Abstract
Efforts are underway for the development of an effective vaccine against Helicobacter pylori infection. We prepared recombinant full length (568 aa) Helicobacter pylori urease B protein (rUreB) and tested it for immunogenicity and protection. BALB/c mice received either rUreB (40 μg) plus CpG (10 μg) intranasally, rUreB (50 μg) plus 3% aluminum hydroxide (50 μL) intramuscularly or rUreB (25 μg) plus Freund’s adjuvant (25 μL) subcutaneously, three times (week 0, 2 and 6). Intranasal rUreB plus CpG was neither immunogenic nor protective; intramuscular rUreB plus aluminum hydroxide was immunogenic and modestly protective; and subcutaneous rUreB plus Freund’s adjuvant was immunogenic and highly protective. The fact that protection was improved with Freund’s adjuvant indicates that recombinant urease B is a good antigen for a vaccine but that it needs a stronger adjuvant than aluminum hydroxide.
Keywords: adjuvant, bacterial vaccine, Helicobacter pylori, mouse model, urease B
Introduction
Helicobacter pylori is one of the most common chronic bacterial infections of humans affecting at least half of the world’s population. Even though asymptomatic most of the times, it leads to peptic ulcer disease in about 10% of the infected individuals and gastric carcinoma in about 1% (Suerbaum & Michetti, 2002). Antibiotics can clear most infections and have a benefit for individual patients, but because of the large number of infected people and the increasing resistance to antibiotics, a more realistic approach is the development of a vaccine. Granted that some experts doubt the possibility of making a protective H. pylori vaccine since the natural infection persists despite the host developing a strong immune response (Blanchard & Czinn, 2000). Yet, the fact that a post-infection immune response is not able to clear an infection does not necessarily negate the possibility that pre-infection immunity may prevent acquisition of a new infection. In fact, experimental animal data suggest that oral administration of Helicobacter-specific antibodies may be effective to prevent as well as to treat Helicobacter infection (Czinn et al., 1993; Casswall et al., 2002; Gorrell et al., 2009).
For 20 years a number of researchers have been working in the development of a vaccine to prevent H. pylori infection (Czinn & Nedrud, 1991). Of the various candidate antigens, the most promising is the B subunit of the urease protein (urease B), a 65 kDa protein encoded in a 1.7 kbp gene. The protein, which is exposed on the surface of the cell membrane, frequently elicits an immune response (Futagami et al., 1998) and its activity (likely by counteracting the gastric acidity) is crucial for the survival of this bacterium, as shown by the fact that urease-deficient H. pylori mutants fail to colonize the gastric mucosa (Eaton et al., 1991). Ferrero et al. (1994) reported that immunization with urease B resulted in 25–60% protection against H. felis (the Helicobacter species that naturally infects mice) challenge, as compared to no protection with urease A. Subsequent work has shown that mice immunized with whole cell lysate or urease B purified protein (either natural or recombinant) results in protection against infection following challenge with either H. pylori SS1 (an H. pylori strain adapted to colonize mice) (Kleanthous et al., 1998) or H. felis (Chen & Lee, 1992; Michetti et al., 1994). Despite these progresses, a vaccine for H. pylori remains elusive. Immunization of mice results in reduction but rarely elimination of Helicobacter organisms in the stomach (Sutton et al., 2000) and the few attempts to immunize human volunteers have not resulted in adequate immunogenicity (Kreiss et al., 1996; Michetti et al., 1999; Kotloff et al., 2001). So, even though urease B remains an attractive candidate, its immunogenicity has to be improved. To achieve this goal, researchers have experimented with various strong adjuvants (such as Freund’s, cholera toxin or Escherichia coli labile toxin), but due to their toxicity they have no human application.
In our group we have worked with urease Band produced a DNA vaccine (Zavala-Spinetti et al., 2006) and a protein vaccine (rUreB) based on the full length urease B (Bégué et al., 2007). Our work showed that the DNA vaccine was not immunogenic, while rUreB was highly immunogenic; and that the prime-boost approach with either rUreB followed by the DNA vaccine or the reverse, did not produce any additional benefit (Bégué et al., 2007). We also showed that rUreB was immunogenic when administered percutaneously but not by mucosal immunization, and that aluminum hydroxide significantly increased the immunogenicity of rUreB alone (Bégué & Moll, 2009). Since aluminum hydroxide is an adjuvant accepted for use in human immunization, we then proceeded to evaluate the protective efficacy of rUreB plus aluminum hydroxide against H. pylori infection and compared to other approaches we had found immunogenic. The results are here reported.
