Abstract
Objective
Although the symptoms of autism exhibit quantitative distributions in nature, estimates of recurrence risk in families have never previously considered or incorporated quantitative characterization of the autistic phenotype among siblings.
Method
We report the results of quantitative characterization of 2,920 children from 1,235 families participating in a national volunteer register who met the criteria of having at least one child clinically-affected by an autism spectrum disorder (ASD) and at least one full biological sibling.
Results
The occurrence of a traditionally-defined ASD in an additional child occurred in 10.9% of the families. An additional 20% of non-ASD-affected siblings had a history of language delay, half of whom had exhibited autistic qualities of speech. Quantitative characterization using the Social Responsiveness Scale (SRS) supported previously-reported aggregation of a wide range of subclinical (quantitative) autistic traits among otherwise unaffected children in multiple-incidence families, and a relative absence of quantitative autistic traits among siblings in single-incidence autism families. Girls whose standardized severity ratings fell above a first percentile severity threshold (relative to the general population distribution) were significantly less likely to have elicited community diagnoses than their male counterparts.
Conclusions
These data suggest that, depending on how it is defined, sibling recurrence in ASD may exceed previously-published estimates, and varies as a function of family type. The results support differences in mechanisms of genetic transmission between simplex and multiplex autism, and advance current understanding of the genetic epidemiology of autism.
Keywords: Genetics, Pervasive Developmental Disorder, Language, Broader Autism Phenotype
Introduction
Aside from its clinical importance in genetic counseling, the characterization of sibling recurrence is pivotal in the elucidation of mechanisms of inheritance for any genetically-influenced condition. Categorical estimates of recurrence risk have previously indicated that the siblings of probands with Autistic Disorder have a 22-fold relative risk of developing Autistic Disorder (1). Recent discoveries in the field of autism research, however, have suggested that there exists a diversity of genetic mechanisms that give rise to the autistic syndrome (2), that each is associated with its own pattern of intergenerational transmission, and that autistic symptomatology exhibits a wide, continuous distribution in both the general population and among clinically ascertained cases (3–5). An additional complexity of the quantitative variation of autistic symptoms is that when considering samples largely comprised of sporadic (non-familial) cases of autism, there appear to exist – within the families – separable, discrete populations of affected and unaffected children whose respective severity distributions partially overlap (6, 7). Thus, a re-examination of the phenomenon of recurrence accounting for these developments is warranted.
For the 10–20% of all autism cases whose origins are attributable to known genetic causes, there is an emerging understanding of how specific molecular mechanisms of transmission might map to a given pattern of recurrence in families. For example, large, de novo chromosomal rearrangements (mutations of typically major effect) have been observed in some 10 % of children with autism (8, 9), compared to substantially lower rates in the general population. Common allelic variations of small but statistically-significant effect have been associated with incremental increases in susceptibility to autism, primarily among multiple-incidence autism families (10–12). Rare mutations in a number of synapse–related genes — singly or in combination (13) — have also been associated with a diverse array of full and intermediate autism phenotypes.
It is with that background that the current clinico-epidemiologic family study attempts to advance understanding of the relative proportions of autism cases in the population that might be attributable to these various mechanisms of genetic transmission, recognizing that the vast majority of cases of autism remain idiopathic at this writing. This study had two primary objectives: 1) to derive an updated estimate of recurrence risk in a large, volunteer registry of autism-affected families in which the children were both categorically and quantitatively characterized, and 2) to explore the distributions of quantitative (sub clinical) autistic traits in families with and without categorically-defined recurrence. Additionally, the study presented the opportunity to consider (in a large sample) whether any aggregation of language delays in ASD-unaffected children might constitute an additional type of “recurrence” among siblings in some families. Lindgren, Folstein, Tomblin and Tager-Flusberg (14) recently summarized the existing literature on the aggregation of language impairment in the first-degree relatives of children with ASD, and reported additional data on 52 families; their findings generally supported estimates of 20–25% from prior studies of comparable sample size (15, 16), with a greater predominance of pragmatic than structural language deficits.
Method
Sample
This report is based on data obtained from the Interactive Autism Network (IAN), a national, internet-based, voluntary autism family register (http://ianproject.org/). Parents who enroll their families complete standardized questionnaires about their autism-affected children and the biological siblings of those children. Families of any U.S. children under the age of 18 diagnosed with an ASD by a professional are eligible to be enrolled in the IAN research database by a willing English-speaking parent or a legal guardian. ASD includes all conditions encompassed within the current epidemiologic surveillance protocols for autism spectrum conditions maintained by the U.S. Centers for Disease Control (CDC), in which current U.S. prevalence is estimated at 9 per 1000 (17).
