Abstract
Aim
To compare canal and isthmus debris debridement efficacies of the manual dynamic irrigation (MDI) and apical negative pressure (ANP) techniques in the mesial root of mandibular first molars with narrow isthmi, using a closed canal design.
Methodology
Micro-computed tomography was employed to select 20 teeth, each containing a narrow isthmus. Each root was sealed at the apex with hot glue and embedded in polyvinylsiloxane to simulate a closed canal system. The teeth were submitted to a standardised instrumentation protocol. Final irrigation was performed with either the MDI or the ANP technique using the EndoVac system (N=10). Masson trichrome-stained sections were prepared from completely demineralised roots at ten canal levels between 1–2.8 mm of the anatomical apices. Areas occupied by canals and isthmus of each root and debris in the corresponding regions were digitised by the NIH Image J software and statistically analysed using two-way repeated measures ANOVA.
Results
For the instrumented canals, there were no differences between the two groups (p=0.131) in the area occupied by debris at all canal levels (p=0.343). Conversely, for the isthmus, less debris was found in the ANP group (p<0.001) but no differences were seen in each group with respect to the ten canal levels (p=0.352).
Conclusion
Neither technique produce completely removed debris from the isthmus regions. However the EndoVac system, which encompasses the ANP concept, removed considerably more debris from narrow isthmi of the mandibular mesial roots.
Keywords: Apical negative pressure, Debris, EndoVac, Isthmus, Manual dynamic irrigation, Canal
INTRODUCTION
Success of root canal treatment is dependent upon the effective removal of bacterial biofilms and their by-products from the root canal system (Chávez de Paz et al. 2007, 2008, Estrela et al. 2009, Ricucci & Siqueira 2010). Current instrumentation techniques are inefficient at cleaning all surfaces and irregularities within the root canal space (Siqueira et al. 1997, Peters 2004, Burleson et al. 2007, Vaudt et al. 2009, Williamson et al. 2009). Hard and soft tissue debris that remains after mechanical instrumentation can harbour microorganisms and decrease the efficacy of the seal created by root filling materials, possibly leading to treatment failure (Bergenholtz and Spångberg 2004, Nair, 2006, Brito et al. 2009, Paqué et al. 2009). Therefore, irrigant agitation is a necessary adjunct to mechanical instrumentation as a method of chemical debridement to remove debris and bacteria from the entire canal system (Nielsen & Baumgartner 2007, Carver et al. 2007, de Gregorio et al. 2009, Gu et al. 2009, Jiang et al. 2010).
Apart from the rational selection of irrigants (Zehnder 2006), a sufficient volume of the irrigant, a high flow rate and unrestricted flow of the irrigant along the canal walls are crucial for thorough debridement of the root canal system (Moser & Heuer 1982, Chow 1983, Hsieh et al. 2007). As traditional needle irrigation delivers solutions no further than 1 mm beyond the needle tip (Boutsioukis et al. 2009, Gao et al. 2009), this irrigant delivery technique is relatively ineffective in flushing hard and soft tissue remnants from the apical third of canal walls in the absence of adjunctive irrigant agitation regimes. Gas entrapment prevents optimal irrigant delivery and flow 0–2 mm from the end-point of canals (Tay et al. 2010). With the exception of studies performed by Baumgartner and coworkers (Baumgartner & Mader 1987, Albrecht et al. 2004, Usman et al. 2004, Nielson & Baumgartner 2007), few ex vivo studies have incorporated experimental set ups that truly produce a closed canal system design to simulate the effect of gas entrapment (Fukumoto et al. 2006, Tay et al. 2010). Other studies that were intended to simulate a closed canal system were non- deliberately flawed as they permitted gas or fluid exchange between the canal and external environment. One example is the procedure of half-embedding demineralized roots in soft silicone, while allowing the other halves to be covered with a clearing fluid that can move in and out of the roots. Iatrogenic creation of an open canal system may also occur when teeth extracted after meticulously-executed in vivo irrigation procedures are flushed through unsealed apical foramen to remove blood that accumulate in the root canals during extraction, or by reassembling split roots back into silicone moulds to create a canal that can be easily examined after the irrigation procedures. A plethora of ex vivo irrigation studies is available in the literature in which the use of a closed system or an open system design had not been specified. Thus, the reported canal debridement efficacy in studies with ambiguous or unspecified designs on how the issue of gas entrapment was addressed has to be viewed with scepticism.
Successful debridement of posterior teeth is hampered by the difficulty in flushing debris effectively from the isthmi of roots containing multiple canals. Although there is no hard tissue debris associated with these non-instrumented regions, dissolution of soft tissue remnants and eradication of microbes associated with infected pulpal tissues must rely on the efficacy of irrigants to access these hard-to-reach regions, as well as the generation of a high flow rate that permits a hydraulic force that is strong enough to detach debris that are caught within the predentine collagen network of the non-instrumented isthmus canal walls. Previous ex vivo and in vivo studies have shown that debridement of the isthmus 1–3 mm from the anatomical apex could be improved with the adjunctive use of ultrasonic agitation (Haidet et al. 1989, Walker & de Rio 1991, Archer et al. 1992, Evans et al. 2001, Gutarts et al. 2005, Burleson et al. 2007). Nevertheless, the adverse effect of the apical gas entrapment on the efficacy of isthmus debridement has not been appropriately addressed in those ex vivo studies. Moreover, a critical issue associated with the aforementioned in vivo studies is the failure to adopt a clinically-applicable selection criterion to define the mesiodistal isthmus width until the teeth were extracted and prepared for histological examination (Mannocci et al. 2005). Perceivably, roots with wider isthmi are more readily accessible to tissue dissolving irrigants than roots with narrow “partial isthmi” (Weller et al. 1995, Teixeira et al. 2003) that are partially-obliterated in a three-dimensional manner by calcified or sclerotic dentine (Mannocci et al. 2005). Thus, in the absence of an appropriate clinical selection criterion on isthmus width, it is difficult to prevent the unintentional introduction of bias during the execution of an in vivo study on isthmus debridement efficacy. Clearly, there is a gap in our current understanding of how the phenomenon apical gas entrapment affects isthmus debridement efficacy in narrow isthmi present in roots with multiple canals.
Different techniques and irrigant delivery devices are available for improving the flow and distribution of irrigating solutions within the root canal system (Gu et al. 2009). Manual dynamic irrigation (MDI) has been described as a simple and cost-effective technique for cleaning the walls of instrumented root canals (McGill et al. 2008). It involves repeated insertion of a well-fitting gutta-percha cone to working length of a previously shaped canal. The gutta-percha cone is applied in short, gentle strokes to hydrodynamically displace and agitate an irrigant by producing eddy currents. This technique may be useful in circumventing apical gas entrapment because the air bubble located at the 0–2 mm of the apical seat will be completely displaced by repeated gutta-percha insertions. However, the hydrodynamic effect of the MDI technique on isthmus debridement efficacy in a closed canal system is unknown.
The EndoVac system (Discus Dental, Culver City, CA, USA) is an apical negative pressure (ANP) irrigation device that is designed to deliver irrigating solution to the apical end of the canal system and remove debris via a negative pressure mechanism (Schoeffel 2008). The EndoVac has been shown to introduce a higher flow of irrigant and produce better debridement at 1 mm from the working length when compared with needle irrigation (Nielson & Baumgartner 2007). Additionally, the EndoVac has been shown to extrude less irrigant and, therefore, create less risk of a NaOCl incident (Desai & Himel 2009). Although the ANP suction mechanism could be a means to overcome the obstacle of apical gas entrapment within instrumented canals in anterior teeth, the efficacy of this strategy in removing debris from the apical part of the canal walls in posterior teeth has not been reported except for anecdotal reports of clinical experiences in non-peer-reviewed magazines. In particular, the effectiveness of the EndoVac system in removing debris from narrow isthmi that are associated with the critical apical 0–2 mm of the canal in roots of posterior teeth with multiple canals is unknown. Thus, the objective of the present ex vivo study was to compare the canal and isthmus debris debridement efficacies of the MDI technique and the ANP technique at 0–2 mm from the apical stop prepared in the mesial root of mandibular first molars with narrow isthmi, using a closed canal design. The null hypotheses tested were that: 1) there is no difference between the canal debridement efficacy of the two irrigant agitation techniques at different canal levels from the anatomical apex in a simulated closed canal system; and 2) there is no difference between the isthmus debridement efficacy of the two irrigant agitation techniques at different canal levels from the anatomical apex in a simulated closed canal system.
