Abstract
Activation of extracellular signal-regulated kinase (ERK) in spinal cord neurons could serve as a marker for sensitization of dorsal horn neurons in persistent pain. ERK is normally activated by high-threshold noxious stimuli. We investigated how low-threshold mechanical stimuli could activate ERK after complete Freund’s adjuvant (CFA)-induced inflammation. Unilateral injection of CFA induced ipsilateral heat hyperalgesia and bilateral mechanical allodynia. CFA-induced ERK activation in ipsilateral dorsal horn neurons declined after 2 days. Interestingly, low threshold mechanical stimulation given by light touch either on the inflamed paw or the contralateral non-inflamed paw dramatically increased ERK phosphorylation (pERK) in the dorsal horn ipsilateral to touch stimulation. Notably, light touch induced pERK mainly in superficial neurons in laminae I-IIo. Intrathecal administration of the astroglial toxin L-α-aminoadipate (L-α-AA) on post-CFA day 2 reversed CFA-induced bilateral mechanical allodynia but not heat hyperalgesia. Furthermore, L-α-AA, the glial inhibitor fluorocitrate, and a peptide inhibitor of c-Jun N-terminal Kinase (JNK) all reduced light touch-evoked ERK activation ipsilateral to touch. Collectively, these data suggest that (a) ERK can be activated in superficial dorsal horn neurons by low threshold mechanical stimulation under pathological condition and (b) ERK activation by light touch is associated with mechanical allodynia and requires an astrocyte network.
Keywords: ERK, Complete Freund’s adjuvant, Mechanical allodynia, Astrocytes, JNK
The extracellular signal-regulated kinase (ERK, including ERK1 and ERK2) is a member of mitogen-activated protein kinase (MAPK) family. Early studies indicated a critical role of ERK in regulating mitosis, proliferation, differentiation, and survival of mammalian cells during development (Widmann et al. 1999). Recently, it has been demonstrated that ERK also plays an important role in neuronal plasticity after peripheral inflammation and nerve injury (Ji and Woolf 2001; Ji et al. 2003; Ji et al. 2009). Normally, ERK activation (phosphorylation) in the spinal cord is only induced by high-threshold noxious stimuli and this activation is specifically localized in dorsal horn neurons of the ipsilateral medial superficial spinal cord where primary nociceptive afferents from the hindpaw terminate (Ji et al. 1999). Of note ERK activation can be suppressed by analgesic compounds (Karim et al. 2001; Ji and Strichartz 2004; Kawasaki et al. 2006). Spinal inhibition of ERK activation by a MEK inhibitor has also been shown to inhibit inflammatory pain hypersensitivity (Ji et al. 2009). Thus, pERK (phosphorylated ERK) expression is regarded as a marker for the sensitization of dorsal horn neurons (central sensitization) following persistent nociceptive activity (Gao and Ji 2009).
Cruz et al. have shown that movement of normal joint does not induce ERK activation in the spinal cord but movement of the inflamed joint in monoarthritic rats elicits a marked ERK activation in dorsal horn neurons (Cruz et al. 2005). Gentle touch also induces pERK expression in dorsal horn neurons after peripheral nerve injury (Wang et al. 2004; Hao et al. 2005). These data suggest innocuous stimulation is also capable of evoking ERK activation under pathological conditions.
Tissue injury or inflammation induces pain hypersensitivity, characterized by both mechanical allodynia (pain in response to normally innocuous stimuli) and heat hyperalgesia (enhanced pain in response to noxious stimuli). Especially, mechanical allodynia is observed not only in the injured region but also in adjacent non-injured regions and even in contralateral side of the body (Koltzenburg et al. 1999; Woolf and Salter 2000; Milligan et al. 2003; Baron 2009; Gao et al. 2010). Unilateral injection of complete Freund’s adjuvant (CFA) has been shown to induce mechanical allodynia in both the ipsilateral and contralateral paws (Bertorelli et al. 1999; Nagakura et al. 2003; Raghavendra et al. 2004; Ambalavanar et al. 2006). Inflammation also produces bilateral increases in the expression of cyclooxygenase-2 (COX2) (Samad et al. 2001), phosphorylation of the transcription factor cAMP response element binding protein (CREB) (Ji and Rupp 1997; Messersmith et al. 1998) and c-Jun N-terminal kinase (JNK) in the spinal cord (Gao et al. 2010). Particularly, JNK is activated in spinal astrocytes after nerve injury and CFA inflammation (Zhuang et al. 2006; Gao et al. 2010). Intrathecal administration of JNK inhibitor (Gao et al. 2010) or astrocyte function inhibitor (Milligan et al. 2003) can prevent and reverse inflammation-induced mechanical allodynia bilaterally. However, ERK activation by low threshold mechanical stimulation under inflammation has not been well characterized and the role of astrocyte network in this activation is also unknown.
Materials and methods
Animals
Male adult Sprague Dawley rats (220–260 g) were used under Harvard Medical School Animal Care institutional guidelines. Peripheral inflammatory pain was induced by an s.c. injection of CFA (Sigma, St. Louis, MO) (100 μl) in the plantar surface of the left hind paws under a brief anesthesia with sevofluorane. On day 2 after CFA injection, the animals were anesthetized with isoflurane, light touch stimuli were applied manually by a cotton tip to the ventral surface of the hindpaw, once every 5 sec for 5 min. This touch stimulus did not elicit withdrawal response in normal animals.
Drug administration
For intrathecal injection, spinal cord puncture was made under brief sevofluorane anesthesia with a 27 gauge needle between the L5 and L6 level to deliver the reagents (20 μl) to the CSF (Zhuang et al. 2006). Immediately after the needle entry into subarachnoid space (change in resistance), a brisk tail flick could be observed. The peptide inhibitor of JNK, D-JNKI-1 was kindly provided by Dr. Christopher Bonny, University of Lausanne, Switzerland (Borsello et al. 2003). L-α-aminoadipate (L-α-AA) and fluorocitrate were purchased from Sigma. The L-α-AA and D-JNKI-1 were dissolved in 0.01 M PBS. The fluorocitrate was dissolved initially in 2 M HCl (0.1 μmol/μL) and then diluted in 0.01 M PBS to attain a final concentration of 0.1 nmol/μl (pH 6.0). The vehicle control for fluorocitrate is 0.1% 2M HCL in 0.01M PBS (pH 6.0) (Milligan et al. 2003).
