Abstract
CD4 help for CD8+ T lymphocytes prevents tolerance and promotes the survival of effector and memory CD8+ T cells. Here we describe additional helper functions that require CD4 T cells within the tumor environment. CD8+ T cell recruitment, proliferation, and effector function within the tumor were greatly enhanced by tumor-specific CD4+ T cells. Recruitment of CD8+ T cells was accelerated by IFN-γ dependent production of chemokines. Production of IL-2 by tumor resident CD4+ T cells enhanced CD8+ T cell proliferation and upregulated expression of granzyme B. These results highlight a novel role for tumor-specific CD4+ T cells in promoting CD8+ T cell recruitment and cytolytic function, two previously unappreciated aspects of tumor-specific CD4 help.
Keywords: CD8 T cells, CD4 help, tumor immunity, recruitment, cytolytic function
Introduction
The requirements for successful cancer immunotherapy are not fully understood. Tumor vaccines that successfully result in the stimulation of large numbers of tumor-specific CTL do not necessarily result in tumor destruction (1–5). Several factors may constrain tumor eradication by specific effector CD8+ T cells. One is the relatively low affinity of the T cell repertoire specific for self/tumor antigens caused by mechanisms of tolerance that delete and inactivate T cells with high affinity for self-antigen (6–8). Also, unlike inflammatory sites initiated by an infectious agent, the tumor milieu is an immunosuppressive environment that prevents the recruitment, survival, and function of tumor-specific effector cells (9–10). Furthermore, the tumor vasculature can be inhibitory to migration of immune effector cells (11–12).
Using a tumor model, in which pancreatic neuroendocrine tumors develop that express influenza hemagglutinin (HA) as a tumor antigen(13), we have shown that CD8+ T cells expressing an HA specific TCR obtained from mice that express HA as a self-antigen (Clone-1, (14)) are unable to eradicate tumor, even when activated by a potent viral vaccine. Co-transfer of HA-specific SFE CD4+ T cells greatly enhanced the accumulation of Clone-1 cells in the tumor milieu and promoted tumor destruction (14–15). The provision of non-tumor-specific CD4 help during CD8 priming had no such effect, suggesting that the benefit of CD4 help was accrued in the tumor milieu and was not due to the programming of CD8+ T cells during initial priming (15).
Previous studies have demonstrated the importance of CD4+ T cells in preventing tolerance of CD8+ T cells in the face of persistent antigen produced by self, tumor or persistently infected tissue (16–22). However, tumor-specific CD4+ T cells may afford additional benefits that assist in tumor eradication. We hypothesized CD4+ T cells may promote recruitment, proliferation, survival, and effector function of CD8 effectors within the tumor milieu. Here we have independently assessed each of these parameters and have identified the cytokines required for such enhanced activities.
Materials and Methods
Mice
B10.D2 rat insulin promotor (RIP)-Tag2-hemagglutinin (HA) mice have been previously described (13) and were used at 8–9 wks of age. B10.D2 Clone-1 TCR transgenic mice, which express a TCR specific for HA518–526 (IYSTVASSL) in the context of HA-2Kd and SFE and SFE IL-2−/− TCR transgenic mice, which express a TCR that recognizes HA110–119 (SFERFEIFPK) in the context of I-Ed, were bred with the congenic markers Thy1.1 and CD45.1 respectively. B10.D2 DO11.10 TCR transgenic mice express a TCR that recognizes OVA323–339 in the context of I-Ad. All mice were bred in our facility. All animal experiments were conducted in accordance with protocols approved by the Institutional Animal Care and Use Committee of the Scripps Research Institute.
Adoptive transfer of naïve transgenic T cells and peptide immunization
Lymph nodes were collected and purified by magnetic cell sorting using CD8+/CD4+ T cell enrichments sets (BD Bioscience). Purified lymphocytes (0.3×106 or 1×105) were injected into RIP-Tag2-HA mice i.v. Recipient mice were immunized with 10 μg HA518–526-Kd peptide, 50 μg SFE110–119 or OVA323–339 peptide and 200 μg poly(I:C) (EMD Biosciences, San Diego) in IFA (DIFCO laboratories, Detroit) s.c. in the right flank. Glucose levels in the blood were measured as described before (15).
In vitro analysis of lymphocytes
The pancreas was minced in medium containing 2 mg/ml collagenase P (Roche Diagnostics) and 2 μg/ml DNase (Sigma-Aldrich). Enzymatic digestion was allowed for 20 min at 37°C. Cells were washed with ice-cold complete RPMI (Gibco) and lymphocytes were purified by density-gradient centrifugation using Histopaque-1077 (Sigma-Aldrich). Cells were stained for FACS analysis in HBSS containing 1% FCS and 2mM EDTA. For intracellular staining of IFN-γ, cells were stimulated overnight with1 μg/ml HA518–526 peptide in the presence of 1 μl/ml GolgiPlug. Antibodies for FACS were used from eBioscience, BD Biosciences and Alexis Biochemicals (BimS/EL/L). Intracellular stainings were performed according to the manufacturer's instructions using the Cytofix/Cytoperm plus kit (BD Biosciences).
In vivo cytokine production
Mice received 0.3×106 SFE cells i.v. and immunized with 50 μg SFE peptide and 200 μg poly(I:C) in IFA s.c. At day 4, 250 μg of Brefeldin A (Sigma-Aldrich) was injected i.p. and after 15 hours spleens and pancreata were isolated and analyzed by FACS.