MATERIALS AND METHODS
Recently extracted non-carious human first molars with closed apices that were extracted for periodontal reasons were collected with patient’s consent under a protocol approved by the Human Assurance Committee of the Medical College of Georgia. The teeth were stored at 4°C in 0.9% NaCl solution supplemented with 0.02% sodium azide to prevent bacteria growth until use.
Criterion of tooth selection
As roots with narrow and wide isthmi differ considerably in their ease of debris removal, only those mandibular first molars with narrow isthmus width between the mesiobuccal and mesiolingual canals were used. The criterion for tooth selection was that the mesiodistal isthmus width of completely patent isthmi or partially-obliterated isthmi had to be less than one-quarter of the diameter of the unshaped canals along the canal levels (i.e. 1–2.8 mm from the anatomical apex) from which histological sections would eventually be prepared after cleaning and shaping procedures. As this criterion could not be fulfilled using conventional radiography, micro-computed tomography (micro-CT) was used for non-destructive screening of those mesial roots prior to actual irreversible hemisectioning of the molar teeth. Each tooth was placed apical-coronally inside a custom-made X-ray transparent Styrofoam specimen holder and stabilised with cotton pellets. The specimen holder was attached perpendicularly to the specimen turntable of a micro-CT scanner (SkyScan 1174, SkyScan N.V., Aartselaar, Belgium). Scanning was performed with a spatial resolution of 15 µm using a 0.5 mm thick aluminum filter at 50 kV and 800 µA, a 0.6° rotation step and 360° rotation. After reconstruction, two-dimensional virtual slices in the axial direction (i.e. apical-coronal axis of the root) were made of the mesial root from 1–3 mm coronal to the anatomic root apex using the CT-analyser software (Skyscan) and saved in a 256 grey scale format. Each series consisted of 320–350 virtual sections. Molars with mesial roots that had mesiodistal isthmus widths wider than one-quarter of the diameter of the unshaped canals anywhere along the scanned regions were excluded from the study. Based on the aforementioned criterion, twenty mandibular molars were selected and randomly divided into two groups.
Preparation of the mesial root
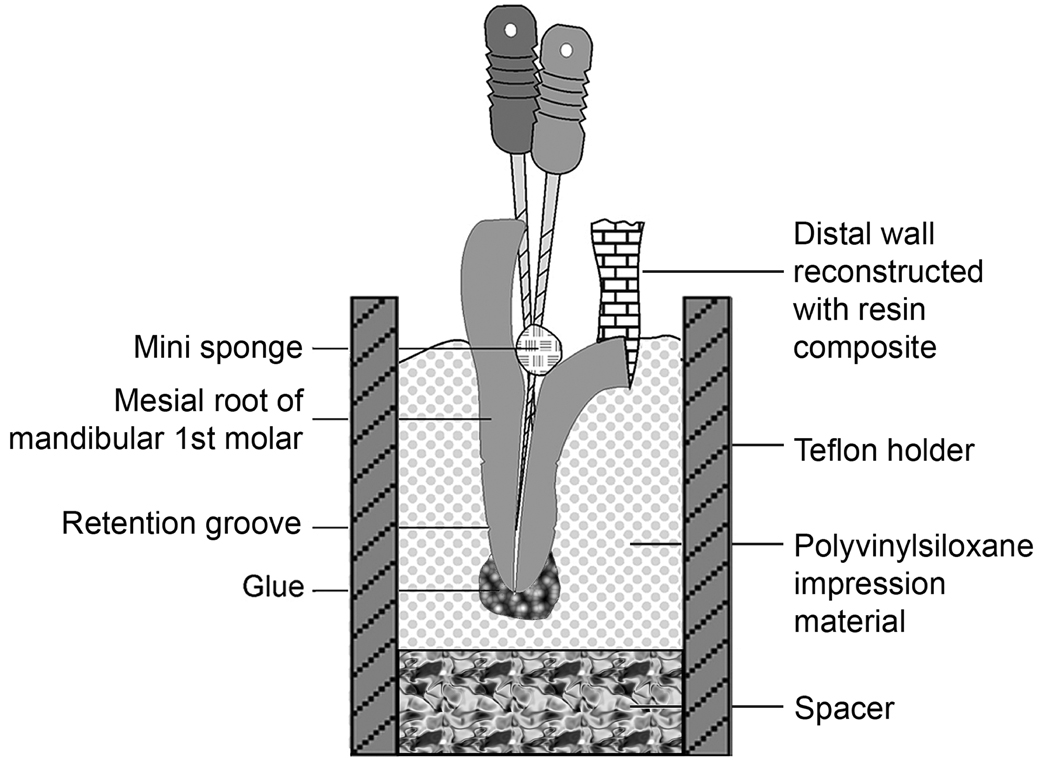
Each molar was hemisectioned using a slow-speed Isomet diamond saw (Buehler Ltd, Lake Bluff, IL, USA) under water-cooling to expose the pulp chamber. The coronal portion of each root was flattened with the Isomet saw for accurate and consistent working length measurements. Pulpal contents were gently removed with a spoon excavator. A size 3 cotton pellet was placed inside the pulp chamber together with two size 10 stainless steel hand files that were inserted into the mesiobuccal and mesiolingual canals to prevent clogging of the canal orifices. Reconstruction of the distal wall of the severed mesial root was performed using a dentine adhesive and a resin composite to provide a reservoir for the irrigants (Figure 1). Working length was determined with the hand files just visible in the apical foramen of each canal. The actual working length was taken to be 1 mm from the anatomic apex. Additionally, shallow horizontal groves were placed circumferentially around the acellular cementum to provide mechanical retention in the experimental set up. Each root had their apical foramina covered by glue expressed from a hot glue gun. The cementum and set glue were coated with tray adhesive (Dentsply Caulk, Milford, DE, USA).
Fig.1.
A schematic depicting a sectioned mesial root of a mandibular first molar with its distal wall reconstructed with a dentine adhesive and resin composite prior to inserting it into the polyvinylsiloxane-filled, simulated closed-end fixture.
