Abstract
The newly discovered T-cell immunoglobulin mucin (TIM) gene family molecules, expressed by T cells, regulate host immunity and tolerance. Although CD4+ T cells mediate innate immunity-dominated liver ischemia-reperfusion injury (IRI), the underlying mechanisms remain obscure. We have recently documented the novel function of TIM-1 pathway in the mechanism of liver IRI and also found that TLR4 activation plays a key triggering role. Using an anti-TIM-3 Ab, we now studied the role of TIM-3 signaling in the model of partial warm liver ischemia followed by reperfusion. Anti-TIM-3 Ab therapy exacerbated the liver damage, as compared with controls. Histological examination has revealed that anti-TIM-3 Ab augmented the hepatocellular damage, increased local neutrophil infiltration, facilitated local accumulation of T cells/macrophages and promoted liver cell apoptosis. Intrahepatic neutrophil activity, induction of pro-inflammatory cytokines/chemokines and expression of cleaved caspase-3/NF-NB/TLR4 were all increased in the treatment group. In parallel, anti-TIM-3 Ab and anti-galectin-9 (Gal-9; TIM-3 ligand) Ab increased IFN-γ production in ConA-stimulated spleen T cells, and TNFα/IL-6 expression in ConA-stimulated macrophage/T cell co-culture system. Interestingly, anti-TIM-3 Ab treatment did not affect liver IRI in TLR4-deficient (KO) mice. In conclusion, TIM-3 blockade exacerbated local inflammation and liver damage, suggesting importance of TIM-3/Gal-9 signaling in the maintenance of hepatic homeostasis. TIM-3-TLR4 cross regulation determined the severity of liver IRI in TLR4-dependent manner, a novel finding of potential importance to modulate tissue innate vs. adaptive responses in liver transplant patients. Thus, harnessing physiological negative T cell co-stimulation signaling on hepatic T cells may minimize innate immunity-mediated liver tissue damage.
Introduction
The ischemia and reperfusion injury (IRI), an exogenous Ag-independent innate immunity-dominated inflammatory event, remains one of the most understudied yet critical problems in clinical organ transplantation. In the case of liver, IRI causes ca. 10% of early graft failure, can lead to a higher incidence of acute and chronic rejection, as well as contributes to the acute shortage of donor organs available for transplantation (1). In agreement with others (2), we have shown that T lymphocytes, particularly of CD4 phenotype, are key mediators in IR-triggered liver inflammation (3,4). In addition, by releasing pro-inflammatory mediators such as TNF-D and IL-6, Kupffer cells are critical players in the pathophysiology of IRI (5). Indeed, CD4+ T-cells may amplify Kupffer cell activity, a key event in IRI cascade (6).
Molecules of T-cell immunoglobulin mucin (TIM) family represent relatively newly described immune regulators. The TIM family is located on chromosome 11B1.1 in mice and consists of four identified members (TIM-1, -2, -3, and -4) and four putative members (TIM-5, -6, -7, and -8). All are predicted to be type I membrane proteins that share a characteristic immunoglobulin V (Ig V), mucin, transmembrane and cytoplasmic domain structure (7). Recently, we have reported that TIM-1–TIM-4 pathway represents one of the key mechanisms underlying T cell-Kupffer cell cross talk in the pathophysiology of liver IRI (8). This finding has catalyzed our present investigation into how other TIM family member, i.e., TIM-3 might regulate/influence the development of IR-mediated liver damage. Importantly, unlike TIM-1, TIM-3 is preferentially expressed on terminally differentiated Th1/Th17 but not Th2 cells (7,9,10). Indeed, interaction of TIM-3 with its galectin-9 (Gal-9) ligand was pivotal in the induction of autoimmune disease states by regulating macrophage activation (9), inhibited Th1-mediated auto/allo-immune responses, and promoted peripheral immune tolerance (10). Interestingly, Tim-3 that is constitutively expressed by mouse and human cells of the innate immune system may synergize with TLR system to influence a range of inflammatory conditions by promoting or terminating Th1-type immunity (11). Hence, TIM-3 may serve opposing roles, and by virtue of divergent expression induce distinct signaling in innate and adaptive immune cells.
The activation of sentinel TLR system represents an important triggering event in the development of infectious and inflammatory diseases (12,13). The liver consists of parenchymal hepatocytes and nonparenchymal cells (NPC) that include Kupffer cells, sinusoidal endothelial, stellate, and hepatic dendritic cells. TLR4 is present on hepatocytes and NPC, and both cell populations possess intact TLR4 signaling (14). We have reported that: (i) TLR4, but not TLR2 signaling initiates innate immunity-driven liver IRI, (ii) the hepatocellular damage proceeds by down-stream interferon regulatory factor-3 (IRF-3)-dependent but MyD88- independent pathway, and (iii) the endogenous TLR4 ligand network is required to facilitate IR-mediated liver inflammation responses (15-18).
This study was designed to examine possible role of TIM-3/Gal-9 pathway in the pathophysiology of liver IRI. Our results demonstrate that TIM-3 blockade exacerbated local inflammation and liver damage, suggesting the importance of TIM-3 signaling in the maintenance of hepatic homeostasis. Moreover, the cross regulation by TIM-3 and TLR4 determined the severity of liver IRI in TLR4-dependent manner, a novel finding of potential importance to modulate tissue innate vs. adaptive responses in liver transplant patients.
Material and Methods
Animals
Male wild type (WT) (C57BL/6) and TLR4-deficient (KO) (B6.B10ScN-Tlr4lps-del/ JthJ) mice (8-10 weeks old) were used (The Jackson Laboratory, Bar Harbor, ME). Mice were housed in the UCLA animal facility under specific pathogen-free conditions. All animals received human care according to the criteria outlined in the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of sciences and published by the National Institute of Health (NIH publication 86-23, revised 1985).
Liver IRI model
We used a mouse model of partial warm hepatic IRI (4,8,19,20). Briefly, mice were anesthetized, injected with heparin (100U/kg), and an atraumatic clip was used to interrupt the artery/portal venous blood supply to the left/middle liver lobes. After 90min of ischemia, the clamp was removed, initiating reperfusion. To investigate the role of TIM-3 signaling, we used anti-mouse TIM-3 mAb (RMT3-23; rat IgG2a,κ) (21). Based on our previous studies with anti-mouse TIM-1 mAb (8), mice were given a single injection of mAb (0.5mg i.p.) one day prior to the ischemia insult, and then sacrificed at 6h or 24h after reperfusion. Controls were treated with irrelevant rat IgG Ab or PBS. Sham-operated mice underwent the same procedure, but without vascular occlusion.
Hepatocellular function
Serum alanine aminotransferase (sALT) levels were measured with an autoanalyzer (ANTECH Diagnostics, Los Angeles, CA).
Histology
Liver paraffin sections (5-μm thick) were stained with hematoxylin/eosin. The severity of liver IRI (necrosis, sinusoidal congestion, and centrilobular ballooning) was blindly graded with modified Suzuki's criteria on a scale from 0-4 (22).
Immunohistochemistry
Primary mAb against mouse Ly-6G (1A8), CD3 (17A2), CD4 (H129.19) and CD11b (Mac-1, M1/70) were used (BD Biosciences, San Jose, CA) on liver cryostat sections. The secondary, biotinylated goat anti-rat IgG (Vector, Burlingame, CA), was incubated with immunoperoxidase (ABC Kit, Vector). Positive cells were counted blindly in 10 HPF/section (×400).
Myeloperoxidase activity assay
The presence of myeloperoxidase (MPO) was used as an index of neutrophil accumulation in the liver. The change in absorbance was measured spectrophotometrically at 655nm (Bio-tek Instruments, Winooski, VT). One unit of MPO activity was defined as the quantity of enzyme degrading 1Pmol peroxide per minute at 25°C per gram of tissue.
Quantitative RT-PCR
Quantitative PCR was performed using the DNA Engine with Chromo 4Detector (MJ Research, Waltham, MA). In a final reaction volume of 20μL, the following were added: 1 × SuperMix (Platinum SYBR Green qPCR Kit, Invitrogen, Carlsbad, CA), complementary DNA, and 10μM of each primer. Amplification conditions were: 50°C (2min), 95°C (5min), followed by 45 cycles of 95°C (15sec), 60°C (30sec). Primers used to amplify specific gene fragments have been listed (8). Target gene expressions were calculated by their ratios to the housekeeping gene HPRT.
