Abstract
Sp proteins are evolutionarily-conserved transcription factors required for the expression of a wide variety of genes that are critical for development and cell-cycle progression. De-regulated expression of certain Sp proteins is associated with the formation of a variety of human tumors, however direct evidence that any given Sp protein is oncogenic has been lacking. Here we report that Sp2 protein abundance in mice increases in concert with the progression of carcinogen-induced murine squamous cell carcinomas. Transgenic mice specifically overexpressing murine Sp2 in epidermal basal keratinocytes were highly susceptible to wound- and carcinogen-induced papillomagenesis. Transgenic animals that were homozygous rather than hemizygous for the Sp2 transgene exhibited a striking arrest in the epidermal differentiation program, perishing within two weeks of birth. Our results directly support the likelihood that Sp2 overexpression occurring in various human cancers has significant functional impact.
Keywords: Sp2, stem cell, differentiation, wound-induced neoplasia, carcinogenesis
Introduction
The Sp-family of mammalian transcription factors includes nine members, Sp1-9, that share a highly-conserved DNA-binding domain (for reviews see 1–4). The promoters of many mammalian genes, including genes controlling cell-cycle progression and development, are regulated by Sp proteins (5, 6). In turn, the activities of Sp proteins are regulated by a variety of growth-related signal transduction pathways as well as mechanisms controlling embryonic development (4). Animals lacking specific Sp proteins exhibit global or tissue-specific defects, suggesting that Sp-family members play essential, non-overlapping roles in development (3, 4). The overexpression of a subset of Sp-family members has also been associated with the formation of a variety of human cancers (6, 7). Yet, it remains uncertain whether the deregulated expression of any given Sp protein is oncogenic.
Although many biochemical and functional properties of Sp-family members have been established, studies focusing on Sp2 have yielded few insights into its roles in cell and organismal physiology. The Sp2 DNA-binding domain is the least conserved (75%) amongst Sp-family members and binds with high affinity (Kd = 225 pM) to a consensus DNA-binding site (5'-GGGCGGGAC-3') that is distinct from that of Sp1 (8–10). Yet, in transient-transfection assays Sp2 only weakly trans-activates promoters carrying consensus Sp2-binding sites or well-characterized Sp-dependent promoters that are readily induced by Sp1 and Sp3 (8, 11). Despite its widespread expression, little or no soluble Sp2 DNA-binding activity has been detected in many human and mouse cell lines (8). Studies using Sp1/Sp2 chimeras have revealed that the Sp2 trans-activation and DNA-binding domains are each negatively-regulated in vitro, and further analyses have shown that each of these domains carry amino acid sequences that independently target Sp2 to the nuclear matrix (12). Recent analyses have shown that Sp2 (i) transcripts are inherited maternally, (ii) is expressed in embryonic and adult tissues, (iii) is essential for the completion of gastrulation, and (iv) transcription is governed by multiple promoters in a cell- and tissue-specific fashion (11, 13, 14). De-regulation of Sp2 expression has also been associated with tumorigenesis. Sp2 abundance is increased in human prostate cancers and correlated directly with pathological grade (15).
Herein we report that Sp2 protein abundance is correlated directly with the progression of murine squamous cell carcinomas induced by DMBA/TPA. To determine whether Sp2 overexpression is oncogenic or merely associated with tumorigenesis, we created a novel mouse model in which Sp2 is overexpressed in cells of the epidermal basal layer via by the bovine keratin 5 promoter. Sp2 overexpression in transgenic hemizygotes induces alopecia, marked susceptibility to wound-induced neoplasia, and increased sensitivity to carcinogen-induced skin tumorigenesis. Levels of Sp2 expression encountered in homozygotes results in post-natal lethality and a striking depletion of terminally differentiated keratinocytes. These results indicate that Sp2 overexpression in this epidermal compartment inhibits keratinocyte differentiation and sensitizes these cells to wound- and carcinogen-induced neoplastic growth.
Materials and Methods
Cells and cell culture
COS-1 cells were obtained from the ATCC (Manassas, VA) and cultured in Dulbecco’s modified Eagle’s medium (DMEM; GIBCO, Inc., Gaithersburg, MD) supplemented with 10% heat-inactivated fetal bovine serum (Atlanta Biologicals, Norcross, GA) and 1% Pipracil at 37°C under 5% CO2.
Animals and generation of transgenic mouse strains
FVB/NJ and K15-EGFP (B6.Cg-Tg(Krt1-15-EGFP)2Cot/J) animals were obtained from The Jackson Laboratory (Bar Harbor, ME) and maintained under standard conditions. A transgene construct carrying the bovine keratin 5 promoter and an epitope-tagged mouse Sp2 cDNA was prepared in plasmid pTg1 (a gift from the Univ. of North Carolina Animal Models Core Facility). The bovine keratin 5 promoter was amplified from plasmid 383 (a kind gift from Dr. Angel Ramirez, Department of Epithelial Biology, CIEMAT, Madrid, Spain) via the PCR utilizing Titanium™ Taq DNA polymerase (Clontech, Inc., Mountain View, CA) and gene-specific primers (5'-ggggcggccgcgatcaaatgcctggtgcaca-3' and 5'-ggggtcgacccgaggtgctggagagaaag-3'). This promoter was subcloned upstream of a splice donor sequence derived from the first exon of the mouse albumin gene. A full-length, epitope-tagged (Influenza hemagglutinin; HA) mouse Sp2 cDNA was isolated via RT-PCR utilizing total mouse heart RNA as a template as reported elsewhere (13). The nucleotide sequence of this cDNA has been deposited in GenBank (accession number GU126673). An HA-epitope tag was appended at the 3' end of mouse Sp2 coding sequences via the PCR utilizing Titanium™ Taq DNA polymerase (Clontech) and gene-specific primers (5'-gaattcagatccgccaccatgagcgcagatccacagatga-3' and 5'-cccacctaggcacgaagggcttgtacccatacgatgttccagattacgctagctgaaagcttg-3'). This tagged cDNA was subcloned downstream of a splice acceptor sequence derived from exon 2 of the mouse albumin gene, creating pTG1-K5-mSp2HA. The integrity of this transgene construct was confirmed by automated DNA sequencing. The transgene was linearized and pronuclear injections (FVB/NJ) and implantations were performed by staff of the Univ. of North Carolina Animal Models Core Facility. Transgene-carrying animals were identified via the PCR using transgene-specific primers (5'-gctgcaaggcaagtttatccctagctgagc-3' and 5'-agggagatggctgggagtcc-3'), and two male founders (designated A and C) were identified via crosses with FVB/NJ females and genotyping of progeny. All animal experiments were conducted with the approval of the Institutional Animal Care and Use Committee of North Carolina State University.
Cloning of transgene integration sites and mouse genotyping
Transgene integration sites were isolated using a procedure described previously (16), subjected to automated DNA sequencing, and compared with the mouse genome sequence. Allele-specific primers were developed that facilitate the identification of wild-type, hemizygous, and homozygous animals amongst progeny within each transgenic strain (termed Sp2-A and Sp2-C). For Sp2-A progeny the primers are : 5'-cgtgtgcaccaggcatttgatc-3', 5'-gggtgactgaggcagagtttg-3', and 5'-caagcagttctgagacctgcac-3'. For Sp2-C progeny the primers are: 5'-acattgcagcacattgcacactatcc-3', 5'-gttctaagcactaacatcatcaggcg-3', and 5'-gttagctgataaccttttgaagaccg-3'. Animals carrying the K15-EGFP transgene were identified with gene-specific primers (5'-aagttcatctgcaccaccg-3' and 5'-tccttgaagaagatggtgcg-3').