Immunohistochemistry
Animals were terminally anesthetized with isoflurane and perfused through the ascending aorta with saline followed by 4% paraformaldehyde/1.5% picric acid. After the perfusion, the spinal cord segments (L4-5) were removed and postfixed in the same fixative overnight, then replaced with 15% sucrose overnight. Spinal sections (transverse, free-floating, 30 μm) were cut in a cryostat. For pERK immunostaining, the sections were blocked with 2% goat serum in 0.3% Triton for 1 h at RT and incubated over night at 4°C with anti-pERK antibody (anti-rabbit, 1:500, Cell Signaling). The sections were then incubated for 1 h at RT with Cy3-conjugated secondary antibody. For double immunostaining of pERK with PKCγ, NeuN, GFAP, or Iba1, the sections were blocked with 2% goat serum and incubated with a mixture of primary antibodies of pERK (mouse or rabbit, 1:500, Cell signaling), PKCγ (rabbit, 1:1000, Santa Cruz), NeuN (mouse, 1:2000, Millipore), GFAP (mouse, 1:5000, Millipore), and Iba1 (rabbit, 1:5000, Wako), followed by a mixture of corresponding secondary antibodies conjugated with either Cy3 or FITC (1:400, Jackson ImmunoResearch). Stained sections were examined with a Nikon (Tokyo, Japan) fluorescence microscope, and images were captured with a CCD Spot camera.
Behavioral analysis
Animals were habituated to the testing environment daily for at least 2 d before baseline testing. The room temperature and humidity remained stable for all experiments. For testing mechanical sensitivity, animals were put under inverted plastic boxes (11×13×24 cm) on an elevated mesh floor and allowed 30 min for habituation, before the threshold testing. Mechanical allodynia was tested using von Frey hairs. The paw was pressed with one of a series of von Frey hairs with logarithmically incrementing stiffness (from 0.6 to 26 g) (Stoelting), presented perpendicular to the plantar surface. The 50% withdrawal threshold was determined using Dixon’s up–down method (Chaplan et al. 1994). For testing heat sensitivity, animals were put in a plastic box placed on a glass plate, and the plantar surface was exposed to a beam of radiant heat through a transparent Perspex surface (Hargreaves et al. 1988). The baseline latencies were adjusted to 10–13 sec with a maximum of 20 sec as cutoff to prevent potential injury.
Paw edema measurement
Animals were briefly anesthetized by sevofluorane and the volume of both hindpaws was measured using the plethysmometer (Ugo Basile, Italy).
Quantification and statistics
For the quantification of pERK-IR profiles, four to five non-adjacent sections (30 μm) from the L5-spinal cord segments were randomly selected and the number of pERK-positive cells in the superficial dorsal horn (laminae I-II) was counted. Three to four rats were included in each group for quantification. All the data were presented as mean ± SEM and analyzed with student’s t-test or ANOVA followed by Newman–Keuls test. P < 0.05 was considered statistically significant in all cases.
Results
Light touch on inflamed (ipsilateral) paw induces ERK activation in the superficial spinal cord
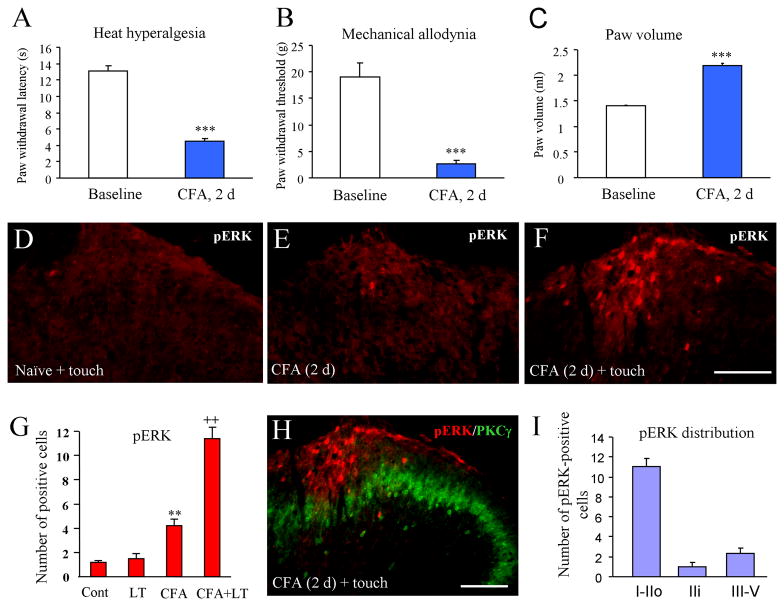
Two days after intraplantar injection of CFA, paw withdrawal latency was significantly decreased (baseline: 13.1 ± 0.67 s vs 4.5 ± 0.4 s, P < 0.05), indicating the development of heat hyperalgesia (Fig. 1A). Paw withdrawal threshold was also dropped from 19.1 ± 2.7 g to 2.6 ± 0.7 g (P < 0.05, Fig. 1B) indicating the development of mechanical allodynia. On post-CFA day 2, the paw volume also increased by 55% (Fig. 1C) indicating a robust edema in the CFA inflammatory pain model.
Fig. 1.
Light touch on the inflamed paw induces ERK activation 2 days after CFA injection. (A–C) CFA induces heat hyperalgesia (A), mechanical allodynia (B), and edema on the ipsilateral paw (C). ***P<0.001, compared to baseline. (D) pERK was not induced by light touch on non-inflamed naïve paw. (E) Low level of pERK in the ipsilateral dorsal horn on post-CFA day 2. (F) Light touch on the inflamed paw induces dramatic increase of pERK expression in the dorsal horn. (G) Histogram shows the number of pERK-positive cells in the superficial dorsal horn (laminae I–II). (H) Double staining of pERK and PKCγ shows the lamina distribution of pERK in the spinal cord. (I) Histogram shows the number of pERK positive cells in different lamina. ** P<0.01, compared to control; ++ P<0.01, compared to CFA only. Scale bar, 100 μm
Our previous study showed that CFA induced a rapid ERK activation in the spinal cord dorsal horn. The number of pERK neurons peaked at 10 min but pERK induction largely declined at 48 hours (Ji et al. 1999). Consistently, pERK expression in the dorsal horn was low on day 2 (Fig. 1E). But light touch of the inflamed paw for 5 min dramatically increased pERK expression (Fig. 1F, G). The pERK-positive cells were mainly located in the medial half of the dorsal horn in L4 and L5 lumbar segments where primary nociceptive afferents from the hindpaw terminate. In contrast, pERK was not induced by light touch in non-inflamed naïve animals (Fig. 1D, G).
In order to identify the lamina distribution of pERK expression, we did pERK and PKCγ double staining, because PKCγ is known to express mainly in neurons of the inner part of lamina II (Malmberg et al. 1997; Neumann et al. 2008). Most pERK-positive cells were found in lamina I and outer lamina II (Fig. 1H), although a few positive cells were also observed in laminae III–IV (Fig. 1I).