Experimental design
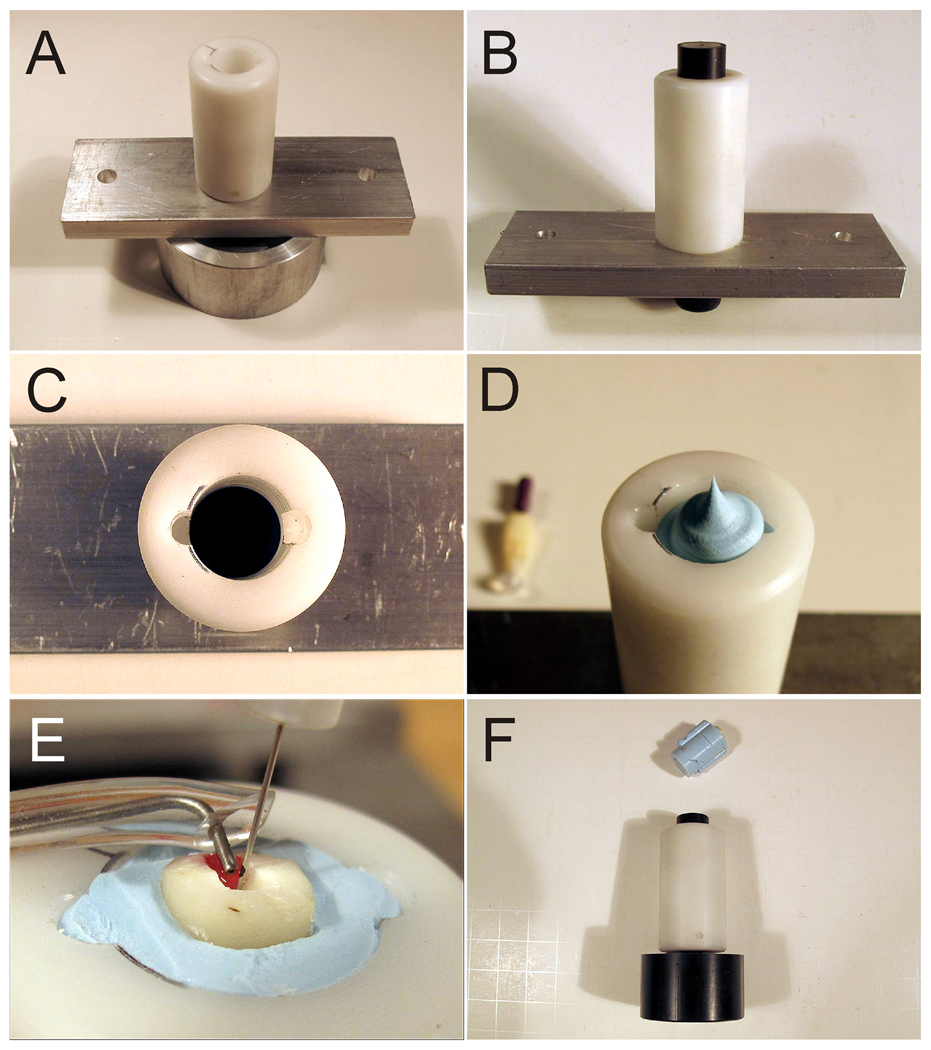
The roots were divided into two groups of ten roots each (N=10). Group I consisted of teeth irrigated with the MDI technique and Group II consisted of teeth irrigated with the ANP technique (i.e. the EndoVac system). A custom-fabricated fixture was used to simulate the effect of apical gas entrapment in a closed canal system (Figure 2). The fixture, in turn, was affixed to the experimental set up (Figure 3), which enabled consistent canal irrigation and suction of irrigants to be performed simultaneously by a single operator. Briefly, a Teflon holder was assembled on top of metal parts that enabled the tube to be attached to the experimental set up (Figures 2A, 3). A tight-fitting metal spacer was inserted into the Teflon holder to form the base of the Teflon holder (Figures 1, 2B) and to prevent extrusion of polyvinylsiloxane (PVS) impression material (Blue Moose, Parkell Inc, Farmington, CT, USA) from the base of the fixture. The Teflon holder with the inserted metal spacer is shown in Figure 2C. The Teflon holder also contained an anti-rotation lock on each side to prevent rotation of the set PVS during cleaning and shaping procedures. After filling the holder with PVS (Figure 2D), the adhesive-coated root was inserted into the PVS. Excess PVS was removed with a razor blade so that 2 mm of the decoronated surface protruded from the surface of the PVS. A slanted groove was created with a high-speed bur on one side of the exposed root surface (Figure 2E) of the reconstructed pulp chamber to facilitate adaptation of the master irrigant delivery tip to the canal orifice. This experimental setup prevented fluid and gases from escaping apically via the apical foramen to the external environment (Baumgartner & Mader 1987, Tay et al. 2010).
Fig.2.
Photographic images of the simulated closed-end fixture employed in the present study. A. The closed canal fixture assembled from its component parts. A Teflon spacer is attached via a metal base to a circular base holder for attachment to the experimental fluid delivery and suction system (see Fig. 3). B. A (black) spacer is inserted into the Teflon holder. C. The spacer is pushed to the bottom of the holder to serve as the base to prevent extrusion of the polyvinylsiloxane impression material from the bottom of the fixture. Two anti-rotation grooves prevent rotation of the set impression material within the holder during canal cleaning and shaping procedures. D. The Teflon holder is filled to the top with impression material. The root is inserted into the impression material before it sets. E. A groove (marked red) is placed on one side of the canal wall dentine to facilitate fitting of the master suction tip. F. After completion of the canal shaping and irrigation procedures, the polyvinylsiloxane impression material is displaced from the Teflon holder with a cap screw for retrieval of the root.
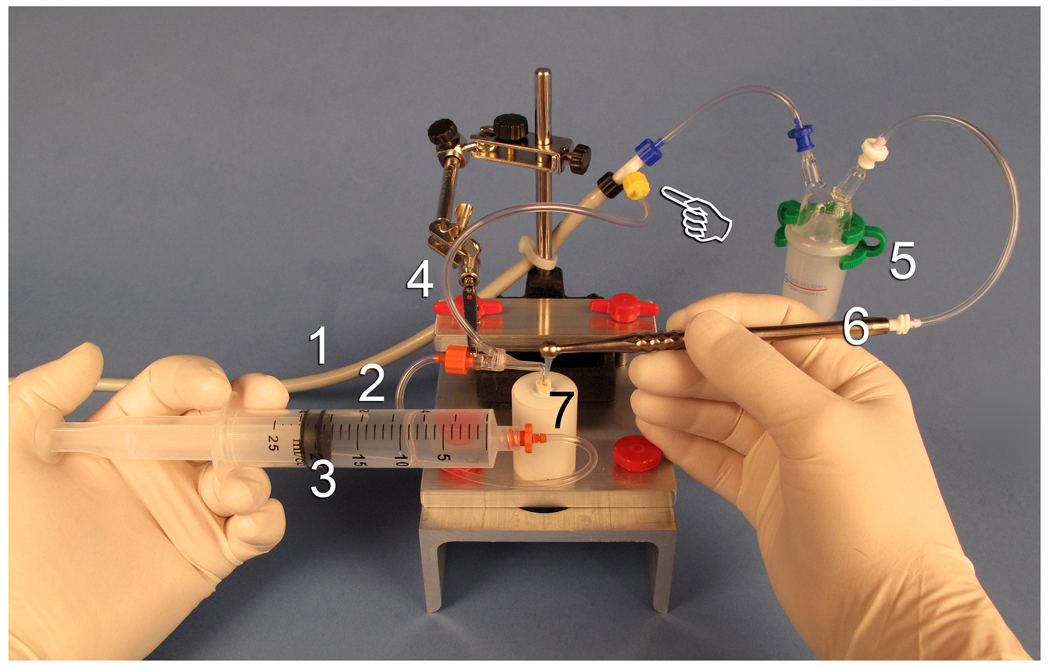
Fig.3.
Custom-fabricated experimental set up to facilitate the application of irrigation procedures in a closed canal system design to be performed by a single operator. The different component parts are: 1. High vacuum suction line. 2. Connector to a 20 mL syringe. 3. Twenty microlitre syringe. 4. The master suction tip is connected to the high vacuum line via a T-connector (pointer). 5. A separate fluid collection trap is attached to the high vacuum line and to the tubing connecting the macro-cannulus and micro-cannulus of the EndoVac hand piece. This trap measured the volume of irrigant suctioned by the EndoVac system. 6. EndoVac hand piece. 7. Teflon holder as shown in Fig. 2.
The experimental set up (Figure 3) had a vacuum tubing that was connected to the high vacuum line (Figure 3 – component 1) for suction of the irrigants. For the ANP group, irrigant was delivered to the canal orifice via the master delivery tube (Figure 3 – component 2) that was connected to a 20 mL syringe (Figure 3 – component 3). For the MDI group, this irrigant delivery technique was replaced by direct delivery of the irrigants to 1 mm short of the working length via a 30-G side-vented needle (Maxi-i-Probe, Dentsply-Rinn, Elgin, IL, USA) connected to the 20 mL syringe (not shown). The rate of irrigant from the 20 mL syringe was consistent for both groups (5 mL per minute). A plastic tubing (Figure 3 – component 4) enclosing the master delivery tube was connected via a “T” connector (Figure 3 – pointer) to a fluid trap (Figure 3 – component 5) and the high vacuum line. The fluid trap, in turn, was connected to the ANP suction device (Figure 3 – component 6). Thus, during irrigation with the ANP technique, irrigant removal was achieved with the apical negative suction, while excess irrigant was simultaneously removed via the plastic suction tubing (component 4) over the reconstructed pulp chamber. During MDI, the ANP component of the experimental set up was disabled so that suction of the irrigant was achieved only via the plastic suction tubing placed over the reconstructed pulp chamber.
