Abstract
Background
Complete lymph node dissection, the current standard treatment for nodal metastasis in melanoma, carries the risk of significant morbidity. Clinically apparent nodal tumor is likely to impact both pre-operative lymphatic function and extent of soft tissue dissection required to clear the basin. We hypothesized that early dissection would be associated with less morbidity than delayed dissection at the time of clinical recurrence.
Methods
The Multicenter Selective Lymphadenectomy Trial I randomized patients to wide excision of a primary melanoma with or without sentinel lymph node biopsy. Immediate completion lymph node dissection (early CLND) was performed when indicated in the SLN arm, while therapeutic dissection (delayed CLND) was performed at the time of clinical recurrence in the wide excision-alone arm. Acute and chronic morbidities were prospectively monitored.
Results
Early CLND was performed in 225 patients, and in the wide excision-alone arm 132 have undergone delayed CLND. The two groups were similar for primary tumor features, body mass index, basin location and demographics except age, which was higher for delayed CLND. The number of nodes evaluated and the number of positive nodes was greater for delayed CLND. There was no significant difference in acute morbidity, but lymphedema was significantly higher in the delayed CLND group (20.4% vs. 12.4%, p=0.04). Length of inpatient hospitalization was also longer for delayed CLND.
Conclusion
Immediate nodal treatment provides critical prognostic information and a likely therapeutic effect for those patients with nodal involvement. These data show that early CLND is also less likely to result in lymphedema.
Background
Complete lymph node dissection (CLND) is the current standard treatment for patients with nodal metastasis in melanoma. The involved surgical procedures are generally safe, provide complete clearance of evident disease and in many cases result in durable disease-free survival for patients. However, CLND carries significant risk of both acute and chronic morbidity which may result in increased suffering, decreased function and quality of life, and increased cost. It is therefore important to take all reasonable steps to decrease the risk of morbidity associated with CLND, while maximizing the benefit derived.1
Sentinel lymph node (SLN) biopsy is a well-established, minimally invasive technique that has become a standard part of the evaluation and treatment of patients with intermediate thickness melanoma. The procedure has been evaluated in several prospective clinical trials, including the Multicenter Selective Lymphadenectomy Trial (MSLT) I.2 This trial randomized 2001 patients to either wide local excision with SLN-directed treatment of regional lymph nodes (early CLND), or wide local excision alone, with CLND only at the time of clinical recurrence (delayed CLND).
The trial has demonstrated the accuracy and prognostic significance of the technique, which leads to an improved disease-free survival.3 These benefits provide a solid basis for the use of SLN and early CLND when indicated, but the impact of early intervention on the surgical morbidity of CLND has not been prospectively studied. The goal of the current analysis is to utilize the prospective data derived from MSLT I to examine which factors are associated with morbidity.
Pre-operative lymphatic function is likely to be compromised by the presence of substantial nodal metastases. This is apparent in patients who develop lymphedema in the setting of bulky regional metastases, but may also be true in patients without detectable lymphedema, but with significant regional nodal disease.
We hypothesized that early CLND would be associated with less morbidity than delayed CLND.
Methods
The design of MSLT I has been previously described.2,3 Briefly, the trial enrolled and randomized 2001 subjects with cutaneous melanoma greater than 1 mm in thickness or at least Clark’s level IV and without clinical evidence of regional or distant metastasis. Eligible subjects were 18–75 years of age and those with prior procedures which might have compromised lymphatic drainage of the primary site were excluded. Those with a history of prior melanoma or other invasive cancer within 5 years, and those with non-melanoma related life expectancy of less than 10 years were also excluded. The protocol was approved by each participating institution’s Institutional Review Board or Ethics Committee.