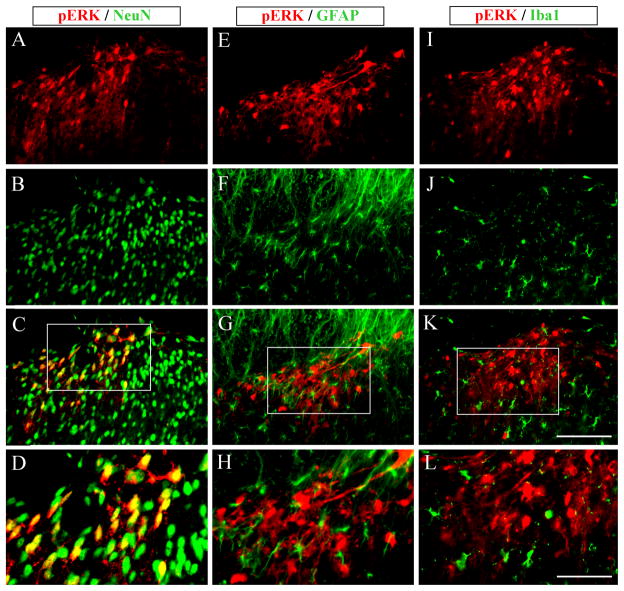
To characterize the cell types expressing pERK in the dorsal horn, we performed double staining of pERK with different cell markers. pERK-IR was colocalized with the neuronal marker NeuN (Fig. 2A–D), but not with astrocytic marker GFAP (Fig. 2E–H) or microglia marker OX-42 (Fig. 2I–L). Thus, light touch-induced ERK activation is restricted to neurons.
Fig. 2.
Cellular distribution of light touch-induced pERK. pERK positive cells are colocalized with NeuN (A–C), a marker for neurons, but not with GFAP (E–G), a marker for astrocytes, or Iba1(I–K), a marker for microglia (I). Scale bar, 100 μm. D, H, and L are higher magnification images of C, G, and K, respectively. Scale bar, 50 μm.
Light touch on non-inflamed (contralateral) paw also induces ERK activation in the superficial spinal cord
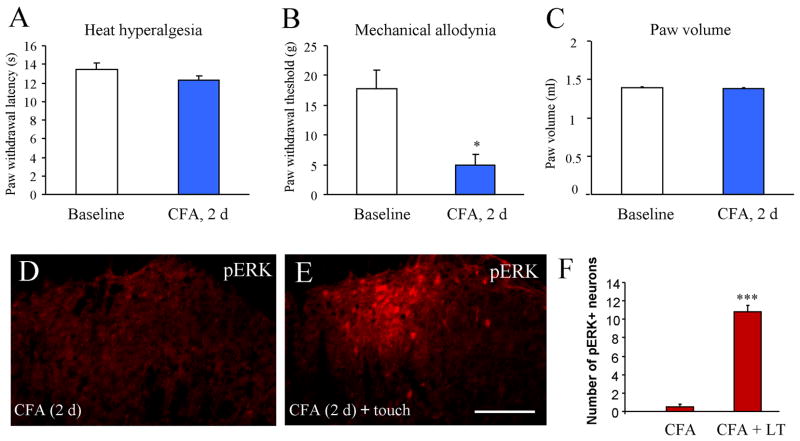
We also checked heat hyperalgesia, mechanical allodynia and paw edema on the non-inflamed (contralateral) paw. Two days after CFA injection, the paw withdrawal latency on the contralateral paw was comparable to that of baseline (13.5 ± 0.6 s vs 12.3 ± 0.4 s, P > 0.05, Fig. 3A). On the contrary, the paw withdrawal threshold was significantly decreased (17.7 ± 3.2 g vs. 5.0 ± 1.7 g, P < 0.01; Fig. 3B). However, the volume of the contralateral paw did not increase (P > 0.05, compared to baseline; Fig. 3C), indicating that there is no edema on the contralateral paw. These results suggest that CFA-induced contralateral mechanical allodynia may be caused by central mechanisms.
Fig. 3.
Light touch on the contralateral non-inflamed paw induces ERK expression in the superficial dorsal horn 2 days after CFA injection. (A) CFA does not induce heat hyperalgesia in the contralateral paw. (B) CFA induces contralateral mechanical allodynia. (C) CFA does not elicit edema in the contralateral paw. *P<0.05, compared to baseline. (D) Very few cells express pERK in the contralateral spinal cord on post-CFA day 2. (E) Light touch on the non-inflamed paw induces pERK expression in the superficial dorsal horn. (F) Histogram shows the number of pERK expression in the dorsal horn with or without light touch (LT). Scale bar, 100 μm
pERK expression was very low in the spinal cord contralateral to the CFA injection (Fig. 3D). Strikingly, light touch of the contralateral paw for 5 min also induced dramatic pERK expression in the spinal cord dorsal horn ipsilateral to touch stimulation (Fig. 3E, F). The distribution pattern of pERK induced by light touch of the non-inflammaed paw was similar to pERK induced by light touch of the inflamed paw: pERK positive neurons were localized in the medial half of the superficial dorsal horn (Fig. 3E)
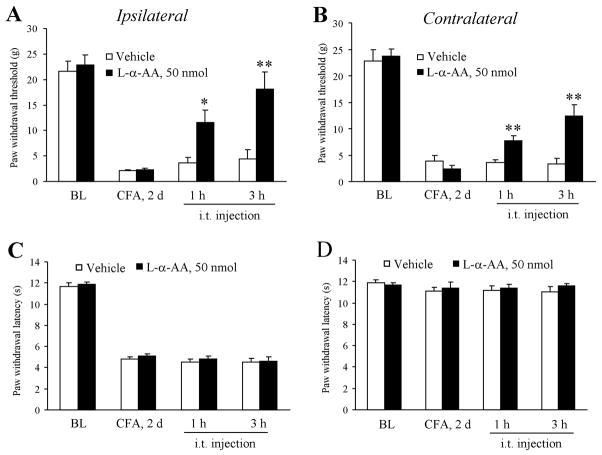
Astroglial toxin L-α-aminoadipate (L-α-AA) reverses CFA-induced mechanical allodynia bilaterally
To test if the astrocytes are involved in CFA-induced bilateral mechanical allodynia, we inhibited spinal cord astrocytes by intrathecal injection of an astroglial toxin L-α-AA at the dose of 50 nmol (20 μl). This dose was chosen according to our previous dose-response study in the nerve injury model (Zhuang et al. 2006). As shown in Fig. 4A,B, CFA-induced mechanical allodynia on the ipsilateral paw on post-CFA day 2 was significantly reduced at both 1 h (P < 0.05, compared to saline control) and 3 h (P < 0.05, compared to saline control) after intrathecal L-α-AA. Similarly, L-α-AA increased paw withdrawal threshold at 1 h (P < 0.01, compared to saline control) and 3 h (P < 0.01, compared to saline control) on the contralateral paw. In sharp contrast, CFA-induced heat hyperalgesia on the ipsilateral paw was not affected by L-α-AA at 1 h (P > 0.05) or 3 h (P > 0.05). Neither did L-α-AA affect paw withdrawal latency on the contralateral paw (Fig. 4C–D).