RT-PCR and Quantitative RT-PCR
Total RNAs were prepared from neonatal and adult animals using TRIzol reagent (Invitrogen, Corp., Carlsbad, CA), first-strand cDNAs were synthesized using oligo-dT (Invitrogen) primers and SuperScript® III reverse transcriptase (Invitrogen), and gene-specific primers (5'-ccagcctaccccaaggaaac-3' and 5'-gggagccctgaatctgaagtat-3') were employed to amplify Sp2 message. Sp2 expression was quantitated using an iQ™5 iCycler (Bio-Rad Laboratories, Inc., Hercules, CA), and QuantiTect SYBR Green (QIAGEN, Inc., Valencia, CA). The Ct value for Sp2 expression in each sample was normalized by subtracting the Ct value for amplification of GAPDH (5'-gggtgtgaaccacgagaaat-3’ and 5’-ccttccacaatgccaaagtt-3’).
Transfection and Western blotting
COS-1 cells were transfected with expression constructs using SuperFect® reagent (Qiagen, Inc., Hilden, Germany). The construction and properties of an epitope-tagged human Sp2 expression construct (pCMV4-hSp2/flu) have been described (8, 11, 12, 17). Western blots were performed as described (8) utilizing anti-HA and anti-actin (sc-805, sc-1616; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) or anti-Sp2 (HPA003357; Sigma-Aldrich, Inc., St. Louis, MO) antibodies and antigen-antibody complexes were detected using an enhanced chemiluminescent kit (ECL™; GE Healthcare Amersham, Piscataway, NJ).
Preparation of tissue sections and immunohistochemical staining
Whole neonates or dorsal skin samples were fixed in 10% formalin, embedded in paraffin, and 10 µm sections were placed on glass slides. Sections were deparaffinized and re-hydrated by consecutive incubations in xylene, 100% ethanol and 95% ethanol, and subjected to staining with hematoxylin and eosin or immunohistochemistry as described (18–20). Antibodies against keratins 5 (PRB-160P), 6 (PRB-169P), 10 (PRB-159P), 14 (PRB-155P), 15 (PCK-153P), and loricrin (PRB-145P) were obtained from Covance Research Products, Inc., Emeryville, CA. A monoclonal anti-keratin 8 antibody has been described (21). CD34 (#553731) and BRdU (#347580) antibodies were obtained from BD Biosciences, Inc., Franklin Lakes, NJ. Antibodies against EGFP (sc-9996) and PCNA (clone PC10; M0879) were obtained from Santa Cruz Biotechnology, Inc., and Dako, Inc., Carpinteria, CA, respectively.
Wounding assay
Mice were anesthetized via isofluorane inhalation, dorsal surfaces were sterilized, and full thickness wounds (4 mm in diameter) were introduced using a dermal biopsy punch (Miltex, Inc. York, PA). Wounded animals were checked daily for healing and papilloma development.
Treatment of animals with DMBA/TPA
Dorsal surfaces of wild-type and transgenic littermates were shaved and animals were replaced into their cages for 48 hours. Shaved surfaces were treated topically with a single dose (200 nmoles) of DMBA in acetone. Two weeks later, DMBA-treated animals were treated topically twice per week with TPA (6.8 nmoles in acetone) for 20 weeks. Animals were checked daily for papilloma development.
Statistical analysis
The statistical significance of results was determined utilizing the Student's t test. Differences with P values of <0.05 are considered significant.
Results and Discussion
Sp2 protein expression increases in concert with DMBA/TPA-induced skin carcinogenesis
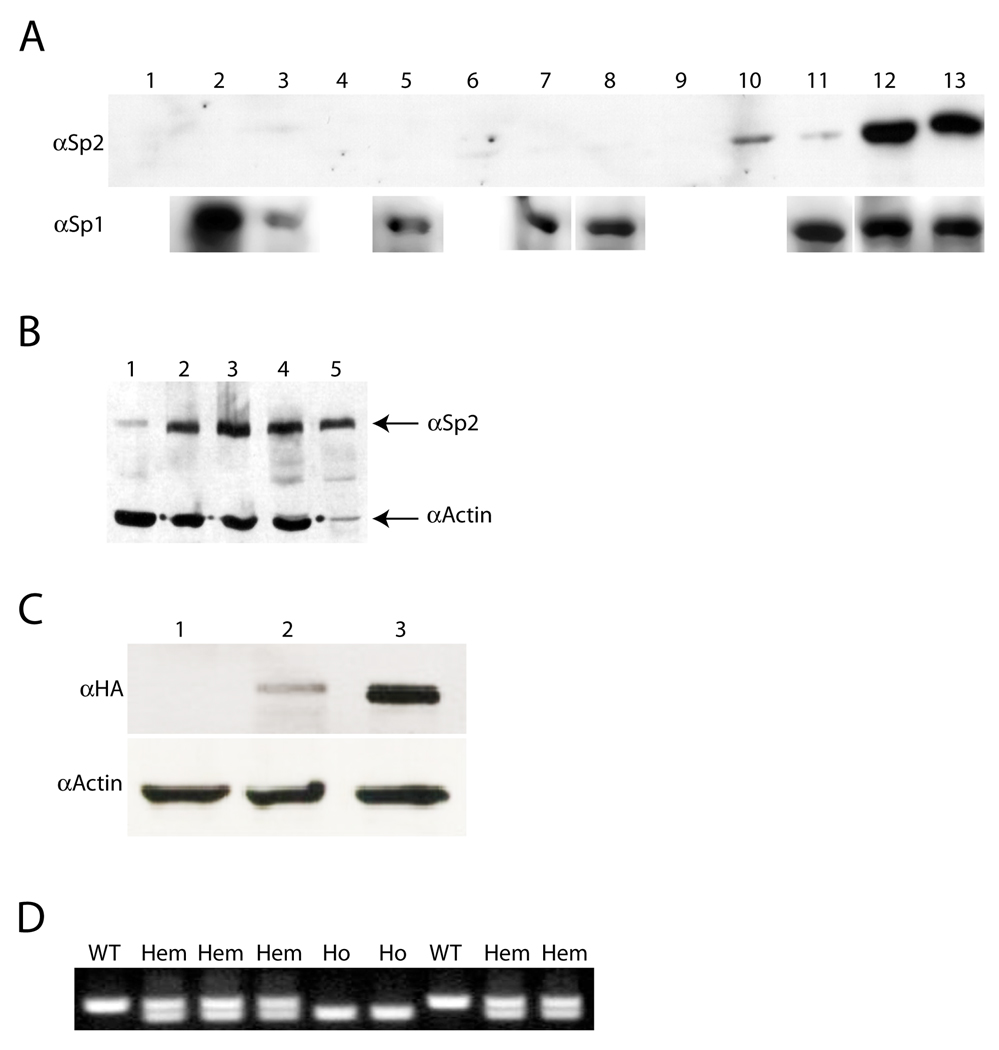
Since Sp2 expression is correlated directly with the progression of human prostate cancers (15), it became of interest to determine if this correlation might extend to additional neoplasms at one or more stages of tumor progression. We chose carcinogen-induced mouse squamous cell carcinomas as our model system for these studies. Protein extracts were prepared from normal whole skin and epidermis, a series of small-, medium-, and large-sized DMBA/TPA-induced papillomas, a mouse cell line derived from a DMBA/TPA-induced squamous cell carcinoma (MT2.6; 18), and a spontaneously-immortalized mouse keratinocyte cell line (BALB/MK2; 22), and equivalent amounts of each extract were examined by Western blotting. As shown in Fig. 1A, Sp2 expression was below the limit of detection in normal mouse skin (lane 1), epidermis (lane 2), or small-sized papillomas (lanes 3–5). Sp2 expression was barely detectable in medium-sized papillomas (lanes 6–8), was expressed to significant levels in two of three large-sized papillomas (lanes 9–11) and was expressed strongly in BALB/MK2 and MT2.6 cells (lanes 12 and 13, respectively). These results indicate that Sp2 expression is up-regulated in this model system, is correlated directly with the progression of DMBA/TPA-induced neoplasms, and is a feature of immortalized (BALB/MK2) keratinocytes. In contrast, Sp1 expression was not correlated with progression in this model system (Fig. 1A). Sp2 expression is also elevated in human squamous carcinoma cell lines relative to primary human keratinocytes (Fig. 1B).