Western blots
Western blots were performed using liver proteins (30μg/sample), and polyclonal rabbit anti-mouse cleaved caspase-3 (Cell Signaling Technology, Danvers, MA), TLR4 (Imgenex, San Diego, CA), NF-κB, heme oxygenase-1 (HO-1; Assay Designs, Inc., Ann Arbor, MI) and β-actin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), as described (3,4). Relative quantities of protein were determined by densitometer and expressed in absorbance units (AU).
Apoptosis assay
Apoptosis in 5-μm liver paraffin sections was detected by TUNEL method (FragELTM DNA Fragmentation Detection kit, Calbiochem, Gibbstown, NJ). Negative control was prepared by omission of terminal deoxynucleotidyl transferase. Positive controls were generated by treatment with DNase. TUNEL-positive cells were counted in 10 HPF/section under light microscopy (×400).
Cell isolation/in vitro splenocytes cultures
Single-cell suspensions were treated with Red Cell Lysing Buffer (Sigma-Aldrich, St. Louis, MO) and re-suspended in RPMI-1640 medium (Invitrogen). Splenocytes, adjusted to 1×106 cells/ml, were plated (150μl) and incubated for 12h at 37°C. For T cell stimulation, cells were incubated for 24-72h with 50μl of ConA (Sigma-Aldrich, St. Louis, MO; final concentration 5μg/ml) with or without anti-mouse TIM-3 Ab (5 and 10μg/ml) (8,23) and anti-Gal-9 mAb (RG9-1; 5, 10, 15 and 20μg/ml) (24).
IFN-γ assay
IFN-γ concentration was evaluated using IFN-J ELISA kit (eBioscience, San Diego, CA). Standard curves starting at 1000pg/ml were performed with serial 2-fold dilutions; optical density (OD) was measured with an ELISA reader (BioTek Instruments).
Macrophage/splenocyte co-culture
Mouse macrophages (RAW 264.7; ATCC, Manassas, VA) were cultured in Dulbucco's Modified Eagle's medium (Invitrogen). Macrophages were co-cultured with C57BL/6 splenocytes at 1:5 (responder/stimulator) ratios (25). The co-cultured cells were incubated for 24-72h with 50μl of ConA (5μg/ml) with or without anti-TIM-3 mAb (effective dose: 10μg/ml) and anti-Gal-9 mAb (effective dose: 20μg/ml). TNF-α and IL-6 in cell-free supernatants was evaluated by ELISA (eBioscience). Standard curves starting at 1000 pg/ml were performed with serial 2-fold dilutions (8).
Statistical analysis
All data are expressed as mean±SD. Differences between experimental groups were analyzed using one-way analysis of variance or Student's t test for unpaired data. All differences were considered statistically significant at the P value of <0.05.
Results
TIM-3 signaling negatively regulates IR-triggered hepatocellular damage
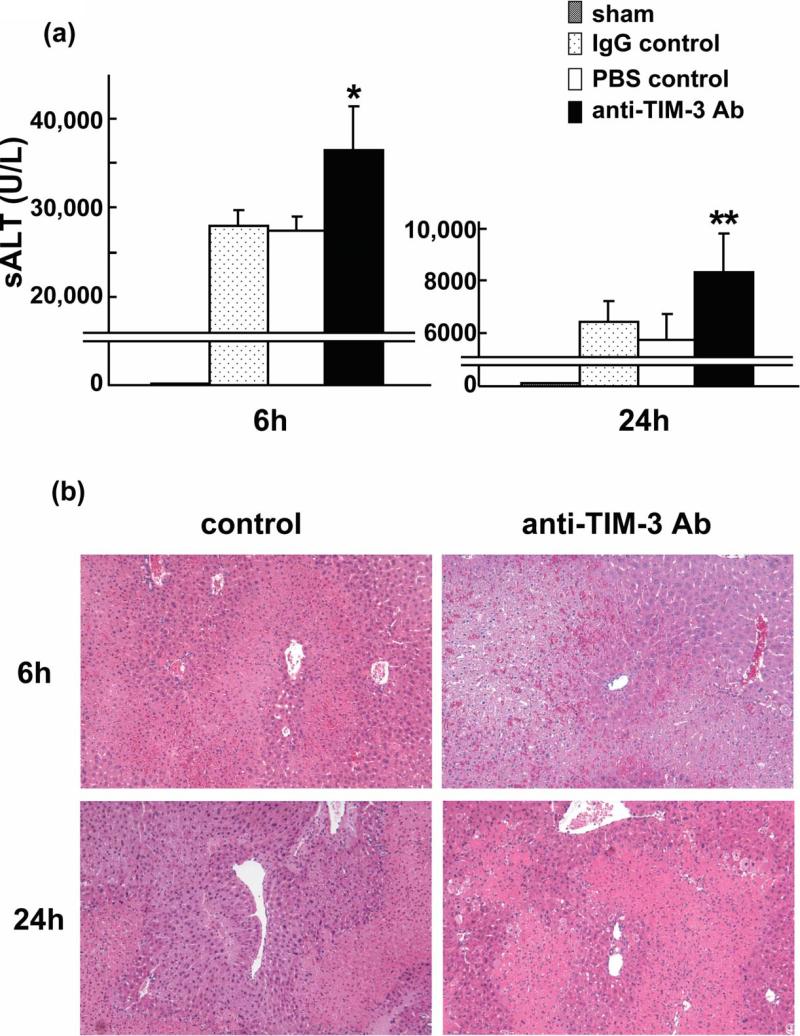
We analyzed hepatocellular function in model of partial liver warm ischemia (90min) followed by reperfusion. Anti-TIM-3 mAb treatment augmented otherwise fulminant IR-induced liver damage. The sALT levels (IU/L) were significantly increased at 6h and 24h after reperfusion, as compared with controls (Figure 1a: [6 h] 36,383±4,995 vs IgG/PBS: 27,967±1,804/27,333±1,607; p<0.01 and [24 h] 8,297±1,514 vs IgG/PBS: 6,467±749/5,796±945; p<0.05).
Figure 1.
Anti-TIM-3 Ab treatment exacerbates liver damage due to warm ischemia (90min) followed by reperfusion (6h and 24h). (a) The hepatocellular function, as evidenced by sALT levels was increased in anti-TIM-3 treatment group, compared with controls (*P<0.01, **P<0.05; n=5-6/group). MeanrSD are shown. (b) Representative liver histology (H&E staining; magnification ×100) of ischemic liver after reperfusion (Upper panel: 6h; Suzuki's score=11.0±0.63 vs 11.83±0.41; p<0.05, Lower panel: 24h; 8.17±0.75 vs 9.17±0.75; p<0.05, n=5-6/group).
We used Suzuki's histological grading to assess the hepatocellular damage (22). Indeed, after disruption of TIM-3 signaling, livers showed severe lobular edema, congestion, ballooning and hepatocellular necrosis, comparable or even higher than in controls (Figure 1b: Suzuki's score = [6 h] 11.83±0.41 vs 11.0±0.63 and [24 h] 9.17±0.75 vs 8.17±0.75; p<0.05).