About 9 months after the registry was initially launched (2007), parents (one per family, usually the mother) were asked to provide quantitative characterizations of autistic symptomatology in each of the 4–18-year-old children in their families using the parent-report version of the SRS (4–18 year old version, see below). This report encompasses those families who had an autism-affected child and at least 1 full biological (non-identical) sibling in the age range from 4–18 years, for whom the SRS was completed. 1,235 families met these inclusion criteria. We note that although it was not possible to specifically cross-identify the subjects with those in the AGRE registry who comprised the multiplex subjects of our earlier report (Virkud et al, 2009), parents in IAN were asked whether their children had ever been in a research study about the genetics of autism; 14.4% of families endorsed this question affirmatively. AGRE exclusively enrolled multiple-incidence families; therefore, a conservative upper limit on the proportion of families in this report who co-participated in our prior study is 2%.
As part of the IAN registration protocol for all families, each reporting parent had indicated specifically whether each child in the family was or was not affected by an ASD diagnosed by a clinician or educational professional in the community. We refer to this as “categorical designation of an ASD diagnosis.” Among the families included in this study, 71% of the reported ASD diagnoses were made by individual doctoral-level professionals, 25% by a team in a health or school system, 4% unspecified. In addition, it was reported that 68% of the children designated by their parents as “affected” had previously undergone standardized assessment using either the Autism Diagnostic Observation Schedule (ADOS) or the Autism Diagnostic Interview-Revised (ADI-R), or both. Among them, 98.5% were scored as ASD-affected by one or both instruments, according to parents’ retrospective reports. Furthermore, in a recent study of verbal children with a history of ASD diagnosis randomly ascertained from the IAN registry and scoring greater than 12 on the Social Communication Questionnaire (see below), 98% were confirmed to have a clinical autism spectrum disorder by ADI-R, expert clinical observation, or both (18).
By parent report, 134 of the 1235 families (10.9%) had more than one child affected by an ASD. In addition, however, among all of the presumed-unaffected children in the sample, 20 %were reported by their parents to have histories of “a diagnosis of language delay or speech problem,” which is at least double the reported general population prevalence, especially when considering community diagnosis (19, 20). This observation prompted an attempt to more carefully characterize language impairment in the sample (see Measures), in order to identify sub sets of language-delayed subjects who might account for the observed excess in language impairment prevalence. Selected sample characteristics are presented in Table 1. A caveat is that the IAN registry is over-representative of Caucasian families (92.7 %).
Table 1.
Selected Sample Characteristics
| n | Mean Age | Gender |
Mean SRS T Scores | Mean SCQ Scores | ||
|---|---|---|---|---|---|---|
| M | F | |||||
| First Affected Child* | 1235 | 9.2±3.4 | 1052 | 183 | 86.3±15.2 | 23.2±7.2 |
| Subsequent Affected Sibs | 138 | 8.3±3.2 | 100 | 38 | 85.8±17.2 | 22.2±7.9 |
| Non-ASD sibs with History of Language Delay with Autistic Speech | 150 | 8.6±3.4 | 80 | 70 | 55.9±15.3 | 6.9±6.0 |
| Unaffected Sibs | 1397 | 9.7±3.8 | 651 | 746 | 45.0±10.4 | 2.6±4.2 |
Linear regression revealed modest effects of a) gender and b) level of functioning (see text)on SRS T-Score: F=63.2, df=2, 1225, p<.0001, R2 = .094; for gender, t=7.2, p<.001; for functioning level, t=-8.4, p<.001
SRS: Social Responsiveness Scale
SCQ: Social Communication Questionnaire
Measures
Categorical Designation of Affected Status
This was provided by the parent and supported by prior clinical diagnosis. The IAN data set also includes parent-report data derived from the Social Communication Questionnaire (SCQ), a developmental history checklist that ascertained whether the child ever manifested the presence of categorically-defined symptoms in fulfillment of DSM-IV criteria for Autistic Disorder (21). A total symptom score of 15 has been used as a clinical cutoff for affected status in previous research (22).
Characterization of Language Disorder Excess among “ASD-Unaffected” Children in IAN
Parent-report data regarding each child’s history of communicative development were retrievable in IAN from the Social Communication Questionnaire, in which a key item set (corresponding to symptoms in fulfillment of the communication criterion domain for a DSM-IV diagnosis of autistic disorder) ascertains whether a child has historically exhibited pathognomonically-autistic qualities of speech, including the use of odd or repetitive phrases, socially-inappropriate questions, pronoun reversal, or invented words (language items in other sections of the SCQ were not used for this purpose since they can be interpreted in ways that are less specific to autistic impairment). In this sample, positive endorsement of any of these characteristics was significantly more pronounced in ASD-unaffected children with versus without parent-reported history of language delay (chi square 36.7, p<.001) was associated with a significantly higher level of sub clinical autistic social impairment than in unaffected children without these characteristics (mean standardized SRS score excluding language items: 49.7 versus 42.2; t= −12.1; df= 722; p<.0001), and occurred in 54% of the children with histories of language delay. For this reason, single-incidence families with one or more “unaffected” children who had histories of diagnosed language delay plus the distinct autistic features of speech described above were considered separately in a subset of the analyses and the children who met this criterion were referred to as having a “History of Language Delay with Autistic Speech.” We note that neither quantitative IQ scores nor the timing of acquisition of language milestones were available on the majority of subjects in our sample.