Experimental groups
Each canal was instrumented to size 35, .04 taper using a crown down approach with Endosequence rotary nickel titanium instruments (Brasseler, Savannah, GA, USA). During all instrumentation, the chamber was flooded with 5.25% NaOCl replenished with 1 mL after each instrument. All post-instrumentation irrigant delivery was performed at an average rate of 5 mL per minute and each group had the same overall total irrigation time of six minutes. The two groups represented the two different agitation techniques that were performed after the use of the last rotary nickel titanium instrument:
Group I
Manual dynamic irrigation that consisted of 5.25% NaOCl for 1 min with short push-pull strokes using a size 35, .04 taper gutta-percha cone followed by 1 min of soaking; 17% EDTA for 1 min with short push-pull strokes using the same gutta-percha cone followed by 1 min of soaking; 5.25% NaOCl for 1 min with short push-pull strokes using the same gutta-percha point followed by 1 min of soaking. The use of an irrigation protocol that consisted of three irrigation cycles (i.e. initial NaOCl, EDTA, final NaOCl) was based on the work of Yamada et al. (1983). The time periods employed for the three irrigation cycles were chosen so that they were similar to those employed for Group II. All push-pull strokes were performed manually at an approximate rate of 100 strokes per minute. The selection of a gutta-percha cone corresponding to the canal preparation size and taper ensured that air inside the apical third of the canal was displaced by the gutta-percha cone when the latter was inserted to the working length.
Group II
Irrigation with the ANP technique also consisted of three irrigation cycles as reported by Nielson & Baumgartner (2007). The canals were first irrigated for 30 sec with 5.25% NaOCl using the macro-cannulus followed by leaving the canal full of irrigant for 30 sec. Three irrigation cycles using the micro-cannulus inserted to full working length followed. The first cycle was 30 sec of 5.25% NaOCl followed by 30 sec of soaking; the second cycle was 1 min of 17% EDTA followed by 1 min of soaking; the third cycle was 1 min of 5.25% NaOCl followed by 1 min of soaking. The combined, post-instrumental NaOCl irrigation and soaking times prior to the use of EDTA (30 sec for macro-cannulus and 30 sec for micro-cannulus) employed in Group II were equivalent to the post-instrumental NaOCl irrigation and soaking times employed for Group I. The same volumes of irrigants were also used in Groups I and II.
Upon completion of the respective irrigation protocol, the canals were rinsed with sterile saline and dried with multiple paper points. The access of each canal was temporized with Tempit (Centrix, Shelton, CT, USA). The PVS was removed from the Teflon holder by inserting a cap screw from the bottom of the holder (Figure 2F). Each instrumented root was removed from the PVS and stored separately in labelled bottles containing 10% formaldehyde to as a fixative for any soft tissue debris remaining within the shaped canals as well as the isthmus area.
Preparation for light microscopy
All teeth were completely demineralised in an aqueous solution of 10 wt% formic acid/sodium formate (pH 2.4) for a minimum of two weeks at 25°C under constant stirring. The end point of demineralisation was assessed using digital radiography. The demineralized roots were embedded in paraffin wax for microtomy. Seven micrometre thick serial histologic sections were prepared from ten canal levels of each root, beginning at 1.0 mm from the anatomic apex. The ten canal levels were (from the anatomic apex): 1.0 mm, 1.2 mm, 1.4 mm, 1.6 mm, 1.8 mm, 2.0 mm, 2.2 mm, 2.4 mm, 2.6 mm and 2.8 mm. Sixteen serial sections were prepared at each canal level. Glass slides containing the serial sections were stained with Masson’s trichrome. All slides derived from the same root were given a random number corresponding to the group to which root belonged and sequentially labelled according to the canal level in which they were sectioned. Only the canal level was known to the evaluator who was assigned to assess the cleanliness of the canal space, who was blind to the group from which the root was derived. Stained sections were examined using an incandescent light microscope (BX 51, Olympus America, Inc., Melville, NY, USA) equipped with a digital camera system (Retiga 4000R OImaging, Burnaby, BC, Canada). Digitised images were taken of the best, technically error-free section as being representative of respective canal level. Images were taken at the highest magnification (20×-40×) in which the mesiobuccal and mesiolingual canals and the isthmus region could be included in the same image, to facilitate subsequent image analysis of these three components of the canal space under the same scale from a single digitised image.
Image analysis
Digitised images were analysed using the Image J software (National Institute of Health, Bethesda, MD, USA) by a collaborator who was not involved in the canal preparation and irrigation procedures. The outlines of the mesiobuccual and mesiolingual canals and the isthmus between the two canals were traced to delineate the relative surface areas of the respective regions of the canal space. Likewise, areas occupied by stained debris in the corresponding regions were determined. For each canal level, the percentage area occupied by debris in the shaped canals was calculated as the quotient of the summation of the relative areas occupied by debris in both canals, over the summation of the mesiobuccal and mesiolingual canals and multiplied by 100. Likewise, the percentage area occupied by debris within the isthmus region was calculated as the quotient of the relative area occupied by debris in the isthmus region over the relative area of the isthmus, multiplied by 100. When a “partial isthmus” was encountered, debris within the partially-exposed isthmus was expressed as the quotient of the partially-exposed isthmus. In the case of a highly sclerotic isthmus with multiple, small, partially-exposed isthmus loci or an isthmus that extended beyond the shaped canals as canal fins, the sum of areas occupied by debris in those regional areas was divided by the total regional areas to generate an overall debris percentage score. For statistical analysis purpose, a completely blocked isthmus or a non-existent isthmus due to merging of the mesiobuccal and mesiolingual canals was given a null percentage score in order to achieve a balanced statistical design.
Statistical Analysis
Data derived from the canals and those derived from the isthmi were analysed separately by a two-factor repeated measures analysis of variance to examine the effects of the factor “Experimental Group” and the repeated factor “Canal Level” and the interaction of these two factors on canal space cleanliness. Pooled data from the ten canal levels were also analysed to examine if there were differences between the MDI group and the ANP group in terms of cleanliness of the instrumented canals and the non-instrumented isthmus regions. As the normality and homoscedasticity assumptions of the data appeared to be violated, the data were expressed as least square means and the common standard error of the least square means. Least square means are the expected values of group or subgroup means for a balanced design involving the group variables with all covariates at their common mean values. All pair-wise multiple comparisons were performed using the Tukey test. Statistical significance was set at α = 0.05.
RESULTS
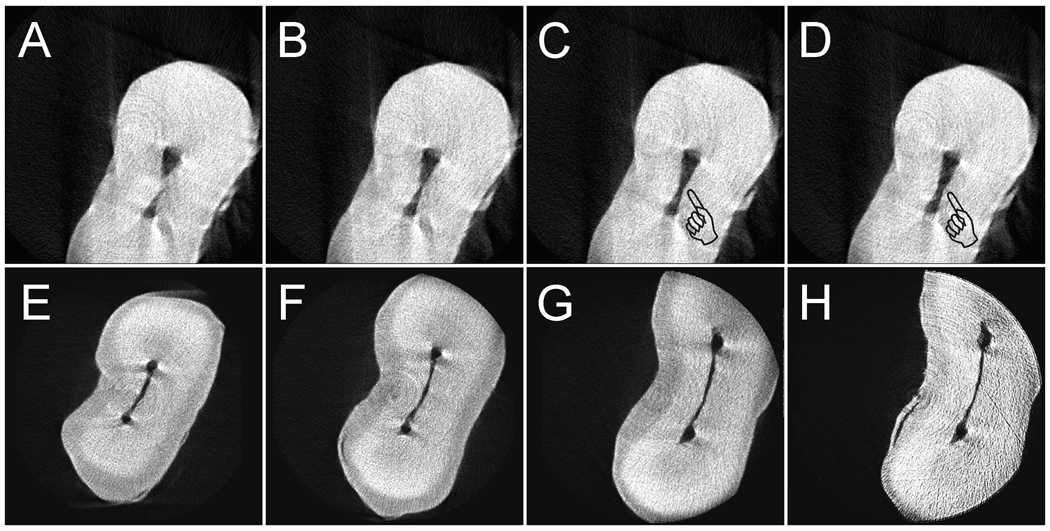
Figure 4 contains two series of micro-CT scans showing the criterion for tooth selection based on non-destructive scanning of the root tips of the mesial roots of mandibular first molars. Each series was taken from the 320–350 images reconstructed from the axial direction commencing at 1.0 mm from the anatomical apex of the mesial root and ending at 3.0 mm from the anatomical apex. In the first series (Figures 4A–4D), the root had a narrow isthmus that was narrower than one-quarter of the mesiodistal diameter of the unshaped root canals. However, axially-reconstructed images taken from positions more coronal to the anatomical apex showed that the isthmus in this part of the root was wider than the pre-established criterion. Thus, this specimen was considered unacceptable for the study. In the second series (Figures 4E–4F), a hairline-type isthmus is illustrated that was barely visible due to its highly sclerotic nature. Since the isthmus was narrower than the pre-established criterion, the specimen was considered acceptable for the study. Although the isthmus was barely visible under micro-CT scanning, it was partially patent (Weller et al. 1995) when examined using histological sections. Indeed, in this type of highly sclerotic “partial isthmus” (an example is shown in Figure 5), the isthmus is partially-obliterated by sclerotic dentine in a three-dimensional manner so that it may appear completely obliterated, partially patent and completely patent at different canal levels. Such an isthmus may also become completely filled with sclerotic dentine at a more coronal canal level after the observation of its complete or partial patency at a more apical level. This makes it very difficult to get irrigants to flow three dimensionally into those patent regions beneath the blocked coronal part of the isthmus.