Subjects were randomized in a 4:6 ratio to wide excision alone or wide excision with SLN biopsy. Dual mapping with a radio-labeled tracer and blue dye was used to locate SLN following pre-operative lymphoscintigraphy. Subjects with metastases in the SLN were treated by early CLND. Those in whom a SLN could not be identified also underwent early CLND. In the wide excision alone group, delayed CLND was performed for those with clinically-detected nodal recurrences. Post-operative monitoring included clinical examination and blood tests at least every 3 months during the first 2 years, every 4 months during year 3, every 6 months during years 4 and 5, and annually until year 10.
Adverse events were prospectively collected including acute toxicity (wound separation, seroma/hematoma, hemorrhage, infection, thrombophlebitis, urinary tract infection, pneumonia, and cardiac complications) and chronic toxicity (lymphedema and nerve dysfunction i.e. motor weakness and/or dysesthesia). Lymphedema was defined as mild, moderate or severe. Mild lymphedema consisted of 2–3 cm enlargement of calf or biceps circumference with minimal or no symptoms. Moderate lymphedema included 3–4 cm enlargement of calf or biceps circumference or with moderate symptoms. Severe lymphedema included ≥4 cm enlargement or disabling symptoms.
Statistical analysis utilized T-test, Chi-square and Wilcoxon rank sum testing as appropriate. Survivals were estimated using the Kaplan-Meier method and compared using log rank. Obesity was defined as a body mass index of ≥30. For multivariate analysis, logistic regression models were developed using stepwise regression and an alpha of 0.1 as entry and retention criterion.
Results
MSLT I enrolled and randomized 2001 subjects. Of these, 800 underwent wide excision alone and 937 underwent wide excision with SLN biopsy. Among those in the SLN arm, 225 had early CLND after the SLN procedure. Among those in the wide excision alone arm, 143 have undergone delayed CLND after recurrence, 132 of whom have complete toxicity data for analysis. Median follow up was 5.1 years in the early CLND group and 4.9 years in the delayed CLND group.
Demographic and primary tumor characteristics were well matched for each dissection group with no statistically significant difference except for age. The mean age of the early CLND group was 50 years, while that of the delayed group was 54.4 years (p=0.004)(Table 1). The number of dissected basins and the anatomic distribution of those basins were also similar between the two dissection groups (Table 2). The number of nodes evaluated was slightly higher in the delayed group (20.9 vs. 18.1, p=0.055), and the number of positive nodes was significantly greater (3.3 vs. 1.5, p<0.001).
Table 1.
Demographic and Pathologic Characteristics
| Immediate (N=225) | Delayed (N=132) | ||
|---|---|---|---|
| Gender | Female | 85 (37.8) | 56 (42.4) |
| Male | 140 (62.2) | 76 (57.6) | |
| Age | Mean ± SD | 50.0 ±14.2 | 54.4 ± 12.9 |
| Median | 50.0 | 54.0 | |
| BMI | 26.8 | 27.0 | |
| Primary Site | Extremity | 91 (40.4) | 56 (42.4) |
| Trunk | 108 (48.0) | 60 (45.5) | |
| Head/Neck | 26 (11.6) | 16 (12.1) | |
| Breslow (mm) | Mean ± SD | 3.17 ± 2.12 | 3.36 ± 2.02 |
| Median | 2.50 | 2.75 | |
| Level | III | 69 (30.7) | 48 (36.4) |
| IV | 142 (63.1) | 77 (58.3) | |
| V | 14 (6.2) | 7 (5.3) | |
| Ulceration | Present | 84 (37.3) | 52 (39.4) |
| Absent | 123 (54.7) | 68 (51.5) | |
| Unknown | 18 (8.0) | 12 (9.1) | |
Table 2.