Cytokine array
Mice were immunized with 50 μg SFE peptide and 200 μg poly(I:C) in IFA s.c. in the right flank with or without the injection of 0.3×106 purified SFE CD4+ cells i.v. Pancreata were isolated 6 days later and after density-gradient centrifugation with Histopaque-1077, cells were resuspended in PBS with protease inhibitors. Cell lysates were prepared according to manufactures instructions and cytokines were analyzed with Mouse Cytokine Array Panel A (R&D Systems).
In vitro generation of effector Clone-1 cells and in vivo recruitment
Lymph node cells from Clone-1 mice were cultured in complete RPMI (Gibco) with 1 μg/ml HA-Kd peptide, 20 ng/ml human IL-2 and splenocytes. After 3 days, live cells were purified by ficoll and cultured for 3 days with 20 ng/ml IL-2. Activated Clone-1 cells (1×106) were injected i.v. into RIP-Tag2-HA mice that were immunized 6 days earlier with 50 μg SFE peptide and 200 μg poly(I:C) in IFA s.c. with or without the injection of 0.3×106 purified SFE CD4+ T cells i.v. Pancreata and spleens were isolated after 40 hours and the number of recruited Clone-1 cells was analyzed by FACS. Neutralizing antibodies against IFN-γ (500 μg/mouse, Clone R4-6A2 BioXcell, West Lebanon NH), CXCL10, CCL2, CCL3, CCL5 (R&D Systems) and CXCL9 (kindly provided by Dr. R. Schreiber, Washington University School of Medicine) were injected 6 hours before and 18 hours after injection of Clone-1 cells (80 μg/mouse per antibody).
Statistical analysis
Differences between group means was determined by a Mann-Whitney test. Data are presented as means ± SEM.
Results
CD4 help within the tumor milieu is required for accumulation of tumor-specific CD8+ T cells
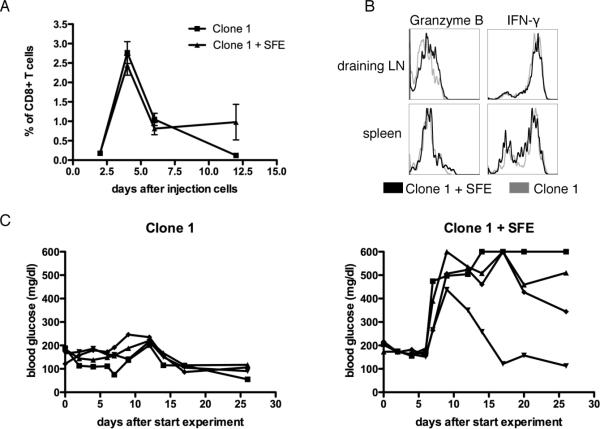
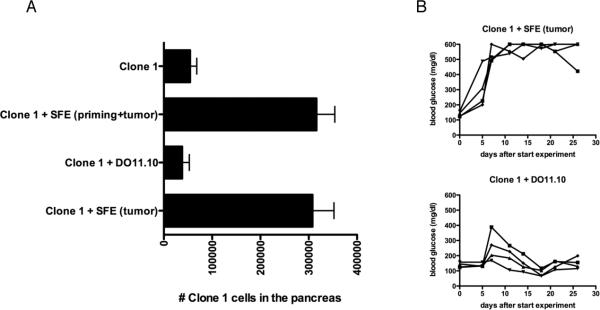
Tumor bearing RIP-Tag2-HA mice received HA specific Clone-1 cells either alone or with HA specific SFE cells, and were immunized with a vaccine containing cognate peptides and poly(I:C) injected subcutaneously in IFA. As previously shown using a viral vaccine, the presence of SFE cells during priming did not significantly increase the expansion of Clone-1 cells in the blood or spleen, and no differences were observed in expression levels of CD25, CD44 and CD62L on Clone-1 cells in the spleen or lymph nodes draining the vaccination site ((15), Fig. 1A and data not shown) and Clone-1 cells developed equally well into effector cells and produced similar levels of IFN-γ and granzyme B ((15) and Fig. 1B). However, in accordance with our earlier studies, tumor-specific CD4+ T cells were required to promote tumor eradication by Clone-1 cells, as evidenced by elevated blood glucose that occurs when the majority of normal and transformed pancreatic islet beta cells are destroyed (Fig. 1C). Successful tumor eradication was paralleled by a 6–10 fold enhancement in the numbers of Clone-1 cells found within the tumors 6 days after immunization (Fig. 2A).
Figure 1. Anti-tumor efficacy of Clone-1 CD8+ T cells.
RIP-Tag2-HA mice were immunized and Clone-1 cells (0.3×106) with or without 0.3×106 SFE cells were injected i.v. A. On the indicated days PBL were analyzed by FACS for Thy1.1+ cells. B. Effector function of Clone-1 cells was measured in the spleen and draining lymph node of the vaccination site at day 6 by intra-cellular staining of Granzyme B and IFN-γ. C. Glucose levels in the blood were measured at the indicated time points and each line represents one mouse. Data are representative of 2 independent experiments with 4 mice per group.
Figure 2. CD4+ T cells are required in the tumor environment, for accumulation of Clone-1 cells and anti-tumor immunity.
A. RIP-Tag2-HA mice were immunized and Clone-1 cells (0.3×106) with or without 0.3×106 SFE or DO11.10 cells were injected i.v. One group of mice received HA-Kd peptide and 200 μg poly(I:C) in IFA s.c. in the left flank and SFE peptide and 200 μg poly(I:C) in IFA s.c. in the right flank. Pancreata were analyzed by FACS at day 6. Data are cumulative over 4 experiments. B. Glucose levels in the blood were measured at the indicated time points and each line represents one mouse. Data are representative of 2 independent experiments with 4 mice per group.
