Abstract
OBJECTIVE
Mast cells are tissue-resident immune sentinels implicated in the pathogenesis of inflammatory joint disease. We hypothesized that complement fragments could be key activators of synovial mast cells in autoimmune arthritis.
METHODS
In vivo studies employed murine K/BxN arthritis, a distal symmetric polyarthritis mediated by IgG immune complexes. Expression of C5aR on synovial mast cells was determined by immunohistochemical and functional studies. C5aR−/− and control mast cells were engrafted into mast cell-deficient W/Wv mice to examine the requirement for this receptor in arthritis. C5aR-dependent activation of mast cells was investigated in C5aR−/− animals and in murine and human mast cell cultures.
RESULTS
Murine synovial mast cells express functional C5aR. Unlike their wild-type counterparts, C5aR−/− mast cells adoptively transferred into W/Wv mice were incompetent to restore arthritis, despite equivalent synovial engraftment. Activation of C5aR−/− mast cells by K/BxN serum in vivo remained intact, indicating that C5aR is dispensable for normal IgG-mediated triggering. Consistent with this result, cultured mast cells treated with C5a failed to modulate expression of Fc γ receptors (FcγR) or otherwise alter activation threshold. In human mast cells, C5a promoted production of the neutrophil chemotaxin interleukin 8, and recruitment of neutrophils at 24h after serum administration was impaired in C5aR−/− mice, suggesting that enhanced neutrophil chemoattractant production underlies the requirement for C5aR on mast cells in arthritis.
CONCLUSION
Stimulation via C5aR is required to unleash the pro-inflammatory activity of synovial mast cells in immune complex arthritis, albeit via a mechanism distinct from C5a-modulated FcγR expression.
The pathogenesis of the idiopathic inflammatory arthritides involves both adaptive and innate immune cells, as well as mesenchymal lineages such as the synovial fibroblast. In some of these conditions, including rheumatoid arthritis (RA), strong evidence has accumulated that antibodies play a key role in the translation of impaired immune tolerance to inflammation in the joint. Autoantibodies such as rheumatoid factor and anti-citrullinated peptide antibodies are common in RA, while the joints of seropositive rheumatoid patients are encrusted with immune complexes and display marked activation of complement via both classical and alternative pathways (reviewed in (1)).
The effector phase of immune complex-driven arthritis has been modeled in multiple murine systems, including collagen-induced arthritis and K/BxN arthritis. These models share many key features, including a dependence on both IgG Fc γ receptors (FcγR) and complement, as well as effector pathways such as the pro-inflammatory cytokine IL-1 (2). Multiple innate immune lineages have been implicated in the inflammatory reaction to articular immune complexes in these models, including neutrophils and macrophages (3, 4). We and others have demonstrated a role for the synovial mast cell in this process, at least in certain genetic backgrounds (5–7). These hematopoietically-derived cells make up approximately 3% of the cells in the normal synovial sublining, where they take up residence in perivascular and perineural tissues and immediately deep to the synovial lining (8, 9). Given their relatively limited numbers, mast cells are thought to act as sentinels, rapidly liberating a broad range of mediators that mobilize adaptive and innate immune responders, including circulating neutrophils. In this way, mast cells have been shown to facilitate resistance to bacterial peritonitis (10, 11), and we have hypothesized that they play a similar role in surveillance of the vulnerable synovial space (9).
In immune complex arthritis, this sentinel function becomes maladaptive. Beyond receptors for IgE, mast cells express FcγR and can readily be activated by IgG immune complexes. In K/BxN serum transfer arthritis, mediated by IgG antibodies that form immune complexes with their autoantigen glucose-6-phosphate isomerase, several strains of mast cell-deficient mice are resistant to disease (5, 6). Resistance can be overcome by engraftment with cultured mast cells, confirming that mast cells can play a key role in arthritis susceptibility (5). Recently, we have elucidated mechanisms underlying this activity, finding that synovial mast cells become activated by immune complex binding to FcγRIII, resulting in release of IL-1 and potentially other mediators that “jump start” inflammation within the joint (12).
However, mast cells can express receptors beyond FcγR that are of potential relevance in arthritis. These include receptors for the complement anaphylatoxins C3a and C5a, generated through the activation of complement by immune complexes and readily demonstrable in rheumatoid synovial fluid (1, 13, 14). We hypothesized that complement receptors could synergize with FcγR to promote the pro-inflammatory activity of the synovial mast cell. Precedent for such an interaction is strong. Rat macrophages activated via both FcγR and C5a produce an expanded repertoire of inflammatory mediators (15). More recently, elegant work in murine macrophages has found that C5a can reciprocally modulate expression of activating (FcγRIII) and inhibitory (FcγRII) Fc γ receptors, leading to a cellular state of enhanced susceptibility to immune complex activation (16–19). This mechanism has been detailed down to the molecular level, where a two base-pair difference in the promoters for these receptors accounts for their differential response to C5aR-initiated intracellular signaling (20, 21). Modulation of FcγR expression represents an important mechanism to limit activation of leukocytes exposed transiently to immune complexes (22). However, it remains unclear whether this mechanism is active in all cells expressing C5aR and FcγR. In particular, mast cells acquire a functional phenotype only upon maturation within the tissues and would be expected to have little “incidental” contact with immune complexes. We therefore studied whether complement receptors participate in mast cell effector activity in inflammatory arthritis, and whether such participation depends on complement-mediated modulation of the threshold to activation via FcγRs.
MATERIALS and METHODS
Mice
C5aR−/− and C3a receptor (C3aR) −/−mice were derived as described (23, 24). WBB6 F1-Kitw/KitW-v (W/Wv), WBB6 littermate controls, C57BL/6J (B6), and B6;129S4-Fcgr2btm1Ttk/J (FcγRII−/−), B6.129P2-Fcgr3tm1Sjv/J (FcγRIII−/−) and B6;129P2-Fcer1g<tm1Rav>/J (FcRγ−/−) animals were purchased from The Jackson Laboratory. C57BL/6-KitW-sh/W-sh (Wsh) mice (25) were maintained at The Jackson Laboratory. Animals were housed in the specific-pathogen-free animal facilities of the Dana-Farber Cancer Institute or Children’s Hospital Boston. All procedures were approved by the appropriate animal care and use committees.
Antibodies and Reagents
Antibodies used for cytofluorimetric analysis in human cells included CD16-PE, CD32A/B-PE (clone AT10), and CD64-PE (Caltag); C5aR-FITC and C3aR-Alexa488 (AbdSerotec); and anti-FcγRIIb-A488 (2B6, gift of MacroGenics, Inc.)(26); and matched isotype controls. Reagents for murine cytofluorimetry included anti-C5aR (20/70, gift of J. Zwirner)(27), anti-FcγRII/III-PE (2.4G2) and anti-CD45-PE-Cy7 (BD Pharmingen), anti-FcγRIII-carboxyfluorescein (Clone 275003, R&D Systems), anti-CD11b-FITC and Gr-1-Alexa647 (Caltag) and matched isotype controls. Unlabeled anti-FcγRIII was employed as a blocking reagent prior to 2.4G2-PE staining to assess surface expression of FcγRII as described (21). Anti-human FcγRIIa antibodies were purified from clone IV.3 supernatants (American Type Culture Collection). Rabbit anti-murine C5aR receptor antiserum for immunohistochemistry was generated as described (28). Recombinant human stem cell factor (hSCF) was purchased from Research Diagnostics, Inc. or Peprotech. Recombinant human C5a, mouse SCF, and ELISA reagents were purchased from R&D Systems. Recombinant mouse IL-3 was purchased from Pierce. Flow cytometry was performed using a FACSDiva cytometer (Becton-Dickenson).