Fig. 4.
Intrathecal injection of the astroglial toxin L-alpha aminoadipate (L-α-AA) reverses CFA-induced mechanical allodynia but not heat hyperalgesia. (A, B) Intrathecal injection of L-α-AA (50 nmol) on post-CFA day 2 reduces mechanical allodynia on the ipsilateral paw (A) and contralateral paw (B). (C–D) L-α-AA fails to change the paw withdrawal latency on the ipsilateral paw (C) and contralateral paw (D). * P<0.05; ** P<0.01 compared to vehicle control.
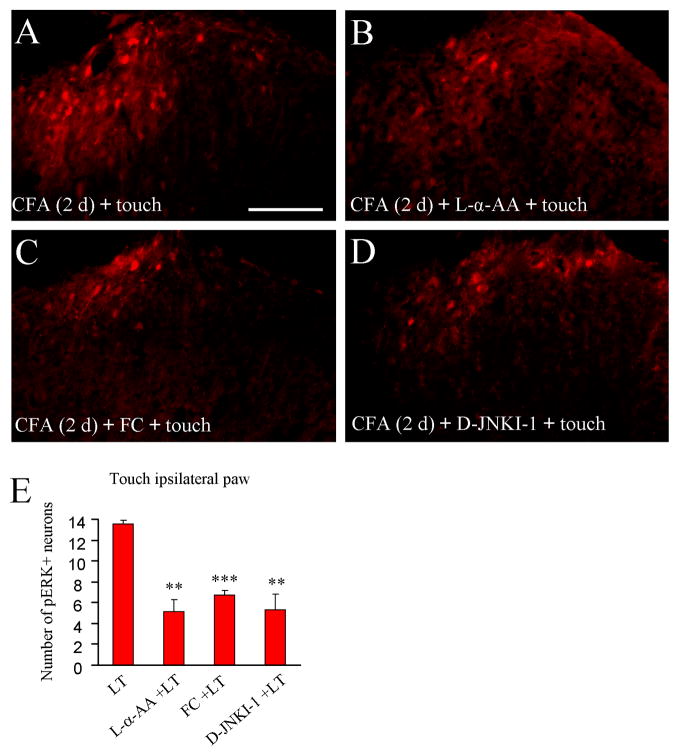
L-α-AA, fluorocitrate, and D-JNKI-1 independently reduce ERK activation by light touch on the inflamed paw
To test if the astrocyte network is involved in touch-evoked pERK expression in the spinal cord after inflammation, we intrathecally injected L-α-AA on post-CFA day 2. Three hours after the drug administration, the animals were given 5-min light touch and perfused immediately after the stimulation. Light touch-induced ERK activation in the superficial dorsal horn was substantially reduced by L-α-AA (P < 0.01, Fig. 5B,E).
Fig. 5.
Intrathecal injection of astrocyte toxins and JNK inhibitor decreases ERK activation following light touch on the inflamed paw. Light touch of the inflamed paw induces pERK expression in the superficial dorsal horn (A), which is inhibited by pretreatment with L-α-AA (B), fluorocitrate (FC, C) and D-JNKI-1 (D). (E) Histogram shows that light touch-induced pERK expression is decreased by pretreatment with L-α-AA, fluorocitrate (FC) or D-JNKI-1 given 3 hours before light touch (LT). ** P<0.01; *** P<0.001 compared to light touch group. Scale bar, 100 μm
Fluorocitrate has been widely used as a glial inhibitor. Low doses of fluorocitrate (< 1 nmol) specifically disrupt glial metabolism by blocking aconitase, an enzyme found in the tricarboxylic acid cycle of glia but not neurons (Hassel et al. 1992; Milligan et al. 2003). Intrathecal fluorocitrate (1 nmol) 3 hours before light touch on post-CFA day 2 also inhibited light touch-induced pERK expression (P < 0.001, Fig. 5C,E).
D-JNKI-1 is a highly specific peptide inhibitor of c-Jun N-terminal kinase (JNK) (Borsello et al. 2003). Interestingly JNK is exclusively activated in the spinal cord astrocytes after spinal nerve ligation and CFA inflammation (Zhuang et al. 2006; Gao et al. 2010). Our previous study showed that intrathecal D-JNKI-1 (4 nmol) reversed CFA-induced mechanical allodynia bilaterally (Gao et al. 2010). Intrathecal D-JNKI-1 (4 nmol) on post-CFA day 2 also suppressed light touch-induced ERK activation (P < 0.01, Fig. 5D,E).
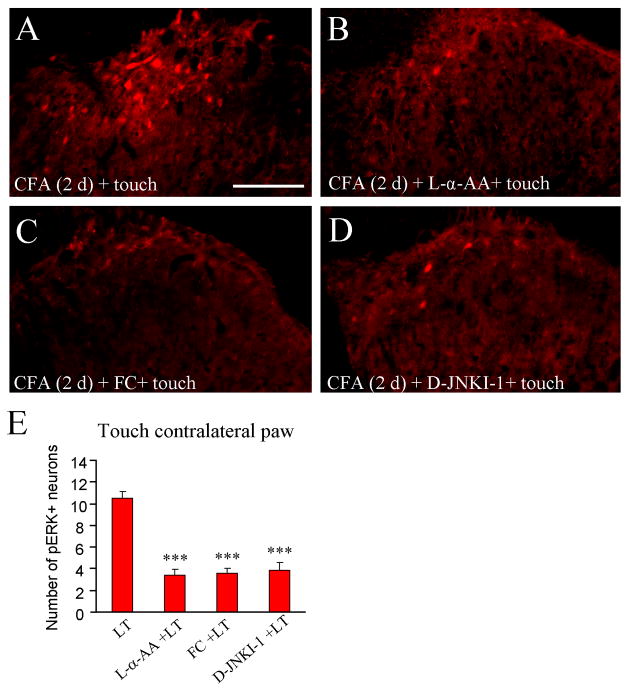
L-α-AA, fluorocitrate, and D-JNKI-1 independently reduce ERK activation by light touch on the non-inflamed paw
We also investigated the effects of L-α-AA, fluorocitrate, and D-JNKI-1 on pERK expression elicited by light touch of the non-inflamed paw contralateral to the CFA injection. As shown in Fig. 6A–E, light touch-induced pERK expression in the spinal cord dorsal horn ipsilateral to touch was significantly reduced by pretreatment of L-α-AA (P < 0.001), fluorocitrate (P < 0.001), and D-JNKI-1 (P < 0.001), given 3 hours before the touch stimulation.