Distribution of Dissected Basins
| Immediate (N=225) | Delayed (N=132) | |||
|---|---|---|---|---|
| # of Basins | 1 | 211 (93.8) | 126 (95.4) | |
| 2 | 14 (6.2) | 6 (4.6) | ||
| Site | Axilla | 117 (52) | 64 (48) | |
| Groin | 75 (33) | 48 (36) | ||
| Neck | 18 (8) | 14 (11) | ||
| Axilla +Axilla | 6 (3) | 0 | ||
| Axilla+Groin | 1 | 3 (2) | ||
| Axilla + Neck | 2 (1) | 0 | ||
| Groin + Groin | 0 | 2 (2) | ||
| Neck + Neck | 5 (2) | 1 | ||
| Missing* | 1 | 0 | ||
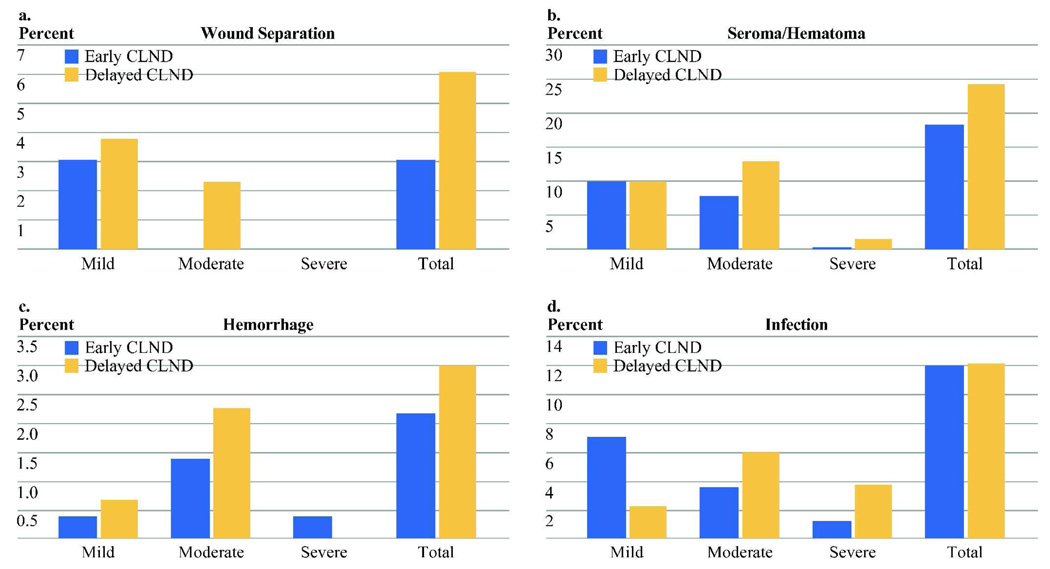
Both regional and systemic acute toxicities were recorded and graded by severity. None were statistically significantly different between groups although there was a non-significant excess in several of the acute toxicities in the delayed group (Figure 1a–d). Systemic toxicities were very low in both groups and included one urinary tract infection, one pneumonia, one cardiac complication, and one case of thrombophlebitis (each <1%).
Figure 1.