Cell culture and stimulation
Murine bone marrow derived mast cells (BMMC) were employed following at least 4 weeks of culture in mSCF 12.5ng/ml and mIL-3 10ng/ml as described (5). To accomplish FcγRIII-mediated stimulation in vitro, LPS-free 2.4G2 was coated onto a high-binding ELISA plate (Costar 9018), 100µL per well in 0.2N carbonate buffer pH8 4C overnight. Plates were washed × 2 with DMEM media. Mast cells re-suspended in 200µL fresh cytokine-free BMMC media per well were spun 900 RPM (160g) × 1 minute to initiate contact between cells and bound antibody. Human skin mast cells (SMC) were cultured from human abdominoplasty tissue in XVIVO media (BioWhittaker) and 100ng/ml hSCF as described (29). To generate the FcγRIIa crosslinking reagent, the anti-hFcγRIIa antibody IV.3 was incubated with goat F(ab’)2 fragments directed against murine F(ab) (Sigma) for 45 minutes at 37°C; the concentration of reagents was optimized for each IV.3 batch, and varied from 1–20µg/ml for IV.3 and 0.6–5µg/ml anti-mouse F(ab’)2. Warm crosslinking reagent was added at the volume indicated for a final well volume of 200µL. β-hexosaminidase release was quantitated by colorimetric assay and expressed as a percentage of total cellular β-hexosaminidase as determined by ultrasonic lysis of parallel wells. For both human and murine mast cells, cells were washed into media containing fresh cytokines one day prior to experimentation. At initiation of each experiment, cells were resuspended in warm cytokine-free media.
Calcium flux
Cells were washed into calcium-containing buffer (Hank’s BSS plus 1g/l BSA, CaCl2 1mM, MgCl2 1mM) and incubated for 30min at 37°C with Fura-2AM (2.5µl/ml, Molecular Probes). Following a further wash, cells were re-suspended at 0.5–1×106 cells/ml in the same buffer, and Ca-sensitive fluorescence was read using a Hitachi F-4500 fluorescence spectrophotometer.
Quantitative polymerase chain reaction (PCR)
Total RNA and cDNA were generated using standard commercial kits (Qiagen). PCR for murine FcγRII and FcγRIII was performed using published primers (30). Alternate validated primers used in some experiments, with similar results, included: FcγRII-forward 5’-AGAAGCTGCCAAAACTGAGG-3’, FcγRII-reverse 5’-CTTCGGGATGCTTGAGAAGTG-3’, FcγRIII-forward 5’-CAGAATGCACACTCTGGAAGC-3’, FcγRIII-reverse 5’-GGGTCCCTTCGCACATCA-3’. Primers for GAPDH were (forward) 5’-AGGTCGGTGTGAACGGATTTG-3’ and (reverse) 5’-TGTAGACCATGTAGTTGAGGTCA-3’, and confirmed to be stable in expression through all experimental conditions. Data were analyzed using the 2−ΔΔCt method.
Induction of arthritis and measurement of paw swelling
Arthritis was induced by i.p. administration of 150µL of serum obtained from arthritic K/BxN mice and scored as described (5). Briefly, each paw was given a score of 0 (no inflammation) to 3 (maximal inflammation) and the results were summed to arrive at an overall clinical index. To measure acute paw swelling, the thickness of each paw was measured immediately prior to serum injection using a spring-loaded caliper (forepaw: dorsal-ventral axis in neutral position; ankle: across malleoli with ankle in full flexion). Measurement was repeated 30 minutes following serum injection to quantitate change in thickness over this interval. A similar measurement technique was used to quantitate acute edema following periarticular instillation of C5a. In these experiments, 20µL of a concentrated C5a stock (400nM in LPS-free PBS) were injected into the dorsal wrist or lateral ankle tissue of otherwise unmanipulated animals. PBS in similar volume was instilled into the contralateral wrist and ankle. The solutions were distributed through the tissues by brief massage and the joints were then measured at baseline and after 10 minutes to assess acute edema.
Engraftment of cultured mast cells
BMMC were engrafted into mast cell deficient W/Wv mice by tail vein injection at 107 cells per recipient as described (5). Experiments were initiated 10 weeks after cell transfer. Engraftment was quantitated by microscopic examination of three 20× high-power fields from each ankle, blinded to treatment group (12).
Microscopy
For immunohistochemical staining, histologic sections were deparaffinized and subjected to antigen retrieval in sodium citrate buffer, pH 6.0 at 86°C for 15 minutes. After blocking with normal goat serum, samples were incubated with primary antibody 10µg/ml at 4C overnight, biotinylated secondary antibody (Vector Labs) for 30 minutes, avidin-biotin alkaline phosphatase ABC reagent (Vector Labs) for 30 minutes, and finally FastRed™ (1 mg/mL) (Sigma) substrate for 20 minutes. Tissues were counterstained with Gill’s II hematoxylin. To assess degranulation, ankles were stained with toluidine blue and examined in blinded fashion to identify mast cells demonstrating granule exocytosis as described (5). Neutrophil infiltration was assessed by staining with the anti-neutrophil antibody NIMP-R14 after antigen retrieval with proteinase K as described (31), followed by microscopic analysis to enumerate the number of infiltration NIMP-R14+ cells in the 3 richest 40× fields.
Neutrophil recruitment assay
To assess functionally for a C5aR-dependent BMMC-derived neutrophil chemoattractant, 250,000 FcγRII−/− BMMC in 1ml 2% FBS BMMC media were spun, in the presence or absence of C5a 50nM, onto a 12 well plate (Costar 3402) that had been coated overnight with carbonate buffer +/− 2.4G2 10µg/ml as described above. After 20min, polycarbonate transwells with 3µm pores were inserted into the wells and whole bone marrow from a C5aR−/− mouse resuspended in 0.1% FBS/DMEM was divided into the upper chambers. After 4h, the transwells were removed and migrated neutrophils (defined as CD45+ CD11bhi Gr-1hi) were enumerated by flow cytometry using added counting beads as a standard (Polysciences #18328).
Statistical analysis
Results were compared using the Student’s t-test. P values ≤ 0.05 were considered significant. Error bars reflect SEM.