Fig.4.
Micro-CT was used for non-destructive screening of the apical third of the mesial root of mandibular molars (1–3 mm from the anatomic apex). Roots with mesiodistal isthmus widths wider than one-quarter of the diameter of the unshaped canals anywhere along the scanned regions were excluded from the study. A–D. Representative images from a root that had acceptable narrow isthmus widths at 1.0 mm (4A) and 1.6 mm (4B) from the anatomic apex but isthmus widths wider than the set selection criterion (pointers) at 2.2 mm (4C) and 2.8 mm (4D). This root was not included in the study. E–H. Representative images from a root that had acceptable isthmus widths at all levels (1.0 mm – 4E; 1.6 mm – 4F; 2.2 mm – 4G; 2.8 mm – 4H) of the scanned regions. This root was included in the study. The partial isthmus was barely visible under micro-CT but could be discerned from histological sections.
Fig.5.
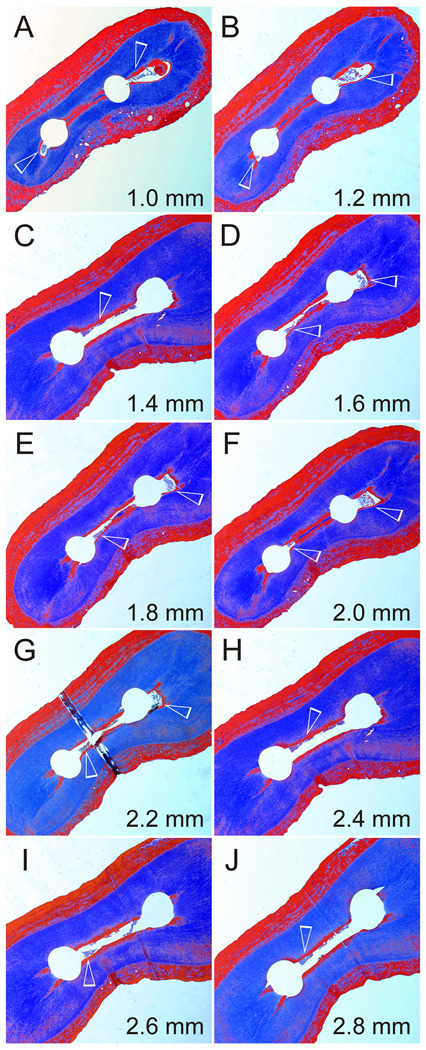
A representative example of a root specimen that was cleaned using the MDI technique. Figures 5A to 5J represent Masson’s trichrome-stained sections of the same root taken at the ten canal levels (original magnification 20–40×). Note that the isthmus between the two canals was sclerotic (stained red) and blocked in Figs 5A (1.0 mm) and 5B (1.2 mm). However, the part of isthmus that extended beyond the canals as canal fins remained patent at these two locations. Debris-containing regions are indicated by open arrows in each section.
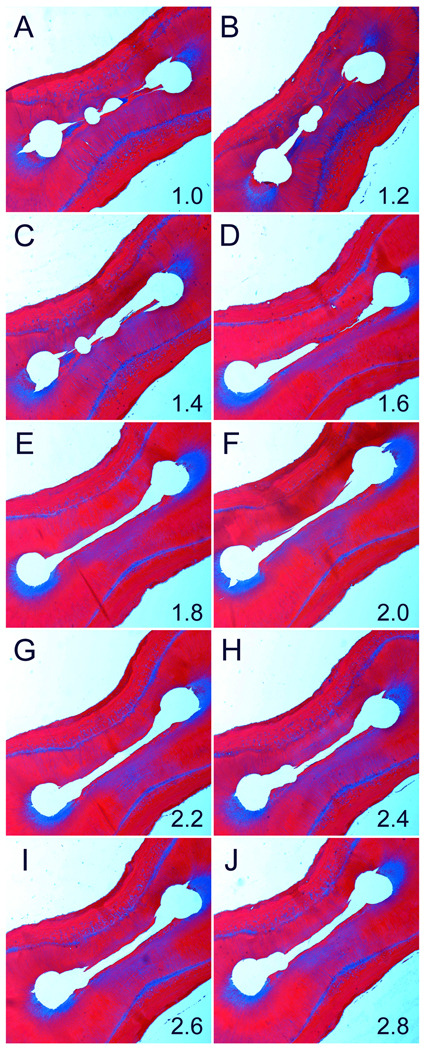
Figure 5 is a representative example from the MDI group showing fairly clean instructed canals but incomplete debris removal from the narrow isthmus. The latter extended across the two canals as canal fins along the buccal and lingual aspect of the mesial root. Incomplete debridement of debris from the isthmus region is evident at all canal levels where apical gas entrapment has been shown to be present in a simulated closed canal system (Tay et al. 2010). Figure 6 is a representative example from the ANP group showing clean instrumented canals and isthmus at all canal levels. Completely debridement of the very narrow isthmus is evident at 1.0 and 1.2 mm from the anatomic apex with the apical negative pressure generated as the microcannulus of the EndoVac system was inserted to working length to eliminate the phenomenon of apical gas entrapment.
Fig.6.
A representative example of a root specimen that was cleaned using the ANP technique. Figures 6A to 6J represent Masson’s trichrome-stained sections of the same root taken at the ten canal levels. No debris could be detected at each canal level. The number at the lower right corner of each image represents canal level in mm (Original magnification 20–4×).
Table I summarises the percentage area occupied by debris in the instrumented canals (i.e. isthmus excluded) of the two experimental groups examined repeatedly at the ten canal levels. Pooled data from all canal levels are also included. The data were presented as least square means with a common standard error of the least square means. Although there appeared to be more debris at most canal levels for the MDI group, two-way repeated measures analysis of variance revealed no significant difference between the two groups at all canal levels (p = 0.131). For each group, there was no difference in the area occupied by debris among the ten canal levels (p = 0.343). There was no statistically significant interaction between the factor “Group” and the repeated factor “Canal Level” (p = 0.227). When the data from the ten canal levels were pooled together, the difference in debris retention between the MDI group and the ANP group was also statistically insignificant (p = 0.092).
Table I.
Percentage of total canal area (mesiobuccal and mesiolingual canals combined) occupied by debris in the MDI and the ANP groups at ten canal levels.
| Canal level (from anatomic apex) |
MDI (%) * |
ANP (%) * |
|---|---|---|
| 1.0 mm | 0.21 | 0.00 |
| 1.2 mm | 0.11 | 0.00 |
| 1.4 mm | 0.02 | 0.00 |
| 1.6 mm | 0.78 | 0.00 |
| 1.8 mm | 0.82 | 0.00 |
| 2.0 mm | 0.13 | 0.00 |
| 2.2 mm | 0.00 | 0.00 |
| 2.4 mm | 0.12 | 0.00 |
| 2.6 mm | 0.00 | 0.16 |
| 2.8 mm | 0.26 | 0.00 |
| Pooled data from the ten canal levels ** |
0.22 1 | 0.02 1 |
Values are expressed as least square means with the common standard error of the least square means = 0.14.
When data from the 10 canal levels of each “Group” are pooled together, the least square mean of the “MDI” group is 0.22% and that of the “ANP” group is 0.02%. The common standard error of the least square means is 0.09%. There is no statistical significant difference (same letter superscripts) between the pooled data from the “MDI” group and the “ANP” group (p = 0.092).