Methods
Recombinant urease B (rUreB) was prepared as previously described (Bégué et al., 2007). Genomic H. pylori DNA (ATCC 43504D, Manassas, VA) was used as template to PCR-amplify the full length ureB gene (GenBank AF352376; bp 1-1710) and cloned into the SalI site of the pQE9 vector (Quiagen, Valencia, CA). Competent XL10 Gold Escherichia coli cells were transformed and protein expression induced with 1 mmolL−1 isopropyl-β-D-thiogalactopyranoside. Cells were lysed with 8 molL−1 urea buffer (ph 8.0) and rUreB was purified by (His)6-tag affinity in a nickel column (Ni-NTA Superflow Column, Qiagen). The product was dialyzed to phosphate buffered saline (PBS, pH 7.4) and concentrated to 1 μgμL−1. Three different adjuvants were used in the experiment: CpG ODN 1826 (5′ – tcc atg acg ttc ctg acg tt – 3′) suspended in PBS to a concentration of 1 μgμL−1; aluminum hydroxide (Al[OH]3 3%, Alhydrogel, Brenntag Biosector, Frederikssund, Denmark) mixed with equal volume rUreB and incubated overnight at 4 °C for absorption; and Freund’s adjuvant (Sigma-Aldrich, St Louis, MO), Complete for first immunization and Incomplete for subsequent ones.
Six-week old female BALB/c mice (Harlan Sprague, Dawley, Indianapolis, IN), 5 per group, were immunized either intranasally (40 μL rUreB plus 10 μL CpG), intramuscularly (50 μL rUreB plus 50 μL aluminum hydroxide) or subcutaneously (25 μL rUreB plus 25 μL Freund’s adjuvant), three times (week 0, 2 and 6). Control mice received no immunization. Before immunization and 2 weeks after the third dose, stool (2 pellets) and blood (100 μL) were obtained from each animal to determine immunogenicity. Stools were suspended in 100 μL PBS, vortexed, centrifuged and the supernatant collected; blood was centrifuged and serum collected. Anti-urease B antibodies were determined by an enzyme-linked immunosorbent assay using rUreB expressed in Saccharomyces cerevisiae as capture antigen (Bégué et al., 2007). Yeast-derived rUreB (0.2 μgμL−1) was diluted 1:100 in PBS and used to coat Immunolon II plates (Dynatech, Chantilly, VA) overnight at 4 °C. The next day, wells were sequentially incubated with 200 μL blocking buffer (PBS solution, 0.5% Tween 20, 4% dry milk, 10% fetal bovine serum), 100 μL specimen (serum 1:50 or stool 1:10 in blocking buffer) and 100 μL of horseradish peroxidase goat anti-mouse (Zyned-Invitrogen, San Francisco, CA) IgG (1:4,000) or IgA (1:2,000) in blocking buffer. Incubations were for 1 hour at room temperature and plates were washed with PBS-Tween 20 (0.05%) between steps. Reaction was developed with 100 μL tetramethylbenzidine substrate (Sigma-Aldrich, St. Louis, MO) for 10 minutes, stopped with 100 μL 1N H2SO4, and the absorbance at a wavelength of 450 nm was determined. All of the specimens were tested in duplicates and the background reading of non-inoculated wells was subtracted from test wells.
Four weeks after the third dose of immunization, animals were challenged with Helicobacter pylori. For that, H. pylori SS1 strain (kindly provided by Dr RM Peek, Vanderbilt University, Nashville, TN) was grown at 37 °C in brucella broth (Becton Dickinson & Co, Sparks, MD) with 10% fetal bovine serum and antibiotics (vancomycin 10 μgmL−1 and amphotericin B 5 μgmL−1) under microaerophilic conditions (GasPak EZ, Becton Dickinson & Co, Sparks, MD) and a suspension of 1–5 × 109 bacteria in PBS administered by gastric gavage every other day for 3 doses. Four weeks after challenge, mice were euthanized and the stomach harvested to determine the presence of H. pylori organisms. Stomachs were homogenized (Tissue Tearor, Biospec Products, Bartlesville, OK), DNA extracted (Dneasy Tissue Kit, Qiagen) and subjected to quantitative real-time PCR (Bégué et al., 2006) using primers previously described by Roussel et al. (2007) and targeted to the H. pylori SS1 16S ribosomal RNA gene (bp: 411-564). Specimens were run in duplicates and positive and negative controls (H. pylori infected and uninfected mice, respectively) were included. In addition, to confirm that the detected signal was due to H. pylori in the specimens, the 16S rRNA gene was amplified (bp: 69-611) by regular PCR using primers described by Thoreson et al. (1995), and the resulting amplicon was sequenced at Louisiana State University Health Sciences Center Genomics Core Facility and compared to the H. pylori SS1 16S rRNA gene (GenBank AY366421).