RESULTS
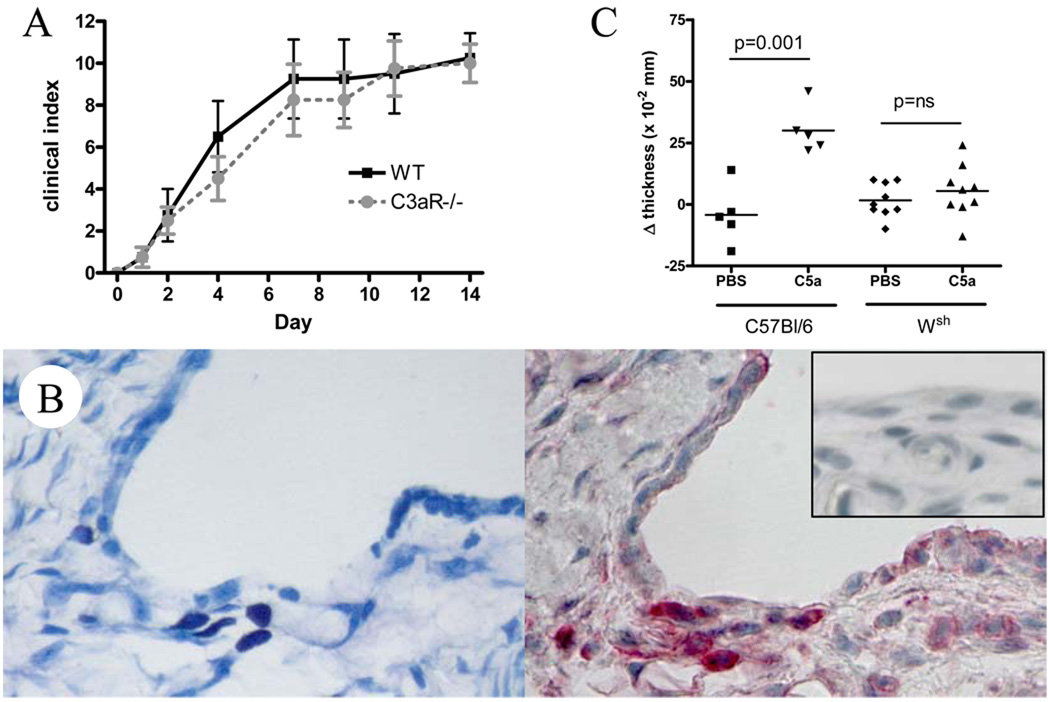
In K/BxN arthritis, C5aR−/− animals are densely resistant to arthritis, a resistance that depends in part on a requirement for C5aR in neutrophils but could include other lineages (32, 33). However, the role of C3aR has not been examined. We therefore initiated arthritis by transfer of K/BxN serum into C3aR−/− mice and found them to be normally susceptible to disease, as has previously been shown in collagen antibody-induce arthritis (34) (Figure 1A). We therefore focused further study on the role of C5aR in synovial mast cells.
Figure 1.
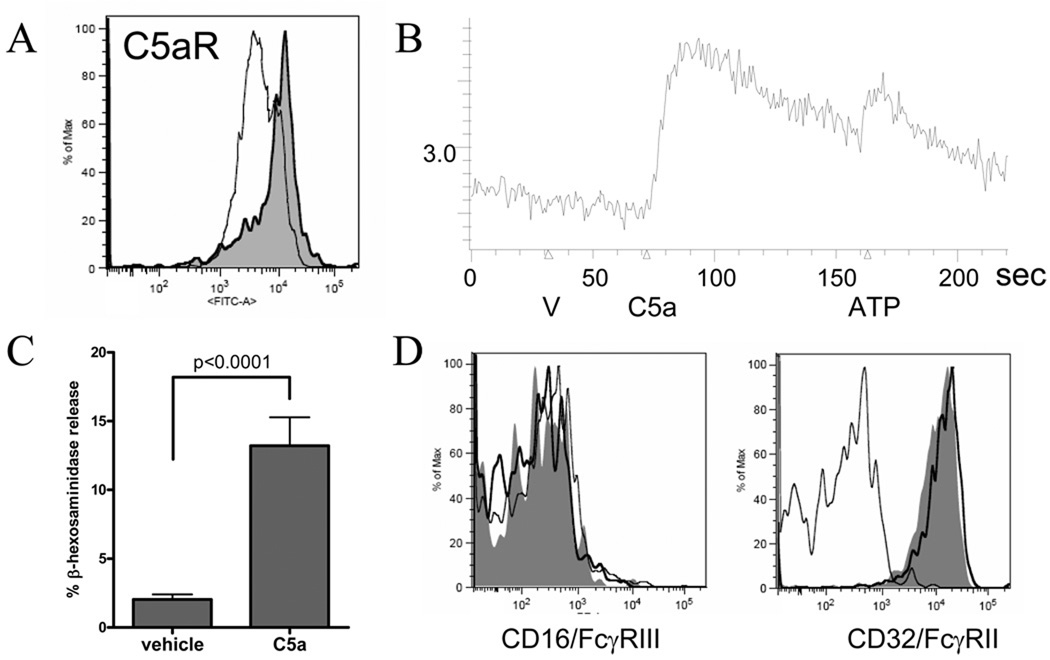
A. C3aR−/− mice and matched controls were injected with K/BxN serum on days 0 and 2, with indistinguishable outcome (n=4 mice per group, representative of 2 experiments). B. Mast cells lying just deep to the synovial lining of a murine tibiotalar joint are identified by toluidine blue staining (left panel) and stain brightly for C5aR in an adjacent section (right panel; inset, isotype). Representative of 4 experiments. C. Instillation of C5a into periarticular tissues of wrist and ankle results in acute edema lacking in mast cell-deficient Wsh mice (n=5 B6 and n=9 Wsh mice pooled from two experiments).
While expression of C5aR by mast cells has been well documented, the characteristics of an individual mast cell population depend markedly on developmental stage and tissue microenvironment (35, 36). Specifically, some murine mast cell populations do not express C5aR until activated by another signal (37). It was therefore important to demonstrate that C5aR was expressed and functional on synovial mast cells. Using immunohistochemistry, we could demonstrate strong expression of C5aR on mast cells in the synovial sublining (Figure 1B). The function of this receptor was confirmed by instillation of C5a into periarticular tissues, resulting in measurable edema within 10 minutes in a mast cell-dependent fashion (Figure 1C). We concluded that C5aR is expressed and functional on normal synovial mast cells and is therefore a potential participant in the activation of mast cells by immune complexes.
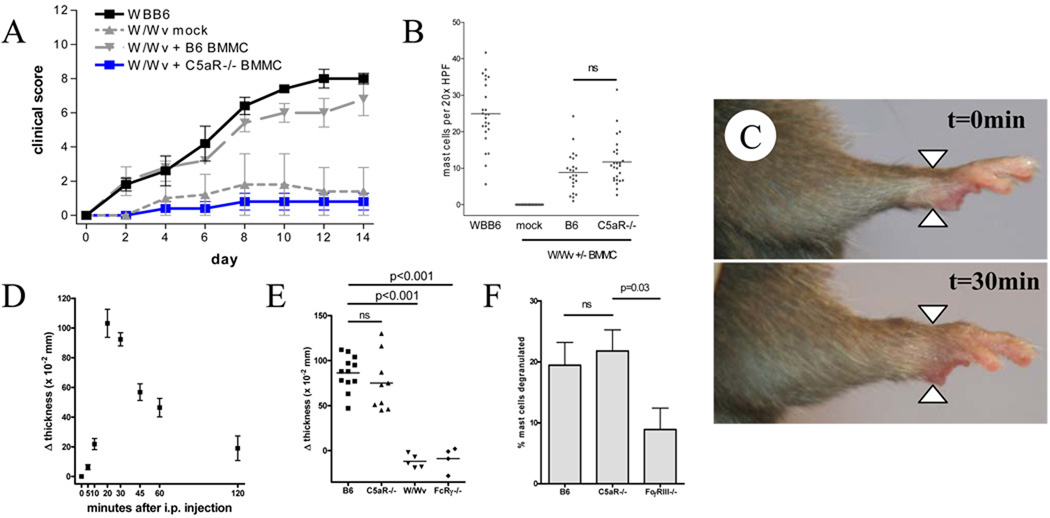
To examine the role of C5aR on mast cells, in isolation from other cell types, we employed a genetic approach. Bone marrow-derived mast cells (BMMC) were cultured from wild-type and C5aR−/− animals and engrafted into mast cell-deficient animals using a standard technique (5, 12, 38). After 10 weeks, animals were injected with K/BxN serum and arthritis susceptibility monitored by clinical examination. Unlike wild-type mast cells, C5aR−/− BMMC failed to restore arthritis susceptibility to W/Wv mice (Figure 2A). To ensure that this phenomenon did not reflect a failure of engraftment, ankle tissue of recipient animals was examined for mast cell density, which was found to be equivalent (Figure 2B). These data indicate that C5aR is dispensable for migration of mast cells into synovial tissue but required for the arthritogenic activity of these cells.