TIM-3 engagement ameliorates neutrophil, T cell and macrophage sequestration in IR livers
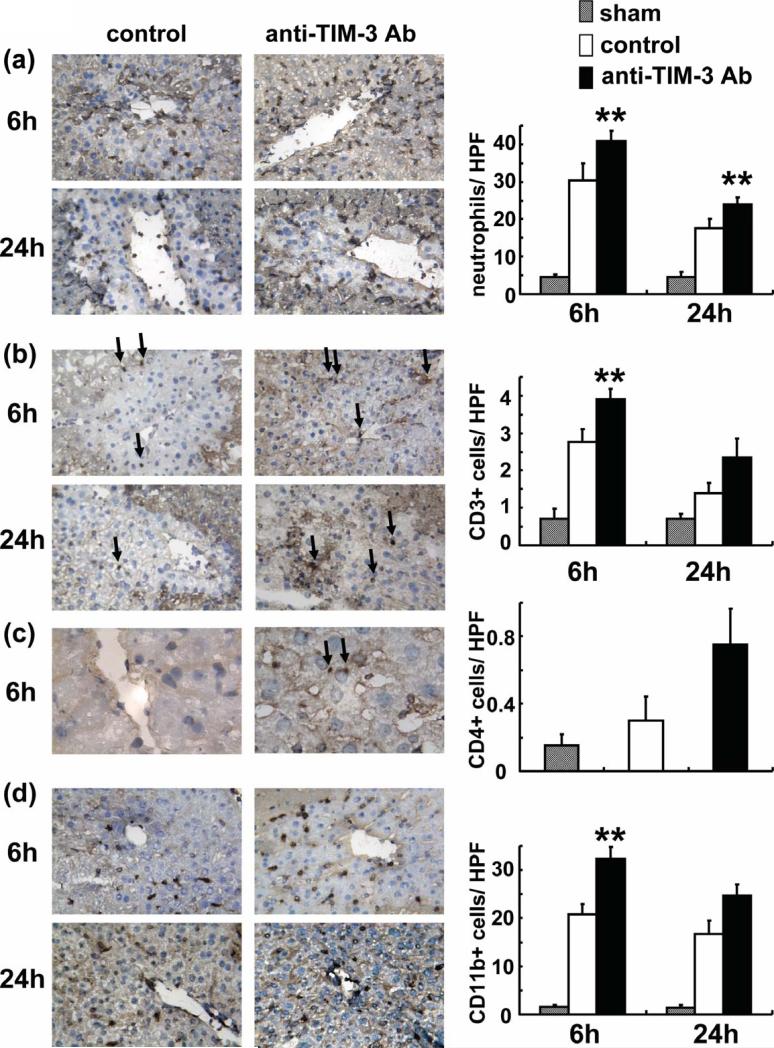
The MPO activity (U/g), an index of liver neutrophil infiltration, was significantly increased in the treatment group, as compared with controls ([6 h] 8.67±0.29 vs 4.10±0.48 and [24 h] 8.04±0.27 vs 3.58±0.45; p<0.01). These results were correlated with the frequency of neutrophils by immunohistology (Figure 2a). Their accumulation in the treated livers was significantly increased, as compared with controls ([6 h] 40.90±2.69 vs 30.27±4.75; p<0.05 and [24 h] 23.90±1.98 vs 17.53±2.47; p<0.05).
Figure 2.
Accumulation of: (a) neutrophils (Ly-6G), T cells (b:CD3, c:CD4) and (d) macrophages (CD11b) in ischemic liver at 6h and 24h of reperfusion after 90min of ischemia. Left panel: Representative liver sections stained by immunohistology (a,b,d:x400, c:x1,000 magnification). Right panel: Cell quantification/HPF. Infiltration of neutrophils, T cells and macrophages (dark spots) in treated livers was decreased compared with controls (**P<0.05; n=2-3/group).
In parallel, we performed immunohistochemical staining for CD3, CD4 and CD11b expression. Although few CD3 positive T cells could be found in control livers, their numbers increased further after treatment with anti-TIM-3 Ab (Figure 2b: [6 h] 2.77±0.35 vs 3.90±0.28; p<0.05 and [24 h] 1.40±0.26 vs 2.35±0.49; p=n.s.). Relatively few CD4 T cells were detected in the treatment group, and almost no positive cells were found in the control livers (Figure 2c: [6h] 0.75±0.21 vs 0.30±0.14; p=ns). The disruption of TIM-3 pathway promoted CD11b positive macrophage infiltration, as compared with controls (Figure 2c: [6 h] 32.3±2.55 vs 20.8±2.12; p<0.05 and [24 h] 24.75±2.33 vs 16.65±2.90; p=n.s.).
TIM-3 downregulates IR-mediated cytokine/chemokine programs
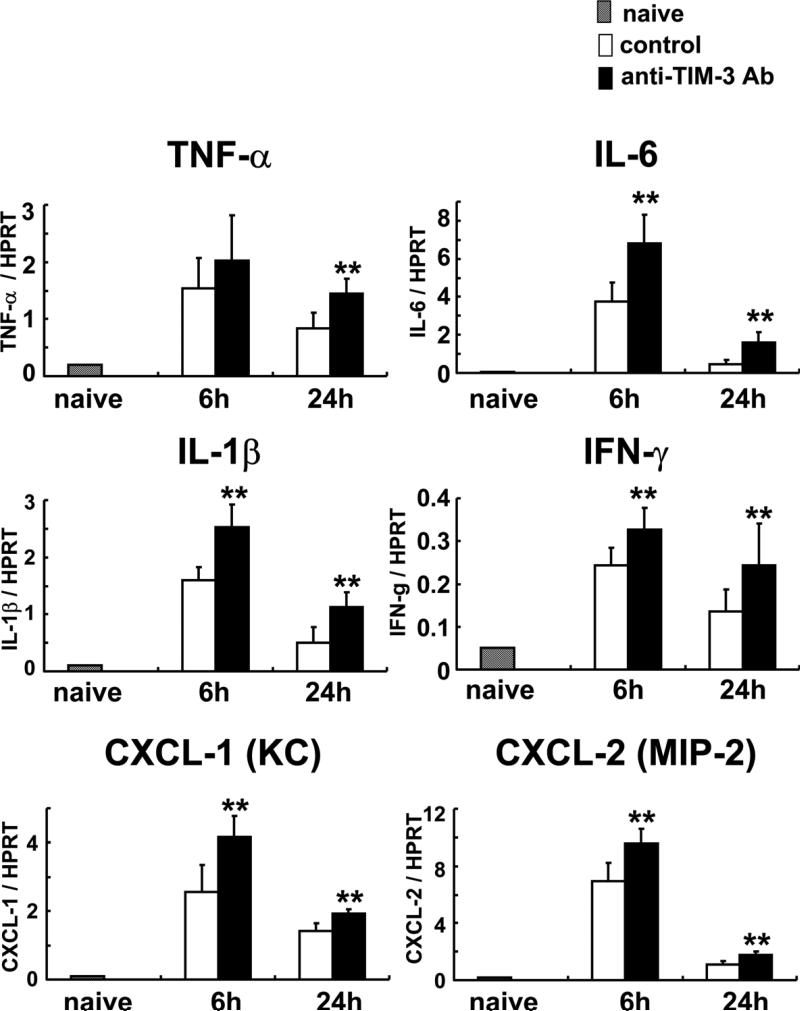
We used qRT-PCR to analyze liver expression of cytokines (TNF-α, IL-6, IL-1β and IFN-γ) and chemokines (CXCL-1 [KC: a mouse homolog of human chemokine gro-α] and CXCL-2 [macrophage inflammatory protein-2: MIP-2]) during IRI, and calculated the ratio between post-IR and basal mRNA levels in each animal (Figure 3). Control livers showed significantly increased induction ratios of TNF-α (TNF-α/HPRT) mRNA: [6 h] 2.03±0.79 vs 1.54±0.53; [24 h] 1.44±0.26 vs 0.84±0.26, p<0.05), IL-6 (IL-6/HPRT mRNA: [6 h] 6.79±1.55 vs 3.73±1.04, p<0.05; [24 h] 1.61±0.53 vs 0.44±0.27, p<0.05), IL-1β (IL-β/HPRT mRNA: [6 h] 2.53±0.40 vs 1.60±0.23, p<0.05; [24 h] 1.13±0.25 vs 0.51±0.28, p<0.05) and IFN-γ (IFN-γ/HPRT mRNA: [6 h] 0.33±0.05 vs 0.24±0.04, p<0.05; [24 h] 0.24±0.10 vs 0.14±0.05, p<0.05) as compared with the treated group. CXC chemokines are known to act predominantly on neutrophils (26). In mice, the two CXC chemokines important in the mechanism of liver IRI are CXCL-1 (KC) and CXCL-2 (MIP-2) (27). The expression of CXCL-1 and -2 increased in anti-TIM-3 treated group, as compared with controls (CXCL-1/HPRT mRNA: [6 h] 4.17±0.61 vs 2.55±0.78, p<0.05; [24 h] 1.92±0.13 vs 1.42±0.23, p<0.05, CXCL-2/HPRT mRNA: [6 h] 9.57±1.00 vs 6.92±1.29, p<0.01; [24 h] 1.75±0.27 vs 1.13±0.20, p<0.05)
Figure 3.
Quantitative RT-PCR-assisted detection of pro-inflammatory cytokines (TNF-α, IL-6, IL-1β, IFN-γ) and chemokines (CXCL-1 and CXCL-2) at 6h and 24h of reperfusion after 90min of ischemia with or without anti-TIM-3 Ab treatment. Data were normalized to HPRT gene expression (**p<0.05; n =3-5/group). MeanrSD are shown.