To determine whether this difference was due to CD4+ T cell help provided during priming and/or the presence of CD4 cells within the tumor environment, we applied 2 different strategies. First, we immunized mice that received Clone-1 cells and non-tumor-specific, OVA-specific DO11.10 CD4+ T cells, with a vaccine containing cognate HA and OVA peptides. This provided CD4 help during priming, but not in the tumor milieu. Such non-tumor-specific CD4 help during priming did not increase the numbers of Clone-1 cells in the tumor (Fig. 2A), and had no effect on tumor growth (Fig. 2B). Thus, help within the tumor environment was critical for tumor eradication. Second, the sites of activation of tumor-specific SFE cells and of Clone-1 were spatially separated in opposite flanks so no CD4 help was present during priming of Clone-1 cells. This resulted in antigen specific CD4 help for Clone-1 cells only at the site of the tumor (Supplementary Fig. 1, CTL peptide right flank/SFE peptide left flank) which was found to be sufficient for enhanced accumulation of Clone-1 cells in the pancreas and resulted in an effective anti-tumor response (Fig. 2A and B, Clone-1 + SFE (tumor)). Thus, as reported previously using virus immunization (15), CD4 help within the tumor milieu greatly enhances the accumulation of tumor-specific CD8+ T cells and promotes tumor eradication.
CD4+ T cells enhance recruitment of CD8+ T cells by an IFN-γ dependent mechanism
To determine the extent to which enhanced accumulation of CD8+ T cells in the tumor was due to recruitment, RIP-Tag2-HA mice received SFE cells and cognate peptide vaccine. Mice were rested (6 days) to allow time for accumulation of tumor-specific CD4+ T cells in the pancreas, then received a bolus of in vitro activated Clone-1 CD8+ T cells. Forty hours later, significant numbers of Clone-1 cells were found only in mice that received SFE cells, suggesting that tumor specific CD4 cells were required for the recruitment of Clone-1 (Fig. 3A). To verify that increased numbers of Clone-1 cells in the tumor were due to recruitment and not proliferation Clone-1 cells were labeled with CFSE to detect division. No proliferation of Clone-1 cells was observed indicating that the increased numbers of Clone-1 cells were due to recruitment by SFE cells (Supplementary Fig. 2).
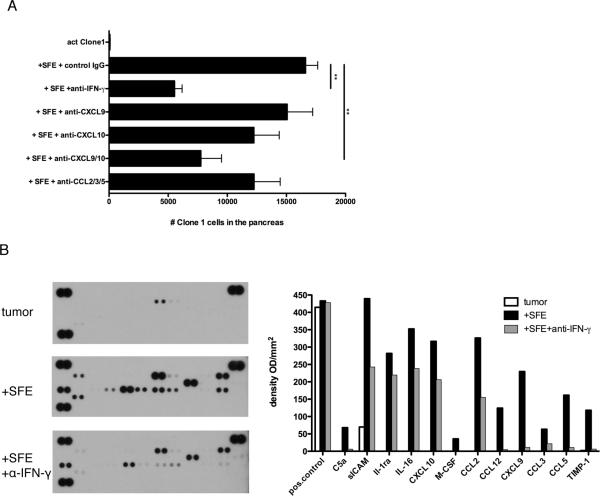
Figure 3. Tumor-specific CD4+ T cells induce an inflammatory environment that promotes recruitment of Clone-1 cells.
A. RIP-Tag2-HA mice were immunized with or without injection of 0.3×106 SFE cells. At day 6, 1×106 in vitro activated Clone-1 cells were injected and recruitment of Clone-1 cells to the pancreas was analyzed 40 hours later. The indicated neutralizing antibodies were injected at day 6 and 7. Data are cumulative over 3 experiments. **, P<0.005. B. RIP-Tag2-HA mice were immunized with or without injection of SFE cells (0.3×106) 6 days prior to isolation of pancreata and one group was injected with IFN-γ neutralizing antibodies at day 4 and 5. 200 μg of cell lysate was used for a cytokine array. Array images are from 30 minute exposures to X-ray film and quantitated data show relative changes between conditions. Data are representative of 2 independent experiments.
We hypothesized that SFE cells enhance recruitment of immune cells to the tumor through their production of inflammatory mediators. One of the major effector functions of CD4+ T cells is the secretion of IFN-γ which induces the secretion of inflammatory mediators, including chemokines (23–26). We confirmed that SFE cells become activated within the tumor to produce IFN-γ (Supplementary Fig. 3). To assess the role of IFN-γ in the recruitment of Clone-1 cells, mice that received SFE cells and vaccine were given an IFN-γ neutralizing antibody on days 6 and 7. This effectively blocked the recruitment of Clone-1 cells to the tumors (Fig. 3A). To identify the cytokines and chemokines produced in the presence of SFE cells we examined total cell lysates of pancreata from RIP-Tag2-HA mice 6 days after they received SFE cells and vaccine (Fig. 3B). The presence of SFE cells induced the production of numerous chemoattractants, all of which were suppressed by IFN-γ neutralizing antibody (Fig. 3B). The expression of CXCL10 (Fig. 3A, B and C), was verified by immunohistochemistry. These data confirm that the expression of this chemoattractant in the pancreatic islets requires SFE cells (Supplementary Fig. 4).
To test the role of specific chemokines in promoting recruitment, neutralizing antibodies were injected prior to transfer of activated Clone-1 cells. Whereas anti-CXCL9 and anti-CXCL10 antibodies alone had only a minor effect, co-injection of these antibodies resulted in a significant inhibition of the recruitment of Clone-1 cells to the site of the tumor (Fig. 3A). Also a mixture of anti-CCL2, -CCL3 and -CCL5 antibodies inhibited recruitment, indicating that multiple chemoattractants acting through distinct chemokine receptors contributed to recruitment of Clone-1 cells to the tumor.
