol Chem. 2009;284:20574–81. doi: 10.1074/jbc.M109.023689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van de Veerdonk FL, Joosten LA, Devesa I, Mora-Montes HM, Kanneganti TD, Dinarello CA, et al. Bypassing pathogen-induced inflammasome activation for the regulation of interleukin-1beta production by the fungal pathogen Candida albicans. J Infect Dis. 2009;199:1087–96. doi: 10.1086/597274. [DOI] [PubMed] [Google Scholar]
- 20.Popa C, Abdollahi-Roodsaz S, Joosten LA, Takahashi N, Sprong T, Matera G, et al. Bartonella quintana lipopolysaccharide is a natural antagonist of Toll-like receptor 4. Infect Immun. 2007;75:4831–7. doi: 10.1128/IAI.00237-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joosten LA, Netea MG, Fantuzzi G, Koenders MI, Helsen MM, Sparrer H, et al. Inflammatory arthritis in caspase-1 gene deficient mice: crucial role of proteinase 3 for caspase-1-independent production of bioactive IL-1β. Arthritis Rheum. 2009;60:3651–62. doi: 10.1002/art.25006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joosten LA, Heinhuis B, Abdollahi-Roodsaz S, Ferwerda G, Lebourhis L, Philpott DJ, et al. Differential function of the NACHT-LRR (NLR) members Nod1 and Nod2 in arthritis. Proc Natl Acad Sci USA. 2008;105:9017–22. doi: 10.1073/pnas.0710445105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdollahi-Roodsaz S, Joosten LA, Koenders MI, Devesa I, Roelofs MF, Radstake TR, et al. Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J Clin Invest. 2008;118:205–216. doi: 10.1172/JCI32639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopes SM, Trimbo SL, Mascioli EA, Blackburn GL. Human plasma fatty acid variations and how they are related to dietary intake. Am J Clin Nutr. 1991;53:628–37. doi: 10.1093/ajcn/53.3.628. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen MT, et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282:35279–92. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 26.Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–6. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–65. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem. 2001;276:16683–9. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 29.Bailey KL, Wyatt TA, Romberger DJ, Sisson JH. Alcohol functionally upregulates Toll-like receptor 2 in airway epithelial cells. Alcohol Clin Exp Res. 2009;33:499–504. doi: 10.1111/j.1530-0277.2008.00862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oak S, Mandrekar P, Catalano D, Kodys K, Szabo G. TLR2- and TLR4-mediated signals determine attenuation or augmentation of inflammation by acute alcohol in monocytes. J Immunol. 2006;176:7628–35. doi: 10.4049/jimmunol.176.12.7628. [DOI] [PubMed] [Google Scholar]
- 31.Dolganiuc A, Bakis G, Kodys K, Mandrekar P, Szabo G. Acute ethanol treatment modulates Toll-like receptor-4 association with lipid rafts. Alcohol Clin Exp Res. 2006;30:76–85. doi: 10.1111/j.1530-0277.2006.00003.x. [DOI] [PubMed] [Google Scholar]
- 32.Kolly L, Karababa M, Joosten LA, Narayan S, Salvi R, Pétrilli V, et al. Inflammatory role of ASC in antigen-induced arthritis is independent of caspase-1, NALP-3, and IPAF. J Immunol. 2009;183:4003–12. doi: 10.4049/jimmunol.0802173. [DOI] [PubMed] [Google Scholar]
- 33.Sozio M, Crabb DW. Alcohol and lipid metabolism. Am J Physiol Endocrinol Metab. 2008;295:10–6. doi: 10.1152/ajpendo.00011.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bouwens M, Afman LA, Müller M. Fasting induces changes in peripheral blood mononuclear cell gene expression profiles related to increases in fatty acid beta-oxidation: functional role of peroxisome proliferator activated receptor alpha in human peripheral blood mononuclear cells. Am J Clin Nutr. 2007;86:1515–23. doi: 10.1093/ajcn/86.5.1515. [DOI] [PubMed] [Google Scholar]
- 35.Darlington LG, Scott JT. Plasma lipid levels in gout. Ann Rheum Dis. 1972;31:487–89. doi: 10.1136/ard.31.6.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mielants H, Veys EM, de Weerdt A. Gout and its relation to lipid metabolism. Ann Rheum Dis. 1973;32:501–5. doi: 10.1136/ard.32.6.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coeshott C, Ohnemus C, Pilyavskaya A, Ross S, Wieczorek M, Kroona H, et al. Converting enzyme-independent release of tumor necrosis factor alpha and IL-1beta from a stimulated human monocytic cell line in the presence of activated neutrophils or purified proteinase 3. Proc Natl Acad Sci USA. 1999;96:6261–6. doi: 10.1073/pnas.96.11.6261. [DOI] [PMC free article] [PubMed] [Google Scholar]