Percentage of acute toxicity in each group. There are no significant differences between early and delayed CLND. (Sample size: Early CLND n=225, Delayed CLND n=132)
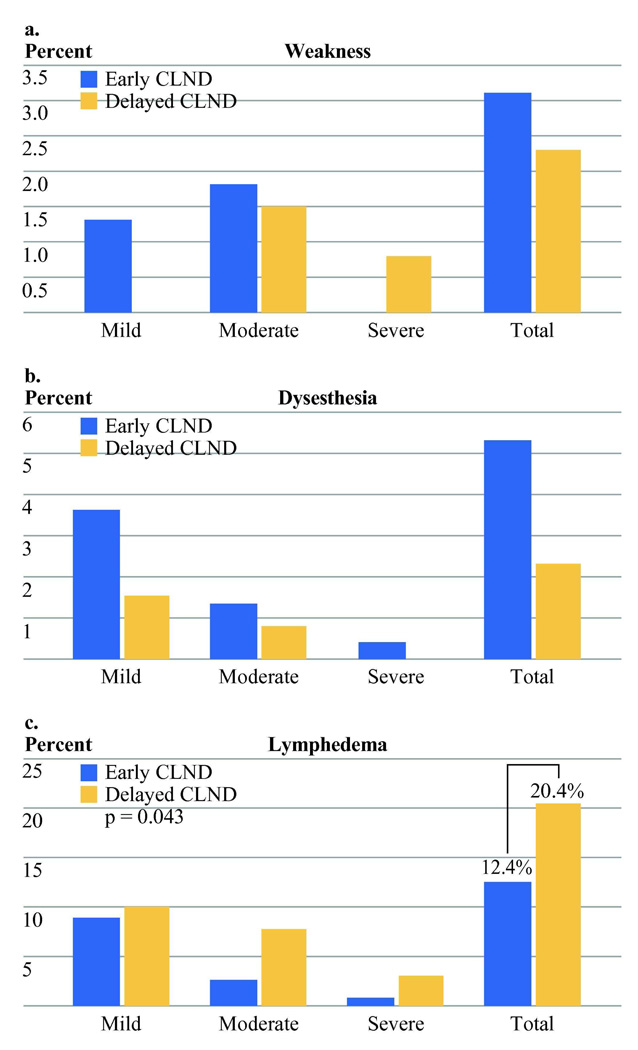
Chronic toxicities evaluated included lymphedema and nerve dysfunction. The latter consisted of motor weakness and dysesthesia (Figure 2a–c). There was a non-significant preponderance of dysesthesia in the early CLND group (5.2% vs. 2.3%).
Figure 2.
Percentage of chronic toxicity. Only lymphedema showed a significant difference between early and delayed CLND. (Sample size: Early CLND n=225, Delayed CLND n=132)
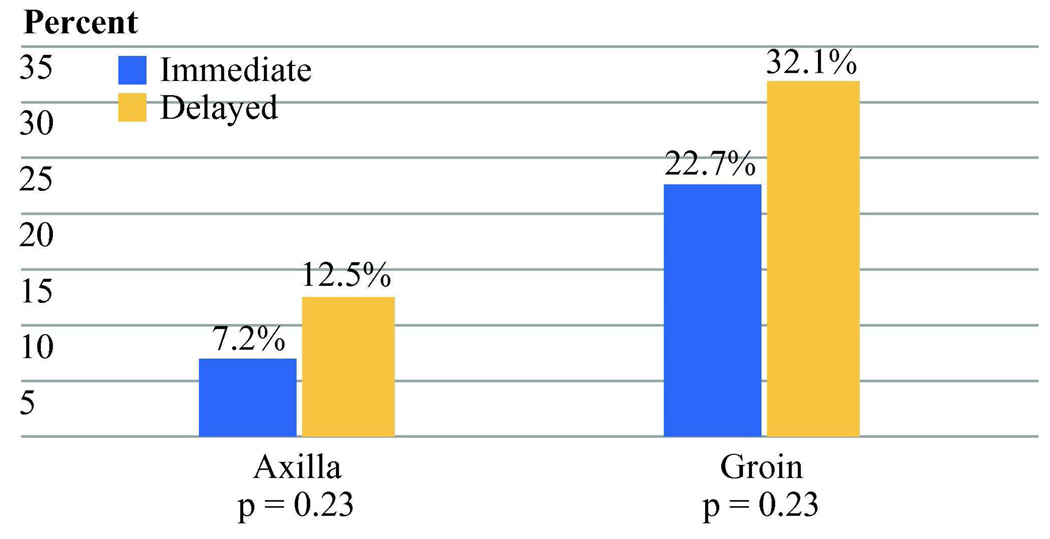
Lymphedema was significantly more common in the delayed CLND group (20.4% vs. 12.4%, Chi-square p=0.04). This difference was also significant when severity was taken into account (Wilcoxon rank sum p = 0.03). Lymphedema was strongly associated with basin site with 9.0% edema after axillary dissection and 26.6% edema after inguinal dissection (Chi-square p<0.001). There was no indication that the benefit to early CLND in lymphedema was limited to either the axillary or inguinal basin (Figure 3).