Difference in antibody and H. pylori infection levels between groups were compared using the non-parametric Mann-Whitney U test (SPSS 14.0; SPSS, Chicago, IL). The animal experimentation protocol was reviewed, approved and supervised by the Institutional Animal Care and Use Committee of the Research Institute for Children, Children’s Hospital, New Orleans, LA
Results
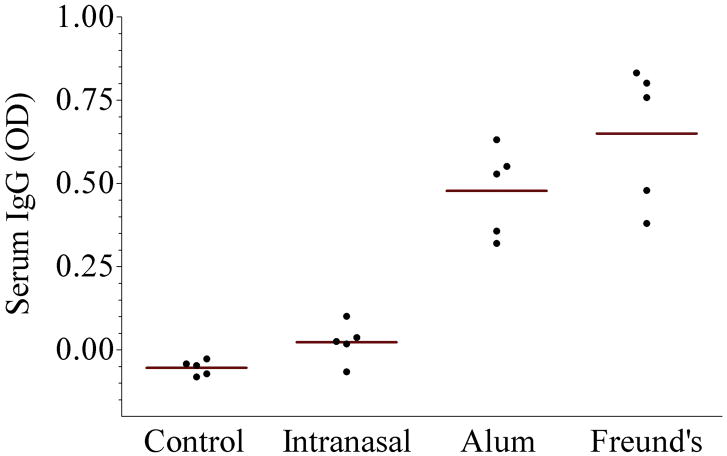
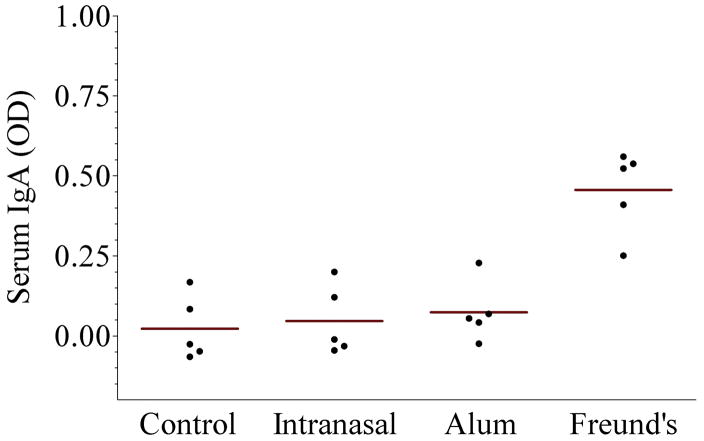
The results of immunogenicity are shown in the Figure. Panel A shows serum anti-urease B IgG antibodies. As noted, intranasal administration of rUreB was poorly immunogenic despite the use of CpG ODN as adjuvant and not different than the control group. On the other hand systemic administration of rUreB, either with aluminum hydroxide or Freund’s adjuvant did result in antibody levels significantly different than the control (p=0.01 for both, as compared to control and intranasal group). Panel B shows serum anti-urease B IgA antibodies, and in this case only rUreB adjuvanted by Freund’s resulted in significant levels of antibodies (p=0.01, as compared to the other 3 groups). Similar testing of stool pellets failed to show any measurable IgG or IgA (data not shown).
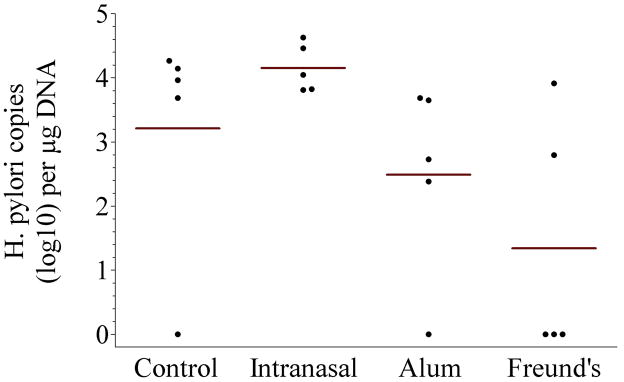
Figure 1.