Figure 1.
Sp2 protein expression is correlated with skin tumor progression and creation of transgenic mouse strains. A, Western blots of proteins derived from normal skin, DMBA/TPA-induced skin papillomas, and keratinocyte cell lines examined with polyclonal antibodies against Sp1 (αSp1) and Sp2 (αSp2). Lane 1, normal whole skin; Lane 2, normal epidermis; Lanes 3–5, small-sized papillomas; Lanes 6–8, medium-sized papillomas; Lanes 9–11, large-sized papillomas; Lane 12, BALB/MK2; Lane 13, MT2.6. B, Western blot of human samples with polyclonal antibodies against Sp2 (αSp2) and actin (αActin). Lane 1, primary human keratinocytes; Lane 2, SCC13; Lane 3, SCC23; Lane 4, SQCCY1; Lane 5, A431. C, Western blot of extracts from control and transfected COS-1 cells examined with polyclonal antibodies against HA (αHA) and actin (αActin). Lane 1, control extracts; Lane 2, extracts prepared from cells transfected with pTG1-K5-mSp2HA; Lane 3, extracts prepared from cells transfected with pCMV4-hSp2/flu. D, PCR-mediated genotyping of Sp2-A litter with allele-specific primers. Amplification reactions produce products of 383 and 290 bp from wild-type (WT) and transgenic alleles (Hem, hemizygous; Ho, homozygous), respectively.
Generation of transgenic mice in which Sp2 is expressed ectopically in basal keratinocytes
To determine if Sp2 expression drives tumor progression or is merely associated with it, we generated a transgene (pTG1-K5-mSp2HA) in which expression of an epitope-tagged (Influenza hemagglutinin; HA) mouse Sp2 cDNA (13) is regulated by the bovine keratin 5 promoter. This promoter has been utilized extensively to express genes of interest in basal keratinocytes, the cell of origin for DMBA/TPA-induced tumors (23–27). To confirm the integrity of this construct, COS-1 cells were transiently transfected with pTG1-K5-mSp2HA or an expression plasmid (pCMV4-hSp2/flu) carrying an epitope-tagged human Sp2 cDNA that has been characterized (8, 11, 12, 17). As shown in Fig. 1C, proteins of the expected size (80 kDa) were detected with an anti-HA antibody in extracts prepared from transfected cells (lanes 2 and 3) but not in an extract prepared from control cells (lane 1).
The transgene carried by pTG1-K5-mSp2HA was injected into FVB/NJ pronuclei, and two male founders (denoted A and C) were identified. One-half of the progeny derived from each founder inherited the transgene, indicating that integration occurred within a single chromosome. We employed a PCR-based strategy (16) to clone transgene integration sites and developed PCR-based assays that identify hemizygous and homozygous descendents (Fig. 1D and data not shown). The integration sites for founders A and C were mapped to mouse chromosomes 6 and 5, respectively. The Sp2-A integration site occurred in an intergenic region 40 Kbp upstream of mouse Olr1 (Oxidized low-density lipoprotein receptor 1), whereas the Sp2-C integration site occurred within intron 15 of the latrophilin 3 (Lphn3/CIRL3) locus. Lphn3/CIRL3 encodes a brain-specific G protein-coupled receptor that is expressed most abundantly immediately after birth (28). It is not known if Lphn3/CIRL3 is an essential gene nor is its physiological significance understood.
Sp2 overexpression in basal keratinocytes causes alopecia in hemizygotes and post-natal lethality in homozygotes
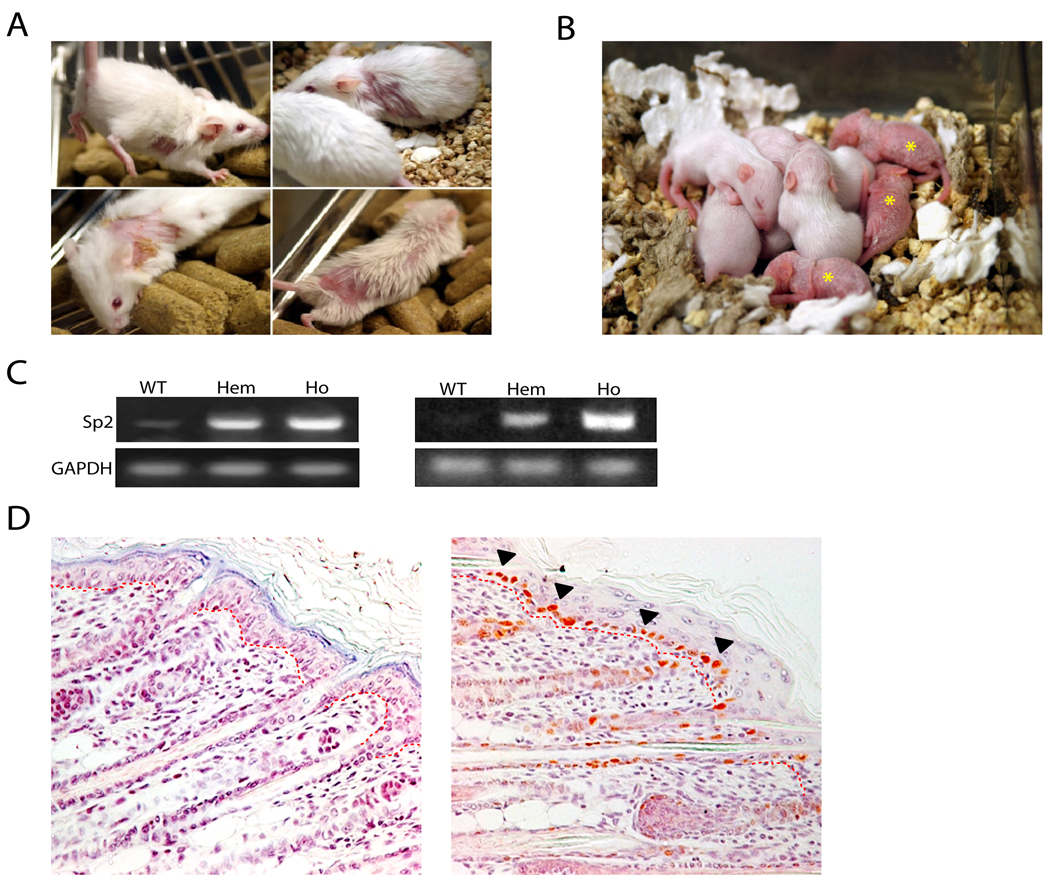
Sp2-A and -C hemizygotes have been bred with FVB/NJ animals for more than 11 generations and exhibit normal life expectancy and fecundity. A majority of Sp2-A hemizygotes develop alopecia and hyperkeratosis beginning at two months of age (Fig. 2A). These skin abnormalities can occur at discrete sites, e.g., sites of abrasion or repetitive movement, or extend throughout the dorsal surface and affected regions increase in severity with age. Sp2-C hemizygotes do not exhibit alopecia or other gross phenotypic abnormalities. Homozygous Sp2-A or -C transgenic pups are produced at expected ratios, and the gross appearance of these animals at birth is indistinguishable from wild-type and hemizygous littermates. However, homozygotes perish within the first two weeks of post-natal life. The skin of Sp2-A homozygotes begins to deteriorate on post-natal day three (PD3), becoming increasingly reddened and hyperkeratotic (Fig. 2B, asterisks). Such pups become runted and developmentally retarded relative to their littermates and succumb prior to PD13. The skin of Sp2-C homozygotes deteriorates more quickly and pups perish prior to PD3.