Quantitative Characterization of the Autistic Phenotype
Affected and unaffected children in each family were assessed by a parent using the Social Responsiveness Scale (SRS). The SRS is an extensively validated (23–27), 65-item questionnaire that capitalizes on observations of children in their naturalistic social contexts, quantitatively measures severity of autistic traits and symptoms, and distinguishes ASD from other psychiatric conditions (27). Norms have been published by gender and rater type (parent versus teacher) in order to standardize ratings which otherwise differ as a function of these variables. SRS scores are highly heritable (3), stable over time (23), exhibit high inter-rater reliability (26), are continuously distributed in the general population (3), are non-significantly correlated with IQ among children representing the normal range of IQ in the general population (26), and exhibit a unitary factor structure (25), which supports the use of a single index score as a quantitative measure of autistic severity. SRS scores greater than 75T (98.8th percentile) indicate a level of autistic social impairment that is generally highly clinically significant. In the IAN sample, the proportion of children at or above the 75T SRS cutoff was highly similar (41.9%) to the proportion of children at or above the Social Communication Questionnaire cutoff of 15 (43.6%). Pearson’s coefficient of correlation between total score on the SRS and total score on the Social Communication Questionnaire was 0.88 in the entire sample, and .60 when considering affected children only.
Data Analysis
We first segregated the sample on the basis of whether or not the first-born ASD-affected child in each family was verbal versus non-verbal (or with parent-reported full scale IQ less than 56). Prior studies have suggested possible differences in recurrence risk among families whose autism-affected children exhibit intellectual disability and dysmorphism (28). In our sample, there was no significant difference in the risk of ASD in later-born siblings, as a function of non-verbal status (14.1% vs. 14.0%), Chi square = 1.58, p<.5 and therefore, in order to optimize the statistical power of the sample, we did not retain this segregation in the analyses presented in this report. Next, we computed recurrence statistics considering the various indices derived from the measures and compared them using chi square statistics. We subsequently tested a quantitative approach to the prospective prediction of sibling recurrence using standard regression methods, in which the index case was defined as the first (oldest) affected child in the family, and we considered the outcome of later-born siblings (one per family chosen at random). Finally, using analysis of variance methods, we compared the distributions of quantitative scores on the SRS across three mutually exclusive groups of families: 1) those in which more than one child was categorically affected (referred to as “multiple-incidence families”); 2) those in which only a single child was affected by a categorical ASD but at least one additional child exhibited a history of language delay with autistic speech; 3) single-incidence families in which no child other than the index case had either ASD or history of language delay with autistic speech.
RESULTS
Recurrence rates, conservatively operationalized as the occurrence of an autistic syndrome in one or more siblings of an index case — using as a denominator all additional children in the family, whether earlier-born or later-born — are presented in Table 2 as a function of recurrence definition. Categorical ASD status in an additional child occurred in 10.9% of the families (8.2% of the individual children in the entire sibling pool). An additional 20% of presumed-unaffected siblings had a history of language delay, and of those, 54% had exhibited autistic qualities of speech ascertained by the Social Communication Questionnaire (see measures). Thus, in addition to the 8.2% of siblings with categorically defined ASD, an additional 8.9% exhibited history of language delay with autistic speech.
Table 2.
Recurrence Rate as a function of “recurrence” definition.
| Recurrence Definition | Number of siblings affected by definition |
Proportion(%) of families with additional affected sib | Proportion (%) of all siblings in the families affected | |
|---|---|---|---|---|
| Male | Female | |||
| Single Criterion | ||||
| Categorical ASD DX | 100 | 38* | 10.9** | 8.2 |
| SCQ > =15 | 108 | 53 | 12.4 | 9.6 |
| SRS-T >= 75 | 99 | 64* | 12.5 | 9.7 |
| History of Language Delay with Autistic Speech | 80 | 70 | 10.8 | 8.9 |
| Category “Change” when switching from categorical to quantitative threshold | ||||
| No ASD DX but SCQ>=15 | 27 | 22 | -- | -- |
| No ASD DX but SRS-T>=75 | 28 | 33 | -- | -- |
| Combined Criteria | ||||
| ASD or SCQ>=15 | 127 | 60 | 14.4 | 11.1 |
| ASD or SRS-T>=75 | 128 | 71 | 15.1 | 11.8 |
| ASD or History of Language Delay with Autistic Speech | 180 | 108 | 21.7** | 17.1 |
| ASD or History of Language Delay with Autistic Speech or SCQ>=15 or SRS-T>=75 | 211 | 142 | 26.0 | 20.9 |
When exclusively considering later-born siblings (of relevance for comparison to high-risk infant sibling studies), the proportion of individual siblings affected by ASD was 14.2%. When including history of language delay with autistic speech and ASD in the definition of affected status, that proportion was 23.2%.