Although no statistical comparisons were made between debris retention in the instrumented canals and non-instrumented isthmus regions, there was a general tendency that more debris were retained within the isthmus regions in both groups, with only very few canal levels being completely free of debris in the isthmus regions. Table II summarises the percentage area occupied by debris within the non-instrumented isthmus regions (i.e. instrumented canals excluded) of the two experimental groups examined repeatedly at the ten canal levels. Highly significant differences (p < 0.001) could be detected between the MDI group and the ANP group for the repeated factor “Canal Level”. Pair-wise comparisons indicate significant differences (p < 0.05) at all canal levels. Conversely, the differences in least square mean values among the different “Canal Level” is not great enough the exclude the possibility that the difference is due to random sampling variability after allowing for the effects of “Group” differences (p = 0.352). Pair-wise comparisons of the canal levels in the MDI group showed that only debris present in the isthmus regions at 1.0 mm and 2.2 mm were significantly different (p < 0.05). There was no difference among other canal levels in the MDI group (p > 0.05), or amongst all canal levels in the ANP group (p > 0.05). The interaction between the factor “Group” and “Canal Level” was not statistically significant (p = 0.559). When the data from the ten canal levels were pooled together, a highly significant difference could be detected between the MDI group and the ANP group with respect to isthmus-retained debris (p < 0.001).
Table II.
Percentage of total isthmus area occupied by debris in the MDI and the ANP groups at ten canal levels.
| Canal level (from anatomic apex) |
MDI (%) * |
ANP (%) * |
|---|---|---|
| 1.0 mm | 15.64 1,B | 0.00 2,a |
| 1.2 mm | 23.59 1,AB | 1.81 2,a |
| 1.4 mm | 24.39 1,AB | 0.00 2,a |
| 1.6 mm | 25.02 1,AB | 0.00 2,a |
| 1.8 mm | 24.76 1,AB | 1.70 2,a |
| 2.0 mm | 25.93 1,AB | 0.70 2,a |
| 2.2 mm | 26.69 1,A | 1.41 2,a |
| 2.4 mm | 21.87 1,AB | 1.84 2,a |
| 2.6 mm | 24.81 1,AB | 2.52 2,a |
| 2.8 mm | 20.81 1,AB | 1.46 2,a |
| Pooled data from the ten canal levels ** |
23.36 1 | 1.15 2 |
Values are expressed as least square means with the common standard error of the least square means = 2.38.
When data from the 10 canal levels of each “Group” are pooled together, the least square mean of the “MDI” group is 23.36% and that of the “ANP” group is 1.15%. The common standard error of the least square means is 2.49%. The difference of the pooled data between the “MDI” group and the “ANP” group is highly significant (p < 0.001).
DISCUSSION
In the present study, two irrigation/agitation techniques were selected for comparison of their canal and isthmus debridement efficacy in the mesial root of mandibular molars. These two techniques differ from other irrigant/agitation regimes (Gu et al. 2009) in that the agitation/negative suction components are inserted to the working length of the instrumented canals, which is not possible or recommended in other irrigant delivery or agitation techniques such as the use of sonic or ultrasonic agitation devices.
The use of an ex vivo closed-end canal model more accurately simulates in vivo situations, in which the tooth’s foramen and outer surface are sealed by the periodontal ligament and further embedded in alveolar bone. Gas entrapment along the end of a closed microchannel (Migun & Shnip 2002, Pesse et al. 2005) is a well-known physical phenomenon in engineering sciences. Although gas entrapment was created by placing small capillaries in a liquid in those studies and may be different from what is encountered in root canal irrigation, it is possible that similar gas entrapment may occur in the most 0–2 mm root canal during the delivery of an irrigant. Tay et al. (2010) compared the smear layer removal and debris clearance potential of NaOCl and EDTA delivered by a side-vented needle to single canal anterior teeth in a closed canal system versus an open canal system. Although the closed end system utilised by Tay et al. (2010) was not as elaborate as the experimental set up employed in the present study, it served the same purpose in preventing fluid and gas communication between the root canal and the external environment, thereby simulating the clinical scenario when a tooth is encased by the periodontal ligament in a bony socket (Gutarts et al. 2005, Burleson et al. 2007). In that study, an open canal system was also employed in which irrigants were allowed to drip through the apical foramen. The results of that study indicated that the canals were considerably cleaner at all levels (i.e. coronal third,, middle third and apical third) of the canal when an open canal design was employed, and that the differences in smear scores and debris scores between the closed canal and open canal systems were most notable in the apical third of the canals. In the same study, the authors opined that it was difficult to use scanning electron microscopy for examining the apical 0.5–1 mm of the canal walls and suggested using light microscopy for critically examining the canal cleanliness (i.e. debris retention) at the different canal levels in the critical zone in which the apical lock phenomenon appeared to exist inside a closed canal (i.e. 0–2 mm from the apical seat). Thus, light microscopy was employed in the present study to examine this critical zone at 0.2 mm intervals for ten canal levels (i.e. 1.0 – 2.8 mm from the anatomical apex, with working length established at 1.0 mm from the anatomical apex). As it has been confirmed that an open canal system overestimates canal debridement efficacy in ex vivo studies, the use of a dual-system experimental design was not repeated in the present study. In a separate study that utilised a dual-system design, also it has been determined that there was no fluid extruded from an open canal system when the ANP technique was used during irrigation of single-rooted teeth.
Understandably, light microscopy does not have the resolution of scanning electron microscopy to identify smear layer remnants from the instrumented canal walls. Nevertheless, smear layers are not present in the non-instrumented isthmus regions. Thus, no attempt was made in the present study to examine the efficacy of smear layer removal. The use of light microscopy in combination with Masson’s trichrome staining, however, did permit easier recognition of sclerotic dentine and acellular cementum, which retained the red, acid fuschin component of the trichrome stain (Flint et al. 1975). Conversely, this acidic dye component was displaced by the subsequently applied aniline blue dye component, which stained the collagen component of the tubular radicular dentine. The loose soft tissue debris within the canals and isthmi were predominantly stained with a blue hue. In some specimens, the entire root section was stained intensely red except for some tubular radicular dentine and the intermediate cementum that were stained with a blue colour (not shown). A detailed discussion of the histochemical mechanisms associated with dye retention is beyond the scope of this discussion. Previous studies using light microscopy (Paqué et al. 2006) and scanning electron microscopy (Tay et al. 2010) have shown that the apical 3 mm of the radicular dentine in anterior teeth were highly sclerotic. Thus, the red-stained radicular dentine in the present study could represent also sclerotic dentine. It is noteworthy that in sections with highly sclerotic dentine, the blue-stained tubular dentine was always located along buccolingual axis of the mesial root, external to the mesiobuccal and mesiolingual canals. A similar phenomenon was also observed when scanning electron microscopy was used to examine the apical 5 mm of premolar teeth with two canals. Patent tubular dentine was predominantly found along the buccolingual axis of the premolar roots and external to the buccal and palatal canals, while the rest of the radicular dentine was highly sclerotic. It is not known what exactly causes dentine sclerosis in the apical part of the roots and why the process of dentine sclerosis is slower along the longer buccolingual axis when compared with the shorter mesiodistal axis. This is an important issue that merits further research.
In the presence of apical gas entrapment associated with a closed canal system (Tay et al. 2010), both MDI with the repeated insertion of a close fitting gutta-percha cone to working length and ANP technique with the placement of the EndoVac micro-cannulus to working length resulted in relatively clean instrumented canal spaces. As there were no differences in the percentage of canal spaces occupied by debris between the two techniques at all canal levels, the first null hypothesis that there is no difference between the canal debridement efficacy of the two irrigant agitation techniques at different canal levels from the anatomical apex in a simulated closed canal system must be accepted. As there is fairly close adaptation between the gutta-percha cone utilised in the MDI technique and the canal walls, it is possible that some of the hard tissue debris may be impregnated on the surface of the gutta-percha cone during repeated insertions into the canal space. Repeated rubbing of a gutta-percha cone with irregular dentine chips impregnated on its surface against a canal wall that has been depleted of the smear layer may generate a new smear layer. This speculation has to be confirmed using scanning electron microscopy of the canal walls that have been treated with the MDI technique. The information would be of interest to those clinicians who ascribe to the philosophy of smear layer removal as their canal debridement objectives (Shahravan et al. 2007).
Contrary to the results obtained from the instrumented canals, both MDI and the ANP technique were unable to completely remove debris from the narrow isthmi present in the mesial root of mandibular molars. Debris ranging from 15–27 area % was found to be present at all the ten canal levels of the MDI group, while debris ranging from 0.7–2.5 area % was observed at 7 of the 10 canal levels of the ANP group. As there was considerably more debris present in the MDI group, either at individual canal levels or when the data from the ten levels were pooled together for statistical analysis, the second null hypothesis that there is no difference between the isthmus debridement efficacy of the two irrigant agitation techniques at different canal levels from the anatomical apex in a simulated closed canal system must be rejected.