CD4+ T cells enhance proliferation of Clone-1 cells in the tumor milieu
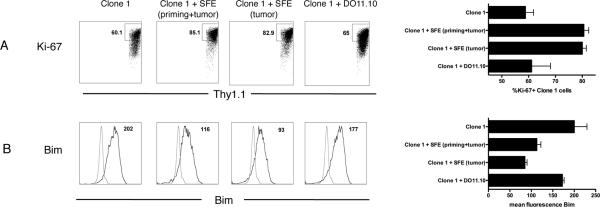
CD4+ T cells could also increase the number of Clone-1 cells within the tumor by enhancing their proliferation. Division was tested using a marker specific for proliferating cells, ki-67. RIP-Tag2-HA mice received either Clone-1 cells or both Clone-1 and SFE cells, and immunized as in Fig. 1. The presence of SFE cells made no difference in the proliferation of Clone-1 cells in the spleen(Supplementary Fig. 5A). However, in the absence of SFE cells, less division of Clone-1 cells occurred in the pancreas as assessed 6 days after immunization (60% vs. 80%, Fig. 4A). This stimulatory effect was the result of CD4 help at the site of the tumor rather than during priming as DO11.10 cells, which help only at the site of priming, did not enhance proliferation of the Clone-1 cells infiltrating the tumor; whereas tumor-specific help, available at the site of the tumor and not during priming, increased the percentage of ki-67+ Clone-1 cells (Fig. 4A).
Figure 4. CD4+ T cells in the tumor environment stimulate proliferation and survival.
RIP-Tag2-HA mice were immunized and Clone-1 cells (0.3×106) with or without 0.3×106 SFE or DO11.10 cells were injected i.v. Tumor infiltrating Clone-1 cells were analyzed at day 6 by FACS for proliferation (A: Ki-67) and Bim expression (B). The gate of the Ki-67 staining was based on the Thy1.1− Ki-67− cells in the pancreas. Control stainings in the histograms show staining with the Bim antibody of Bim−/− splenocytes. Dot plots and histograms are representative examples of each condition and bar graphs depict cumulative data of 3 experiments with 2–4 mice per group.
CD4+ T cells may also promote survival of CD8+ T cells in the tumor milieu. Attempts to detect apoptosis of Clone-1 cells in the pancreas by annexin staining were unsuccessful (data not shown). We therefore examined expression of Bim, a pro-apoptotic Bcl-2 family member, which plays a key role in T cell death in vivo (27). No differences were observed in the spleen (Supplementary Fig. 5B), however, SFE cells significantly reduced Bim expression by Clone-1 cells in the pancreas. The presence of DO11.10 cells did not reduce Bim expression, suggesting CD4 help in the tumor milieu rather than during priming improves T cell survival (Fig. 4B).
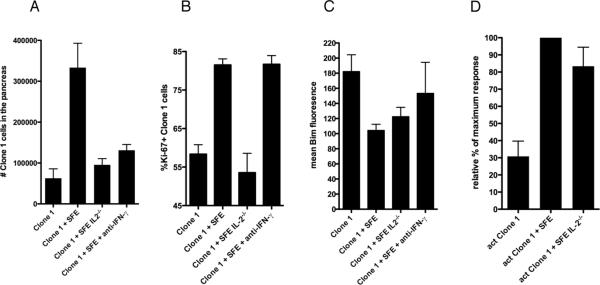
IL-2 is critical for Clone-1 proliferation in the tumor
Production of IL-2 by CD4 cells promotes expansion and survival of CD8+ T cells (28–29). To determine the effect of IL-2 on the accumulation of Clone-1 cells in the tumor we compared the numbers of Clone-1 cells infiltrating the pancreas in mice that received SFE cells or IL-2 deficient SFE cells. Because IL-2 deficiency (and neutralization of IFN-γ) reduced the number of CD4+ T cells in the pancreas, we injected a larger number of CD4+ T cells in these groups of mice to compensate for this difference (Supplementary Fig. 6). The lack of IL-2 production by SFE cells greatly reduced the number of Clone-1 cells found in the pancreas 6 days after immunization (Fig. 5A). We also found decreased numbers of infiltrating Clone-1 cells after blocking of IFN-γ, confirming the role of IFN-γ on recruitment (Fig. 5A). Comparison of ki-67 expressed by Clone-1 cells in the presence of IL-2 deficient and sufficient SFE cells indicated that IL-2 was necessary to increase proliferation of Clone-1 cells (Fig. 5B). In contrast, blocking IFN-γ had no effect on cell division. We also assessed whether IL-2 expression by SFE cells was required for downregulation of Bim expression by Clone-1 cells. IL-2 deficient SFE cells were almost as effective as IL-2 sufficient cells in promoting Bim downregulation (Fig. 5C). There was an increase in Bim expression by Clone-1 cells in mice in which IFN-γ was blocked, but this was found to be variable and not statistically significant.
Figure 5. The role of IL-2 production by CD4+ T cells.