TIM-3 suppresses liver cell apoptosis and TLR4 expression during IRI
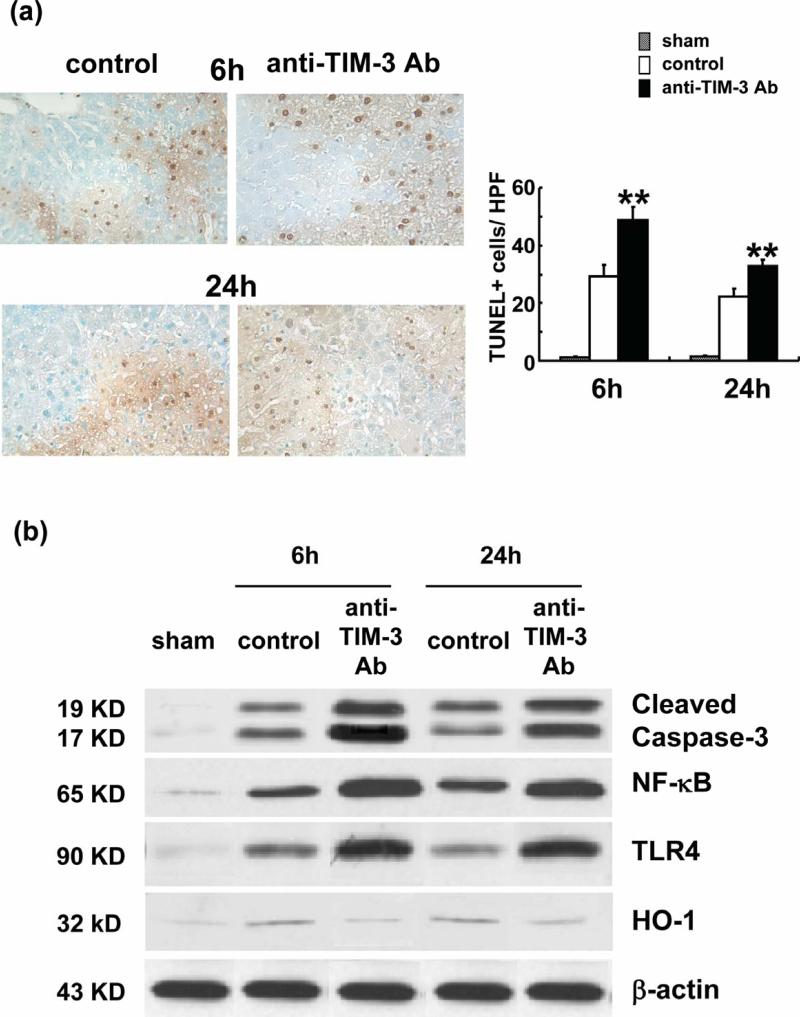
The disruption of TIM-3 signaling increased the number of TUNEL positive cells (Figure 4a: [6 h] 48.7±4.67 vs 29.15±4.17; p<0.05 and [24 h] 32.75±2.19 vs 22.25±2.62; p<0.05). Western blot analysis (Figure 4b) has revealed that treatment with anti-TIM-3 Ab promoted the expression of cleaved caspase-3 and NF-κB, as compared with controls (cleaved caspase-3: sham [0.05-0.1 AU], control 6h [1.1-1.3 AU], anti-TIM-3- 6h [2.8-3.0 AU], control 24h [1.0-1.2 AU], anti-TIM-3- 24h [2.6-2.8 AU], NF-κB: sham [0.1-0.2 AU], control 6h [1.1-1.2 AU], anti-TIM-36h [2.7-2.8 AU], control 24h [1.0-1.1 AU], anti-TIM-3- 24h [2.6-2.7 AU]). Treatment with anti-TIM-3 Ab simultaneously increased the expression of TLR4 (sham [0.05-0.1 AU], control 6h [0.8-0.9 AU], anti-Tim-3- 6h [2.3-2.5 AU], control 24h [0.7-0.8 AU], anti-TIM-3- 24h [2.4-2.6 AU]). In addition, anti-TIM-3 Ab decreased the expression of HO-1 (Figure 4b: sham [0.05-0.1 AU], control 6h [0.7-0.8 AU], anti-Tim-3- 6h [0.2-0.3 AU], control 24h [0.6-0.7 AU], anti-TIM-324h [0.3-0.4 AU]).
Figure 4.
Anti-TIM-3 Ab treatment promotes apoptosis. (a) Representative TUNEL-assisted detection of hepatic apoptosis in ischemic liver. Left panel: Stained liver sections (×400 magnification). Right panel: Quantification of hepatic apoptosis. Anti-TIM-3 treatment group with high frequency of TUNEL+ cells (dark spots), compared with controls (**P<0.05; n=2-3/group). MeanrSD are shown. (b) Western blot-assisted expression of cleaved caspase-3, NF-NB, TLR4 and HO-1 at 6h and 24h of reperfusion after 90min of ischemia. E-actin was used as internal control.
TIM-3/Gal-9 pathway regulates T cell-macrophage crosstalk
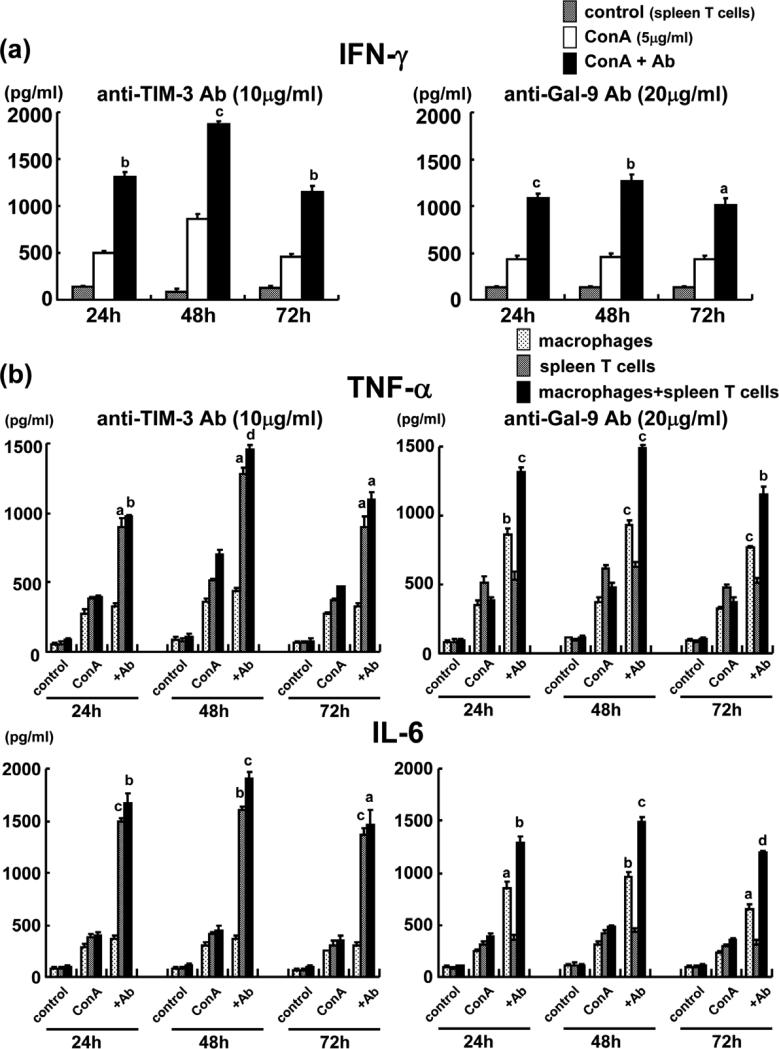
We further analyzed the immunomodulatory function of TIM-3 pathway in well-controlled cell culture experiments, designed to mimic in vivo liver IRI settings. Addition of anti-TIM-3 Ab (effective dose: 10μg/ml) or anti-Gal-9 Ab (effective dose: 20μg/ml) significantly increased Con A-stimulated IFN-γ production by murine spleen T cells in vitro at three different time-points (Figure 5a: anti-TIM-3: [24h] 1,311 vs 502; p<0.001, [48h] 1,873 vs 860; p<0.0001 and [72h] 1,154 vs 453; p<0.001, anti-Gal-9: [24h] 1,081 vs 431; p<0.0001, [48h] 1,260 vs 455; p<0.001 and [72h] 1,012 vs 428; p<0.01). Interestingly, in macrophage - T cell co-culture system, anti-TIM-3 and anti-Gal-9 supplement (effective dose: 10μg/ml and 20μg/ml, respectively) did increase Con A-mediated production of TNF-α and IL-6, the “signature” macrophage-derived mediators of IR-mediated hepatocellular damage (Figure 5b: [TNF-α] anti-TIM-3: [24h] 396 vs 980; p<0.001, [48h] 704 vs 1,457; p<0.00001 and [72h] 467 vs 1,100; p<0.01, anti-Gal-9: [24h] 385 vs 1,310; p<0.0001, [48h] 477 vs 1,487; p<0.0001 and [72h] 378 vs 1,154; p<0.001, [IL-6] anti-TIM-3: [24h] 393 vs 1,674; p<0.001, [48h] 446 vs 1,899; p<0.0001 and [72h] 348 vs 1,464; p<0.01, anti-Gal-9: [24h] 390 vs 1,282; p<0.001, [48h] 476 vs 1,495; p<0.0001 and [72h] 352 vs 1,188; p<0.00001). In marked contrast, although anti-TIM-3 enhanced TNF-α and IL-6 production in purified spleen T cells cultures (TNF-α/IL-6: [24h] 385 vs 892/ 387 vs 1,485; p<0.01/ p<0.0001, [48h] 519 vs 1,281/ 417 vs 1,600; p<0.01/p<0.001 and [72h] 372 vs 892/ 301 vs 1,371; p<0.01/ p<0.0001), it did not affect macrophage cultures devoid of T cells (TNF-α/IL-6: [24h] 278 vs 328/ 293 vs 360; [48h] 357 vs 435/ 299 vs 360 and [72h] 270 vs 328/ 247 vs 298. Although anti-Gal-9 enhanced TNF-α and IL-6 production in purified macrophage cultures (TNF-α/IL-6: [24h] 353 vs 863/ 254 vs 846; p<0.001/ p<0.01, [48h] 375 vs 927/ 307 vs 965; p<0.0001/p<0.001 and [72h] 321 vs 762/ 230 vs 652; p<0.0001/p<0.01), it did not affect spleen T cells cultures devoid of macrophages (TNF-α/IL-6: [24h] 508 vs 536/ 317 vs 358; [48h] 617 vs 627/ 412 vs 439 and [72h] 476 vs 510/ 290 vs 307). Ab dose needed to achieve these effects was twice as high for anti-Gal-9 (20ug/ml) as compared with anti-TIM-3 (10ug/ml). These results document the key regulatory function of TIM-3/Gal-9 signaling is macrophage activation through IFN-γ production, as evidenced by in vitro IFN-γ and TNF-α/IL-6 profiles, respectively.