For difference in proportion of all female sibs affected as a function of recurrence classification, comparing standardized T-score threshold to community diagnosis, McNemar’s Test, Two-Tail p=.000042
Difference in recurrence rate with versus without inclusion of siblings with History of Language Delay with Autistic Speech, p<.001.
The family-based recurrence rate was uniformly higher than that calculated for all individual siblings in the sample, which reflects the possible effects of stoppage (a tendency for families who have a child with a serious clinical condition to reduce subsequent childbearing). When considering families with more than two children affected by ASD and the existence of at least one additional sibling, ASD status in the third child occurred in 8% of these families. Linear regression revealed statistically significant effects of both proband gender and sib gender on sib standardized quantitative trait scores on the SRS (F=5.80, df=4, 622, p<.001, R2 = .036); however, the effects were very modest in magnitude and there was no appreciable effect of proband level of functioning (non-verbal status or IQ < 56) on sib SRS score.
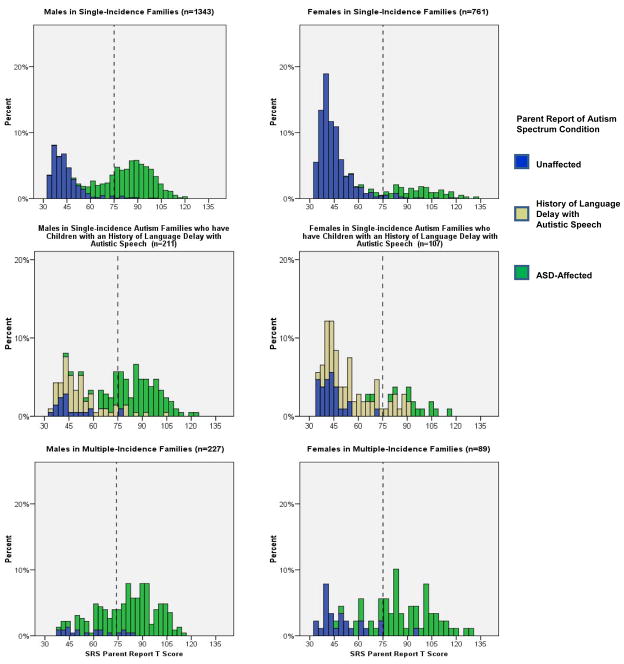
Table 3 lists means and standard deviations for the quantitative trait scores of specific groupings of index cases and siblings, segregated by gender and family type. The respective quantitative trait distributions are depicted in the histograms presented in Figure 1. Most striking across all subject groups, and in keeping with our previous report (7), we observed an absence of quantitative autistic traits in the unaffected siblings of ASD-affected children in single-incidence families. Also in keeping with our previous report, we observed a relative aggregation of quantitative autistic traits in the “unaffected” siblings in multiple-incidence families. This was manifested by an elevated mean and a contrasting shape of the distribution, especially for presumed-unaffected males in those families. Unaffected siblings with a history of language delay with autistic speech contributed to an intermediate distribution, with quantitative trait scores of females significantly overlapping with those of ASD-affected girls. The differences in mean SRS scores of presumed-unaffected siblings across the three groups were highly statistically significant, and remained so for males when multiple incidence families were directly compared to single incidence families.
Table 3.
Analysis of Variance for SRS Parent Report T-scores across three family types as a function of diagnosis and family type.
| Single-incidence Autism Families |
Single-incidence Autism Families who have children with History of Language Delay with Autistic Speech |
Multiple-incidence Autism Families |
F | P Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | |||
| ASD-Affected Boys | 826 | 85.4 | 14.0 | 119 | 86.2 | 14.5 | 206 | 82.2 | 17.1 | 4.4 | 0.01 |
| Presumed Unaffected Boys | 517 | 44.3* | 9.9 | 92 | 51.5 | 13.9 | 21 | 57.5* | 15.5 | 30.4 | 0.000001 |
| ASD-Affected Girls | 142 | 95.3 | 16.5 | 14 | 92.6 | 14.5 | 63 | 88.6 | 18.2 | 3.4 | 0.04 |
| Presumed Unaffected Girls | 619 | 45.2** | 10.5 | 93 | 53.6 | 15.5 | 26 | 49.7** | 14.5 | 23.2 | 0.000001 |
t=−3.9, df=21, p=.001
t=−1.6, df=26, p=.124
Figure 1.