Figure 2.
A. W/Wv mice were engrafted with B6 or C5aR−/− BMMC × 10 weeks followed by initiation of K/BxN arthritis (n=5 mice per group, representative of 5 independent experiments). B. Quantitation of engrafted mast cells in ankles from A, n=23–26 mice/group, B6 vs. C5aR−/− p=ns. C. Paw edema (flare) 30 minutes after i.p. injection of 150µL K/BxN serum into a B6 mouse. D. Timecourse of flare, n=5 mice representative of 4 experiments. E. Flare 30m after injection of K/BxN serum in B6, C5aR−/−, mast cell-deficient (W/Wv) and FcRγ−/− mice lacking FcγRIII (mice, n=4–12 mice/group from 3 experiments. Of these, 5 B6 and 4 C5aR−/− mice received a second serum injection on day 2 with day 7 clinical scores of 11.6+/−0.4 and 0+/−0 respectively (p<0.0001). F. Degranulation of synovial mast cells assessed histologically 24h after administration of K/BxN serum.
The impact of C5aR on macrophage FcγR expression is well established. We therefore anticipated that C5aR on synovial mast cells would play a key permissive role in immune complex-mediated activation, otherwise constrained by the presence of the inhibitory receptor FcγRII (39). We examined this hypothesis in two ways. First, we employed a functional approach. Mice injected intravenously with K/BxN serum develop an acute but transient increase in paw microvascular permeability, visible by the technically demanding technique of intravital microscopy in anesthestized mice administered high molecular-weight intravascular dye (40). This vascular leak is dependent on mast cells (40). We observed that this phenomenon could be quantitated in animals receiving serum via the usual intraperitoneal route by measurement of paw thickness before and 30 minutes after injection (Figure 2C–D). Accordingly, we injected K/BxN serum into C5aR−/− and control B6 animals, as well as negative control mast cell-deficient W/Wv and FcRγ−/− mice lacking FcγRIII. Despite profound arthritis resistance in C5aR−/− animals, we found that vascular leak remained intact in these animals (Figure 2E). This finding is consistent with the published observation that C5aR−/− mice exhibit typical dye extravasation after i.v. serum administration in the more involved assay (40).
We also assessed mast cell activation by a second approach, direct histological examination for granule exocytosis. We examined sections of ankles harvested from wild-type, C5aR−/− and negative control FcγRIII−/− animals 24 hours after administration of K/BxN serum, a timepoint at which degranulation is readily apparent (5). Consistent with the vascular leak result, degranulation of C5aR−/− mast cells was indistinguishable from B6 controls, and greater than that of mast cells lacking the relevant activating FcγR (Figure 2F). These studies confirm that IgG-dependent activation of synovial mast cells in K/BxN serum transfer arthritis does not require co-stimulation via C5a, and suggested that C5aR-mediated changes in FcγR expression may be less important in mast cells than in macrophages.
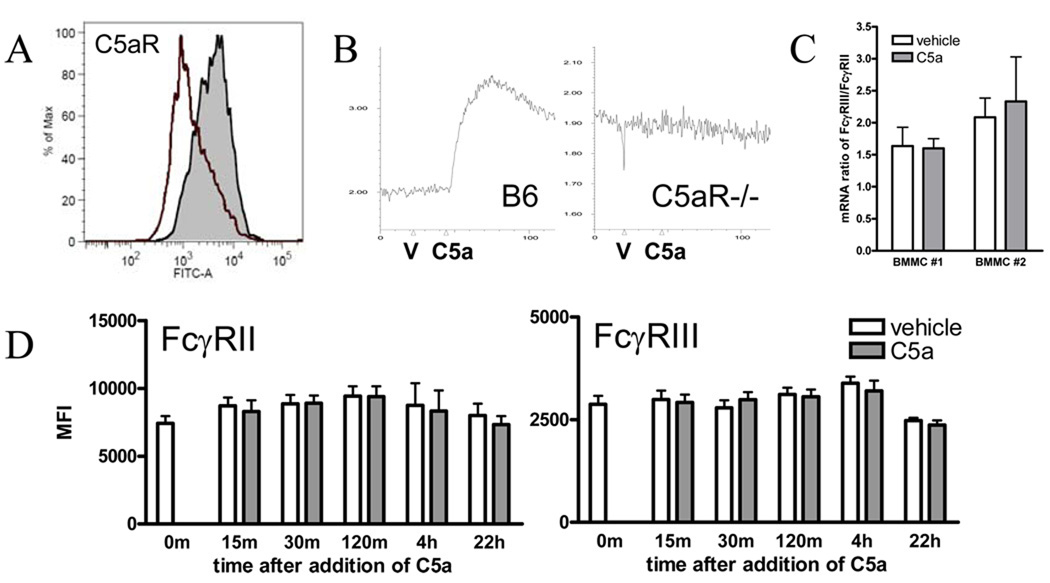
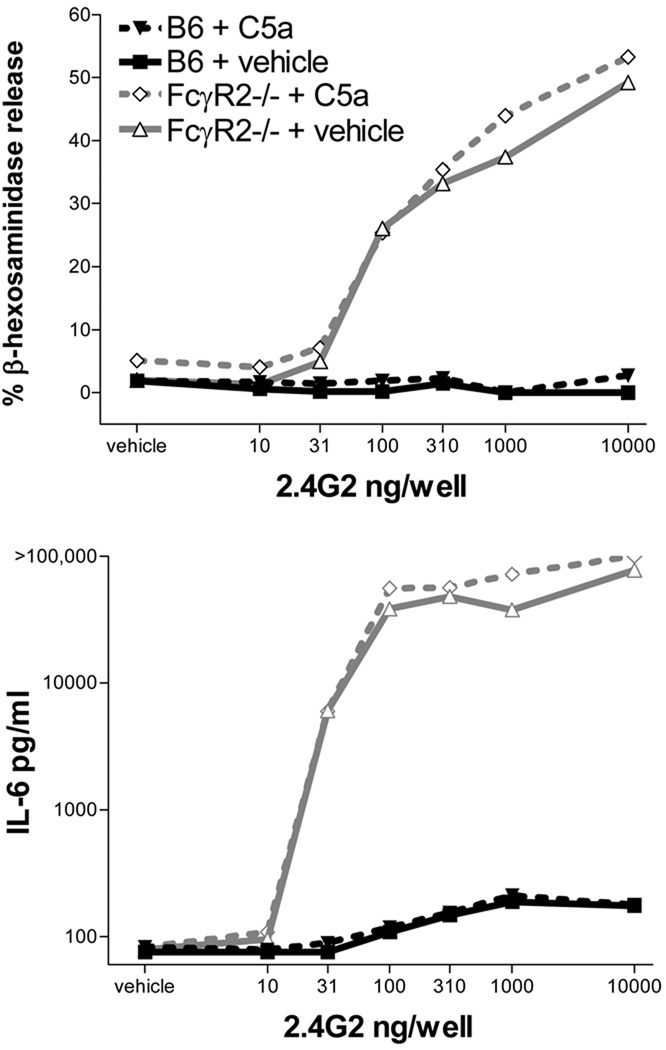
To corroborate this observation, we investigated C5aR-mediated FcγR expression and activation in cultured mast cells. Murine BMMC express both the inhibitory receptor FcγRII and the activating receptor FcγRIII (39). Expression of C5aR on these cells was confirmed by flow cytometry (Figure 3A). Function was confirmed by a calcium flux assay, in which C5a uniformly induced a brisk signal in B6 BMMC assessed within 1 day of media change that was absent in C5aR−/− BMMC (Figure 3B). Contrary to our expectations, stimulation with C5a did not alter FcγRII or FcγRIII expression as assessed by qPCR or specific surface staining (Figure 3C–D) (16, 17). Consistent with this result, C5a did not promote FcγRIII-mediated activation of B6 or FcγRII−/− BMMC, as assessed by the β-hexosaminidase assay for degranulation or by production of the cytokine IL-6 (Figure 4). Note that C5a alone was not a potent activator of BMMC, inducing degranulation of 0–5% and no appreciable production of IL-6 in unstimulated cells (Figure 4, “vehicle”). Multiple experiments failed to identify conditions in which C5a could modulate BMMC activation as assessed by these parameters, including dose ranging (C5a 1–500nM), time course (stimulation with C5a up to 24h prior or 4h after FcγR ligation), and conditioning or co-stimulation with other potentially relevant ligands, including C3a (0–100nM), LPS (0–10µg/ml), IL-1 β (10ng/ml), IL-4 (10ng/ml), TGF-β (10ng/ml), IL-33 (10ng/ml), and IL-3-deficient and/or high-SCF (250ng/ml) culture conditions (data not shown). Similarly, stimulation of the phenotypically more mature peritoneal-derived mast cell (41) with C5a together with IgG immune complexes failed to demonstrate a change in activation parameters, though expression of functional C5aR could not be established conclusively in this model cell (data not shown).