Figure 5.
Cytokine production in murine spleen T cells and RAW 264.7 macrophage cultures. (a) Increased IFN-γ production in ConA-stimulated T cells. Marked increase of IFN-γ after addition of anti-TIM-3 or anti-Gal-9 Ab. (b) Upregulated TNFαand IL-6 production in ConA-stimulated spleen T cell+macrophage co-cultures. Anti-TIM-3 and anti-Gal-9 Ab treatment increased TNFα and IL-6 levels (aP<0.01; bP<0.001; cP<0.0001; dP<0.00001; n=3/group).
TIM-3-TLR4 cross regulation in liver IRI
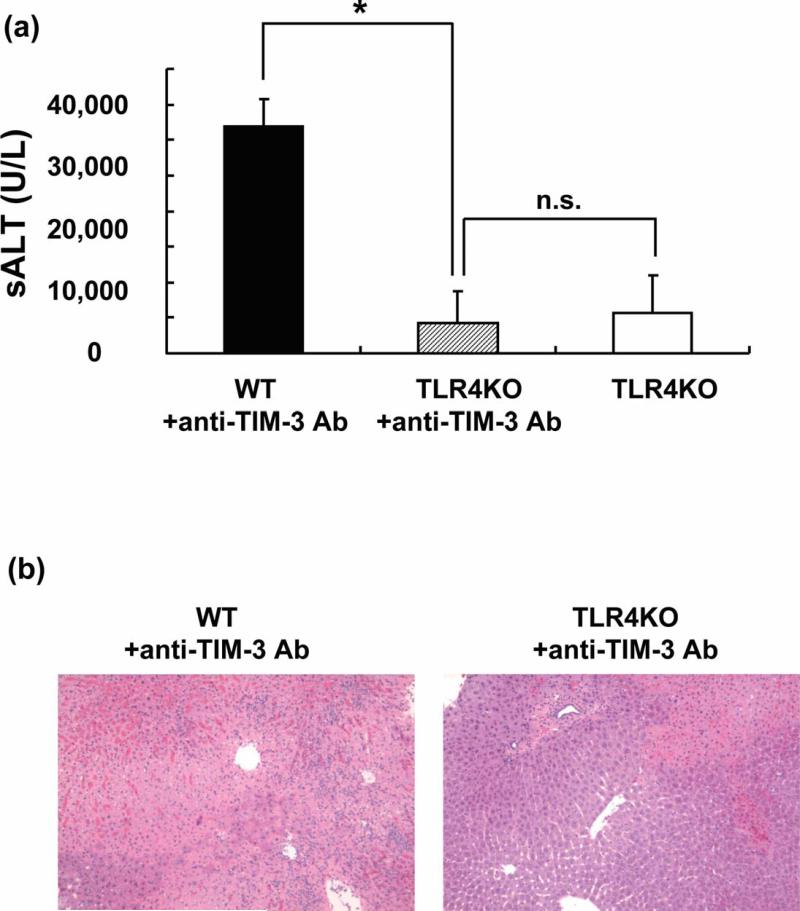
Having demonstrating increased TLR4 expression in livers after TIM-3 blockade (Figure 4b), and documenting the key role of TLR4 in the mechanism of liver IRI in our previous study (15,16), we attempted to clarify putative significance of TLR4 - TIM-3 cross talk in our model. Accordingly, separate cohorts of WT and TLR4 KO mice underwent standard 90min of warm liver ischemia with or without anti-TIM-3 Ab pre-treatment. Unlike in WT counterparts, disruption of TIM-3 signaling failed to exacerbate IR-triggered liver damage in TLR4-deficient hosts, as evidenced by diminished sALT levels (U/L; Figure 6a: WT + anti-TIM-3=31,975±3,738 ; TLR4 KO+anti-TIM-3= 4,325±4,370; p<0.0001), and well-preserved liver histology detail (Figure 6b: Suzuki's score: WT+anti-TIM-3=11.5±1.0; TLR4KO+anti-TIM-3=5.25±2.22; p<0.01) at 6h post-reperfusion. Indeed, unlike in WT, and consistent with our previous studies (28), IR-induced liver damage in TLR4 KO mice with or without TIM-3 Ab treatment was comparable, as evidenced by both sALT levels (Fig. 6a) and liver histology (data not shown).
Figure 6.
Anti-TIM-3 Ab treatment does not exacerbate liver IRI in TLR4 KO mice. (a) The hepatocellular function, evidenced by sALT levels after liver warm ischemia (90min) followed by reperfusion (6h), remained diminished in anti-TIM-3 Ab treated TLR4 KO mice, compared with treated TLR4-proficient controls (*P<0.0001; n=4/group). MeanrSD are shown. (b) Representative liver histology (magnification ×100) of ischemic liver lobes.
Discussion
This is the first study, which documents the functional significance of TIM-3 pathway in the innate immunity-dominated liver IRI cascade. The disruption of TIM-3 signaling exacerbated the hepatocellular damage, consistent with increased neutrophil infiltration/ activity; augmented T cell/macrophage sequestration; enhanced apoptosis through caspase-3 pathway; TLR4 and NF-κB expression; and upregulated pro-inflammatory cytokine/chemokine gene programs. The parallel in vitro studies corroborate in vivo data, and suggest that IR-triggered liver damage resulting from activated T cell–macrophage cross talk proceeded through TIM-3 signaling. Moreover, TIM-3-TLR4 regulation determined the severity of liver IRI in TLR4-dependent manner, a novel finding of potential importance to modulate tissue innate vs. adaptive responses in liver transplant patients.
Others have reported that TIM-3 blockade promotes Th1-mediated auto- and allo-immune responses in mice, as evidenced by acceleration of experimental autoimmune encephalomyelitis (9), diabetes (10), graft-versus-host-disease (29), and prevention of immunological tolerance (10). Moreover, dysregulated TIM-3 signaling has been reported in multiple sclerosis patients (30). However, others have shown that TIM-3 blockage suppressed the induction/progression of murine allergic conjunctivitis (21) and ameliorated IFN-J production from hepatic CD8+ T cells in a mouse model of hepatitis B virus infection (31). Although TIM-3 may serve a dual role in the innate and adaptive immune systems (11), it remains unknown how a single molecule can exert such broad activities with distinctive proand anti-inflammatory functions.