Distributions of parent-report SRS T-scores, each encompassing all assessed children in the family for the respective gender and family type represented by each panel.
The quantitative distributions depicted in the histograms reveal the manner in which parents distinguished their children with versus without an ASD diagnosis in this sample. The nadir in these distributions, effectively the point at which parents (on average) differentiated affected versus unaffected children in their families, fell below the SRS score of 75T. We observed also that the distributions for unaffected children in simplex families were slightly non-pathologically-shifted in comparison to previously-published general population distributions (3) suggestive of subtle rater contrast effects. In the entire sample, there were 207 families with more than one ASD-unaffected child for whom parent-report SRS data were available. For these unaffected children (predominantly from single-incidence autism families in the sample), the sibling correlation for parent-report SRS was .38, in keeping with previously-published estimates (3, 29).
DISCUSSION
The results of this study of recurrence are notable in several respects and provide new information on the genetic epidemiology of autism spectrum conditions. First, there exists an aggregation of quantitative autistic traits among unaffected children in multiple-incidence ASD families—most pronounced in males, but an absence of such traits in single-incidence families, as initially observed in a prior study that included teacher-report data involving a smaller number of single-incidence families (7). The absence of such aggregation in single-incidence families is also consistent with our recent taxometic analysis of the entire IAN data set (a predominantly single-incidence sample), indentifying categorical discontinuity (ASD vs. non-ASD) rather than graded levels of symptoms within a predominantly single-incidence family sample (6). Second, we observed minimal effects of proband gender and level of functioning on the rate of sibling recurrence, but when using standardized quantitative criteria for designation of affected status, many more females are identified, and the gender ratio narrows to 3:2. Third, across all family types, and highly consistent with prior family studies, some 20% of presumed-unaffected siblings carry an historic diagnosis of language delay, over half of whom exhibited distinctly autistic speech. This may constitute a form of recurrence in a substantial minority of autism-affected families. Finally, the rate of sibling recurrence of categorically-defined ASD in this sample is in keeping with prior estimates, though distinctly lower than that reported for nonidentical twins from the same registry (31% concordance rate reported by Rosenberg et al., 2009) (30); whether this difference is explainable on the basis of a) factors that might raise recurrence risk in twins versus b) ascertainment bias favoring the enrollment of concordant over discordant twin pairs in this volunteer register will be a critical issue to resolve via future research in independent samples.
In summary we observed a range of manifestations of sibling recurrence in autism, to include: 1) categorically-defined ASD; 2) history of language delay with autistic speech qualities; and 3) the aggregation of quantitative (sub clinical) autistic traits. This third manifestation appears to be absent in single-incidence autism families. These disparate manifestations of recurrence may reflect differential mechanisms of genetic transmission of autism in the population, including (respectively): 1) rare recessive or de novo mutations (including chromosomal rearrangements) of substantial effect, which, in some cases, have accounted for sporadic cases of autism; 2) inherited mutations that may be variably expressed and result in varying degrees of social and language impairment (i.e. categorically-defined ASD, history of language delay with autistic speech) and/or sub clinical autistic impairment; and 3) common susceptibility alleles or rare variants of minor effect, which may operate in additive or epistatic fashion. We note that even among single-incidence families in which affectation status appears categorical, the distribution of quantitative trait scores for affected children extends well into the range of the distribution for the general population. Thus the continuum observed for autistic symptoms in nature may be composed of highly overlapping “segments”, each with its own mechanism (or mechanisms) of genetic transmission. Finally, the observation of a narrowing of the gender ratio when standardized quantitative criteria for affectation status are applied suggests the possibility that affected females may be under-ascertained when using traditional categorical methods for diagnostic assignment.
Limitations of the study are that the sample was not fully epidemiologic (rather a large volunteer register), not fully representative of the ethnicity of the population of U.S. children affected by ASD, and that the data were provided exclusively by parents, which potentially introduce a variety of biases including rater contrast effects. Higher levels of rater contrast are expected in parent-report data in clinically-ascertained families (a reason for the use of teacher-report data in our previous study (7)) and may have actually resulted in underestimation of the magnitude of familial aggregation among multiple-incidence families in this report. We note also that we were unable to directly compare the proportion of children with language delay in this sample with a population-based sample in which the same ascertainment methods were employed.