Immunogenicity and protection of recombinant urease B (rUreB). Groups of BALB/c mice (n = 5) were vaccinated with rUreB either intranasally (40 μg rUreB plus 10 μg CpG), intramuscularly (50 μg rUreB plus 50 μL 2% aluminum hydroxide [alum]) or subcutaneously (25 μg rUreB plus 25 μL Freund’s adjuvant), three times (week 0, 2 and 6); control group received no immunization. A significant rise in serum anti-urease B IgG antibodies was detected in mice immunized with rUreB and either aluminum hydroxide (alum) or Freund’s adjuvant, while specific serum IgA was detected only in those receiving rUreB and Freund’s. Mice immunized with rUreB and either aluminum hydroxide (alum) or Freund’s adjuvant had a significantly lower level of H. pylori colonization than control and intranasally immunized mice (p = 0.01).
Protection is shown on panel C and expressed as the number of H. pylori copies detected in the stomach of challenged mice. Except for 1 that was negative, control mice had high levels of H. pylori infection (defined as > 1,000 copies per μg DNA), with an overall geometric mean of 1,627 copies per μg DNA. Intranasal inoculation resulted in no protection with all mice having high levels of infection and a geometric mean of 14,256 copies per μg DNA. Administration of rUreB with aluminum hydroxide had a modest effect with 1 mouse being negative, 2 positive at low levels of infection (defined as < 1,000 copies per μg DNA) and 2 at high levels, and a geometric mean of 309 copies per μg DNA (p=0.01 as compared to intranasal inoculation). rUreB adjuvanted with Freund’s had a more marked effect with 3 mice testing negative, 1 showing low level of infection and only 1 with high level of infection, for a geometric mean of 22 copies per μg DNA (p=0.01 as compared to intranasal inoculation). There was no statistically significant difference in the level of infection between the group that received rUreB and aluminum hydroxide and the group that received rUreB and Freund’s adjuvant (p=0.55).
Discussion
Similar to what others have described we found that rUreB had a partial efficacy against H. pylori infection, with 1 animal protected and 2 partially protected. What is original about our study is the use of aluminum hydroxide as adjuvant. We elected to test aluminum hydroxide because it is the only adjuvant approved for routine immunization of humans in the US. Few other groups have evaluated aluminum hydroxide as an adjuvant to either natural (Lee et al., 1999; Weltzin et al., 2000; Londoño-Arcila et al., 2002) or recombinant (Moschos et al., 2006; Wu et. al., 2008) urease. Similar to our findings, the immunogenicity has been good but the protective efficacy unclear. The better protection that we found with Freund’s adjuvant makes the point that rUreB is potentially a good antigen that can be made even more protective, provided better adjuvants are used or the antigen is presented in a more immunogenic manner. Other adjuvants such as MF59, approved in Europe for use with influenza vaccine in humans, can also be tested in the future.
Serum IgG and IgA levels were very similar among mice in specific vaccination groups. The resulting protection, however, had a much wider distribution. Most variability was given by uninfected mice; i.e., mice with no H. pylori detected in the stomach. The challenge procedure is relatively well established in the literature but its efficiency varies at different institutions and is mainly dependent on the infecting strain utilized. In our laboratory, the H. pylori challenge has been effective in inducing infection in ~ 80% of mice. Infected mice tended to have either high number (~ 1 × 104) H. pylori copies per μg DNA – which likely indicates no protection since that was the level shown by unvaccinated mice – or low number (~ 1 × 102.5 104) H. pylori copies per μg DNA – which likely indicates partial protection. The challenge method utilizes high dose (1 × 109) H. pylori organisms over a brief period, which is unlike natural human infection that occurs through exposure to low levels H. pylori over a prolonged period. This artificial way of infection may partially explain why some properly immunized mice missed protection.
We could not find a good serological correlate of protection. Even though as a group, those with the highest serum IgG and IgA had the lowest geometric mean H. pylori copies per μg DNA, the correlation was very poor at the individual level (r2 = 0.3037 and 0.0577 for IgG and IgA, respectively). This finding suggests that serum antibodies are markers for immune response but by themselves play limited role in protection, and that other arms of the immune system (innate, cellular, mucosal) are more important. Unfortunately, in this set of experiments we could not detect any stool antibodies.