Figure 2.
Gross phenotypes of hemizygous and homozygous Sp2-A animals and ectopic expression of mouse Sp2 in basal keratinocytes. A, alopecia in adult hemizygotes. Representative affected animals are illustrated at two- to four-months of age. B, F2 litter of wild-type, hemizygous, and homozygous animals on PD12. Homozygous animals are indicated with an asterisk. C, amplification of endogenous and exogenous Sp2 mRNAs in wild-type and transgenic animals via RT-PCR. Total RNAs were prepared from dorsal whole skin from wild-type (WT), hemizygous (Hem), and homozygous (Ho) littermates on PD1 and analyzed by RT-PCR with Sp2- or GAPDH-specific primers. Left panel, Sp2-A; Right panel, Sp2-C. D, immunohistochemical detection of ectopic mouse Sp2 expression in basal keratinocytes of Sp2A homozygotes. Paraffin-embedded dorsal skin sections prepared from wild-type (left) and homozygous transgenic (right) littermates on PD6 were analyzed with an anti-HA antibody. HA-positive basal keratinocytes are indicated by a filled arrowhead. The basement membrane separating the epidermis from dermis is indicated by a dotted red line.
Histological examinations of PD1 Sp2-A homozygous pups revealed a well-structured stratified epidermis with an intact stratum corneum, as well as developing sebaceous glands and hair follicles similar to wild-type animals. Multifocal apoptosis within the epidermal basal layer was marginally elevated relative to wild-type littermates with scattered disorganization of basal cells that became significantly more pronounced by PD4. A loss of normal epidermal architecture was noted by PD6 with a disorganization of cells in all layers and occasional areas of partial to complete epidermal collapse where the stratum spinosum contacted the dermis. Loss of laminar epidermal architecture was accompanied by scattered apoptosis, hypertrophy and hydropic swelling of keratinocytes, as well as orthokeratotic laminated hyperkeratosis and patchy areas of parakeratosis. The severity and extent of these aforementioned features worsened progressively through PD13. Histological examinations of Sp2-C pups revealed identical epidermal defects.
To assess levels of Sp2 expression in transgenic animals two experiments were performed. First, RNAs were harvested from whole skin of wild-type, hemizygous, and homozygous post-natal pups and Sp2 expression was detected via RT-PCR. As shown in Fig. 2C, robust levels of Sp2 message were detected in hemizygous and homozygous Sp2-A (left panel) and Sp2-C (right panel) animals relative to wild-type littermates. Second, levels of Sp2 expression were quantified by real-time PCR as a function of animal age. Real-time PCR assays performed with RNAs from whole skin of three-month old hemizygotes indicated that Sp2 expression was nearly 20-fold above endogenous levels in Sp2-A animals and elevated by 200-fold in Sp2-C animals (data not shown). Levels of exogenous Sp2 expression in Sp2-C hemizygotes increased with age whereas transgene expression levels in Sp2-A hemizygotes remained unchanged (data not shown).
To determine if proteins of expected sizes were synthesized in transgenic animals, denatured extracts were prepared from whole skin and Western blots were performed using an anti-HA antibody. Consistent with results obtained in transfection experiments (Fig. 1C), a single protein of 80 kDa was detected (data not shown). To determine if the bovine keratin 5 promoter directed expression of Sp2 to basal keratinocytes, paraffin-embedded sections were prepared from whole skin harvested from wild-type and homozygous Sp2-A littermates and exogenous Sp2 expression was detected via immunohistochemistry using an anti-HA antibody. As shown in Fig. 2D, basal cells within the interfollicular epidermis as well as the hair follicle outer root sheath stained strongly with an anti-HA antibody whereas sections prepared from wild-type animals lacked staining. We conclude that the Sp2 transgene is expressed as anticipated, and Sp2 overexpression in basal keratinocytes results in alopecia in Sp2-A hemizygotes and post-natal lethality in Sp2-A and –C homozygotes.
Sp2 overexpression causes arrested differentiation of the interfollicular epidermis
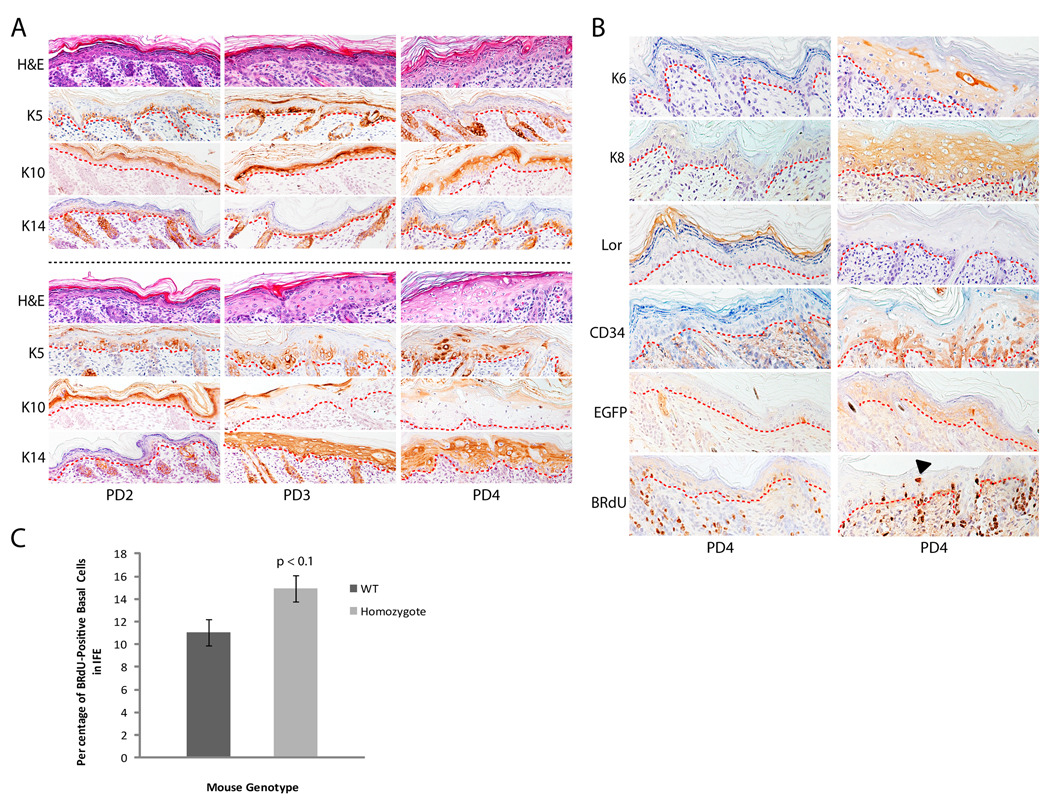
To determine the consequence of Sp2 overexpression for epidermal differentiation, paraffin-embedded whole skin sections were prepared from Sp2-A homozygotes and wild-type littermates on successive post-natal days. Pups were injected with BRdU one hour prior to euthanasia to label proliferating cells, and skin sections were examined with anti-BRdU antibodies as well as antibodies against differentiation-specific markers. As shown in Fig. 3A, sections stained with hematoxylin and eosin revealed that the epidermis of wild-type and homozygotes is similar in cell stratification and thickness on PD2 but diverge markedly on subsequent post-natal days. The epidermis of homozygotes thickened increasingly on PD3-4 relative to wild-type littermates with hypertrophic and hydropically swollen cells accumulating in disorganized epidermal layers. To determine whether markers of basal (keratins 5 and 14) and suprabasal (keratin 10) keratinocytes were expressed on these post-natal days, paraffin-embedded sections were analyzed by immunohistochemistry. Keratins 5, 10, and 14 were expressed as expected in wild-type animals (Fig. 3A, top panels), whereas the expression of these markers was altered profoundly in homozygotes (Fig. 3A, bottom panels). Basal keratinocytes of homozygotes expressed keratin 5 on PD2 with sporadic keratin 5-stained cells noted in suprabasal layers. The abundance of keratin 5-positive cells in all epidermal cell layers increased significantly on PD3-4. Similarly, keratin 14 expression was detected in basal keratinocytes on PD2 and in all epidermal layers on subsequent days. Keratin 10 expression was detected in all suprabasal layers on PD2, and diminished to low levels or was absent in the granular and cornified layers during subsequent post-natal days.