Several aspects of the data validate the reports of parents in this study, however, including the very high rate of reported diagnostic confirmation (98%) in families whose children underwent standardized testing for ASD (18), the fact that parents’ report of a diagnosis corresponded closely with quantitative characterizations of social deficiency in their children (with nadirs closely corresponding to established “cutoffs” for clinical-level symptomatology), and that these results replicate what was observed by both teacher-report data (minimizing the likelihood of rater contrast) and parent-report data in a smaller independent sample (7). It is important to note that elevations in quantitative autistic traits ascertained by the SRS and Social Communication Questionnaire have been observed in samples of children seriously affected by other primary psychiatric conditions not ascertained in the IAN data collection (31, 32). Future research will need to explore the extent to which the quantitative distribution of autistic traits in these populations represent distinct or overlapping continua with those that characterize ASD.
On the basis of these findings, we propose careful reconsideration of what constitutes “recurrence”, informed by an understanding of the range of symptoms that aggregate in the siblings of ASD-affected probands (including females or twins) (30), and that may more closely correspond to the manner in which autistic syndromes are intergenerationally transmitted. Among families of ASD subjects in this sample, fully 21.7% exhibited a recurrence of either ASD or history of language delay with autistic speech, with a broad distribution of sub clinical autistic traits among unaffected males in multiple incidence families.
Studies examining the association between autistic phenotypes and their underlying genetic (33) or neurobiologic (34, 35) determinants may be optimized by including information about recurrence of the autistic syndrome and the aggregation of relevant subclinical phenotypes among first-degree relatives. The data from the current study provide new perspectives on the relative proportions of autism cases in the general population that manifest distinct patterns of familial aggregation, and should alert clinicians to the presence of both clinical and sub clinical ASD-related-syndromes that occur in the siblings of children affected by autism.
Acknowledgments
This research was supported by a grant to Dr. Constantino from the National Institute of Child Health and Human Development (HD42541). The IAN volunteer register is supported by Autism Speaks.
Footnotes
Financial Disclosures: Dr. Constantino receives royalties from Western Psychological Services for the commercial distribution of one of the metrics used in this study, the Social Responsiveness Scale; however, no royalties were generated by any of the assessments performed in this research. For this work, the instrument was provided at no cost to the Interactive Autism Network, a national volunteer autism registry. The authors otherwise declare no competing financial interests.
References
- 1.Lauritsen MB, Pedersen CB, Mortensen PB. Effects of familial risk factors and place of birth on the risk of autism: a nationwide register-based study. J Child Psychol Psychiatry. 2005;46(9):963–71. doi: 10.1111/j.1469-7610.2004.00391.x. [DOI] [PubMed] [Google Scholar]
- 2.Weiss LA. Autism genetics: emerging data from genome-wide copy-number and single nucleotide polymorphism scans. Expert Rev Mol Diagn. 2009;9(8):795–803. doi: 10.1586/erm.09.59. [DOI] [PubMed] [Google Scholar]
- 3.Constantino JN, Todd RD. Autistic traits in the general population: a twin study. Arch Gen Psychiatry. 2003;60(5):524–30. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- 4.Ronald A, Happe F, Price TS, Baron-Cohen S, Plomin R. Phenotypic and genetic overlap between autistic traits at the extremes of the general population. J Am Acad Child Adolesc Psychiatry. 2006;45(10):1206–14. doi: 10.1097/01.chi.0000230165.54117.41. [DOI] [PubMed] [Google Scholar]
- 5.Skuse DH, Mandy W, Steer C, Miller LL, Goodman R, Lawrence K, Emond A, Golding J. Social communication competence and functional adaptation in a general population of children: preliminary evidence for sex-by-verbal IQ differential risk. J Am Acad Child Adolesc Psychiatry. 2009;48(2):128–37. doi: 10.1097/CHI.0b013e31819176b8. [DOI] [PubMed] [Google Scholar]