It is pertinent to note that debris removal was much more difficult in the narrow isthmus regions than in the instrumented canals. Although the two examples shown in Figures 5 and 6 were completely patent isthmi, up to 30% of the isthmi in each group were partially obliterated (not shown). These “partial isthmi” were obliterated by sclerotic dentine in a three-dimensional manner, so that completely obliterated, partially obliterated and completely patent versions of the same isthmus could be observed at different canal levels of the apical 1–2.8 mm region of the roots. This complicated morphology rendered it extremely difficult for the delivery of a large volume of irrigant with a high flow rate to the entire isthmus region even with the use of the ANP technique. It is worth mentioning that the presence of a completely patent isthmus did not result in better debris removal, as illustrated in the MDI group (Figure 5). With the MDI technique, it is possible, as previously mentioned, that displacement of the apically entrapped gases within the canal space was achieved at the expense of displacing gases to the dead-ends of the isthmus regions. It is also possible that the gutta-percha cone, being closely-adapted only to circular canal space, was incapable of displacing the gases that was originally present within the isthmus region. Irrespective of the cause, this resulted in a greater amount of debris remaining within the narrow isthmi in the MDI group. Using micro-CT to examine the distribution of isthmi in the apical 5 mm of the mesial root of mandibular molars, Mannocci et al. (2005) reported that isthmi recognisable by micro-CT were present at all canal levels with prevalence between 17–50%. As the micro-CT employed in that study had a lower resolution than what could be achieved using light microscopy, the present study clearly demonstrated that even under the circumstance where a hairline-type isthmus was barely visible under the micro-CT, the isthmus could be identified as partially patent under light microscopy. Teixeira et al. (2003) reported that 22% of the isthmi present in mandibular first molars were completely patent while 37% were partially patent. Although only narrow isthmi were selected for examination in the present study, it would be interesting to compare the effect of isthmus debridement between roots with narrow and wide isthmi using the ANP technique in a future study.
Within the limits of the present study, it may be concluded that both the MDI technique and the ANP technique produced fairly clean canals that had minimal debris recognisable by light microscopy. Both techniques did not completely remove light microscopy-recognisable debris from the narrow isthmus between the canals due to the difficulty in getting irrigating solutions to reach the isthmus and to create a strong enough current to flow through the isthmus. Although both techniques involves the placement of the agitation component directly to working length, the use of the ANP technique resulted in considerably cleaner isthmi when compared with MDI.
ACKNOWLEDGEMENTS
Set ups for the closed canal system for simulation the effect of apical gas entrapment were generously provided by Dr. John Schoeffel. Preparation of histological sections was generously supported by the Department of Endodontics, School of Dentistry, Medical College of Georgia. The micro-CT scanner employed in one part of the study was acquired via Grant R21 DE019213-01 from the National Institute of Dental and Craniofacial Research (PI. Franklin R. Tay). The authors thank Dr. Jongruhl Kim for demineralization of the root specimens, Mrs. Donna Kumiski for cutting, staining and mounting of the histological sections, Dr. Ulf Wikesjo for use of his light microscope for acquisition of the digitised images, Mr. Thomas Bryan for assembly of the fixtures and for cleaning up after completion of the experiments by endodontic residents and Mrs. Marie Churchville for secretarial support.
REFERENCES
- Albrecht LJ, Baumgartner JC, Marshall JG. Evaluation of apical debris removal using various sizes and tapers of ProFile GT files. Journal of Endodontics. 2004;30:425–428. doi: 10.1097/00004770-200406000-00012. [DOI] [PubMed] [Google Scholar]
- Archer R, Reader A, Nist R, Beck M, Meyers WJ. An in vivo evaluation of the efficacy of ultrasound after step-back preparation in mandibular molars. Journal of Endodontics. 1992;18:549–552. doi: 10.1016/S0099-2399(06)81212-4. [DOI] [PubMed] [Google Scholar]
- Baumgartner JC, Mader CL. A scanning electron microscopic evaluation of four root canal irrigation regimens. Journal of Endodontics. 1987;13:147–157. doi: 10.1016/s0099-2399(87)80132-2. [DOI] [PubMed] [Google Scholar]
- Bergenholtz G, Spångberg L. Controversies in endodontics. Critical Reviews in Oral Biology and Medicine. 2004;15:99–114. doi: 10.1177/154411130401500204. [DOI] [PubMed] [Google Scholar]
- Boutsiokis C, Lambrianidis T, Kastrinakis E. Irrigant flow within a prepared root canal using various flow rates: a Computational Fluid Dynamics study. International Endodontic Journal. 2009;42:144–155. doi: 10.1111/j.1365-2591.2008.01503.x. [DOI] [PubMed] [Google Scholar]
- Brito PR, Souza LC, Machado de Oliveira JC, et al. Comparison of the effectiveness of three irrigation techniques in reducing intracanal Enterococcus faecalis populations: an in vitro study. Journal of Endodontics. 2009;35:1422–1427. doi: 10.1016/j.joen.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Burleson A, Nusstein J, Reader A, Beck M. The in vivo evaluation of hand/rotary/ultrasound instrumentation in necrotic, human mandibular molars. Journal of Endodontics. 2007;33:782–787. doi: 10.1016/j.joen.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Carver K, Nusstein J, Reader A, Beck M. In vivo antibacterial efficacy of ultrasound after hand and rotary instrumentation in human mandibular molars. Journal of Endodontics. 2007;33:1038–1043. doi: 10.1016/j.joen.2006.11.026. [DOI] [PubMed] [Google Scholar]
- Chávez de Paz LE, Bergenholtz G, Dahlén G, Svensäter G. Response to alkaline stress by root canal bacteria in biofilms. International Journal of Endodontics. 2007;40:344–355. doi: 10.1111/j.1365-2591.2006.01226.x. [DOI] [PubMed] [Google Scholar]
- Chávez de Paz LE, Hamilton IR, Svensäter G. Oral bacteria in biofilms exhibit slow reactivation from nutrient deprivation. Microbiology. 2008;154:1927–1938. doi: 10.1099/mic.0.2008/016576-0. [DOI] [PubMed] [Google Scholar]
- Chow TW. Mechanical effectiveness of root canal irrigation. Journal of Endodontics. 1983;9:475–479. doi: 10.1016/S0099-2399(83)80162-9. [DOI] [PubMed] [Google Scholar]
- de Gregorio C, Estevez R, Cisneros R, Heilborn C, Cohenca N. Effect of EDTA, sonic, and ultrasonic activation on the penetration of sodium hypochlorite into simulated lateral canals: an in vitro study. Journal of Endodontics. 2009;35:891–895. doi: 10.1016/j.joen.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Desai P, Himel V. Comparative safety of various intracanal irrigation systems. Journal of Endodontics. 2009;35:545–549. doi: 10.1016/j.joen.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Estrela C, Sydney GB, Figueiredo JA, Estrela CR. Antibacterial efficacy of intracanal medicaments on bacterial biofilm: a critical review. Journal of Applied Oral Sciences. 2009;17:1–7. doi: 10.1590/S1678-77572009000100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GE, Speight PM, Gulabivala K. The influence of preparation technique and sodium hypochlorite on removal of pulp and predentine from root canals of posterior teeth. International Endodontic Journal. 2001;34:322–330. doi: 10.1046/j.1365-2591.2001.00398.x. [DOI] [PubMed] [Google Scholar]
- Flint MH, Lyons MF, Meaney MF, Williams DE. The Masson staining of collagen – an explanation of an apparent paradox. Histochemical Journal. 1975;7:529–546. [Google Scholar]