Fig. 6.
Intrathecal injection of astrocyte toxins and JNK inhibitor decreases ERK activation following light touch (LT) on the non-inflamed paw. Light touch on the non-inflamed paw induces pERK expression (A), which is decreased by pretreatment with Lα-AA (B), fluorocitrate (FC, C) and D-JNKI-1 (D). E. Histogram shows the number of pERK-positive cells in the superficial dorsal horn. *** P<0.001 compared to light touch group. Scale bar, 100 μm
Discussion
In this study, we examined ERK activation in the spinal cord following light touch on the inflamed or non-inflamed paw in the rat CFA model. We have three interesting findings. First, light touch of either inflamed or non-inflamed paw induced marked ERK activation in the medial superficial dorsal horn neurons. Second, intrathecal injection of the astrocyte inhibitor L-α-AA inhibited CFA-induced bilateral mechanical allodynia and ERK activation induced by light touch on either inflamed paw or non-inflamed paw. Third, the glial inhibitor fluorocitrate and the JNK inhibitor D-JNKI-1 also inhibited light touch-induced ERK activation. These findings indicate under pathological condition low-threshold mechanical stimulation can also activate ERK and spinal cord astrocytes are required for this activation.
Light touch induces ERK activation in superficial dorsal horn neurons after inflammation
Under normal conditions, only noxious stimuli, such as noxious chemical stimulation (e.g., capsaicin), heat, and noxious electrical stimulation (activation of C- and Aδ fibers) are able to activate ERK, whereas innocuous stimuli such as light touch, warm water, and electrical stimulation of Aβ-fibers fail to activate ERK (Ji et al. 1999). Noxious stimuli-induced ERK activation is very rapid, reaching to a peak with 2–5 min (Ji et al. 1999). CFA also elicits rapid ERK activation in neurons in the superficial layers (laminae I–IIo) of the dorsal horn, peaking at 10 min, maintaining at 6 h, but declining at 48 h (Ji et al. 2002). CFA-induced ERK activation was only found in the ipsilateral side of the spinal cord (Ji et al. 2002). However, after CFA-induced monoarthritis, innoxious stimulation of the inflamed joint increases ERK activation in spinal cord neurons (Cruz et al. 2005). Gentle touch stimulation also induces pERK expression in dorsal horn neurons after peripheral nerve injury (Wang et al. 2004; Hao et al. 2005). In parallel, our data showed that light touch of the inflamed paw also induced rapid and marked ERK activation in spinal cord neurons. These data support an important role of ERK activation in neuroplasticity in the spinal cord under persistent pain conditions. Although we found ERK activation only in neurons 2 days after CFA inflammation, we should not exclude the possibility that ERK may also be activated in glial cells such as astrocytes in later time points in this inflammatory pain model (Weyerbacher et al. 2010).
CFA-induced mechanical allodynia is not only limited in the ipsilateral side but also found in the contralateral side (Bertorelli et al. 1999; Nagakura et al. 2003; Raghavendra et al. 2004; Ambalavanar et al. 2006; Gao et al. 2010). In numerous clinical pain syndromes, pain hypersensitivity not only occurs in the ipsilateral side, but also arises from the body side contralateral to the injury (Maleki et al. 2000; Woda and Pionchon 2000). In this study, we are the first group to report that light touch on non-inflamed contralateral paw is also able to induce strong pERK expression in the spinal cord neurons. Because we did not find edema and heat hyperalgesia in the contralateral paw, ERK activation by touching the contralateral non-inflamed paw is likely to be driven by central mechanisms such as spinal cord mechanisms.
The contribution of astrocytes to CFA-induced mechanical allodynia
Low-threshold mechanical stimulation such as light touch is conducted by large myelinated Aβ fibers that project to the deep dorsal horn (laminae II–IV) (Woolf et al. 1992). After intense noxious stimulation, tissue injury and inflammation, low-threshold Aβ input can activate nociceptive pathways. Innocuous input may activate PKCγ interneurons in the dorsal horn via myelinated afferent fibers (Neumann et al. 2008). Loss of inhibitory input to PKCγ neurons that also receive innocuous input from Aβ fibers contributes to the activation of nociceptive specific neurons in the superficial dorsal horn after intracisternal injection of strychnine (Miraucourt et al. 2007; Miraucourt et al. 2009). However, it would be difficult to explain contralateral pain by the disinhibition mechanism.
Mounting evidence indicates that astrocytes play an important role in enhancing and maintaining chronic pain (Hansson 2006; Ji et al. 2006; Ren and Dubner 2008; Romero-Sandoval et al. 2008; Hald 2009; McMahon and Malcangio 2009). Astrocytes are persistently activated in the spinal cord in chronic pain conditions under nerve injury, spinal cord injury, and cancer (Ji et al. 2006). Astrocytes have strong structural interrelationship with neurons by enwrapping synaptic terminals, enabling signaling between glia and neurons. Also, astrocytes form widespread networks via gap junctions and propagate calcium waves to a long distance (Giaume and McCarthy 1996; Haydon 2001). Inhibition of astroglial function by fluorocitrate (Milligan et al. 2003; Clark et al. 2007; Okada-Ogawa et al. 2009) or L-α-AA (Zhuang et al. 2006) has been shown to attenuate nerve injury- or nerve inflammation-induced mechanical allodynia. Furthermore, fluorocitrate inhibits sciatic inflammatory neuropathy-induced mechanical allodynia on the contralateral side (Milligan et al. 2003). Blockade of astrocytic gap junction also suppresses mechanical allodynia in the contralateral paw (Spataro et al. 2004). In this study, our results showed that intrathecal injection of L-α-AA reversed CFA-induced mechanical allodynia in both the ipsilateral side and contralateral side but had no effect on the CFA-induced heat hyperalgesia. Collectively, these data suggest that astrocytes and astrocyte network play an important role in regulating mechanical allodynia and contralateral pain.