A. RIP-Tag2-HA mice were immunized and Clone-1 cells (0.3×106) with or without 0.3×106 SFE, 2×106 IL-2−/− SFE or 2×106 SFE cells were injected i.v. The latter group was injected with IFN-γ neutralizing antibodies at day 4 and 5. Pancreata were analyzed by FACS at day 6. Data are cumulative over 2 experiments with 7 mice per group. B. Mice were injected as described in A. Expression of markers for proliferation (B: Ki-67) and survival (C: Bim) on Clone-1 cells were analyzed by FACS at day 6. Data are cumulative over 3 experiments with 9 mice per group. D. RIP-Tag2-HA mice were immunized with or without i.v. injection of 0.3×106 SFE or 2×106 SFE IL-2−/−. At day 6, 1×106 in vitro activated Clone-1 cells were injected and recruitment of Clone-1 cells to the pancreas was analyzed 4 days later. Data are cumulative over 3 experiments with 9 mice per group.
To determine whether IL-2 also affected the recruitment of CD8+ T cells, previously activated Clone-1 cells were injected into RIP-Tag2-HA mice that received IL-2 deficient SFE cells and vaccine 6 days earlier. IL-2 deficiency did not affect the numbers of Clone-1 cells recruited to the tumor (Fig. 5D).
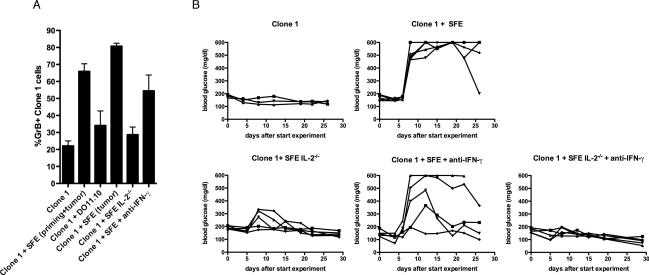
CD4+ T cells in the tumor milieu enhance granzyme B expression by Clone-1 cells through an IL-2 dependent mechanism
By day 6 following transfer, significant numbers of Clone-1 cells could be found in the tumor even in the absence of transferred CD4+ T cells (Fig. 2A). Nevertheless, as shown in Figs. 1–2, this did not result in tumor destruction. This observation prompted us to examine the effector function of Clone-1 cells in the pancreas. The presence of SFE cells did not significantly enhance production of IFN-γ after in vitro peptide re-stimulation (data not shown and (15). However, we did observe a higher frequency of granzyme B+ Clone-1 cells in the pancreas of mice that received SFE cells (Fig. 6A). This difference was detectable in the pancreas and not in the spleen (Supplementary Fig. 5C). In accordance with the anti-tumor efficacy of Clone-1 cells shown in Figure 1, CD4 help by DO11.10 cells did not enhance granzyme B expression, and help was only required at the site of the tumor and not during priming(Fig. 6A). The enhanced frequency of granzyme B+ Clone-1 cells observed in the presence of SFE cells was dependent on expression of IL-2, but did not require IFN-γ (Fig. 6A).
Figure 6. IL-2 production by CD4+ T cells is critical for increased effector function and anti-tumor efficacy of Clone-1 cells.
A. RIP-Tag2-HA mice were immunized and Clone-1 cells (0.3×106) with or without 0.3×106 SFE, 0.3×106 DO11.10, 2×106 IL-2−/− SFE or 2×106 SFE cells were injected i.v. The latter group was injected with IFN-γ neutralizing antibodies at day 4 and 5. Pancreata were isolated at day 6. Data are cumulative over 3 experiments with 9 mice per group. B. RIP-Tag2-HA mice were immunized and Clone-1 cells (1×105) with or without 1×105 SFE, 7×105 IL-2−/− SFE or 7×105 SFE cells were injected i.v. The latter group was injected with IFN-γ neutralizing antibodies daily starting at day 4 through 13 followed by injections every 2 days. Glucose levels in the blood were measured at the indicated time points. Data are representative of 2 independent experiments with 3–5 mice per group. Each line represents one mouse.
The role of IL-2 and IFN-γ in tumor eradication
To determine which of these mechanisms of CD4 help contributed to tumor eradication, we tested the anti-tumor efficacy of Clone-1 cells in RIP-Tag2-HA mice that received SFE cells and neutralizing IFN-γ antibodies and/or IL-2 deficient SFE cells (Fig. 6B). When tumor bearing mice received Clone-1 cells and IL-2 deficient SFE cells, tumor eradication was greatly reduced. Blocking IFN-γ had less of an effect on tumor eradication, as most of the mice demonstrated evidence of tumor destruction, although in most mice this was less effective than when IFN-γ was present. When both cytokines were absent, CD4 help was completely abrogated as indicated by a lack of tumor killing.
Discussion
In the present study, we examined the molecular mechanisms of CD4 help for tumor-specific CD8+ T cells within the tumor environment. It was reported that the innate immune activator, poly(I:C), can substitute for CD4 help during priming by upregulating DC expression of CD70 (30–31). Consistent with those results, we found that immunization with poly(I:C) obviates the need for CD4 help with respect to the numbers and function of the resultant CD8 effectors in the periphery. However, as previously reported using virus as a tumor vaccine, this was not sufficient to either promote the accumulation of Clone-1 cells in the pancreas or to achieve tumor eradication ((15) and this manuscript). Furthermore, this was not improved by providing CD4 help during the priming of CD8+ T cells in the form of non-tumor-specific DO11.10 CD4+ T cells, yet was improved when tumor-specific help was provided. Thus, tumor-specific CD4+ T cells play a unique role post-priming in promoting tumor eradication. Here we have identified the benefits of CD4 help that are exclusive to the tumor milieu and that are required for effective immunotherapy.
Whereas the numbers of Clone-1 cells in the blood were comparable in the absence or presence of SFE cells, far greater numbers of Clone-1 cells were found in the pancreas of mice that received SFE cells. Our study shows that enhanced intra-tumoral accumulation of tumor-specific CD8+ T cells is the result of multiple effects by tumor-specific CD4+ T cells involving the recruitment, proliferation and possibly survival of the CD8+ T cells.