Figure 3.
Percentage of lymphedema by nodal basin site. There was no indication that the difference between early and delayed CLND was limited to axillary or inguinal sites. (Axilla: early CLND 9 of 125 (7.2%) delayed CLND 8 of 64 (12.5%). Inguinal: early CLND 17 of 75 (22.7%), delayed CLND 17 of 53 (32.1%)).
We also examined other previously reported risk factors for lymphedema including body mass index and number of lymph nodes evaluated. The mean BMI of subjects with lymphedema was slightly higher than that of subjects without (27.7 vs. 26.7, p=0.21), but this was not significant. Similarly, the risk for lymphedema among obese subjects (BMI >30), was non-significantly greater than for non-obese subjects (20% vs. 13.9%, p =0.21).
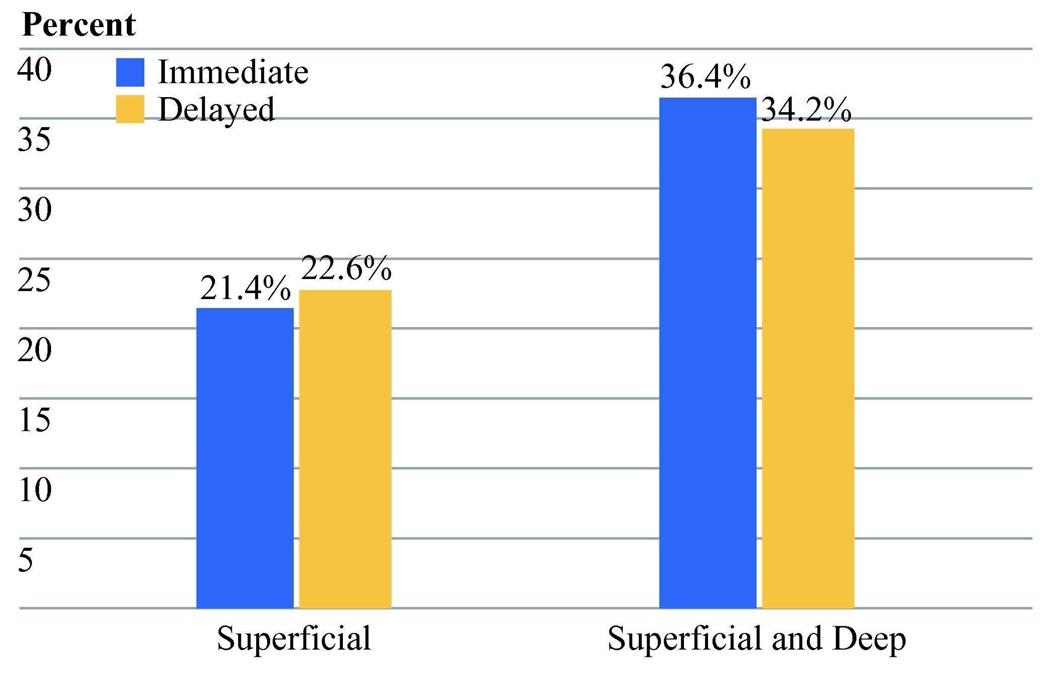
The extent of dissection for the inguinal basin was also examined. The mean number of nodes evaluated in patients with lymphedema was not different from the number in patients without lymph edema for either the axilla (mean: edema 19.6, no edema 21.2, p=0.61) or inguinal (mean: edema 14.9, no edema 14.2, p=0.36) basin. In addition, there was no apparent effect of the addition of a deep inguinal dissection to the risk of lymphedema (Figure 4). This was true for early and delayed CLND groups as well as the trial overall.
Figure 4.