Recently we showed that CFA induced persistent bilateral activation of JNK in the spinal cord. Inhibition of spinal JNK by intrathecal D-JNKI-1 dose-dependently reduced CFA-induced mechanical allodynia bilaterally, but only high dose of D-JNKI-1 decreased ipsilateral heat hyperalgesia (Gao et al. 2010). In the spinal cord, pJNK was exclusively expressed in the astrocytes, although not all astrocytes express pJNK (Zhuang et al. 2006). Thus, activation of JNK in astrocytes is involved in bilateral mechanical allodynia after inflammation.
The contribution of astrocyte network and JNK signaling pathway to light touch-evoked neuronal activation in the spinal cord
Similar to CFA-induced bilateral mechanical allodynia, light touch either the inflamed paw or non-inflamed paw induced ERK activation in dorsal horn neurons. It is worthy to note that pERK-positive neurons were mainly distributed in laminae I and IIo of the superficial dorsal horn, where Aδ- and C-fibers terminate. In contrast, Ma and Woolf (Ma and Woolf 1996) showed that low intensity touch stimuli applied at 24 h and 48h after CFA injection elicited significant increase of Fos-immunoreactive neurons in both the superficial (laminae I and II) and deep laminae (laminae III–VI) of the dorsal horn. Electrical stimulation of the sciatic nerve 24h post-CFA injection, at a strength only sufficient to activate Aβ-afferents fibers also elicited a significant c-fos increase in laminae V–VI (Ma and Woolf 1996). Since light touch only activates ERK in the superficial dorsal horn and Aβ-afferents normally do not project to the superficial dorsal horn [but see (Boada and Woodbury 2008)], we explored a possible involvement of astrocyte network in activating ERK in superficial dorsal horn neurons. However, we should not exclude the following possibilities. First, Aβ-afferent stimulation after CFA may activate superficial dorsal horn neurons via polysynaptic mechanisms (Baba et al. 1999). Second, Aβ-afferent may activate laminae I and IIo neurons via activation of PKCγ neurons in lamina IIi (Neumann et al. 2008). Third, activation of unmyelinated, low threshold mechanoreceptors (C-LTMRs) that express vesicle glutamate transporter-3 (VGLT3) after inflammation may direct activate ERK in the laminae I and IIo (Seal et al. 2009).
Our results showed that light touch-induced ERK activation in laminae I and IIo neurons was independently inhibited by astroglial toxins L-α-AA and fluorocitrate and the JNK inhibitor D-JNKI-1, in support of a role of astrocytes and astrocyte network in the activation of spinal cord neurons by light touch. How can astrocytes activate ERK in dorsal horn neurons? Activation of JNK in astrocytes may plays a role in the release of inflammatory mediators such as nitric oxide, PGE2 and IL-6 (Falsig et al. 2004). These mediators can potentially activate ERK in neurons. Astrocytes are also known to produce IL-1β (Guo et al. 2007; Zhang et al. 2008; Weyerbacher et al. 2010), which can activate ERK too. In particular, JNK activation in astrocytes results in the synthesis and release of the chemokines such as monocyte chemoattractant protein-1 (MCP-1 or CCL2) (Gao et al. 2010). MCP-1 can strongly activate ERK in superficial dorsal horn neurons both in vivo and ex vivo in isolated spinal cord slices (Gao et al. 2009).
Concluding remarks
Accumulating evidence supports a critical role of ERK activation in spinal cord neurons for the induction and maintenance of central sensitization (Ji et al. 1999; Karim et al. 2001; Ji et al. 2003; Ji et al. 2009). ERK can induce central sensitization via increasing the activity of excitatory glutamate receptors (AMPA and NMDA) (Kohno et al. 2008; Xu et al. 2010) and suppressing the activity of inhibitory potassium channels (Kv4.2) (Hu et al. 2006) in dorsal horn neurons. ERK can also maintain central sensitization via CREB-mediated gene transcription (Ji and Rupp 1997; Ji et al. 2002; Kawasaki et al. 2004). Intrathecal injection of MEK inhibitor has been shown to reduce inflammatory pain (Ji et al. 2002; Adwanikar et al. 2004; Cruz et al. 2005).
In this study, we have shown that low-threshold mechanical stimulation (light touch) could also activate ERK in superficial dorsal horn neurons after persistent inflammatory pain. This ERK activation could occur not only after touching the inflamed paw but also after touching the contralateral non-inflamed paw, in strong support of the presence of contralateral mechanical allodynia. Moreover, we have demonstrated a critical role of astrocytes and astrocyte network for light touch-evoked ERK activation and bilateral mechanical allodynia. Taken together, our data suggest spinal astrocytes play an important role in mediating mechanical allodynia and the spread pain after inflammation via possible activation of the ERK signaling pathway.
Acknowledgments
This study was supported by NIH grants NS54932 and DE17794 to RRJ and Program for New Century Excellent Talents in University (NCET-09-0164) to YJG.