Figure 3.
A BMMC were stained with 20/70 (rat anti-mouse C5aR, shaded histogram) or isotype (open histogram), followed by an anti-rat-FITC. B. B6 but not C5aR−/− BMMC exhibited Ca flux (V=vehicle, C5a=C5a 100nM). Ca flux was absent in BMMC from “spent” media (data not shown), so refreshed cells were used for all experiments. C. B6 BMMC exposed to C5a 100nM or vehicle were harvested at 2h for measurement of FcγR mRNA by qPCR. Shown are results from two separate BMMC cultures, stimulated in triplicate, expressed as a ratio of FcγRIII to FcγRII, representative of 3 experiments at 2h and 3 experiments at 4h. D. B6 BMMC stimulated with C5a 50nM or vehicle were harvested at intervals and tested for FcγR expression by flow cytometry. No effect of stimulation was observed. Data pooled from 2 experiments, each with 2 separate BMMC cultures.
Figure 4.
B6 or FcγRII−/− BMMC were spun onto a plate coated with the anti-FcγRII/III antibody 2.4G2, in the presence of vehicle or C5a 100nM, and supernatants from duplicate wells harvested after overnight incubation. Note logarithmic scale for IL-6. Degranulation remained suppressed in B6 BMMC, though low levels of IL-6 production could be detected in some experiments, as shown here. Data representative of at least 3 separate experiments.
While these results mirrored our in vivo findings, we wished to replicate our experiments in a model cell relevant for human disease. We therefore tested the effect of C5a on human connective tissue mast cells cultured from skin explants (skin mast cells, SMC). These cells display a mature mast cell protease phenotype and can be activated via surface FcγRIIa, though they do not express the inhibitory receptor FcγRIIb (29, 42). Mast cells resident in human skin express C5aR (43), and we confirmed that this phenotype was maintained in cultured SMC by cytofluorimetry and C5a-mediated calcium flux (Figure 5A–B). Further, C5a was competent to activate SMC, if modestly (Figure 5C). However, as in BMMC, C5a failed to modulate surface FcγR expression (Figure 5D). Stimulation with C5a did not shift the dose-response curve of activation via FcγRIIa, though degranulation effects were additive (Figure 6A).
Figure 5.
A. Human SMC were stained with anti-C5aR (shaded) or isotype and assessed by flow cytometry. C3aR was not detectable (data not shown). B. Ca flux in SMC (V=vehicle, C5a=C5a 100nm, ATP=adensine triphosphate positive control). A and B representative of 2 experiments using distinct SMC cultures. C. Degranulation in SMC treated with C5a 10nM for 4–6h, pooled from 7 experiments (n=14/group). Degranulation was observed with as little as 1nM C5a and did not increase measurably from 10–300nM (data not shown). D. SMC stained for FcγR expression 2h after incubation with C5a 100nM (shaded) or vehicle (dark line) compared to isotype (light line). FcγRI (CD64) remained absent, as did 2B6 staining for FcγRIIB (data not shown). Data confirmed in 3 SMC cultures in 2 experiments.
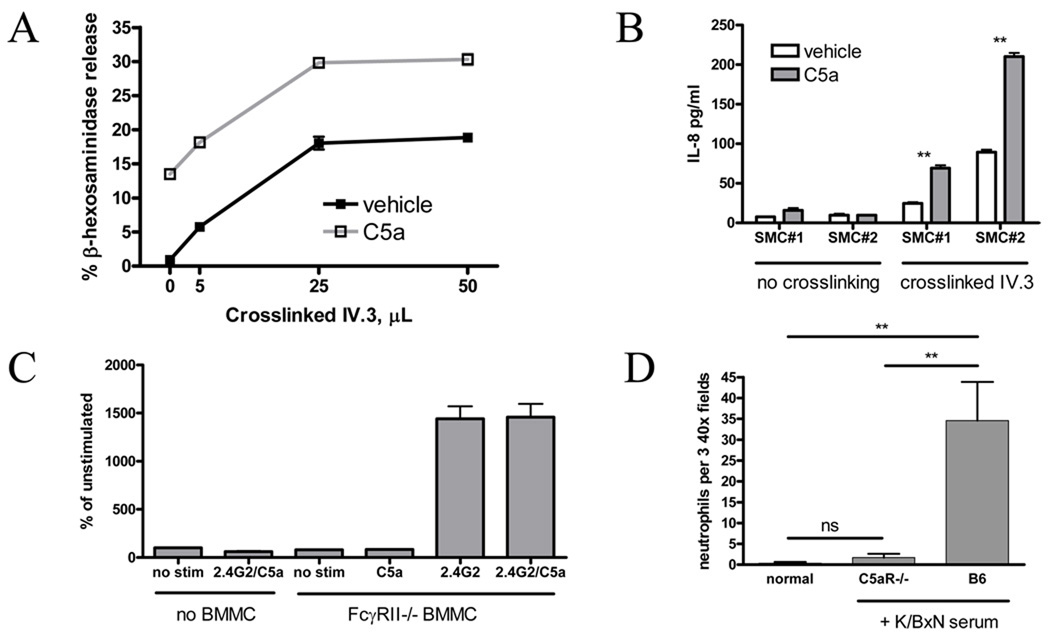
Figure 6.
A. SMC were stimulated with graded concentrations of FcγRIIa crosslinker with or without C5a 10nM × 4h. C5a contributed to SMC degranulation but did not alter responsiveness to FcγRIIa. B. SMC were stimulated × 4h with C5a 10nM, FcγRIIa crosslinker (5µL), or both; ** p<0.01. Data in A and B representative of 5 independent experiments using SMC from 2 donors. C. FcγRII−/− BMMC in the bottom of a transwell system were stimulated via C5a 50nM, 2.4G2 10ug/ml or both, and migration of C5aR−/− neutrophils from the upper chamber quantitated at 4h by flow cytometry. C5a did not result in enhanced migration. Representative of 2 experiments. D. Presence of extravasated NIMP-R14+ neutrophils was assessed 24h after i.p. instillation of 200uL K/BxN serum; n=5 mice (9 ankles)/group.