Figure 3.
Histochemical and immunohistochemical characterization of post-natal Sp2-A homozygotes. A, paraffin-embedded dorsal skin sections from wild-type (top panels) and homozygous transgenic (bottom panels) littermates on PD2-4. Sections were stained with hematoxylin and eosin (H&E) or with antibodies against keratins 5 (K5), 10 (K10), or 14 (K14). Dashed red lines indicate the position of the epidermal basement membrane. B, paraffin-embedded dorsal skin sections from K15-EGFP transgenic animals (left panels) and [K15-EGFP, Sp2-A/Sp2-A] double-transgenic (right panels) littermates on PD4. Sections were stained with antibodies against keratin 6 (K6) or 8 (K8), loricrin (Lor), CD34, EGFP, or BRdU. A filled arrowhead indicates a BRdU-positive suprabasal keratinocyte and dashed red lines indicate the position of the epidermal basement membrane. C, enumeration of BRdU-positive basal keratinocytes within the interfollicular epidermis of K15-EGFP and [K15-EGFP, Sp2-A/Sp2-A] double-transgenic littermates on PD4. Columns, mean (K15-EGFP, n = >3400 cells/group; [K15-EGFP, Sp2-A/Sp2-A], n = >7300 cells/group); bars, SE.
To extend this analysis, paraffin-embedded sections on PD4 were examined for the expression of a bevy of additional markers (Fig. 3B). The expression of keratin 6, a marker associated with neoplastic, inflamed, and/or wounded epidermis, was detected in the epidermis of homozygotes but not wild-type animals (29, 30). Keratin 8, an alternative heterodimeric partner of keratin 14 and a keratin normally restricted to "simple" epithelia, was detected in the epidermis of homozygotes and absent in wild-type animals (31–34). Finally, a marker characteristic of the stratum corneum, loricrin, was not detected in homozygotes.
To determine if epidermal distress induced the recruitment of stem cells from the hair follicle "bulge" region to the interfollicular epidermis, two experiments were performed. First, PD4 sections were stained for the expression of CD34, a well-characterized marker of this stem cell population (35, 36). Second, a lineage tracing experiment was performed in which Sp2-A mice were inter-crossed with animals that express a transgene, K15-EGFP, restricted to "bulge"-derived stem cells (37, 38). CD34- and EGFP-positive cells were detected within the basal and suprabasal layers of Sp2-A homozygotes, but not wild-type littermates, on PD4 indicating the recruitment of "bulge"-derived cells to the interfollicular epidermis (Fig. 3B).
Finally, sections were stained with an anti-BRdU antibody to identify proliferating cells. BRdU-positive cells were detected in the basal layers of both wild-type and homozygous animals, however BRdU-positive cells were also noted in suprabasal layers of homozygotes (arrowhead, Fig. 3B). To quantify basal cell proliferation, BRdU-positive cells within the interfollicular epidermis of wild-type and homozygous post-natal animals were enumerated and compared. As shown in Fig. 3C, BRdU-positive basal cells were more numerous in homozygotes however this level of increased cell proliferation was not statistically significant (p<0.1). Taken together, we conclude from these immunohistochemical analyses that Sp2 overexpression in basal keratinocytes produces a population of phenotypically immature keratinocytes that appear unable to commit to the epidermal differentiation program.
Sp2 overexpression renders hemizygous animals susceptible to wound-induced neoplasia
In the course of these studies we noted that Sp2-C hemizygotes developed occasional papillomas at sites of ear punches or minor wounds sustained from littermates. To quantify this apparent susceptibility to wound-induced neoplasia, full-thickness surgical wounds (4 mm diameter) were introduced into the dorsal skin of Sp2-C hemizygotes and wild-type littermates and these animals were monitored for the development of papillomas. As shown in Fig. 4A, surgery-induced papillomas developed within weeks following wounding of Sp2-C hemizygotes. Whereas surgical wounding of wild-type animals did not induce the formation of a single papilloma, 27% of wounds sustained by Sp2-C hemizygotes induced papillomagenesis (p=0.001; Fig. 5A). To determine if the incidence of wound-induced papillomagenesis is influenced by animal age, results presented in Fig. 5A were plotted as a function of the age of Sp2-C animals at the time of surgery. Whereas young animals (one to four months of age) were only mildly susceptible to wound-induced papillomas, 70% of animals developed papillomas when wounded at six to ten months of age and this increased incidence of papillomagenesis is statistically significant (p<0.01; Fig. 5B). We conclude from these results that Sp2 overexpression in basal keratinocytes induces a marked susceptibility to wound-induced neoplasms. Moreover, this susceptibility to papillomagenesis increases in concert with the age-dependent increase in Sp2 expression noted in these animals.