- Fukumoto Y, Kikuchi I, Yoshioka T, Kobayashi C, Suda H. An ex vivo evaluation of a new root canal irrigation technique with intracanal aspiration. International Endodontic Journal. 2006;39:93–99. doi: 10.1111/j.1365-2591.2006.01050.x. [DOI] [PubMed] [Google Scholar]
- Gao Y, Haapasalo M, Shen Y, Wu H, Li B, Ruse ND, Zhou X. Development and validation of a three-dimensional computational fluid dynamics model of root canal irrigation. Journal of Endodontics. 2009;35:1282–1287. doi: 10.1016/j.joen.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Gu LS, Kim JR, Ling J, Choi KK, Pashley DH, Tay FR. Review of contemporary irrigant agitation techniques and devices. Journal of Endodontics. 2009;35:791–804. doi: 10.1016/j.joen.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Gutarts R, Nusstein J, Reader A, Beck M. In vivo debridement efficacy of ultrasonic irrigation following hand-rotary instrumentation in human mandibular molars. Journal of Endodontics. 2005;31:166–170. doi: 10.1097/01.don.0000137651.01496.48. [DOI] [PubMed] [Google Scholar]
- Haidet J, Reader A, Beck M, Meyers W. An in vivo comparison of the step-back technique versus a step-back/ultrasonic technique in human mandibular molars. Journal of Endodontics. 1989;15:195–199. doi: 10.1016/S0099-2399(89)80234-1. [DOI] [PubMed] [Google Scholar]
- Harrison JW, Svec TA, Baumgartner JC. Analysis of clinical toxicity of endodontic irrigants. Journal of Endodontics. 1978;4:6–11. doi: 10.1016/S0099-2399(78)80241-6. [DOI] [PubMed] [Google Scholar]
- Hsieh YD, Gau CH, Kung Wu SF, Shen EC, Hsu PW, Fu E. Dynamic recording of irrigating fluid distribution in root canals using thermal image analysis. International Endodontic Journal. 2007;40:11–17. doi: 10.1111/j.1365-2591.2006.01168.x. [DOI] [PubMed] [Google Scholar]
- Jiang LM, Verhaagen B, Versluis M, van der Sluis LW. Evaluation of a sonic device designed to activate irrigant in the root canal. Journal of Endodontics. 2010;36:143–146. doi: 10.1016/j.joen.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Mannocci F, Peru M, Sherriff M, Cook R, Pitt Ford TR. The isthmuses of the mesial root of mandibular molars: a micro-computed tomographic study. International Endodontic Journal. 2005;38:558–563. doi: 10.1111/j.1365-2591.2005.00994.x. [DOI] [PubMed] [Google Scholar]
- McGill S, Gulabivala K, Mordan N, Ng YL. The efficacy of dynamic irrigation using a commercially available system (RinsEndo) determined by removal of a collagen 'bio-molecular film' from an ex vivo model. International Endodontic Journal. 2008;41:602–608. doi: 10.1111/j.1365-2591.2008.01408.x. [DOI] [PubMed] [Google Scholar]
- Migun NP, Shnip AI. Model of film flow in a dead-end conic capillary. Journal of Engineering Physics and Thermophysics. 2002;75:1422–1428. [Google Scholar]
- Moser JB, Heuer MA. Forces and efficacy in endodontic irrigation systems. Oral Surgery Oral Medicine Oral Pathology. 1982;53:425–428. doi: 10.1016/0030-4220(82)90446-7. [DOI] [PubMed] [Google Scholar]
- Nair PN. On the causes of persistent apical periodontitis: a review. International Endodontic Journal. 2006;39:249–281. doi: 10.1111/j.1365-2591.2006.01099.x. [DOI] [PubMed] [Google Scholar]
- Nielsen BA, Baumgartner CJ. Comparison of the EndoVac system to needle irrigation of root canals. Journal of Endodontics. 2007;33:611–615. doi: 10.1016/j.joen.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Paqué F, Laib A, Gautschi H, Zehnder M. Hard-tissue debris accumulation analysis by high-resolution computed tomography scans. Journal of Endodontics. 2009;35:1044–1047. doi: 10.1016/j.joen.2009.04.026. [DOI] [PubMed] [Google Scholar]
- Paqué F, Luder HU, Sener B, Zehnder M. Tubular sclerosis rather than the smear layer impedes dye penetration into the dentine of endodontically instrumented root canals. International Endodontic Journal. 2006;29:18–25. doi: 10.1111/j.1365-2591.2005.01042.x. [DOI] [PubMed] [Google Scholar]
- Pesse AV, Warrier GR, Dhir VK. An experimental study of the gas entrapment process in closed-end microchannels. International Journal of Heat and Mass Transfer. 2005;48:5150–5165. [Google Scholar]
- Peters OA. Current challenges and concepts in the preparation of root canal systems: a review. Journal of Endodontics. 2004;30:559–567. doi: 10.1097/01.don.0000129039.59003.9d. [DOI] [PubMed] [Google Scholar]
- Ricucci D, Siqueira JF., Jr Fate of the tissue in lateral canals and apical ramifications in response to pathologic conditions and treatment procedures. Journal of Endodontics. 2010;36:1–15. doi: 10.1016/j.joen.2009.09.038. [DOI] [PubMed] [Google Scholar]
- Schoeffel GJ. The EndoVac method of endodontic irrigation: part 2—efficacy. Dentistry Today. 2008;27:82,84,86–87. [PubMed] [Google Scholar]
- Senia ES, Marshall FJ, Rosen S. The solvent action of sodium hypochlorite on pulp tissue of extracted teeth. Oral Surgery Oral Medicine Oral Pathology. 1971;31:96–103. doi: 10.1016/0030-4220(71)90040-5. [DOI] [PubMed] [Google Scholar]
- Shahravan A, Haghdoost AA, Adl A, Rahimi H, Shadifar F. Effect of smear layer on sealing ability of canal obturation: a systematic review and meta-analysis. Journal of Endodontics. 2007;33:96–105. doi: 10.1016/j.joen.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Siqueira JF, Jr, Araújo MC, Garcia PF, Fraga RC, Dantas CJ. Histological evaluation of the effectiveness of five instrumentation techniques for cleaning the apical third of root canals. Journal of Endodontics. 1997;23:499–502. doi: 10.1016/S0099-2399(97)80309-3. [DOI] [PubMed] [Google Scholar]
- Tay FR, Gu LS, Schoeffel JG, et al. The effect of vapor lock on root canal debridement using a side-vented needle for positive-pressure irrigant delivery. Journal of Endodontics. 2010 doi: 10.1016/j.joen.2009.11.022. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira FB, Sano CL, Gomes BP, Zaia AA, Ferraz CC, Souza-Filho FJ. A preliminary in vitro study of the incidence and position of the root canal isthmus in maxillary and mandibular first molars. International Endodontic Journal. 2003;36:276–280. doi: 10.1046/j.1365-2591.2003.00638.x. [DOI] [PubMed] [Google Scholar]
- Usman N, Baumgartner JC, Marshall JG. Influence of instrument size on root canal debridement. Journal of Endodontics. 2004;30:110–112. doi: 10.1097/00004770-200402000-00012. [DOI] [PubMed] [Google Scholar]
- Vaudt J, Bitter K, Neumann K, Kielbassa AM. Ex vivo study on root canal instrumentation of two rotary nickel-titanium systems in comparison to stainless steel hand instruments. International Journal of Endodontics. 2009;42:22–33. doi: 10.1111/j.1365-2591.2008.01489.x. [DOI] [PubMed] [Google Scholar]
- Walker TL, del Rio CE. Histological evaluation of ultrasonic debridement comparing sodium hypochlorite and water. Journal of Endodontics. 1991;17:66–71. doi: 10.1016/S0099-2399(06)81610-9. [DOI] [PubMed] [Google Scholar]
- Weller RN, Niemczyk SP, Kim S. Incidence and position of the canal isthmus. Part 1. Mesiobuccal root of the maxillary first molar. Journal of Endodontics. 1995;21:380–383. doi: 10.1016/s0099-2399(06)80975-1. [DOI] [PubMed] [Google Scholar]
- Williamson AE, Sandor AJ, Justman BC. A comparison of three nickel titanium rotary systems, EndoSequence, ProTaper universal, and profile GT, for canal-cleaning ability. Journal of Endodontics. 2009;35:107–109. doi: 10.1016/j.joen.2008.09.022. [DOI] [PubMed] [Google Scholar]
- Yamada R, Armas A, Goldman M, Pin A. A scanning electron microscopic comparison of a high volume final flush with several irrigating solutions. Journal of Endodontics. 1983;9:137–142. doi: 10.1016/S0099-2399(83)80032-6. [DOI] [PubMed] [Google Scholar]
- Zehnder M. Root canal irrigants. Journal of Endodontics. 2006;32:389–398. doi: 10.1016/j.joen.2005.09.014. [DOI] [PubMed] [Google Scholar]