We expressed the level of infection as number of H. pylori copies per μg purified DNA. This is unconventional since most studies express the level of infection as H. pylori copies per mg of stomach. We decided to use DNA as denominator because our detection method was based on PCR of purified DNA and the purification efficiency may have varied for each specimen. Indeed, even though there was a good correlate between the weight of the stomach and the amount of DNA purified, it was less than perfect (r2 = 0.59). So that our results can be compared to the ones reported in other studies, in our experiments on average 3.4 H. pylori copies per μg DNA corresponded to 1 copy per mg stomach.
In conclusion, our study adds to the evidence that rUreB is a promising H. pylori vaccine candidate, that aluminum hydroxide has a significant but modest adjuvant effect and that better adjuvants must be pursued.
Acknowledgments
This work was partially funded by NIH grant R03CA128048.
Footnotes
Competing Interests: The authors have no competing interests.
References
- Bégué RE, Manning J, Correa H. Real-time PCR for quantification of Helicobacter felis in mouse stomach. Southern Med J. 2006;99:1306–1307. doi: 10.1097/01.smj.0000247299.17826.a7. [DOI] [PubMed] [Google Scholar]
- Bégué RE, Cruz AR, Ramgoolam A, Breslin MB. Immunogenicity of Helicobacter pylori urease B protein and DNA vaccines in a mouse model. J Pediatr Gastroenterol Nutr. 2007;45:493–496. doi: 10.1097/MPG.0b013e31806c7bf8. [DOI] [PubMed] [Google Scholar]
- Bégué RE, Moll A. Immunogenicity of recombinant Helicobacter pylori urease B administered by various routes and with different adjuvants. The Open Vaccine Journal. 2009;2:28–32. doi: 10.2174/1875035400902010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard TG, Czinn SJ. Immunology of Helicobacter pylori and prospects for a vaccine. Gastroenterol Clin NA. 2000;29:671–685. doi: 10.1016/s0889-8553(05)70137-0. [DOI] [PubMed] [Google Scholar]
- Casswall TH, Nillson HO, Björck L, Sjostedt S, Xu L, Nord CK, Boren T, Wadstrom T, Hammarstrom L. Bovine anti-Helicobacter pylori antibodies for oral immunotherapy. Scand J Gastroenterol. 2002;37:1380–1385. doi: 10.1080/003655202762671242. [DOI] [PubMed] [Google Scholar]
- Chen M, Lee A, Hazell S. Immunization against helicobacter infection in a mouse/Helicobacter felis model. Lancet. 1992;339:1120–1121. doi: 10.1016/0140-6736(92)90720-n. [DOI] [PubMed] [Google Scholar]
- Czinn SJ, Nedrud JG. Oral immunization against Helicobacter pylori. Infect Immun. 1991;59:2359–2363. doi: 10.1128/iai.59.7.2359-2363.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czinn SJ, Cai A, Nedrud JG. Protection of germ-free mice from infection by Helicobacter felis after active oral or passive IgA immunization. Vaccine. 1993;11:637–642. doi: 10.1016/0264-410x(93)90309-l. [DOI] [PubMed] [Google Scholar]
- Eaton KA, Brooks CL, Morgan DR, Krakowka S. Essential role of urease in pathogenesis of gastritis induced by Helicobacter pylori in gnotobiotic piglets. Infect Immun. 1991;59:2470–2475. doi: 10.1128/iai.59.7.2470-2475.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrero RL, Thiberge JM, Huerre M, Labigne A. Recombinant antigens prepared from urease subunits of Helicobacter spp: evidence of protection in a mouse model of gastric infection. Infect Immun. 1994;62:4981–4989. doi: 10.1128/iai.62.11.4981-4989.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futagami S, Takahashi H, Norose Y, Kobayashi M. Systemic and local immune responses against Helicobacter pylori urease in patients with chronic gastritis: distinct IgA and IgG productive sites. Gut. 1998;43:168–175. doi: 10.1136/gut.43.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorell RJ, Robins-Browne RM. Antibody-mediated protection against infection with Helicobacter pylori in a suckling mouse model of passive immunity. Infect Immun. 