In the acute phase of liver IR-triggered liver damage at 3-6h after reperfusion, the hepatocellular injury associates with T lymphocyte/Kupffer cell activation and apoptosis (2,32). The subacute phase at 18-24h is accompanied by massive neutrophil accumulation in the liver (33). Activated Kupffer cells can increase the oxidative stress by the release of superoxide radicals, TNFα and IL-1 during the early reperfusion stages (5,34-36). The recruited neutrophils lead to cellular responses that culminate in the tissue damage (5,37). We and others have documented that T-lymphocytes, especially of CD4 phenotype, are the key regulators initiating IR-induced liver inflammation (2-4). The initiation of IR-induced inflammatory cascade in the liver is dependent on CD1d-mediated IFN-γ production by NKT cells, which comprise a subset of CD4+ T lymphocytes through Adenosine2A receptor activation (38). Recent findings suggest the important contribution of TIM family molecules (8) in T cell-Kupffer cell interactions that on their own constitute a pivotal event in local IR-induced cascade (2,6).
In this study, treatment with blocking anti-TIM-3 Ab increased IR-triggered hepatocellular damage, as evidenced by increased sALT levels and cardinal histological features of liver injury, i.e., lobular edema, ballooning, hepatocyte necrosis and sinusoidal congestion. One of the striking effects TIM-3 blockade was the marked increase in MPO activity and Ly-6G neutrophil infiltration, in parallel with increased expression of CXCL-1 (KC), CXCL-2 (MIP-2) and IFN-γ. Indeed, both CXCL-1 and CXCL-2 have been recognized as the key neutrophil chemoattractants facilitating their recruitment in the hepatic inflammation response (27). As Th1-derived IFN-γ acts directly on neutrophils to enhance their sequestration in the damaged liver (2), TIM-3 signaling can regulate neutrophil function through cytokine/chemokine networks during liver IRI. Anti-TIM-3 Ab significantly increased TNFα, IL-6 and IL-1 induction ratios in the livers, as compared with controls. These cytokines also influence T cell/macrophage trafficking patterns, as evidenced by increased numbers of infiltrating CD3+, CD4+ cells and CD11b+ cells after reperfusion. Consistent with our previous study (8), IFN-γ may trigger not only neutrophil but also Kupffer cell/macrophage activation in IR inflammatory cascade.
Apoptosis represents a key event after liver reperfusion, the severity of which correlates with the degree of local injury (32). Anti-TIM-3 Ab treated livers showed increased frequency of TUNEL+ cells, accompanied by increased cleaved caspase-3 expression. Activation of NF-κB, an important regulator in the early stages of liver IRI, affects cell growth and programmed cell death (39). Indeed, multiple pro-inflammatory cytokine programs leading to neutrophil-mediated inflammation have been linked to NF-κB activation (40). In this study, dysregulated TIM-3 signaling augmented NF-κB expression in association with enhanced hepatocellular damage. Hence, TIM-3 pathway can regulate liver apoptosis through cytokine/chemokine/NF-κB.
In our previous liver IRI study (8), we have shown that TIM-1 on Th2 cells cross-links TIM-4, its ligand, to activate macrophages via “direct pathway”. In addition, TIM-1 expressed on activated Th1 cells triggers IFN-γ that may also activate macrophages via “indirect pathway”. As a result, activated macrophages produce cytokine/ chemokine programs that ultimately facilitate liver damage. The Gal-9, identified as the TIM-3 ligand, has been associated with tolerance induction and as a negative regulator of Th1-driven immune responses (41-43). Indeed, administration of Gal-9 resulted in selective loss of IFN-γ producing cells (42) and prolonged survival of fully allogeneic cardiac allografts, which was accompanied by suppression of Th1/Th17 responses (24). Although infusion of anti-Gal-9 Abs did not modify the severity of allergic conjunctivitis in mice (44), it did affect the severity of the disease, if cultured in vitro with primed restimulated cells that adoptively transfer the disease state into naïve test recipients. As Gal-9 is ubiquitously expressed in variety of tissues and particularly abundant in the liver (42,45,46), Gal-9 Ab-mediated blockade of TIM-3 pathway may not only regulate T cells but also affect an array of diverse biological functions.
The question arises as to how TIM-3 signaling may affect IR-induced liver damage? To mimic in vivo scenario, we used ConA-activated cell cultures to stimulate lymphocyte TIM-3 expression (8,47,48). Indeed, the amount of ConA-stimulated spleen T cell (predominantly Th1) derived IFN-γ almost doubled after addition of anti-TIM-3 Ab or anti-Gal-9 Ab (Figure 5a). This is consistent with our in vivo data where anti-TIM-3 treatment increased post liver reperfusion local IFN-γ levels. Furthermore, we also used RAW 264.7 mouse macrophage and spleen T cell co-culture system to investigate T cell–macrophage interactions (49). Interestingly, although anti-TIM-3 and anti-Gal-9 had little or no effect upon TNFα/IL-6 levels in pure macrophage and spleen T cell cultures, respectively, the cytokine elaboration in the co-culture system profoundly increased after anti-TIM-3 or anti-Gal-9 supplement (Figure 5b). Our findings suggest that macrophage cross-linking through TIM-3/Gal-9 interaction results in TNFα/IL-6 secretion, and IFN-γ as one of transmitter molecules that stimulates macrophage activation with resultant exacerbation of liver damage. Moreover, in our preliminary studies, anti Gal-9 Ab treatment in vivo only slightly exacerbated liver damage due to IR, as compared with controls (Uchida, unpublished data). We believe that Gal-9 Ab-mediated blockade of TIM-3 not only regulates T cells per se, but may also affect an array of diverse biological functions due to broad Gal-9 cellular distribution. Although macrophages, which express Gal-9, represent the major target for TIM-3 on T cells, the net effect of TIM-3 ligation on cell-to-cell basis might be weaker, as compared with that of TIM-4 ligation via TIM-1. Indeed, two-fold higher dose of anti-Gal-9 than anti-TIM-3 Ab was required to achieve the biological effects in vitro in the present study.
Our current in vivo and in vitro data, consistent with previous reports (10,41,42), suggests that TIM-3 blockade positively regulates Th1 cells through IFN-γ, and hence indicates TIM-3 signaling may exert “cytoprotective” function in liver IRI. In addition, IFN-γ is the major inducer of TLR expression and enhancer of TLR signaling in immune cells during responses to infection and inflammation, and can also induce TLR4 expression (50-52). We and others have documented the requirement for the intact TLR4 system, primarily on Kupffer cells, in the pathogenesis of liver IRI (14,15). Our present results confirm that HO-1 may modulate proinflammatory responses triggered through TLR4, a putative HO-1 repressor (28), and suggest the importance of novel crosstalk interactions between HO-1, TLR4 and TIM-3 signaling. Indeed, as shown in Figure 6, unlike in WT counterparts, IR-induced hepatocellular damage in TLR4 KO mice was minimal despite anti-TIM-3 treatment, yet comparable with otherwise untreated TLR4-deficient mice subjected to IR. Hence, our results are in agreement with previously reported cross regulation by TLR4 and TIM-3 in inflammatory heart disease murine model (53). Moreover, our findings show that TIM-3 signaling dictates the severity of IR-mediated hepatocellular damage in TLR4-dependent manner.