Abbreviations use
- AMPA
alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate
- CFA
complete Freund’s adjuvant
- COX2
cyclooxygenase-2
- CREB
cAMP response element binding protein
- ERK
extracellular signal-regulated kinase
- FC
fluorocitrate
- GFAP
glial fibrillary acidic protein
- L-α-AA
L-α-aminoadipate
- JNK
c-Jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- VGLT
vesicle glutamate transporter
References
- Adwanikar H, Karim F, Gereau RWt. Inflammation persistently enhances nocifensive behaviors mediated by spinal group I mGluRs through sustained ERK activation. Pain. 2004;111:125–135. doi: 10.1016/j.pain.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Ambalavanar R, Dessem D, Moutanni A, Yallampalli C, Yallampalli U, Gangula P, Bai G. Muscle inflammation induces a rapid increase in calcitonin gene-related peptide (CGRP) mRNA that temporally relates to CGRP immunoreactivity and nociceptive behavior. Neuroscience. 2006;143:875–884. doi: 10.1016/j.neuroscience.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Baba H, Doubell TP, Woolf CJ. Peripheral inflammation facilitates Abeta fiber-mediated synaptic input to the substantia gelatinosa of the adult rat spinal cord. J Neurosci. 1999;19:859–867. doi: 10.1523/JNEUROSCI.19-02-00859.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R. Neuropathic pain: a clinical perspective. Handbook of experimental pharmacology. 2009:3–30. doi: 10.1007/978-3-540-79090-7_1. [DOI] [PubMed] [Google Scholar]
- Bertorelli R, Corradini L, Rafiq K, Tupper J, Calo G, Ongini E. Nociceptin and the ORL-1 ligand [Phe1psi (CH2-NH)Gly2]nociceptin(1-13)NH2 exert anti-opioid effects in the Freund’s adjuvant-induced arthritic rat model of chronic pain. British journal of pharmacology. 1999;128:1252–1258. doi: 10.1038/sj.bjp.0702884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boada MD, Woodbury CJ. Myelinated skin sensory neurons project extensively throughout adult mouse substantia gelatinosa. J Neurosci. 2008;28:2006–2014. doi: 10.1523/JNEUROSCI.5609-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsello T, Clarke PG, Hirt L, Vercelli A, Repici M, Schorderet DF, Bogousslavsky J, Bonny C. A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nature medicine. 2003;9:1180–1186. doi: 10.1038/nm911. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Clark AK, Gentry C, Bradbury EJ, McMahon SB, Malcangio M. Role of spinal microglia in rat models of peripheral nerve injury and inflammation. Eur J Pain. 2007;11:223–230. doi: 10.1016/j.ejpain.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Cruz CD, Neto FL, Castro-Lopes J, McMahon SB, Cruz F. Inhibition of ERK phosphorylation decreases nociceptive behaviour in monoarthritic rats. Pain. 2005;116:411–419. doi: 10.1016/j.pain.2005.05.031. [DOI] [PubMed] [Google Scholar]
- Falsig J, Porzgen P, Lotharius J, Leist M. Specific modulation of astrocyte inflammation by inhibition of mixed lineage kinases with CEP-1347. J Immunol. 2004;173:2762–2770. doi: 10.4049/jimmunol.173.4.2762. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Ji RR. c-Fos and pERK, which is a better marker for neuronal activation and central sensitization after noxious stimulation and tissue injury? Open Pain J. 2009;2:11–17. doi: 10.2174/1876386300902010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Xu ZZ, Liu YC, Wen YR, Decosterd I, Ji RR. The c-Jun N-terminal kinase 1 (JNK1) in spinal astrocytes is required for the maintenance of bilateral mechanical allodynia under a persistent inflammatory pain condition. Pain. 2010;148:309–319. doi: 10.1016/j.pain.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ, Park JY, Lind AL, Ma Q, Ji RR. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci. 2009;29:4096–4108. doi: 10.1523/JNEUROSCI.3623-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaume C, McCarthy KD. Control of gap-junctional communication in astrocytic networks. Trends Neurosci. 1996;19:319–325. doi: 10.1016/0166-2236(96)10046-1. [DOI] [PubMed] [Google Scholar]
- Guo W, Wang H, Watanabe M, Shimizu K, Zou S, LaGraize SC, Wei F, Dubner R, Ren K. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J Neurosci. 2007;27:6006–6018. doi: 10.1523/JNEUROSCI.0176-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hald A. Spinal astrogliosis in pain models: cause and effects. Cell Mol Neurobiol. 2009;29:609–619. doi: 10.1007/s10571-009-9390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson E. Could chronic pain and spread of pain sensation be induced and maintained by glial activation? Acta Physiol (Oxf) 2006;187:321–327. doi: 10.1111/j.1748-1716.2006.01568.x. [DOI] [PubMed] [Google Scholar]
- Hao S, Mata M, Wolfe D, Huang S, Glorioso JC, Fink DJ. Gene transfer of glutamic acid decarboxylase reduces neuropathic pain. Ann Neurol. 2005;57:914–918. doi: 10.1002/ana.20483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hassel B, Paulsen RE, Johnsen A, Fonnum F. Selective inhibition of glial cell metabolism in vivo by fluorocitrate. Brain Res. 1992;576:120–124. doi: 10.1016/0006-8993(92)90616-h. [DOI] [PubMed] [Google Scholar]
- Haydon PG. GLIA: listening and talking to the synapse. Nat Rev Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- Hu HJ, Carrasquillo Y, Karim F, Jung WE, Nerbonne JM, Schwarz TL, Gereau RWt. The kv4.2 potassium channel subunit is required for pain plasticity. Neuron. 2006;50:89–100. doi: 10.1016/j.neuron.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Ji RR, Rupp F. Phosphorylation of transcription factor CREB in rat spinal cord after formalin-induced hyperalgesia: relationship to c-fos induction. J Neurosci. 1997;17:1776–1785. doi: 10.1523/JNEUROSCI.17-05-01776.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Woolf CJ. Neuronal plasticity and signal transduction in nociceptive neurons: implications for the initiation and maintenance of pathological pain. Neurobiol Dis. 2001;8:1–10. doi: 10.1006/nbdi.2000.0360. [DOI] [PubMed] [Google Scholar]
- Ji RR, Strichartz G. Cell signaling and the genesis of neuropathic pain. Sci STKE. 2004;2004:reE14. doi: 10.1126/stke.2522004re14. [DOI] [PubMed] [Google Scholar]
- Ji RR, Baba H, Brenner GJ, Woolf CJ. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci. 1999;2:1114–1119. doi: 10.1038/16040. [DOI] [PubMed] [Google Scholar]