A strong inflammatory environment that is induced by the presence of SFE cells relies mainly on the production of IFN- γ and facilitates early recruitment to the tumor. In the presence of SFE cells, large numbers of Clone-1 cells were found in the pancreas two days after injection and a significant reduction was observed when IFN-γ was blocked. Furthermore, blocking of several chemokines that are induced by IFN-γ (CXCL10, CXCL9, CCL2, CCL3, and CCL5) decreased the numbers of infiltrating Clone-1 cells. We have shown that CD4+ T cells can produce IFN-γ in the tumor milieu (Supplementary Fig. S3); however, this does not exclude a role of other cell types, such as NK cells and macrophages, in contributing to production of this cytokine. Others have also shown the importance of an IFN-γ dependent increase in the expression of Th1 chemokines within the tumor environment (9) and these chemokines have been described to be preferentially expressed in human tumors that contain T cells (32). A direct role for production of IFN-γ by CD4+ T cells in the recruitment of virus specific CD8+ T cells was recently reported in a model of herpes virus infected vaginal tissue (33). These results highlight the importance of CD4+ T cells for recruitment of CD8+ T cells in a variety of different tissues that are not able to directly recruit effector CD8+ T cells. This brings up the interesting question of why CD4+ T cells, but not CD8+ T cells, are effectively recruited into the transformed islets. The underlying basis for such differences in recruitment of CD4+ and CD8+ T cells is an unexplored area of investigation.
Our data indicate that recruitment of effector CD8+ T cells by CD4+ T cells in the tumor milieu is not sufficient to explain tumor eradication. Another major effector function of CD4+ T cells, the production of IL-2, was required to optimize tumor eradication by infiltrating CD8+ T cells. Although a number of studies have demonstrated a critical role for IL-2 during CD8 priming, (34–36) there has been less emphasis on the importance of IL-2 in sustaining a CD8 response within infected parenchymal tissue or in the tumor tissue. Lefrancois and coworkers compared the numbers of CD8+ T cells sufficient or deficient in the high affinity IL-2 receptor (CD25) following viral infection. They observed comparable numbers of cells in lymphoid tissue; however the numbers of CD25 deficient cells were greatly reduced in the lamina propria (37). Mescher and co-workers examined the role of IL-2 in sustaining tumor immunity. In their model, delivery of IL-2 to tumor bearing mice on days 4 and 5 following injection of tumor-specific CD8+ T cells was required to prevent anergy of tumor-specific CD8+ T cells (38). However, they saw no difference in effector function in the presence or absence of IL- 2 (39). Our experiments highlight the important role for IL-2 production by CD4+ T cells in the tumor environment, and further demonstrates that IL-2 affects both CD8+ T cell proliferation and effector function. Importantly, SFE cells deficient in IL-2 did not support tumor eradication by Clone-1 CD8+ T cells. Whether this is due to a need for proliferation or enhanced cytolytic function is not directly distinguished by these studies. However, previous experiments in which 10-fold larger numbers of Clone-1 cells were transferred to RIP-Tag2HA mice in the absence of CD4 help did not result in tumor eradication, despite the fact that very significant numbers of Clone-1 infiltrates were observed in the islets (14). In addition, most Clone-1 cells undergo at least some division in the tumor even in the absence of IL-2. IL-2 has been shown to promote the induction of granzyme B via STAT5 activation (40–41) as well as upregulating expression of perforin (42).
Taken together, these observations suggest that the main function of IL-2 in this model is most likely the upregulated cytolytic activity as indicated by enhanced granzyme B. It is striking that even in the presence of CD4 help, granzyme B levels are low in the periphery but show highly increased levels (3–4 fold increase) at the site of the tumor, emphasizing the importance of the local effects of IL-2. Other approaches to deliver cytokines like IL-2 and/or IFN-γ into the tumor environment that improved anti-tumor responses have also been described (43–46). Considering our results, it will be of great interest to determine whether the local delivery of IL-2 and IFN-γ into the tumor environment may replace CD4 help in our model.
An important outstanding question is how CD4 help is delivered to CD8+ T cells in the tissue. As the tumor cells do not themselves express MHC class II, it is likely that an antigen presenting dendritic cell is required for stimulation of CD4+ T cells in the tumor. MHC class II positive cells were observed in the absence of CD4+ SFE cells, but this number was greatly increased when CD4+ SFE cells were present (Supplementary Fig. 7). This increase could be due to the recruitment of MHC class II positive cells to the site of the tumor or upregulation of MHC class II on resident cells. CD4+ T cells are able to activate DC's in tumors by CD40-CD40L interactions, a process often referred to as licensing which may be important for preventing CD8+ T cell tolerance (22). However, such `licensed' DC's would not provide the IL-2 and IFN-γ required for tumor eradication in our model. Whether in our model the CD8+ T cells also require DC's for their stimulation in the tissue is not known.
These data clearly illustrate the importance of CD4 help at the site of the tumor and the different mechanisms by which CD4+ T cells increase the number and function of tumor-specific CD8+ T cells activated by a tumor vaccine. Of interest, we also find that previously activated CD8+ T cells, such as those used for adoptive transfer in tumor immunotherapy, also greatly benefit from the presence of tumor-specific CD4+ T cells which enhances CD8 recruitment to the tumor milieu. Taken together, these results provide strong evidence that tumor-specific CD4+ T cells uniquely support tumor-specific CD8+ T cells activated by either tumor vaccines or adoptive immunotherapy. Whether the numerous functions provided by tumor-specific CD4+ T cells, can be provided by alternative means, remains to be determined.