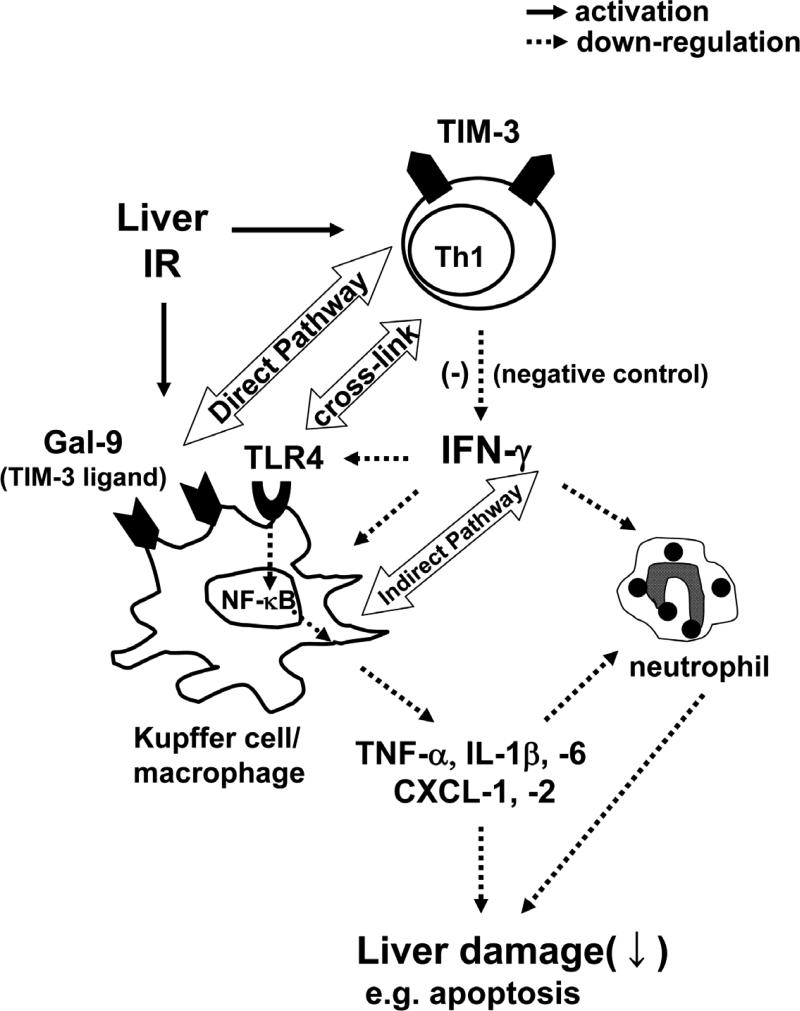
Figure 7 summarizes potential mechanisms by which TIM-3–Gal-9 pathway controls liver IR-triggered innate immunity-driven inflammation accompanied by Th1 cell and macrophage activation (2,36). TIM-3 blockade on activated Th1 cells induces IFN-γ, which in turn activates Kupffer cells, macrophages, neutrophils, and upregulates TLR4 expression. Activated macrophages elaborate cytokine/chemokine programs through TLR4 pathway, which is critical for dysregulated TIM-3 signaling to promote the ultimate organ damage. We favor the notion that TIM-3 pathway may exert a “protective” function by depressing IFN-γ production, and hence sparing liver from IR-mediated damage in TLR4-dependent manner. Thus, although the blockade of TIM-1/TIM-4 and enhancement of TIM-3/Gal-9 signaling might be essential, further studies are needed to accurately assess their therapeutic potential given the opposing effects of TIM-1 and TIM-3 in liver IRI.
Figure 7.
Scheme of cross-talk interactions between TIM-3, Gal-9 and TLR4 signaling networks. IR-triggered liver damage leads to the activation of macrophages and Th1 cells, which express Gal-9 and TIM-3, respectively. TIM-3-Gal-9 signaling negatively regulates Th1 cells by suppressing TLR4-NF-κB pathway via IFN-γ, which in turn stimulates Gal-9 and prevents macrophage activation. Diminished elaboration of pro-inflammatory cytokine and chemokine programs mitigates the hepatocellular damage and ultimately facilitates liver homeostasis.
Hence, our results suggest that TIM-3-Gal-9 pathway is instrumental in the maintenance of hepatic homeostasis in dysregulated liver immune response, such as during IRI, a finding of potential importance to modulate tissue innate vs. adaptive responses in liver transplant patients.
Acknowledgments
This work was supported by NIH Grants RO1 DK062357, AI23847, and The Dumont Research Foundation. We have no financial arrangements and potential conflicts.
Abbreviations
- ALT
alanine aminotransferase
- IRI
ischemia/reperfusion injury
- MPO
myeloperoxidase
- TIM-3
T Cell Ig Mucin-3
- TNF
tumor necrosis factor
- Gal-9
galectin-9
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Farmer DG, Amersi F, Kupiec-Weglinski JW, et al. Current status of ischemia and reperfusion injury in the liver. Transplant Rev. 2000;14:106–126. [Google Scholar]
- 2.Zwacka RM, Zhang Y, Halldorson J, et al. CD4(+) T-lymphocytes mediate ischemia/reperfusion-induced inflammatory responses in mouse liver. J Clin Invest. 1997;100:279–289. doi: 10.1172/JCI119533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen XD, Ke B, Zhai Y, et al. CD154-CD40 T-cell costimulation pathway is required in the mechanism of hepatic ischemia/reperfusion injury, and its blockade facilitates and depends on heme oxygenase-1 mediated cytoprotection. Transplantation. 2002;74:315–319. doi: 10.1097/00007890-200208150-00005. [DOI] [PubMed] [Google Scholar]
- 4.Shen XD, Ke B, Zhai Y, et al. Stat4 and Stat6 signaling in hepatic ischemia/reperfusion injury in mice: HO-1 dependence of Stat4 disruption-mediated cytoprotection. Hepatology. 2003;37:296–303. doi: 10.1053/jhep.2003.50066. [DOI] [PubMed] [Google Scholar]
- 5.Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol. 1991;260:G355–G362. doi: 10.1152/ajpgi.1991.260.3.G355. [DOI] [PubMed] [Google Scholar]
- 6.Hanschen M, Zahler S, Krombach F, et al. Reciprocal activation between CD4+ T cells and Kupffer cells during hepatic ischemia-reperfusion. Transplantation. 2008;86:710–718. doi: 10.1097/TP.0b013e3181821aa7. [DOI] [PubMed] [Google Scholar]
- 7.Kuchroo VK, Umetsu DT, DeKruyff RH, et al. The TIM gene family: emerging roles in immunity and disease. Nat Rev Immunol. 2003;3:454–462. doi: 10.1038/nri1111. [DOI] [PubMed] [Google Scholar]
- 8.Uchida Y, Ke B, Freitas MC, et al. The emerging role of T cell immunoglobulin mucin-1 in the mechanism of liver ischemia and reperfusion injury in the mouse. Hepatology. 2010;51:1463–1472. doi: 10.1002/hep.23442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monney L, Sabatos CA, Gaglia JL, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 10.Sánchez-Fueyo A, Tian J, Picarella D, et al. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat Immunol. 2003;4:1093–1101. doi: 10.1038/ni987. [DOI] [PubMed] [Google Scholar]
- 11.Anderson AC, Anderson DE, Bregoli L, et al. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 2007;318:1141–1143. doi: 10.1126/science.1148536. [DOI] [PubMed] [Google Scholar]
- 12.O'Neill LA. Therapeutic targeting of Toll-like receptors for inflammatory and infectious diseases. Curr Opin Pharmacol. 2003;3:396–403. doi: 10.1016/s1471-4892(03)00080-8. [DOI] [PubMed] [Google Scholar]
- 13.Obhrai J, Goldstein DR. The role of toll-like receptors in solid organ transplantation. Transplantation. 2006;81:497–502. doi: 10.1097/01.tp.0000188124.42726.d8. [DOI] [PubMed] [Google Scholar]
- 14.Tsung A, Hoffman RA, Izuishi K, et al. Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in nonparenchymal cells. J Immunol. 2005;175:7661–7668. doi: 10.4049/jimmunol.175.11.7661. [DOI] [PubMed] [Google Scholar]
- 15.Zhai Y, Shen XD, O'Connell R, et al. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol. 2004;173:7115–7119. doi: 10.4049/jimmunol.173.12.7115. [DOI] [PubMed] [Google Scholar]
- 16.Zhai Y, Qiao B, Shen XD, et al. Evidence for the pivotal role of endogenous toll-like receptor 4 ligands in liver ischemia and reperfusion injury. Transplantation. 2008;85:1016–1022. doi: 10.1097/TP.0b013e3181684248. [DOI] [PubMed] [Google Scholar]
- 17.Zhai Y, Qiao B, Gao F, et al. Type I, but not type II, interferon is critical in liver injury induced after ischemia and reperfusion. Hepatology. 2008;47:199–206. doi: 10.1002/hep.21970. [DOI] [PubMed] [Google Scholar]
- 18.Zhai Y, Shen XD, Gao F, et al. CXCL10 regulates liver innate immune response against ischemia and reperfusion injury. Hepatology. 2008;47:207–214. doi: 10.1002/hep.21986. [DOI] [PubMed] [Google Scholar]
- 19.Uchida Y, Freitas MC, Zhao D, et al. The inhibition of neutrophil elastase ameliorates mouse liver damage due to ischemia and reperfusion. Liver Transpl. 2009;15:939–947. doi: 10.1002/lt.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uchida Y, Freitas MC, Zhao D, et al. The Protective Function of Neutrophil Elastase Inhibitor in Liver Ischemia/Reperfusion Injury. Transplantation. 2010;89:1050–1056. doi: 10.1097/TP.0b013e3181d45a98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukushima A, Sumi T, Fukuda K, et al. Antibodies to T-cell Ig and mucin domain-containing proteins (Tim)-1 and -3 suppress the induction and progression of murine allergic conjunctivitiṣ. Biochem Biophys Res Commun. 2007;353:211–216. doi: 10.1016/j.bbrc.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, et al. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation. 1993;55:1265–1272. doi: 10.1097/00007890-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Schulz SM, Köhler G, Holscher C, et al. IL-17A is produced by Th17, gammadelta T cells and other CD4- lymphocytes during infection with Salmonella enterica serovar Enteritidis and has a mild effect in bacterial clearance. Int Immunol. 2008;20:1129–1138. doi: 10.1093/intimm/dxn069. [DOI] [PubMed] [Google Scholar]