If C5aR is not required to enable IgG-mediated activation of mast cells, what is the contribution of this receptor to the function of mast cells in arthritis? The most likely possibility is that C5a enables production of a mediator vital to the evolution of synovitis, either alone or in conjunction with FcγR ligation. We have previously shown that mast cells are a key source of IL-1 in early synovitis, and this mediator is elaborated by BMMC upon activation via FcγR (12). However, despite extensive study in this model cell, we were unable to demonstrate an effect of C5a, with or without concomitant FcγR ligation, on synthesis of IL-1α or IL-1 β protein, processing of IL-1 β from its 31kD precursor to active 17kD cytokine, or release of active cytokine into the environment (data not shown). In analogous experiments, we could not demonstrate an effect of C5a on BMMC production of arachidonate metabolites (leukotriene B4, prostaglandin D2) or, via dedicated ELISA assays, PCR or multiplex techniques (Pierce SearchLight™, R&D Systems Proteome Profiler™ Mouse Cytokine Antibody Array), a large set of candidate cytokines and chemokines (data not shown). However, in human SMC stimulated via FcγRIIa, C5a could reproducibly enhance production of the neutrophil chemoattractant IL-8 (Figure 6B). This effect was specific, in that TNF production remained unaffected; IL-1β could not be detected (data not shown). These results suggest that the interaction between C5a and FcγR in synovial mast cells is likely to be one of synergy in the production of key pro-inflammatory factors, potentially including one or more neutrophil chemoattractants.
We therefore returned to the murine system to evaluate neutrophil chemoattractants as potential C5a-driven mast cell mediators. IL-8 is not expressed in mouse, but is instead represented by a family of related neutrophil chemoattractants that are ligands for the murine counterpart of the human IL-8 receptors CXCR1 and CXCR2, including MIP-2, GCP-2, NAP2, KC and LIX. Individual investigation of each of these mediators in BMMC stimulated via C5a +/− FcγR ligation revealed no consistent changes in production of protein (MIP-2, KC; the CCR5 ligand MIP-1α also tested) or in gene expression where no ELISA was commercially available (GCP-2, NAP2, LIX) (data not shown). To ensure we had not missed a chemoattractant using this candidate approach, we addressed the question functionally. BMMC stimulated via C5a, FcγR ligation, or both were situated in the bottom of a transwell chamber, with C5aR−/− neutrophils (as whole bone marrow) in the upper chamber. Whereas FcγR-stimulated BMMC elaborated potent chemoattractants, no effect of C5a alone was observed, and no increment was noted with combination stimulus (Figure 6C). We concluded that we were unable to demonstrate a C5a-dependent neutrophil chemoattractant from our model cell.
We therefore sought supportive evidence for a role for C5aR in neutrophil infiltration in vivo, via immunohistological staining of ankle tissues with the neutrophil marker NIMP-R14 (31). Tissues harvested from B6 and C5aR−/− mice 30min after serum administration, at the peak of the “flare,” demonstrated no infiltration of neutrophils or other recognizable lineages into ankle tissues of either B6 or C5aR−/− mice (data not shown). However, at 24h, scattered foci of infiltrating neutrophils could be demonstrated in most WT mice (7 of 9 ankles examined), while these remained absent in C5aR−/− animals (Figure 6D). We cannot exclude that this effect represents simply impaired migration of C5aR-deficient neutrophils. However, we have recently shown that C5aR−/− neutrophils can migrate to inflamed joints if drawn by a non-C5a stimulus (33). Thus these data support the possibility that C5aR is involved broadly in the generation of neutrophil chemoattractants in arthritis. Together with our engraftment data, they are consistent with the hypothesis that C5aR on mast cells promotes formation of one or more neutrophil chemoattractants. The identity of such chemoattractants, or other C5aR-dependent mast cell mediators, remains undefined.
DISCUSSION
Innate immune cells are key players in autoimmune synovitis. Among these are mast cells, which populate the normal joint in small numbers but can expand tenfold as arthritis progresses (44). The contribution of these cells to joint inflammation is likely complex. At initiation of disease, mast cells can serve as first sensors of immune complexes, contributing both to rapid increases in local vasopermeability and to the recruitment of inflammatory cells, an activity we have termed the “jump start” (12, 40). In established disease, where an expanded population of mast cells is often present, their contribution is as yet largely unexplored. Mast cell-derived mediators such as heparin and proteases are elevated in synovial fluid from both rheumatoid and osteoarthritic joints, and proteases of the tryptase family have been implicated experimentally in injury to cartilage (9, 31). Other potential functions of mast cells in the diseased joint include recruitment of inflammatory cells, promotion of antigen-directed adaptive immune responses, growth of new blood vessels, development of fibrosis, and degradation of pro-inflammatory mediators to help terminate inflammation (reviewed in (9)). Indeed, since mast cells from rheumatoid joints are phenotypically diverse (45, 46), different populations of mast cells could exert distinct effects.
The current work focuses on the role of the C5aR in murine synovial mast cells at initiation of arthritis. We find that C5aR expression is required to allow mast cells to accomplish their pro-inflammatory activity. We find no evidence that C5aR modulates the susceptibility of mast cells to immune complex-mediated activation in the joint, or in model cells studied in vitro, and therefore conclude that mast cells can diverge in this respect from murine macrophages. Since the phenotype of mast cells is strongly conditioned by the microenvironment, our results do not exclude the possibility that mast cells exhibit such modulation under other conditions. However, in the context of the arthritic “jump start” studied here, C5aR plays a different role. Most probably, C5aR synergizes with FcγRIII to promote synthesis of critical mediators, as has been reported in rat alveolar macrophages (15). We could not demonstrate this phenomenon in BMMC, perhaps reflecting the developmental immaturity of these cells (35). However, synergy between C5a and FcγR on IL-8 production by human SMC confirms that such a mechanism can occur in mast cells, and histological data from the mouse are consistent with the hypothesis that C5aR on mast cells participates in neutrophil recruitment to the joint.
The relevance of mast cell C5aR to human disease remains to be determined. Mast cells isolated from rheumatoid synovium, but not osteoarthritis samples, have been shown to express C5aR and to be capable of mediator release upon activation via C5a (46). Expression of C5aR on mast cells in healthy synovium is unknown. Blockade of C5a/C5aR in established rheumatoid arthritis has to date proved disappointing, though effective antagonism at the level of synovial tissue was not confirmed (47, 48). Since C5a is present at physiologically relevant levels in human RA synovial fluid, it would be surprising if this mediator had no effect on C5aR-bearing synovial mast cells (14).
Interestingly, while immune complexes fix complement and are likely a major source of synovial C5a, this anaphylatoxin can be generated in other ways. Hepatic macrophages can elaborate C5, and synthesis of C5 within rheumatoid synovial tissue has been detected in some studies, although its cellular source is unclear (1, 19). Cleavage into C5a can be accomplished by proteases beyond C3 convertase, including mast cell tryptase (49). This finding may explain why mice lacking C3 (and therefore incapable of complement-mediated C5 proteolysis) are less densely resistant to K/BxN arthritis than mice lacking C5aR (32). Similar results have been observed in the cutaneous immune complex-mediated Arthus reaction, immune complex alveolitis, and autoimmune hemolytic anemia (18, 19, 50). The implication of these results is that C5aR on mast cells could contribute to joint inflammation even in disease not mediated by immune complexes.