Supplementary Material
Acknowledgements
We thank Dr. Robert Schreiber for generously providing neutralizing Mig (CXCL9) antibody and Kristi Marquardt and Judith A. Biggs for breeding mice used in these experiments and technical assistance.
This work was supported by grant R01 CA57855 from the NIH. Rinke Bos was funded by a Rubicon fellowship from the Netherlands Organization for Scientific Research.
References
- 1.Peterson AC, Harlin H, Gajewski TF. Immunization with Melan-A peptide-pulsed peripheral blood mononuclear cells plus recombinant human interleukin-12 induces clinical activity and T-cell responses in advanced melanoma. J Clin Oncol. 2003;21:2342–8. doi: 10.1200/JCO.2003.12.144. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Sherry RM, Morton KE, Scharfman WJ, Yang JC, Topalian SL, et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol. 2005;175:6169–76. doi: 10.4049/jimmunol.175.9.6169. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–15. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–7. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mocellin S, Mandruzzato S, Bronte V, Lise M, Nitti D. Part I: Vaccines for solid tumours. Lancet Oncol. 2004;5:681–9. doi: 10.1016/S1470-2045(04)01610-9. [DOI] [PubMed] [Google Scholar]
- 6.Theobald M, Biggs J, Hernandez J, Lustgarten J, Labadie C, Sherman LA. Tolerance to p53 by A2.1-restricted cytotoxic T lymphocytes. J Exp Med. 1997;185:833–41. doi: 10.1084/jem.185.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heath WR, Kurts C, Miller JF, Carbone FR. Cross-tolerance: a pathway for inducing tolerance to peripheral tissue antigens. J Exp Med. 1998;187:1549–53. doi: 10.1084/jem.187.10.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Redmond WL, Sherman LA. Peripheral tolerance of CD8 T lymphocytes. Immunity. 2005;22:275–84. doi: 10.1016/j.immuni.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Weiss JM, Back TC, Scarzello AJ, Subleski JJ, Hall VL, Stauffer JK, et al. Successful immunotherapy with IL-2/anti-CD40 induces the chemokine-mediated mitigation of an immunosuppressive tumor microenvironment. Proc Natl Acad Sci U S A. 2009;106:19455–60. doi: 10.1073/pnas.0909474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–96. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryschich E, Schmidt J, Hammerling GJ, Klar E, Ganss R. Transformation of the microvascular system during multistage tumorigenesis. Int J Cancer. 2002;97:719–25. doi: 10.1002/ijc.10074. [DOI] [PubMed] [Google Scholar]
- 12.Buckanovich RJ, Facciabene A, Kim S, Benencia F, Sasaroli D, Balint K, et al. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat Med. 2008;14:28–36. doi: 10.1038/nm1699. [DOI] [PubMed] [Google Scholar]
- 13.Lyman MA, Aung S, Biggs JA, Sherman LA. A spontaneously arising pancreatic tumor does not promote the differentiation of naive CD8+ T lymphocytes into effector CTL. J Immunol. 2004;172:6558–67. doi: 10.4049/jimmunol.172.11.6558. [DOI] [PubMed] [Google Scholar]
- 14.Lyman MA, Nugent CT, Marquardt KL, Biggs JA, Pamer EG, Sherman LA. The fate of low affinity tumor-specific CD8+ T cells in tumor-bearing mice. J Immunol. 2005;174:2563–72. doi: 10.4049/jimmunol.174.5.2563. [DOI] [PubMed] [Google Scholar]
- 15.Wong SB, Bos R, Sherman LA. Tumor-specific CD4+ T cells render the tumor environment permissive for infiltration by low-avidity CD8+ T cells. J Immunol. 2008;180:3122–31. doi: 10.4049/jimmunol.180.5.3122. [DOI] [PubMed] [Google Scholar]
- 16.Kirberg J, Bruno L, von Boehmer H. CD4+8− help prevents rapid deletion of CD8+ cells after a transient response to antigen. Eur J Immunol. 1993;23:1963–7. doi: 10.1002/eji.1830230835. [DOI] [PubMed] [Google Scholar]
- 17.Battegay M, Moskophidis D, Rahemtulla A, Hengartner H, Mak TW, Zinkernagel RM. Enhanced establishment of a virus carrier state in adult CD4+ T-cell-deficient mice. J Virol. 1994;68:4700–4. doi: 10.1128/jvi.68.7.4700-4704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–63. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cardin RD, Brooks JW, Sarawar SR, Doherty PC. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J Exp Med. 1996;184:863–71. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurts C, Carbone FR, Barnden M, Blanas E, Allison J, Heath WR, et al. CD4+ T cell help impairs CD8+ T cell deletion induced by cross-presentation of self-antigens and favors autoimmunity. J Exp Med. 1997;186:2057–62. doi: 10.1084/jem.186.12.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marzo AL, Kinnear BF, Lake RA, Frelinger JJ, Collins EJ, Robinson BW, et al. Tumor-specific CD4+ T cells have a major “post-licensing” role in CTL mediated anti-tumor immunity. J Immunol. 2000;165:6047–55. doi: 10.4049/jimmunol.165.11.6047. [DOI] [PubMed] [Google Scholar]