Percentage of lymphedema by extent of inguinal dissection. There was no significant difference with the addition of the deep nodal basin. (Immediate CLND: Superficial =9 of 42 [21.4%], with deep = 7 of 31 [22.6%], p=0.90. Delayed CLND: Superficial = 4 of 11 [36.4%], with deep = 13 of 38 [34.2%], p=0.89.)
A multivariate analysis was conducted on potential risk factors for development of lymphedema. Stepwise logistic regression examined age at CLND, BMI, basin site, number of nodes evaluated and type of CLND (delayed vs. early.) The model identified basin site (groin vs. other) as the most powerful factor (odds ratio 3.64, 95% CI 1.93–6.86, p<0.001). Both BMI (odds ratio 1.06, 95% CI 1.00–1.13, p=0.069) and delayed CLND (odds ratio 1.74, 95% CI 0.93–3.25, p=0.083) showed trends toward an independent adverse effect on edema risk.
The relationship of hospital length of stay was also examined as it related to early (n=217) versus delayed (n=113) CLND. Length of stay varied considerably between continents. Mean length of stay in the United States, Europe, and Australia were 2.8, 10.6, and 9.5 days respectively. Mean stay for the early and delayed CLND groups were 8.3 and 9.9 days, with medians of 7.0 and 9.0 respectively. By Wilcoxon rank sum test this difference was significant (p=0.021). The length of stay for patients undergoing groin dissections was longer if the deep basin was dissected (13.9 vs. 10.2 days, p=0.009). Since this was more common for patients in the delayed group (78% vs. 42%), this is the likely source of the increased length of stay. For those undergoing only superficial dissection, the length of stay was longer in the delayed group but not significantly (9.8 vs. 12.3 days, p=0.48). Length of stay was also directly related to age, but the relationship with timing of dissection remained significant even after adjusting for age (p=0.038).
Discussion
Complete lymph node dissection is an important tool in the care of patients with melanoma and can generally be performed quite safely.4 The present report confirms the extremely low risk of systemic toxicity associated with nodal dissection. However, these procedures are associated with the risk of both acute and chronic morbidity as well as the increased costs related to inpatient hospitalization.5,6 Minimizing these risks and costs should be beneficial to both for patient quality of life and economically for the health care system.
While studies performed in the elective lymph node era did suggest a decreased toxicity associated with early CLND,7 this came at the cost of performing many dissections in patients with no nodal disease. Since approximately 80% of intermediate-thickness melanoma patients will not have nodal involvement, only a minority of patients could possibly derive benefit, while all risked the morbidity of the operation. Development of SLN biopsy has allowed selection of patients so that only those with nodal disease undergo the larger procedure.8 The toxicity associated with SLN biopsy is markedly less than that of CLND, as has been demonstrated in both melanoma and breast cancer.2,9,10 SLN biopsy, then, has already eliminated much of the potential surgical morbidity in the regional evaluation and treatment of melanoma patients.
Among patients in whom CLND is indicated based on regional metastasis, there has also been considerable interest in decreasing morbidity, particularly lymphedema. Several modifications of surgical technique have been suggested as means of reducing risk including preservation of the muscular fascia, preservation of the saphenous vein, utilization of an incision above the groin crease, and most recently minimally invasive, videoscopic technique.1,11–13 In addition, the use of post-operative prophylactic and therapeutic decompression using compression garments, positioning, and massage are considered helpful in limiting lymphedema and its effects. The effect of the extent of dissection, for example inclusion of level 3 axillary nodes and deep inguinal basin, has been controversial.14–16
The issue of early nodal dissection, prior to the development of clinically apparent nodal involvement has recently been examined in a retrospective study by Sabel and colleagues.12 They examined 212 patients who had undergone complete inguinal nodal dissection at the University of Michigan, 132 of whom had early CLND and 80 of whom had CLND for clinically palpable disease. In this study, morbidity was significantly associated with body mass index (odds ratio 1.1), and with the presence of palpable disease (odds ratio 2.2). The populations undergoing CLND in that study were well matched. Similar to our current report, there were no significant differences in demographic variables or BMI, except in age. There were also no obvious differences in primary tumor histology, although the retrospective nature of the study limited the availability of some of those data. Similarly, there is also the potential for unmeasured selection or referral biases among patients referred to a tertiary care center after melanoma recurrence.