Together with previous results (12), the current findings demonstrate that participation of mast cells in immune complex-driven murine inflammatory arthritis is dependent co-dominantly upon FcγR and C5aR. The need for activation via both pathways does not reside in alteration of FcγR expression or activation threshold, but reflects the contribution of C5a to the elaboration of arthritogenic mediators, including neutrophil chemoattractants. These results define a novel pathway of interaction between complement and FcγR ligation in mast cells that may play an important part in the pathogenesis of immune-complex mediated disease, including inflammatory arthritis.
ACKNOWLEDGEMENTS
We gratefully acknowledge the technical assistance of Blair Chaletzky, Teri Bowman, Nicholas Calderone, and the gift of antibodies from Professor J. Zwirner and MacroGenics, Inc.
Supported by an Arthritis Foundation Physician Scientist Development Award and K08-AR051321 (to PAN), R01-GM083274-01 and R21 ES015696 (CLK), and an Arthritis Foundation Arthritis Investigator Award, the Cogan Family Foundation, and R01-AI059745 (to DML).
REFERENCES
- 1.Nigrovic PA, Lee DM. Immune complexes and innate immunity in rheumatoid arthritis. In: Firestein GS, Panayi GS, Wollheim FA, editors. Rheumatoid Arthritis: new frontiers in pathogenesis and treatment. 2nd ed. Oxford: Oxford University Press; 2006. pp. 135–156. [Google Scholar]
- 2.Monach PA, Benoist C, Mathis D. The role of antibodies in mouse models of rheumatoid arthritis, and relevance to human disease. Adv Immunol. 2004;82:217–248. doi: 10.1016/S0065-2776(04)82005-4. [DOI] [PubMed] [Google Scholar]
- 3.Wipke BT, Allen PM. Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. J Immunol. 2001;167(3):1601–1608. doi: 10.4049/jimmunol.167.3.1601. [DOI] [PubMed] [Google Scholar]
- 4.Van Lent PL, Holthuysen AE, Van Rooijen N, Van De Putte LB, Van Den Berg WB. Local removal of phagocytic synovial lining cells by clodronate-liposomes decreases cartilage destruction during collagen type II arthritis. Ann Rheum Dis. 1998;57(7):408–413. doi: 10.1136/ard.57.7.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002;297(5587):1689–1692. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- 6.Corr M, Crain B. The role of FcgammaR signaling in the K/B x N serum transfer model of arthritis. J Immunol. 2002;169(11):6604–6609. doi: 10.4049/jimmunol.169.11.6604. [DOI] [PubMed] [Google Scholar]
- 7.Kneilling M, Hultner L, Pichler BJ, Mailhammer R, Morawietz L, Solomon S, et al. Targeted mast cell silencing protects against joint destruction and angiogenesis in experimental arthritis in mice. Arthritis Rheum. 2007;56(6):1806–1816. doi: 10.1002/art.22602. [DOI] [PubMed] [Google Scholar]
- 8.Castor W. The microscopic structure of normal human synovial tissue. Arthritis Rheum. 1960;3:140–151. doi: 10.1002/art.1780030205. [DOI] [PubMed] [Google Scholar]
- 9.Nigrovic PA, Lee DM. Synovial mast cells: role in acute and chronic arthritis. Immunol Rev. 2007;217:19–37. doi: 10.1111/j.1600-065X.2007.00506.x. [DOI] [PubMed] [Google Scholar]
- 10.Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature. 1996;381(6577):77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 11.Echtenacher B, Mannel DN, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381(6577):75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- 12.Nigrovic PA, Binstadt BA, Monach PA, Johnsen A, Gurish M, Iwakura Y, et al. Mast cells contribute to initiation of autoantibody-mediated arthritis via IL-1. Proc Natl Acad Sci U S A. 2007;104(7):2325–2330. doi: 10.1073/pnas.0610852103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson AR, Hugli TE, Muller-Eberhard HJ. Release of histamine from rat mast cells by the complement peptides C3a and C5a. Immunology. 1975;28(6):1067. [PMC free article] [PubMed] [Google Scholar]
- 14.Jose PJ, Moss IK, Maini RN, Williams TJ. Measurement of the chemotactic complement fragment C5a in rheumatoid synovial fluids by radioimmunoassay: role of C5a in the acute inflammatory phase. Ann Rheum Dis. 1990;49(10):747–752. doi: 10.1136/ard.49.10.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Czermak BJ, Lentsch AB, Bless NM, Schmal H, Friedl HP, Ward PA. Synergistic enhancement of chemokine generation and lung injury by C5a or the membrane attack complex of complement. Am J Pathol. 1999;154(5):1513–1524. doi: 10.1016/S0002-9440(10)65405-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shushakova N, Skokowa J, Schulman J, Baumann U, Zwirner J, Schmidt RE, et al. C5a anaphylatoxin is a major regulator of activating versus inhibitory FcgammaRs in immune complex-induced lung disease. J Clin Invest. 2002;110(12):1823–1830. doi: 10.1172/JCI200216577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Godau J, Heller T, Hawlisch H, Trappe M, Howells E, Best J, et al. C5a initiates the inflammatory cascade in immune complex peritonitis. J Immunol. 2004;173(5):3437–3445. doi: 10.4049/jimmunol.173.5.3437. [DOI] [PubMed] [Google Scholar]
- 18.Skokowa J, Ali SR, Felda O, Kumar V, Konrad S, Shushakova N, et al. Macrophages induce the inflammatory response in the pulmonary Arthus reaction through G alpha i2 activation that controls C5aR and Fc receptor cooperation. J Immunol. 2005;174(5):3041–3050. doi: 10.4049/jimmunol.174.5.3041. [DOI] [PubMed] [Google Scholar]
- 19.Kumar V, Ali SR, Konrad S, Zwirner J, Verbeek JS, Schmidt RE, et al. Cell-derived anaphylatoxins as key mediators of antibody-dependent type II autoimmunity in mice. J Clin Invest. 2006;116(2):512–520. doi: 10.1172/JCI25536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konrad S, Engling L, Schmidt RE, Gessner JE. Characterization of the murine IgG Fc receptor III and IIB gene promoters: a single two-nucleotide difference determines their inverse responsiveness to C5a. J Biol Chem. 2007;282(52):37906–37912. doi: 10.1074/jbc.M707937200. [DOI] [PubMed] [Google Scholar]
- 21.Konrad S, Ali SR, Wiege K, Syed SN, Engling L, Piekorz RP, et al. Phosphoinositide 3-kinases gamma and delta, linkers of coordinate C5a receptor-Fcgamma receptor activation and immune complex-induced inflammation. J Biol Chem. 2008;283(48):33296–33303. doi: 10.1074/jbc.M804617200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ravetch JV. A full complement of receptors in immune complex diseases. J Clin Invest. 2002;110(12):1759–1761. doi: 10.1172/JCI200217349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hopken UE, Lu B, Gerard NP, Gerard C. The C5a chemoattractant receptor mediates mucosal defence to infection. Nature. 1996;383(6595):86–89. doi: 10.1038/383086a0. [DOI] [PubMed] [Google Scholar]
- 24.Humbles AA, Lu B, Nilsson CA, Lilly C, Israel E, Fujiwara Y, et al. A role for the C3a anaphylatoxin receptor in the effector phase of asthma. Nature. 2000;406(6799):998–1001. doi: 10.1038/35023175. [DOI] [PubMed] [Google Scholar]