- 22.Shafer-Weaver KA, Watkins SK, Anderson MJ, Draper LJ, Malyguine A, Alvord WG, et al. Immunity to murine prostatic tumors: continuous provision of T-cell help prevents CD8 T-cell tolerance and activates tumor-infiltrating dendritic cells. Cancer Res. 2009;69:6256–64. doi: 10.1158/0008-5472.CAN-08-4516. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Cole KE, Strick CA, Paradis TJ, Ogborne KT, Loetscher M, Gladue RP, et al. Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J Exp Med. 1998;187:2009–21. doi: 10.1084/jem.187.12.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farber JM. HuMig: a new human member of the chemokine family of cytokines. Biochem Biophys Res Commun. 1993;192:223–30. doi: 10.1006/bbrc.1993.1403. [DOI] [PubMed] [Google Scholar]
- 25.Luster AD, Unkeless JC, Ravetch JV. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 1985;315:672–6. doi: 10.1038/315672a0. [DOI] [PubMed] [Google Scholar]
- 26.Gouwy M, Struyf S, Proost P, Van Damme J. Synergy in cytokine and chemokine networks amplifies the inflammatory response. Cytokine Growth Factor Rev. 2005;16:561–80. doi: 10.1016/j.cytogfr.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Strasser A. The role of BH3-only proteins in the immune system. Nat Rev Immunol. 2005;5:189–200. doi: 10.1038/nri1568. [DOI] [PubMed] [Google Scholar]
- 28.Smith KA. Interleukin-2: inception, impact, and implications. Science. 1988;240:1169–76. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- 29.Cousens LP, Orange JS, Biron CA. Endogenous IL-2 contributes to T cell expansion and IFN-gamma production during lymphocytic choriomeningitis virus infection. J Immunol. 1995;155:5690–9. [PubMed] [Google Scholar]
- 30.Taraban VY, Rowley TF, Tough DF, Al-Shamkhani A. Requirement for CD70 in CD4+ Th cell-dependent and innate receptor-mediated CD8+ T cell priming. J Immunol. 2006;177:2969–75. doi: 10.4049/jimmunol.177.5.2969. [DOI] [PubMed] [Google Scholar]
- 31.Hervas-Stubbs S, Olivier A, Boisgerault F, Thieblemont N, Leclerc C. TLR3 ligand stimulates fully functional memory CD8+ T cells in the absence of CD4+ T-cell help. Blood. 2007;109:5318–26. doi: 10.1182/blood-2006-10-053256. [DOI] [PubMed] [Google Scholar]
- 32.Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69:3077–85. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009;462:510–3. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–3. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson EB, Livingstone AM. Cutting edge: CD4+ T cell-derived IL-2 is essential for help-dependent primary CD8+ T cell responses. J Immunol. 2008;181:7445–8. doi: 10.4049/jimmunol.181.11.7445. [DOI] [PubMed] [Google Scholar]
- 36.Lai YP, Lin CC, Liao WJ, Tang CY, Chen SC. CD4+ T cell-derived IL-2 signals during early priming advances primary CD8+ T cell responses. PLoS One. 2009;4:e7766. doi: 10.1371/journal.pone.0007766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D'Souza WN, Lefrancois L. IL-2 is not required for the initiation of CD8 T cell cycling but sustains expansion. J Immunol. 2003;171:5727–35. doi: 10.4049/jimmunol.171.11.5727. [DOI] [PubMed] [Google Scholar]
- 38.Shrikant P, Khoruts A, Mescher MF. CTLA-4 blockade reverses CD8+ T cell tolerance to tumor by a CD4+ T cell- and IL-2-dependent mechanism. Immunity. 1999;11:483–93. doi: 10.1016/s1074-7613(00)80123-5. [DOI] [PubMed] [Google Scholar]
- 39.Deeths MJ, Kedl RM, Mescher MF. CD8+ T cells become nonresponsive (anergic) following activation in the presence of costimulation. J Immunol. 1999;163:102–10. [PubMed] [Google Scholar]
- 40.Verdeil G, Puthier D, Nguyen C, Schmitt-Verhulst AM, Auphan-Anezin N. STAT5-mediated signals sustain a TCR-initiated gene expression program toward differentiation of CD8 T cell effectors. J Immunol. 2006;176:4834–42. doi: 10.4049/jimmunol.176.8.4834. [DOI] [PubMed] [Google Scholar]
- 41.Verdeil G, Marquardt K, Surh CD, Sherman LA. Adjuvants targeting innate and adaptive immunity synergize to enhance tumor immunotherapy. Proc Natl Acad Sci U S A. 2008;105:16683–8. doi: 10.1073/pnas.0805054105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32:79–90. doi: 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tjuvajev J, Gansbacher B, Desai R, Beattie B, Kaplitt M, Matei C, et al. RG-2 glioma growth attenuation and severe brain edema caused by local production of interleukin-2 and interferon-gamma. Cancer Res. 1995;55:1902–10. [PubMed] [Google Scholar]
- 44.Fearon ER, Pardoll DM, Itaya T, Golumbek P, Levitsky HI, Simons JW, et al. Interleukin-2 production by tumor cells bypasses T helper function in the generation of an antitumor response. Cell. 1990;60:397–403. doi: 10.1016/0092-8674(90)90591-2. [DOI] [PubMed] [Google Scholar]
- 45.Bramson JL, Hitt M, Addison CL, Muller WJ, Gauldie J, Graham FL. Direct intratumoral injection of an adenovirus expressing interleukin-12 induces regression and long-lasting immunity that is associated with highly localized expression of interleukin-12. Hum Gene Ther. 1996;7:1995–2002. doi: 10.1089/hum.1996.7.16-1995. [DOI] [PubMed] [Google Scholar]
- 46.Addison CL, Braciak T, Ralston R, Muller WJ, Gauldie J, Graham FL. Intratumoral injection of an adenovirus expressing interleukin 2 induces regression and immunity in a murine breast cancer model. Proc Natl Acad Sci U S A. 1995;92:8522–6. doi: 10.1073/pnas.92.18.8522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.