- 6.Frazier TW, Youngstrom EA, Sinclair L, Kubu CS, Law P, Rezai A, Constantino JN, Eng C. Autism spectrum disorders as a qualitatively distinct category from typical behavior in a large, clinically ascertained sample. Assessment. 2009 Dec 29; doi: 10.1177/1073191109356534. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Virkud YV, Todd RD, Abbacchi AM, Zhang Y, Constantino JN. Familial aggregation of quantitative autistic traits in multiplex versus simplex autism. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(3):328–34. doi: 10.1002/ajmg.b.30810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, Yamrom B, Yoon S, Krasnitz A, Kendall J, Leotta A, Pai D, Zhang R, Lee YH, Hicks J, Spence SJ, Lee AT, Puura K, Lehtimaki T, Ledbetter D, Gregersen PK, Bregman J, Sutcliffe JS, Jobanputra V, Chung W, Warburton D, King MC, Skuse D, Geschwind DH, Gilliam TC, Ye K, Wigler M. Strong association of de novo copy number mutations with autism. Science. 2007;316(5823):445–9. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss LA, Shen Y, Korn JM, Arking DE, Miller DT, Fossdal R, Saemundsen E, Stefansson H, Ferreira MA, Green T, Platt OS, Ruderfer DM, Walsh CA, Altshuler D, Chakravarti A, Tanzi RE, Stefansson K, Santangelo SL, Gusella JF, Sklar P, Wu BL, Daly MJ. Association between microdeletion and microduplication at 16p11.2 and autism. N Engl J Med. 2008;358(7):667–75. doi: 10.1056/NEJMoa075974. [DOI] [PubMed] [Google Scholar]
- 10.Arking DE, Cutler DJ, Brune CW, Teslovich TM, West K, Ikeda M, Rea A, Guy M, Lin S, Cook EH, Chakravarti A. A common genetic variant in the neurexin superfamily member CNTNAP2 increases familial risk of autism. Am J Hum Genet. 2008;82(1):160–4. doi: 10.1016/j.ajhg.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell DB, Warren D, Sutcliffe JS, Lee EB, Levitt P. Association of MET with social and communication phenotypes in individuals with autism spectrum disorder. Am J Med Genet B Neuropsychiatr Genet. 2009 doi: 10.1002/ajmg.b.30998. [Epub ahead of print, Jun 22] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang K, Zhang H, Ma D, Bucan M, Glessner JT, Abrahams BS, Salyakina D, Imielinski M, Bradfield JP, Sleiman PM, Kim CE, Hou C, Frackelton E, Chiavacci R, Takahashi N, Sakurai T, Rappaport E, Lajonchere CM, Munson J, Estes A, Korvatska O, Piven J, Sonnenblick LI, Alvarez Retuerto AI, Herman EI, Dong H, Hutman T, Sigman M, Ozonoff S, Klin A, Owley T, Sweeney JA, Brune CW, Cantor RM, Bernier R, Gilbert JR, Cuccaro ML, McMahon WM, Miller J, State MW, Wassink TH, Coon H, Levy SE, Schultz RT, Nurnberger JI, Haines JL, Sutcliffe JS, Cook EH, Minshew NJ, Buxbaum JD, Dawson G, Grant SF, Geschwind DH, Pericak-Vance MA, Schellenberg GD, Hakonarson H. Common genetic variants on 5p14.1 associate with autism spectrum disorders. Nature. 2009;459(7246):528–33. doi: 10.1038/nature07999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vernes SC, Newbury DF, Abrahams BS, Winchester L, Nicod J, Groszer M, Alarcon M, Oliver PL, Davies KE, Geschwind DH, Monaco AP, Fisher SE. A functional genetic link between distinct developmental language disorders. N Engl J Med. 2008;359(22):2337–45. doi: 10.1056/NEJMoa0802828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindgren KA, Folstein SE, Tomblin JB, Tager-Flusberg H. Language and reading abilities of children with autism spectrum disorders and specific language impairment and their first-degree relatives. Autism Research. 2009;2:22–38. doi: 10.1002/aur.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartak L, Rutter M, Cox A. A comparative study of infantile autism and specific developmental receptive language disorders. III. Discriminant function analysis. J Autism Child Schizophr. 1977;7(4):383–96. doi: 10.1007/BF01540396. [DOI] [PubMed] [Google Scholar]
- 16.Piven J, Palmer P, Jacobi D, Childress D, Arndt S. Broader autism phenotype: evidence from a family history study of multiple-incidence autism families. Am J Psychiatry. 1997;154(2):185–90. doi: 10.1176/ajp.154.2.185. [DOI] [PubMed] [Google Scholar]
- 17.Rice C. Autism and Developmental Disabilities Monitoring Network Surveillance Year 2006 Principal Investigators. Center for Disease Control and Prevention: Prevalence of Autism Spectrum Disorders --- Autism and Developmental Disabilities Monitoring Network, United States 2006 United States. Morb Mortal Wkly Rep Surveill Summ. 2009;58 (SS10):1–20. [PubMed] [Google Scholar]