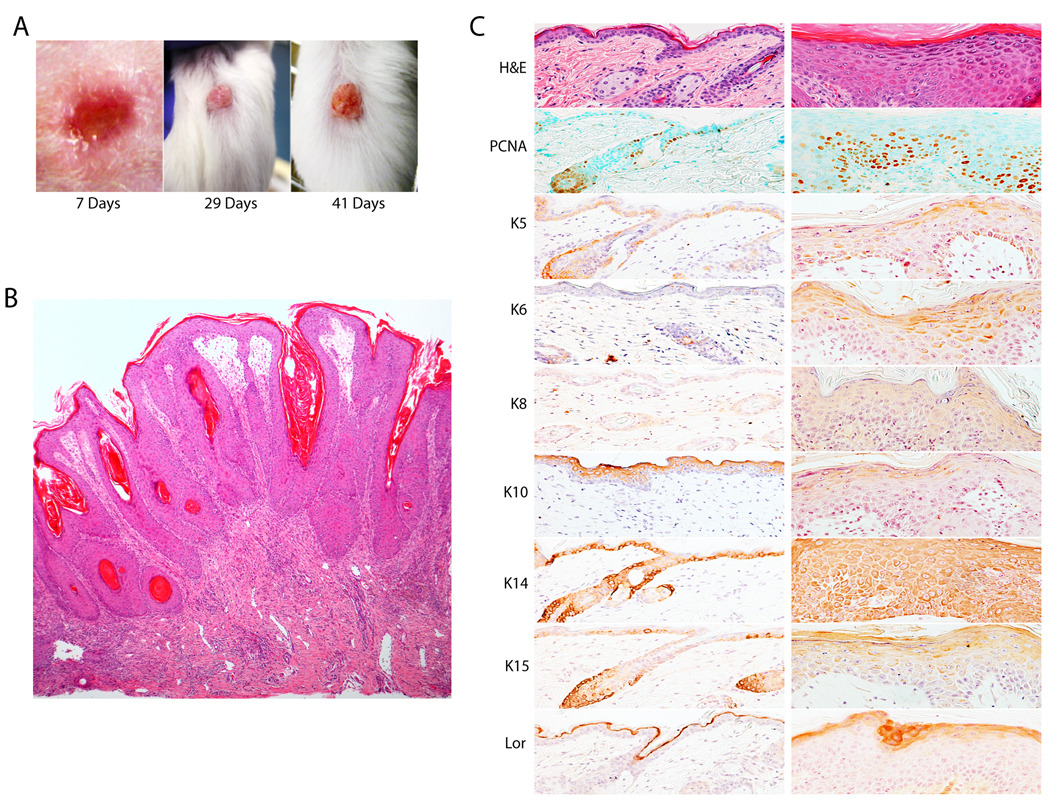
Figure 4.
Characterization of wound-induced papillomas in Sp2-C hemizygotes. A, papilloma development on the dorsal surface of a surgically-wounded Sp2-C hemizygote. The number of days following wounding are indicated below each image. B, low magnification image of wound-induced papilloma. C, histochemical and immunohistochemical characterization of a wound-induced papilloma. Paraffin-embedded dorsal skin tissue sections from the papilloma margin (left column) and papilloma (right column) were stained with hematoxylin and eosin (H&E) or various antibodies. Antibodies employed are indicated as in Fig. 3 with the exception of the addition of antibodies against PCNA and keratin 15 (K15).
Figure 5.
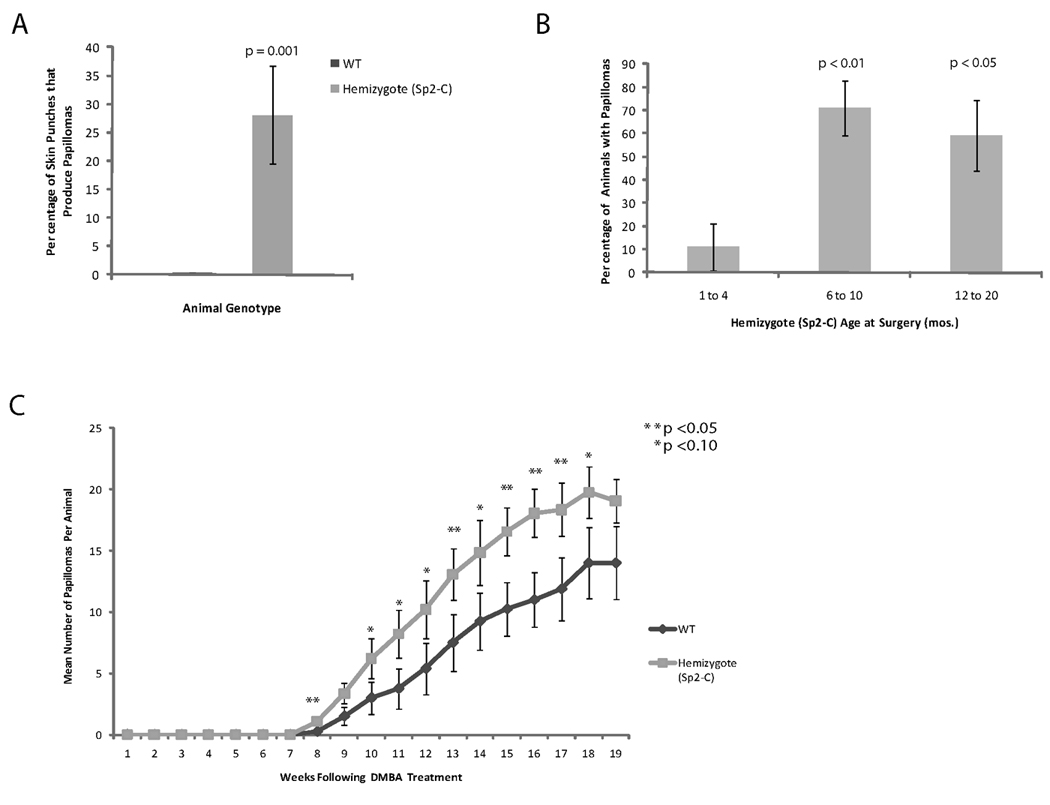
Incidence of wound- and DMBA/TPA-induced papillomas in wild-type and Sp2-C hemizygotes. A, percentage of surgical wounds that produced papillomas in wild-type and Sp2-C hemizygous littermates in animals between 1 and 20 months of age. Columns, mean (wild-type, n = 11 mice/group; Sp2-C, n = 48 mice/group); bars, SE. B, percentage of Sp2-C hemizygotes that developed wound-induced papillomas as a function of age at time of surgery. Columns, mean (1 to 4 months, n = 9 mice/group; 6 to 10 months, n = 17 mice/group; 12 to 20 months, n = 22 mice/group); bars, SE. C, mean number of DMBA/TPA-induced papillomas per animal (wild-type, n = 8 mice/group; Sp2-C, n = 14 mice/group) are plotted as a function of age at time of DMBA treatment. Bars, SE.
Histological examinations of wound-induced lesions revealed them to be pedunculated to sessile cutaneous papillomas, composed of epidermal hyperplasia and fibrovascular stroma that often contained mixed neutrophilic and lymphoplasmacytic inflammation (Fig. 4B). Multifocal areas of mild epidermal dysplasia were accompanied by mild to moderate keratinocyte apoptosis in the basal and immediate suprabasal layers, where lymphocyte satellitosis was occasionally noted. Skin sections prepared from wound-induced papillomas were examined by immunohistochemistry for markers of cell proliferation (PCNA) and keratinocyte differentiation (keratins 5, 6, 8, 10, 14, 15 and loricrin). Consistent with expectations, only a minority of basal cells within the epidermis at the margins of wound-induced papillomas stained with PCNA antibodies (Fig. 4C, left column). In stark contrast, PCNA-positive cells were detected throughout wound-induced papillomas in basal as well as suprabasal cell layers (Fig. 4C, right column). Differentiation markers (keratins 5, 14, and 15) expressed within cells of the basal cell layer in margin tissue were detected largely in suprabasal layers of wound-induced papillomas (Fig. 4C). Keratin 10 was detected in all suprabasal layers in margin tissue yet was detected weakly in the most superficial suprabasal layers of papillomas (Fig. 4C). Consistent with results noted earlier for post-natal transgenic homozygotes, keratins 6 and 8 were detected throughout the epidermis of wound-induced neoplasms (Fig. 4C). Finally, diffuse loricrin expression was detected in papillomas within an expanded suprabasal zone relative to its restricted expression within the cornified layer of margin tissue (Fig. 4C). We conclude from immunohistochemical results that wound-induced neoplasms are composed of highly proliferative, phenotypically immature keratinocytes that exhibit a profound disruption of the epidermal differentiation program.