- 25.Wolters PJ, Mallen-St Clair J, Lewis CC, Villalta SA, Baluk P, Erle DJ, et al. Tissue-selective mast cell reconstitution and differential lung gene expression in mast cell-deficient Kit(W-sh)/Kit(W-sh) sash mice. Clin Exp Allergy. 2005;35(1):82–88. doi: 10.1111/j.1365-2222.2005.02136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veri MC, Gorlatov S, Li H, Burke S, Johnson S, Stavenhagen J, et al. Monoclonal antibodies capable of discriminating the human inhibitory Fcgamma-receptor IIB (CD32B) from the activating Fcgamma-receptor IIA (CD32A): biochemical, biological and functional characterization. Immunology. 2007;121(3):392–404. doi: 10.1111/j.1365-2567.2007.02588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soruri A, Kim S, Kiafard Z, Zwirner J. Characterization of C5aR expression on murine myeloid and lymphoid cells by the use of a novel monoclonal antibody. Immunol Lett. 2003;88(1):47–52. doi: 10.1016/s0165-2478(03)00052-x. [DOI] [PubMed] [Google Scholar]
- 28.Riedemann NC, Guo RF, Neff TA, Laudes IJ, Keller KA, Sarma VJ, et al. Increased C5a receptor expression in sepsis. J Clin Invest. 2002;110(1):101–108. doi: 10.1172/JCI15409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kambe N, Kambe M, Kochan JP, Schwartz LB. Human skin-derived mast cells can proliferate while retaining their characteristic functional and protease phenotypes. Blood. 2001;97(7):2045–2052. doi: 10.1182/blood.v97.7.2045. [DOI] [PubMed] [Google Scholar]
- 30.Radeke HH, Janssen-Graalfs I, Sowa EN, Chouchakova N, Skokowa J, Loscher F, et al. Opposite regulation of type II and III receptors for immunoglobulin G in mouse glomerular mesangial cells and in the induction of anti-glomerular basement membrane (GBM) nephritis. J Biol Chem. 2002;277(30):27535–27544. doi: 10.1074/jbc.M200419200. [DOI] [PubMed] [Google Scholar]
- 31.Shin K, Nigrovic PA, Crish J, Boilard E, McNeil HP, Larabee KS, et al. Mast cells contribute to autoimmune inflammatory arthritis via their tryptase/heparin complexes. J Immunol. 2009;182(1):647–656. doi: 10.4049/jimmunol.182.1.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ji H, Ohmura K, Mahmood U, Lee DM, Hofhuis FM, Boackle SA, et al. Arthritis critically dependent on innate immune system players. Immunity. 2002;16(2):157–168. doi: 10.1016/s1074-7613(02)00275-3. [DOI] [PubMed] [Google Scholar]
- 33.Monach PA, Nigrovic PA, Chen M, Hock H, Lee DM, Benoist C, et al. Neutrophils in a mouse model of autoantibody-mediated arthritis: critical producers of Fc receptor gamma, the receptor for C5a, and lymphocyte function-associated antigen 1. Arthritis Rheum. 2010;62(3):753–764. doi: 10.1002/art.27238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grant EP, Picarella D, Burwell T, Delaney T, Croci A, Avitahl N, et al. Essential role for the C5a receptor in regulating the effector phase of synovial infiltration and joint destruction in experimental arthritis. J Exp Med. 2002;196(11):1461–1471. doi: 10.1084/jem.20020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gurish MF, Austen KF. The diverse roles of mast cells. J Exp Med. 2001;194(1):F1–F5. doi: 10.1084/jem.194.1.f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as "tunable" effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 37.Soruri A, Grigat J, Kiafard Z, Zwirner J. Mast cell activation is characterized by upregulation of a functional anaphylatoxin C5a receptor. BMC Immunol. 2008;9:29. doi: 10.1186/1471-2172-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakano T, Sonoda T, Hayashi C, Yamatodani A, Kanayama Y, Yamamura T, et al. Fate of bone marrow-derived cultured mast cells after intracutaneous, intraperitoneal, and intravenous transfer into genetically mast cell-deficient W/Wv mice. Evidence that cultured mast cells can give rise to both connective tissue type and mucosal mast cells. J Exp Med. 1985;162(3):1025–1043. doi: 10.1084/jem.162.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature. 1996;379(6563):346–349. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- 40.Binstadt BA, Patel PR, Alencar H, Nigrovic PA, Lee DM, Mahmood U, et al. Particularities of the vasculature can promote the organ specificity of autoimmune attack. Nat Immunol. 2006;7(3):284–292. doi: 10.1038/ni1306. [DOI] [PubMed] [Google Scholar]
- 41.Malbec O, Roget K, Schiffer C, Iannascoli B, Dumas AR, Arock M, et al. Peritoneal cell-derived mast cells: an in vitro model of mature serosal-type mouse mast cells. J Immunol. 2007;178(10):6465–6475. doi: 10.4049/jimmunol.178.10.6465. [DOI] [PubMed] [Google Scholar]
- 42.Zhao W, Kepley CL, Morel PA, Okumoto LM, Fukuoka Y, Schwartz LB. Fc gamma RIIa, not Fc gamma RIIb, is constitutively and functionally expressed on skin-derived human mast cells. J Immunol. 2006;177(1):694–701. doi: 10.4049/jimmunol.177.1.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oskeritzian CA, Zhao W, Min HK, Xia HZ, Pozez A, Kiev J, et al. Surface CD88 functionally distinguishes the MCTC from the MCT type of human lung mast cell. J Allergy Clin Immunol. 2005;115(6):1162–1168. doi: 10.1016/j.jaci.2005.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crisp AJ, Chapman CM, Kirkham SE, Schiller AL, Krane SM. Articular mastocytosis in rheumatoid arthritis. Arthritis Rheum. 1984;27(8):845–851. doi: 10.1002/art.1780270802. [DOI] [PubMed] [Google Scholar]
- 45.Gotis-Graham I, McNeil HP. Mast cell responses in rheumatoid synovium. Association of the MCTC subset with matrix turnover and clinical progression. Arthritis Rheum. 1997;40(3):479–489. doi: 10.1002/art.1780400314. [DOI] [PubMed] [Google Scholar]
- 46.Kiener HP, Baghestanian M, Dominkus M, Walchshofer S, Ghannadan M, Willheim M, et al. Expression of the C5a receptor (CD88) on synovial mast cells in patients with rheumatoid arthritis. Arthritis Rheum. 1998;41(2):233–245. doi: 10.1002/1529-0131(199802)41:2<233::AID-ART7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 47.Vergunst CE, Gerlag DM, Dinant H, Schulz L, Vinkenoog M, Smeets TJ, et al. Blocking the receptor for C5a in patients with rheumatoid arthritis does not reduce synovial inflammation. Rheumatology (Oxford) 2007;46(12):1773–1778. doi: 10.1093/rheumatology/kem222. [DOI] [PubMed] [Google Scholar]
- 48.Mojcik C, Kremer J, Bingham C, et al. Results of a phase 2b study of the humanized anti-C5 antibody eculizumab in patients with rheumatoid arthritis [abstract] Ann Rheum Dis. 2004;63:FRI0170. [Google Scholar]
- 49.Fukuoka Y, Xia HZ, Sanchez-Munoz LB, Dellinger AL, Escribano L, Schwartz LB. Generation of anaphylatoxins by human beta-tryptase from C3, C4, and C5. J Immunol. 2008;180(9):6307–6316. doi: 10.4049/jimmunol.180.9.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baumann U, Chouchakova N, Gewecke B, Kohl J, Carroll MC, Schmidt RE, et al. Distinct tissue site-specific requirements of mast cells and complement components C3/C5a receptor in IgG immune complex-induced injury of skin and lung. J Immunol. 2001;167(2):1022–1027. doi: 10.4049/jimmunol.167.2.1022. [DOI] [PubMed] [Google Scholar]