- Ji RR, Befort K, Brenner GJ, Woolf CJ. ERK MAP kinase activation in superficial spinal cord neurons induces prodynorphin and NK-1 upregulation and contributes to persistent inflammatory pain hypersensitivity. J Neurosci. 2002;22:478–485. doi: 10.1523/JNEUROSCI.22-02-00478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Ji RR, Gereau RWt, Malcangio M, Strichartz GR. MAP kinase and pain. Brain research reviews. 2009;60:135–148. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Kawasaki Y, Zhuang ZY, Wen YR, Decosterd I. Possible role of spinal astrocytes in maintaining chronic pain sensitization: review of current evidence with focus on bFGF/JNK pathway. Neuron Glia Biol. 2006;2:259–269. doi: 10.1017/S1740925X07000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim F, Wang CC, Gereau RWt. Metabotropic glutamate receptor subtypes 1 and 5 are activators of extracellular signal-regulated kinase signaling required for inflammatory pain in mice. J Neurosci. 2001;21:3771–3779. doi: 10.1523/JNEUROSCI.21-11-03771.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y, Kohno T, Ji RR. Different effects of opioid and cannabinoid receptor agonists on C-fiber-induced extracellular signal-regulated kinase activation in dorsal horn neurons in normal and spinal nerve-ligated rats. The Journal of pharmacology and experimental therapeutics. 2006;316:601–607. doi: 10.1124/jpet.105.093583. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Kohno T, Zhuang ZY, Brenner GJ, Wang H, Van Der Meer C, Befort K, Woolf CJ, Ji RR. Ionotropic and metabotropic receptors, protein kinase A, protein kinase C, and Src contribute to C-fiber-induced ERK activation and cAMP response element-binding protein phosphorylation in dorsal horn neurons, leading to central sensitization. J Neurosci. 2004;24:8310–8321. doi: 10.1523/JNEUROSCI.2396-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno T, Wang H, Amaya F, Brenner GJ, Cheng JK, Ji RR, Woolf CJ. Bradykinin enhances AMPA and NMDA receptor activity in spinal cord dorsal horn neurons by activating multiple kinases to produce pain hypersensitivity. J Neurosci. 2008;28:4533–4540. doi: 10.1523/JNEUROSCI.5349-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltzenburg M, Wall PD, McMahon SB. Does the right side know what the left is doing? Trends Neurosci. 1999;22:122–127. doi: 10.1016/s0166-2236(98)01302-2. [DOI] [PubMed] [Google Scholar]
- Ma QP, Woolf CJ. Basal and touch-evoked fos-like immunoreactivity during experimental inflammation in the rat. Pain. 1996;67:307–316. doi: 10.1016/0304-3959(96)03132-6. [DOI] [PubMed] [Google Scholar]
- Maleki J, LeBel AA, Bennett GJ, Schwartzman RJ. Patterns of spread in complex regional pain syndrome, type I (reflex sympathetic dystrophy) Pain. 2000;88:259–266. doi: 10.1016/S0304-3959(00)00332-8. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Chen C, Tonegawa S, Basbaum AI. Preserved acute pain and reduced neuropathic pain in mice lacking PKCgamma. Science. 1997;278:279–283. doi: 10.1126/science.278.5336.279. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Malcangio M. Current challenges in glia-pain biology. Neuron. 2009;64:46–54. doi: 10.1016/j.neuron.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Messersmith DJ, Kim DJ, Iadarola MJ. Transcription factor regulation of prodynorphin gene expression following rat hindpaw inflammation. Brain research. 1998;53:260–269. doi: 10.1016/s0169-328x(97)00308-2. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Twining C, Chacur M, Biedenkapp J, O’Connor K, Poole S, Tracey K, Martin D, Maier SF, Watkins LR. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J Neurosci. 2003;23:1026–1040. doi: 10.1523/JNEUROSCI.23-03-01026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miraucourt LS, Dallel R, Voisin DL. Glycine inhibitory dysfunction turns touch into pain through PKCgamma interneurons. PLoS ONE. 2007;2:e1116. doi: 10.1371/journal.pone.0001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miraucourt LS, Moisset X, Dallel R, Voisin DL. Glycine inhibitory dysfunction induces a selectively dynamic, morphine-resistant, and neurokinin 1 receptor-independent mechanical allodynia. J Neurosci. 2009;29:2519–2527. doi: 10.1523/JNEUROSCI.3923-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagakura Y, Okada M, Kohara A, Kiso T, Toya T, Iwai A, Wanibuchi F, Yamaguchi T. Allodynia and hyperalgesia in adjuvant-induced arthritic rats: time course of progression and efficacy of analgesics. The Journal of pharmacology and experimental therapeutics. 2003;306:490–497. doi: 10.1124/jpet.103.050781. [DOI] [PubMed] [Google Scholar]
- Neumann S, Braz JM, Skinner K, Llewellyn-Smith IJ, Basbaum AI. Innocuous, not noxious, input activates PKCgamma interneurons of the spinal dorsal horn via myelinated afferent fibers. J Neurosci. 2008;28:7936–7944. doi: 10.1523/JNEUROSCI.1259-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada-Ogawa A, Suzuki I, Sessle BJ, Chiang CY, Salter MW, Dostrovsky JO, Tsuboi Y, Kondo M, Kitagawa J, Kobayashi A, Noma N, Imamura Y, Iwata K. Astroglia in medullary dorsal horn (trigeminal spinal subnucleus caudalis) are involved in trigeminal neuropathic pain mechanisms. J Neurosci. 2009;29:11161–11171. doi: 10.1523/JNEUROSCI.3365-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur J Neurosci. 2004;20:467–473. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- Ren K, Dubner R. Neuron-glia crosstalk gets serious: role in pain hypersensitivity. Curr Opin Anaesthesiol. 2008;21:570–579. doi: 10.1097/ACO.0b013e32830edbdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Sandoval EA, Horvath RJ, DeLeo JA. Neuroimmune interactions and pain: focus on glial-modulating targets. Curr Opin Investig Drugs. 2008;9:726–734. [PMC free article] [PubMed] [Google Scholar]
- Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, Bonventre JV, Woolf CJ. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- Seal RP, Wang X, Guan Y, Raja SN, Woodbury CJ, Basbaum AI, Edwards RH. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature. 2009;462:651–655. doi: 10.1038/nature08505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spataro LE, Sloane EM, Milligan ED, Wieseler-Frank J, Schoeniger D, Jekich BM, Barrientos RM, Maier SF, Watkins LR. Spinal gap junctions: potential involvement in pain facilitation. J Pain. 2004;5:392–405. doi: 10.1016/j.jpain.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Wang H, Dai Y, Fukuoka T, Yamanaka H, Obata K, Tokunaga A, Noguchi K. Enhancement of stimulation-induced ERK activation in the spinal dorsal horn and gracile nucleus neurons in rats with peripheral nerve injury. Eur J Neurosci. 2004;19:884–890. doi: 10.1111/j.0953-816x.2004.03203.x. [DOI] [PubMed] [Google Scholar]
- Weyerbacher AR, Xu Q, Tamasdan C, Shin SJ, Inturrisi CE. N-Methyl-D-aspartate receptor (NMDAR) independent maintenance of inflammatory pain. Pain. 2010;148:237–246. doi: 10.1016/j.pain.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- Woda A, Pionchon P. A unified concept of idiopathic orofacial pain: pathophysiologic features. J Orofac Pain. 2000;14:196–212. [PubMed] [Google Scholar]
- Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Shortland P, Coggeshall RE. Peripheral nerve injury triggers central sprouting of myelinated afferents. Nature. 1992;355:75–78. doi: 10.1038/355075a0. [DOI] [PubMed] [Google Scholar]
- Xu ZZ, Zhang L, Liu T, Park JY, Berta T, Yang R, Serhan CN, Ji RR. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nature medicine. 2010;16:592–597. 591–597. doi: 10.1038/nm.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RX, Li A, Liu B, Wang L, Ren K, Zhang H, Berman BM, Lao L. IL-1ra alleviates inflammatory hyperalgesia through preventing phosphorylation of NMDA receptor NR-1 subunit in rats. Pain. 2008;135:232–239. doi: 10.1016/j.pain.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang ZY, Wen YR, Zhang DR, Borsello T, Bonny C, Strichartz GR, Decosterd I, Ji RR. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J Neurosci. 2006;26:3551–3560. doi: 10.1523/JNEUROSCI.5290-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]