Our report shows strikingly similar findings with multivariate odds ratios for BMI (1.06) and early vs. delayed CLND (1.74) nearly identical to the Michigan report. The prospective nature of the trial eliminates referral bias and helps limit the effects of other potential sources of bias. Indeed only age differed between the early and late groups. Since age was not associated with lymphedema, this is unlikely to have affected the difference between the groups.
Other factors which did not seem to impact lymphedema risk included the extent of dissection. Although the number of evaluated nodes was slightly higher in the delayed CLND group, the number of nodes evaluated was not related to lymphedema for either the axilla or groin. Similarly, the inclusion of the deep inguinal nodes did not increase the risk of lymphedema.
Other than the expected effect of basin site (axillary vs. inguinal) the presence of clinically evident metastases had the strongest relationship to post-operative edema. While it is not possible to determine the mechanism behind this effect, it seems reasonable to suggest two possible causes. First, there is likely dysfunction of lymphatic drainage pre-operatively in patients with macroscopic nodal involvement. Both the size of metastasis and the number of involved lymph nodes are greater in the delayed CLND patients, which would be expected to result in relatively obstructed lymphatic flow. Second, the extent of soft tissue disruption required during the surgical procedure to provide safe clearance of the basin would be expected to be increased in the setting of clinically evident disease. This disruption might obliterate potential collateral drainage pathways, which may have otherwise saved the limb from edema.
Overall this suggests that post-operative lymphatic function is not salvaged by leaving lymph nodes behind at the operative site with a more limited dissection, but preservation of pre-existing lymphatic function and minimizing collateral tissue damage is important to post-operative function.
Finally, this trial demonstrated that the length of inpatient hospitalization was increased by a small but significant amount in the delayed CLND group. We are not able to determine the exact cause of this increase. Although several of the acute toxicities were numerically increased in frequency or severity in the delayed cohort, none of these differences achieved significance. Obviously neither the patients nor the surgeons were blinded to the clinical status of the basin, so it is possible this knowledge biased length of stay. Alternatively, the tendency toward increased acute toxicity or an unquantified variable such as pain may have played a role. In any case, the decreased length of stay among early CLND patients would be expected to result in significant savings in costs related to the procedure.
Early CLND, guided by SLN biopsy is now a cornerstone of treatment for patients with intermediate thickness melanoma. Benefits of this treatment paradigm include the unequaled prognostic value of regional nodal status, the ability to select patients for adjuvant therapy or clinical trials, improved disease-free survival, and, for those with regional micrometastases at presentation, improved melanoma-specific survival. If these benefits were not sufficient, the current data also demonstrate that early CLND decreases both lymphedema and length of stay.
Acknowledgments
Supported by grant P01 CA29605 from the National Cancer Institute.
Footnotes
Synopsis
Acute and chronic morbidities of early and delayed complete lymph node dissection were compared in this prospective, multicenter clinical trial. The risk of lymphedema was significantly less when dissection was performed early, prior to clinical recurrence of melanoma.