- 24.He W, Fang Z, Wang F, et al. Galectin-9 significantly prolongs the survival of fully mismatched cardiac allografts in mice. Transplantation. 2009;88:782–790. doi: 10.1097/TP.0b013e3181b47f25. [DOI] [PubMed] [Google Scholar]
- 25.Marguti I, Yamamoto GL, da Costa TB, et al. Expansion of CD4+ CD25+ Foxp3+ T cells by bone marrow-derived dendritic cells. Immunology. 2009;127:50–61. doi: 10.1111/j.1365-2567.2008.02927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 27.Lentsch AB, Yoshidome H, Cheadle WG, et al. Chemokine involvement in hepatic ischemia/reperfusion injury in mice: Roles for macrophage inflammatory protein-2 and KC. Hepatology. 1998;27:1172–1177. doi: 10.1002/hep.510270440. [DOI] [PubMed] [Google Scholar]
- 28.Shen XD, Ke B, Zhai Y, et al. Toll-like receptor and heme oxygenase-1 signaling in hepatic ischemia/reperfusion injury. Am J Transplant. 2005;5:1793–1800. doi: 10.1111/j.1600-6143.2005.00932.x. [DOI] [PubMed] [Google Scholar]
- 29.Oikawa T, Kamimura Y, Akiba H, et al. Preferential involvement of Tim-3 in the regulation of hepatic CD8+ T cells in murine acute graft-versus-host disease. J Immunol. 2006;177:4281–4287. doi: 10.4049/jimmunol.177.7.4281. [DOI] [PubMed] [Google Scholar]
- 30.Yang L, Anderson DE, Kuchroo J, et al. Lack of TIM-3 immunoregulation in multiple sclerosis. J Immunol. 2008;180:4409–4414. doi: 10.4049/jimmunol.180.7.4409. [DOI] [PubMed] [Google Scholar]
- 31.Ju Y, Hou N, Zhang XN, et al. Blockade of Tim-3 pathway ameliorates interferon-gamma production from hepatic CD8+ T cells in a mouse model of hepatitis B virus infection. Cell Mol Immunol. 2009;6:35–43. doi: 10.1038/cmi.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rüdiger HA, Graf R, Clavien PA. Liver ischemia: apoptosis as a central mechanism of injury. J. Invest. Surg. 2003;16:149–159. [PubMed] [Google Scholar]
- 33.Hernandez LA, Grisham MB, Twohig B, et al. Role of neutrophils in ischemia-reperfusion induced microvascular injury. Am J Physiol. 1987;253:H699–H703. doi: 10.1152/ajpheart.1987.253.3.H699. [DOI] [PubMed] [Google Scholar]
- 34.Jaeschke H. Kupffer cell-induced oxidant stress during hepatic ischemia-reperfusion: does the controversy continue? Hepatology. 1999;30:1527–1528. doi: 10.1002/hep.510300630. [DOI] [PubMed] [Google Scholar]
- 35.Rudiger HA, Clavien PA. Tumor necrosis factor-alpha, but not Fas, mediates hepatocellular apoptosis in the murine ischemic liver. Gastroenterology. 2002;122:202–210. doi: 10.1053/gast.2002.30304. [DOI] [PubMed] [Google Scholar]
- 36.Colletti LM, Remick DG, Burtch GD, et al. Role of tumor necrosis factor-alpha in the pathophysiologic alterations after hepatic ischemia/reperfusion injury in the rat. J Clin Invest. 1990;85:1936–1943. doi: 10.1172/JCI114656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jaeschke H, Smith CW. Mechanisms of neutrophil-induced parenchymal cell injury. J Leukoc Biol. 1997;61:647–653. doi: 10.1002/jlb.61.6.647. [DOI] [PubMed] [Google Scholar]
- 38.Lappas CM, Day YJ, Marshall MA, et al. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med. 2006;203:2639–2648. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taub R. Blocking NF-kappaB in the liver: the good and bad news. Hepatology. 1998;27:1445–1446. doi: 10.1002/hep.510270538. [DOI] [PubMed] [Google Scholar]
- 40.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 41.Sabatos CA, Chakravarti S, Cha E, et al. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat. Immunol. 2003;4:1102–1110. doi: 10.1038/ni988. [DOI] [PubMed] [Google Scholar]
- 42.Zhu C, Anderson AC, Schubart A, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 43.Anderson AC, Anderson DE. TIM-3 in autoimmunity. Curr Opin Immunol. 2006;18:665–669. doi: 10.1016/j.coi.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 44.Fukushima A, Sumi T, Fukuda K, et al. Roles of galectin-9 in the development of experimental allergic conjunctivitis in mice. Int Arch Allergy Immunol. 2008;146:36–43. doi: 10.1159/000112501. [DOI] [PubMed] [Google Scholar]
- 45.Wada J, Kanwar YS. Identification and characterization of galectin-9, a novel β-galactoside-binding mammalian lectin. J. Biol. Chem. 1997;272:6078–6086. doi: 10.1074/jbc.272.9.6078. [DOI] [PubMed] [Google Scholar]
- 46.Matsumoto R, Matsumoto H, Seki M, et al. Human ecalectin, a variant of human galectin-9, is a novel eosinophil chemoattractant produced by T lymphocytes. J. Biol. Chem. 1998;273:16976–16984. doi: 10.1074/jbc.273.27.16976. [DOI] [PubMed] [Google Scholar]
- 47.Meyers JH, Chakravarti S, Schlesinger D, et al. TIM-4 is the ligand for TIM-1, and the TIM-1-TIM-4 interaction regulates T cell proliferation. Nat Immunol. 2005;6:455–464. doi: 10.1038/ni1185. [DOI] [PubMed] [Google Scholar]
- 48.McIntire JJ, Umetsu SE, Akbari O, et al. Identification of Tapr (an airway hyperreactivity regulatory locus) and the linked Tim gene family. Nat Immunol. 2001;2:1109–1116. doi: 10.1038/ni739. [DOI] [PubMed] [Google Scholar]
- 49.Hein RM, Woods ML. TIM-1 regulates macrophage cytokine production and B7 family member expression. Immunol Lett. 2007;108:103–108. doi: 10.1016/j.imlet.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 50.Wolfs TG, Buurman WA, van Schadewijk A, et al. In vivo expression of Toll-like receptor 2 and 4 by renal epithelial cells: IFN-gamma and TNF-alpha mediated up-regulation during inflammation. J Immunol. 2002;168:1286–1293. doi: 10.4049/jimmunol.168.3.1286. [DOI] [PubMed] [Google Scholar]
- 51.Schroder K, Sweet MJ, Hume DA. Signal integration between IFN γ and TLR signalling pathways in macrophages. Immunobiology. 2006;211:511–524. doi: 10.1016/j.imbio.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 52.Tang SC, Arumugam TV, Xu X, et al. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci USA. 2007;104:13798–13803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frisancho-Kiss S, Davis SE, Nyland JF, et al. Cutting edge: cross-regulation by TLR4 and T cell Ig mucin-3 determines sex differences in inflammatory heart disease. J Immunol. 2007;178:6710–6714. doi: 10.4049/jimmunol.178.11.6710. [DOI] [PubMed] [Google Scholar]