Sp2 overexpression increases the sensitivity of hemizygous animals to skin carcinogenesis
To determine if Sp2 overexpression increases the sensitivity of basal keratinocytes to transformation by an environmental carcinogen, papillomagenesis in Sp2-C hemizygotes and control animals was analyzed using a "two-stage" model of skin carcinogenesis. Wild-type and hemizygous Sp2-C littermates were treated with a single application of DMBA followed by twice weekly treatments with TPA for 20 weeks. Sp2-C hemizygotes and wild-type littermates developed papillomas 5.5 and 7.5 weeks following DMBA treatment, respectively (data not shown), and DMBA/TPA-treated Sp2-C hemizygotes exhibited greater numbers of papillomas per animal throughout the course of this study (Fig. 5C). Treated animals were sacrificed prior to the progression of papillomas to squamous cell carcinomas and thus it was not possible to determine whether ectopic Sp2 expression affects the incidence of tumor progression. We conclude from these results that Sp2 overexpression in basal keratinocytes increases their sensitivity to an environmental carcinogen.
This study establishes that Sp2 overexpression inhibits the differentiation of epidermal keratinocytes, rendering these cells susceptible to oncogenesis. Indeed, the striking incidence of wound-induced papillomagenesis in Sp2-C hemizygotes indicates that Sp2 overexpression is sufficient, in the appropriate physiological milieu, to subvert mechanisms controlling basal cell proliferation and differentiation. Similar susceptibilities to wound-induced neoplasia have been reported in transgenic animals expressing potent oncogenes, e.g., Ha-ras or v-jun, in this same epidermal compartment (39–41). It will be of interest to determine whether wound-induced neoplasms in Sp2-C hemizygotes are dependent on inflammatory growth factors and cytokines released following wounding, as has been noted in other systems (42–45). Since stem cells supporting the interfollicular epidermis are located within the basal layer, our results suggest that Sp2 may regulate the commitment of progenitors in this, and perhaps additional, stem cell compartments. In keeping with this speculation, Sp2 overexpression is associated with the progression of human prostatic carcinoma and thus Sp2 may regulate the proliferation/differentiation of progenitor cells in tissues beyond the epidermis (14). To our knowledge this study provides the first direct evidence that Sp-family members can function as oncogenes and suggests that therapeutic strategies targeting Sp2 may prove efficacious.
Acknowledgments
Grant Support: National Cancer Institute grant CA105313, National Institute of General Medical Sciences grant GM065405, and funds supplied by the Jimmy V–NCSU Cancer Therapeutics Training Program.
This work was conducted in part in the Intramural Research Division of the NIH and NIEHS. The authors wish to thank members of the Horowitz, Smart, and Rodriguez-Puebla laboratories for reagents, protocols and helpful discussions. The authors also thank Dr. Angel Ramirez for plasmid 383, the Univ. of North Carolina Animal Models Core Facility for plasmid pTG1, and the veterinarians and technical staff of North Carolina State University associated with this project.
References
- 1.Phillipsen S, Suske G. A tale of three fingers: the family of mammalian Sp/XKLF transcription factors. Nucl Acids Res. 1999;27:2991–3000. doi: 10.1093/nar/27.15.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suske G. The Sp-family of transcription factors. Gene. 1999;238:291–300. doi: 10.1016/s0378-1119(99)00357-1. [DOI] [PubMed] [Google Scholar]
- 3.Suske G, Bruford E, Philipsen S. Mammalian SP/KLF transcription factors: Bring in the family. Genomics. 2005;85:551–556. doi: 10.1016/j.ygeno.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Zhao C, Meng A. Sp1-like transcription factors are regulators of embryonic development in vertebrates. Develop Growth Differ. 2005;47:201–211. doi: 10.1111/j.1440-169X.2005.00797.x. [DOI] [PubMed] [Google Scholar]
- 5.Bouwman P, Philipsen S. Regulation of the activity of Sp1-related transcription factors. Mol Cell Endocrinol. 2002;195:27–38. doi: 10.1016/s0303-7207(02)00221-6. [DOI] [PubMed] [Google Scholar]
- 6.Safe S, Abdelrahim M. Sp transcription factor family and its role in cancer. Eur J Cancer. 2005;41:2438–2448. doi: 10.1016/j.ejca.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Black AR, Black JD, Azizkhan-Clifford J. Sp1 and kruppel-like factor family of transcription factors in cell growth regulation and cancer. J Cell Physiol. 2001;188:143–160. doi: 10.1002/jcp.1111. [DOI] [PubMed] [Google Scholar]
- 8.Moorefield KS, Fry SJ, Horowitz JM. Sp2 DNA binding activity and trans-activation are negatively regulated in mammalian cells. J Biol Chem. 2004;279:13911–13924. doi: 10.1074/jbc.M313589200. [DOI] [PubMed] [Google Scholar]
- 9.Shi Y, Berg JM. A direct comparison of the properties of natural and designed zinc-finger proteins. Chem Biol. 1995;2:83–89. doi: 10.1016/1074-5521(95)90280-5. [DOI] [PubMed] [Google Scholar]
- 10.Thiesen HJ, Bach C. Target Detection Assay (TDA): a versatile procedure to determine DNA binding sites as demonstrated on SP1 protein. Nucleic Acids Res. 1990;18:3203–3209. doi: 10.1093/nar/18.11.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie J, Nichols TD, Yoder JA, Horowitz JM. Sp2 is a maternally inherited transcription factor required for embryonic development. J Biol Chem. 2010;285:4153–4164. doi: 10.1074/jbc.M109.078881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moorefield KS, Yin H, Nichols TD, Cathcart C, Simmons SO, Horowitz JM. Sp2 localizes to sub-nuclear foci associated with the nuclear matrix. Mol Biol Cell. 2006;17:1711–1722. doi: 10.1091/mbc.E05-11-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yin H, Nichols TD, Horowitz JM. Transcription of mouse Sp2 yields alternatively spliced and sub-genomic mRNAs in a tissue- and cell type-specific fashion. Biochim Biophys Acta. 2010 doi: 10.1016/j.bbagrm.2010.03.002. 10.1016/j.bbagrm.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baur F, Nau K, Sadic D, Allweiss L, Elsasser HP, Gillemans N, et al. Specificity protein 2 (Sp2) is essential for mouse development and autonomous proliferation of mouse embryonic fibroblasts. PLoS One. 2010;5:e9587. doi: 10.1371/journal.pone.0009587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phan D, Cheng CJ, Galfione M, Vakar-Lopez F, Tunstead J, Thompson NE, et al. Identification of Sp2 as a transcriptional repressor of carcinoembryonic antigen-related cell adhesion molecule 1 in tumorigenesis. Cancer Res. 2004;64:3072–3078. doi: 10.1158/0008-5472.can-03-3730. [DOI] [PubMed] [Google Scholar]
- 16.Pillai MM, Venkataraman GM, Kosak S, Torok-Storb B. Integration site analysis in transgenic mice by thermal asymmetric interlaced (TAIL)-PCR: segregating multiple-integrant founder lines and determining zygosity. Transgenic Res. 2007;17:749–754. doi: 10.1007/s11248-007-9161-4. [DOI] [PubMed] [Google Scholar]