References
- 1.Sarnaik AA, Puleo CA, Zager JS, Sondak VK. Limiting the morbidity of inguinal lymphadenectomy for metastatic melanoma. Cancer Control. 2009;16(3):240–247. doi: 10.1177/107327480901600306. [DOI] [PubMed] [Google Scholar]
- 2.Morton D, Cochran A, Thompson J, et al. Sentinel node biopsy for early-stage melanoma: accuracy and morbidity in MSLT-1, an interanational multicenter trial. Ann Surg. 2005;242(3):302–311. doi: 10.1097/01.sla.0000181092.50141.fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morton DL, Thompson JF, Cochran AJ, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355(13):1307–1317. doi: 10.1056/NEJMoa060992. [DOI] [PubMed] [Google Scholar]
- 4.Beitsch P, Balch C. Operative morbidity and risk factor assessment in melanoma patients undergoing inguinal lymph node dissection. Am J Surg. 1992;164(5):462–465. doi: 10.1016/s0002-9610(05)81181-x. discussion 465–6. [DOI] [PubMed] [Google Scholar]
- 5.de Vries M, Hoekstra HJ, Hoekstra-Weebers JE. Quality of life after axillary or groin sentinel lymph node biopsy, with or without completion lymph node dissection, in patients with cutaneous melanoma. Ann Surg Oncol. 2009;16(10):2840–2847. doi: 10.1245/s10434-009-0602-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brouns E, Donceel P, Stas M. Quality of life and disability after ilio-inguinal lymphadenectomy. Acta Chir Belg. 2008;108(6):685–690. doi: 10.1080/00015458.2008.11680316. [DOI] [PubMed] [Google Scholar]
- 7.Ingvar C, Erichsen C, Jonsson PE. Morbidity following prophylactic and therapeutic lymph node dissection for melanoma--a comparison. Tumori. 1984;70(6):529–533. doi: 10.1177/030089168407000610. [DOI] [PubMed] [Google Scholar]
- 8.Morton D, Wen D, Wong J, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127(4):392–399. doi: 10.1001/archsurg.1992.01420040034005. [DOI] [PubMed] [Google Scholar]
- 9.Olson JA, Jr., McCall LM, Beitsch P, et al. Impact of immediate versus delayed axillary node dissection on surgical outcomes in breast cancer patients with positive sentinel nodes: results from American College of Surgeons Oncology Group Trials Z0010 and Z0011. J Clin Oncol. 2008;26(21):3530–3535. doi: 10.1200/JCO.2007.15.5630. [DOI] [PubMed] [Google Scholar]
- 10.Wrightson WR, Wong SL, Edwards MJ, et al. Complications associated with sentinel lymph node biopsy for melanoma. Ann Surg Oncol. 2003;10(6):676–680. doi: 10.1245/aso.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Lawton G, Rasque H, Ariyan S. Preservation of muscle fascia to decrease lymphedema after complete axillary and ilioinguinofemoral lymphadenectomy for melanoma. J Am Coll Surg. 2002;195(3):339–351. doi: 10.1016/s1072-7515(02)01230-9. [DOI] [PubMed] [Google Scholar]
- 12.Sabel MS, Griffith KA, Arora A, et al. Inguinal node dissection for melanoma in the era of sentinel lymph node biopsy. Surgery. 2007;141(6):728–735. doi: 10.1016/j.surg.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 13.Delman KA, Kooby DA, Ogan K, et al. Feasibility of a Novel Approach to Inguinal Lymphadenectomy: Minimally Invasive Groin Dissection for Melanoma. Ann Surg Oncol. 2009 doi: 10.1245/s10434-009-0816-7. [DOI] [PubMed] [Google Scholar]
- 14.Karakousis CP. Surgical procedures and lymphedema of the upper and lower extremity. J Surg Oncol. 2006;93(2):87–91. doi: 10.1002/jso.20349. [DOI] [PubMed] [Google Scholar]
- 15.Roaten JB, Pearlman N, Gonzalez R, et al. Identifying risk factors for complications following sentinel lymph node biopsy for melanoma. Arch Surg. 2005;140(1):85–89. doi: 10.1001/archsurg.140.1.85. [DOI] [PubMed] [Google Scholar]
- 16.Strobbe LJ, Jonk A, Hart AA, et al. Positive iliac and obturator nodes in melanoma: survival and prognostic factors. Ann Surg Oncol. 1999;6(3):255–262. doi: 10.1007/s10434-999-0255-5. [DOI] [PubMed] [Google Scholar]