- 18.Lee H, Marvin AR, Watson T, Piggot J, Law JK, Law PA, Constantino JN, Nelson SF. Accuracy of phenotyping of autistic children based on internet implemented parent report. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. doi: 10.1002/ajmg.b.31103. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shriberg LD, Tomblin JB, McSweeny JL. Prevalence of speech delay in 6-year-old children and comorbidity with language impairment. J Speech Lang Hear Res. 1999;42(6):1461–81. doi: 10.1044/jslhr.4206.1461. [DOI] [PubMed] [Google Scholar]
- 20.Tomblin JB, Records NL, Buckwalter P, Zhang X, Smith E, O’Brien M. Prevalence of specific language impairment in kindergarten children. J Speech Lang Hear Res. 1997;40(6):1245–60. doi: 10.1044/jslhr.4006.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rutter M, Bailey A, Lord C. Social Communication Questionnaire (SCQ) Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- 22.Charman T, Baird G, Simonoff E, Loucas T, Chandler S, Meldrum D, Pickles A. Efficacy of three screening instruments in the identification of autistic-spectrum disorders. Br J Psychiatry. 2007;191:554–9. doi: 10.1192/bjp.bp.107.040196. [DOI] [PubMed] [Google Scholar]
- 23.Constantino JN, Abbacchi AM, Lavesser PD, Reed H, Givens L, Chiang L, Gray T, Gross M, Zhang Y, Todd RD. Developmental course of autistic social impairment in males. Dev Psychopathol. 2009;21(1):127–38. doi: 10.1017/S095457940900008X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Constantino JN, Gruber CP. The Social Responsiveness Scale Manual. California: Western Psychological Services; 2005. [Google Scholar]
- 25.Constantino JN, Gruber CP, Davis S, Hayes S, Passanante N, Przybeck T. The factor structure of autistic traits. J Child Psychol Psychiatry. 2004;45(4):719–26. doi: 10.1111/j.1469-7610.2004.00266.x. [DOI] [PubMed] [Google Scholar]
- 26.Constantino JN, Lavesser PD, Zhang Y, Abbacchi AM, Gray T, Todd RD. Rapid quantitative assessment of autistic social impairment by classroom teachers. J Am Acad Child Adolesc Psychiatry. 2007;46(12):1668–76. doi: 10.1097/chi.0b013e318157cb23. [DOI] [PubMed] [Google Scholar]
- 27.Constantino JN, Przybeck T, Friesen D, Todd RD. Reciprocal social behavior in children with and without pervasive developmental disorders. J Dev Behav Pediatr. 2000;21(1):2–11. doi: 10.1097/00004703-200002000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Miles JH, Takahashi TN, Bagby S, Sahota PK, Vaslow DF, Wang CH, Hillman RE, Farmer JE. Essential versus complex autism: definition of fundamental prognostic subtypes. Am J Med Genet A. 2005;135(2):171–80. doi: 10.1002/ajmg.a.30590. [DOI] [PubMed] [Google Scholar]
- 29.Constantino JN, Lajonchere C, Lutz M, Gray T, Abbacchi A, McKenna K, Singh D, Todd RD. Autistic social impairment in the siblings of children with pervasive developmental disorders. Am J Psychiatry. 2006;163(2):294–6. doi: 10.1176/appi.ajp.163.2.294. [DOI] [PubMed] [Google Scholar]
- 30.Rosenberg RE, Law JK, Yenokyan G, McGready J, Kaufmann WE, Law PA. Characteristics and Concordance of Autism Spectrum Disorders Among 277 Twin Pairs. Arch Pediatr Adolesc Med. 2009;163(10):907–914. doi: 10.1001/archpediatrics.2009.98. [DOI] [PubMed] [Google Scholar]
- 31.Pine DS, Guyer AE, Goldwin M, Towbin KA, Leibenluft E. Autism spectrum disorder scale scores in pediatric mood and anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2008;47(6):652–61. doi: 10.1097/CHI.0b013e31816bffa5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reiersen AM, Constantino JN, Todd RD. Co-occurrence of motor problems and autistic symptoms in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2008;47(6):662–72. doi: 10.1097/CHI.0b013e31816bff88. [DOI] [PubMed] [Google Scholar]
- 33.Duvall JA, Lu A, Cantor RM, Todd RD, Constantino JN, Geschwind DH. A quantitative trait locus analysis of social responsiveness in multiplex autism families. Am J Psychiatry. 2007;164(4):656–62. doi: 10.1176/ajp.2007.164.4.656. [DOI] [PubMed] [Google Scholar]
- 34.Dalton KM, Nacewicz BM, Alexander AL, Davidson RJ. Gaze-fixation, brain activation, and amygdala volume in unaffected siblings of individuals with autism. Biol Psychiatry. 2007;61(4):512–20. doi: 10.1016/j.biopsych.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 35.Di Martino A, Shehzad Z, Kelly C, Roy AK, Gee DG, Uddin LQ, Gotimer K, Klein DF, Castellanos FX, Milham MP. Relationship between cingulo-insular functional connectivity and autistic traits in neurotypical adults. Am J Psychiatry. 2009;166(8):891–9. doi: 10.1176/appi.ajp.2009.08121894. [DOI] [PMC free article] [PubMed] [Google Scholar]