- 17.Simmons SO, Horowitz JM. Nkx3.1 binds and negatively regulates the transcriptional activity of Sp-family members in prostate-derived cells. Biochem J. 2005;393:397–409. doi: 10.1042/BJ20051030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shim M, Powers KL, Ewing SJ, Zhu S, Smart RC. Diminished expression of C/EBPalpha in skin carcinomas is linked to oncogenic Ras and reexpression of C/EBPalpha in carcinoma cells inhibits proliferation. Cancer Res. 2005;65:861–867. [PubMed] [Google Scholar]
- 19.Oh HS, Smart RC. Expression of CCAAT/enhancer binding proteins (C/EBP) is associated with squamous differentiation in epidermis and isolated primary keratinocytes and is altered in skin neoplasms. J Invest Dermatol. 1998;110:939–945. doi: 10.1046/j.1523-1747.1998.00199.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhu S, Oh HS, Shim M, Sterneck E, Johnson PF, Smart RC. C/EBPbeta modulates the early events of keratinocyte differentiation involving growth arrest and keratin 1 and keratin 10 expression. Mol Cell Biol. 1999;19:7181–7190. doi: 10.1128/mcb.19.10.7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Owens DM, Wei S, Smart RC. A multihit, multistage model of chemical carcinogenesis. Carcinogenesis. 1999;20:1837–1844. doi: 10.1093/carcin/20.9.1837. [DOI] [PubMed] [Google Scholar]
- 22.Weissman BE, Aaronson SA. BALB and Kirsten murine sarcoma viruses alter growth and differentiation of EGF-dependent balb/c mouse epidermal keratinocyte lines. Cell. 1983;32:599–606. doi: 10.1016/0092-8674(83)90479-8. [DOI] [PubMed] [Google Scholar]
- 23.Paulson QX, McArthur MJ, Johnson DG. E2F3a stimulates proliferation, p53-independent apoptosis and carcinogenesis in a transgenic mouse model. Cell Cycle. 2006;5:184–190. doi: 10.4161/cc.5.2.2307. [DOI] [PubMed] [Google Scholar]
- 24.Ramirez F, Bravo A, Jorcano JL, Vidal M. Sequences 5' of the bovine keratin 5 gene direct tissue-and-cell-type-specific expression of a lacZ gene in the adult and during development. Differentiation. 1994;58:53–64. doi: 10.1046/j.1432-0436.1994.5810053.x. [DOI] [PubMed] [Google Scholar]
- 25.Robles AI, Larcher F, Whalin RB, Murillas R, Richie E, Gimenez-Conti IB, et al. Expression of cyclin D1 in epithelial tissues of transgenic mice results in epidermal hyperproliferation and severe thymic hyperplasia. Proc Natl Acad Sci USA. 1996;93:7634–7638. doi: 10.1073/pnas.93.15.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DiGiovanni J, Bol DK, Wilker E, Beltran L, Carbajal S, Moats S, et al. Constitutive expression of insulin-like growth factor-1 in epidermal basal cells of transgenic mice leads to spontaneous tumor promotion. Cancer Res. 2000;60:1561–1570. [PubMed] [Google Scholar]
- 27.Diamond I, Owolabi T, Marco M, Lam C, Glick A. Conditional gene expression in the epidermis of transgenic mice using the tetracycline-regulated transactivators tTA and rTA linked to the keratin 5 promoter. J Invest Dermatol. 2000;115:788–794. doi: 10.1046/j.1523-1747.2000.00144.x. [DOI] [PubMed] [Google Scholar]
- 28.Kreienkamp HJ, Zitzer H, Gundelfinger ED, Richter D, Bockers TM. The calcium-independent receptor for alpha-latrotoxin from human and rodent brains interacts with members of the ProSAP/SSTRIP/Shank family of multidomain proteins. J Biol Chem. 2000;275:32387–32390. doi: 10.1074/jbc.C000490200. [DOI] [PubMed] [Google Scholar]
- 29.Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- 30.Lane EB, McLean WH. Keratins and skin disorders. J Pathol. 2004;204:355–366. doi: 10.1002/path.1643. [DOI] [PubMed] [Google Scholar]
- 31.Oshima RG, Baribault H, Caulin C. Oncogenic regulation and function of keratins 8 and 18. Cancer Metastasis Rev. 1996;15:445–471. doi: 10.1007/BF00054012. [DOI] [PubMed] [Google Scholar]
- 32.Lu H, Hesse M, Peters B, Magin TM. Type II keratins precede type I keratins during early embryonic development. Eur J Cell Biol. 2005;84:709–718. doi: 10.1016/j.ejcb.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Yamada S, Wirtz D, Coulombe PA. Pairwise assembly determines the intrinsic potential for self-organization and mechanical properties of keratin filaments. Mol Biol Cell. 2002;13:382–391. doi: 10.1091/mbc.01-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Omary MB, Ku NO, Strnad P, Hanada S. Toward unraveling the complexity of simple epithelial keratins in human disease. J Clin Invest. 2009;119:1794–1805. doi: 10.1172/JCI37762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trempus CS, Morris RJ, Ehinger M, Elmore A, Bortner CD, Ito M, et al. CD34 expression by hair follicle stem cells is required for skin tumor development in mice. Cancer Res. 2007;67:4173–4181. doi: 10.1158/0008-5472.CAN-06-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trempus CS, Morris RJ, Bortner CD, Cotsarelis G, Faircloth RS, Reece JM, et al. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J Invest Dermatol. 2003;120:501–511. doi: 10.1046/j.1523-1747.2003.12088.x. [DOI] [PubMed] [Google Scholar]
- 37.Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, Lyle S, Yang Z, Cotsarelis G. Keratin 15 promoter targets putative epithelial stem cells in the hair follicle bulge. J Invest Dermatol. 2003;121:963–968. doi: 10.1046/j.1523-1747.2003.12600.x. [DOI] [PubMed] [Google Scholar]
- 39.Schuh AC, Keating SJ, Monteclaro FS, Vogt PK, Breitman ML. Obligatory wounding requirement for tumorigenesis in v-jun transgenic mice. Nature. 1990;346:756–760. doi: 10.1038/346756a0. [DOI] [PubMed] [Google Scholar]
- 40.Cannon RE, Spalding JW, Trempus CS, Szczesniak CJ, Virgil KM, Humble MC, et al. Kinetics of wound-induced v-Ha-ras transgene expression and papilloma development in transgenic Tg.AC mice. Mol Carcinog. 1997;20:108–114. doi: 10.1002/(sici)1098-2744(199709)20:1<108::aid-mc12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 41.Battalora MS, Spalding JW, Szczesniak CJ, Cape JE, Morris RJ, Trempus CS, et al. Age-dependent skin tumorigenesis and transgene expression in the Tg.AC (v-Ha-ras) transgenic mouse. Carcinogenesis. 2001;22:651–659. doi: 10.1093/carcin/22.4.651. [DOI] [PubMed] [Google Scholar]
- 42.Vassar R, Fuchs E. Transgenic mice provide new insights into the role of TGF-alpha during epidermal development and differentiation. Genes Dev. 1991;5:714–727. doi: 10.1101/gad.5.5.714. [DOI] [PubMed] [Google Scholar]
- 43.Martins-Green M, Boudreau N, Bissell MJ. Inflammation is responsible for the development of wound-induced tumors in chickens infected with Rous sarcoma virus. Cancer Res. 1994;54:4334–4341. [PubMed] [Google Scholar]
- 44.Sieweke MH, Thompson NL, Sporn MB, Bissell MJ. Mediation of wound-related Rous sarcoma virus tumorigenesis by TGF-beta. Science. 1990;248:1656–1660. doi: 10.1126/science.2163544. [DOI] [PubMed] [Google Scholar]
- 45.Stuelten CH, Barbul A, Busch JI, Sutton E, Katz R, Sato M, et al. Acute wounds accelerate tumorigenesis by a T cell-dependent mechanism. Cancer Res. 2008;68:7278–7282. doi: 10.1158/0008-5472.CAN-08-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]