2009;77:5116–5129. doi: 10.1128/IAI.00547-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleanthous H, Myers GA, Georgakopoulos KM, et al. Rectal and intranasal immunizations with recombinant urease induce distinct local and serum immune responses in mice and protect against Helicobacter pylori infection. Infect Immun. 1998;66:2879–2886. doi: 10.1128/iai.66.6.2879-2886.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotloff KL, Szeiten MB, Wasserman SS, Losonsky GA, DiLorenzo SC, Walker RI. Safety and immunogenicity of oral inactivated whole-cell Helicobacter pylori vaccine with adjuvant among volunteers with or without subclinical infection. Infect Immun. 2001;69:3581–3590. doi: 10.1128/IAI.69.6.3581-3590.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiss C, Buclin T, Cosma M, Corthesy-Theulauz I, Michetti P. Safety of oral immunization with recombinant urease in patients with Helicobacter pylori infection. Lancet. 1996;347:1630–1631. doi: 10.1016/s0140-6736(96)91119-8. [DOI] [PubMed] [Google Scholar]
- Lee CK, Soike K, Giannasca P, Hill J, Weltzin R, Kleanthous H, Blanchard J, Monath TP. Immunization of rhesus monkeys with a mucosal prime, parenteral boost strategy protects against infection with Helicobacter pylori. Vaccine. 1999;17:3072–3082. doi: 10.1016/s0264-410x(99)00144-9. [DOI] [PubMed] [Google Scholar]
- Londoño-Arcila P, Freeman D, Kleanthous H, et al. Attenuated Salmonella enteric serovar Typhi expressing urease effectively immunizes mice against Helicobacter pylori challenge as part of a heterologous mucosal priming-parenteral boosting vaccination regimen. Infect Immun. 2002;70:5096–5106. doi: 10.1128/IAI.70.9.5096-5106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschos SA, Bramwell VW, Somavarapu S, Alpar HO. Modulating the adjuvanticity by co-administration of muramyl di-peptide (MDP) or Quil-A. Vaccine. 2006;24:1081–1086. doi: 10.1016/j.vaccine.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Michetti P, Corthesy-Theulaz I, Davin C, Haas R, Vaney AC, Heitz M, Bille J, Kraehenbuhl JP, Saraga E, Blum AL. Immunization of BALB/c mice against Helicobacter felis infection with Helicobacter pylori urease. Gastroenterology. 1994;107:1002–1011. doi: 10.1016/0016-5085(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Michetti P, Kreiss C, Kotloff KL, et al. Oral immunization with urease and Escherichia coli heat-labile enterotoxin is safe and immunogenic in Helicobacter pylori-infected adults. Gastroenterology. 1999;116:804–812. doi: 10.1016/s0016-5085(99)70063-6. [DOI] [PubMed] [Google Scholar]
- Roussel Y, Harris A, Lee MH, Wilks M. Novel methods of quantitative real-time PCR data analysis in a murine Helicobacter pylori vaccine model. Vaccine. 2007;25:2919–2929. doi: 10.1016/j.vaccine.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- Sutton P, Wilson J, Lee A. Further development of a Helicobacter pylori mouse vaccination model. Vaccine. 2000;18:2677–2685. doi: 10.1016/s0264-410x(00)00052-9. [DOI] [PubMed] [Google Scholar]
- Thoreson ACE, Borre MB, Andersen LP, Elsborg L, Holck S, Conway P, Henrichsen J, Vuust J, Krogfelt KA. Development of a PCR-based technique for detection of Helicobacter pylori. FEMS Immunol Med Microbiol. 1995;10:325–334. doi: 10.1111/j.1574-695X.1995.tb00051.x. [DOI] [PubMed] [Google Scholar]
- Weltzin R, Guy B, Thomas WD, Jr, Giannasca PJ. Parenteral adjuvant activities of Escherichia coli heat-labile toxin and its B subunit for immunization of mice against gastric Helicobacter pylori infection. Infect Immun. 2000;68:2775–2782. doi: 10.1128/iai.68.5.2775-2782.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Shi Y, Guo H, Zhou WY, Guo G, Xie QH, Mao XH, Tong WD, Zou QM. Protection against Helicobacter pylori infection in Mongolian gerbil by intragastric or intramuscular administration of H. pylori multicomponent vaccine. Helicobacter. 2008;13:191–199. doi: 10.1111/j.1523-5378.2008.00609.x. [DOI] [PubMed] [Google Scholar]
- Zavala-Spinetti L, Breslin MB, Correa H, Bégué RE. Construction and evaluation of a Helicobacter pylori urease B DNA vaccine. Helicobacter. 2006;11:517–522. doi: 10.1111/j.1523-5378.2006.00453.x. [DOI] [PubMed] [Google Scholar]