Summary
In human monocytes, Toll-like receptor (TLR) 2/1 activation leads to vitamin D3-dependent antimycobacterial activities, but the molecular mechanisms by which TLR2/1 stimulation induces antimicrobial activities against mycobacteria remain unclear. Here we show that TLR2/1/CD14 stimulation by mycobacterial lipoprotein LpqH can robustly activate antibacterial autophagy through vitamin D receptor signalling activation and cathelicidin induction. We found that CCAAT/enhancer-binding protein (C/EBP)-β-dependent induction of 25-hydroxycholecalciferol-1α-hydroxylase (Cyp27b1) hydroxylase was critical for LpqH-induced cathelicidin expression and autophagy. In addition, increases in intracellular calcium following AMP-activated protein kinase (AMPK) activation played a crucial role in LpqH-induced autophagy. Moreover, AMPK-dependent p38 mitogen-activated protein kinase (MAPK) activation was required for LpqH-induced Cyp27b1 expression and autophagy activation. Collectively, these data suggest that TLR2/1/CD14-Ca2+-AMPK-p38 MAPK pathways contribute to C/EBP-β-dependent expression of Cyp27b1 and cathelicidin, which played an essential role in LpqH-induced autophagy. Furthermore, these results establish a previously uncharacterized signalling pathway of antimycobacterial host defence through a functional link of TLR2/1/CD14-dependent sensing to the induction of autophagy.
Introduction
Autophagy is a major cellular mechanism for the lysosomal degradation of cytoplasmic organelles and long-lived proteins (Swanson et al., 2006; Levine and Deretic, 2007). Autophagosomes or autophagic vacuoles proceed through a stepwise maturation into degradative autolysosomes, thereby playing an important role for housekeeping or protective functions against intracellular microbes (Eskelinen and Saftig, 2009). Recent studies have identified microbial inducers of autophagy and have revealed that autophagy is an important effector of innate immunity against invasive bacteria (Deretic et al., 2006; Deuretzbacher et al., 2009) and an essential linker between innate and adaptive immunity through MHC-mediated antigen presentation (Crotzer and Blum, 2009). Mycobacterium tuberculosis (Mtb) is a facultative intracellular pathogen characterized by intracellular survival in host macrophages. Mtb persists within immature phagosomes by interfering with maturation into phagolysosomes (Deretic et al., 2006). Induction of autophagy, however, allows elimination of intracellular Mtb through enhanced interaction between mycobacterial phagosomes and autophagosomes (Deretic et al., 2006; Yuk et al., 2009).
Toll-like receptor (TLR) 2/1 stimulation leads to direct antimicrobial activities through vitamin D3-dependent cathelicidin activation in human monocytes/macrophages (Liu et al., 2006; Liu et al., 2007; Yang et al., 2009). Recently, we have provided evidence that the addition of the exogenous active vitamin D3 hormone 1,25-dihydroxyvitamin D3 (1,25D3) triggers autophagy and antimicrobial responses against Mtb in human monocytes/macrophages, both dependent on the expression of the antimicrobial peptide cathelicidin hCAP18/LL-37 (Yuk et al., 2009). In addition, monocyte stimulation via a TLR2/1 ligand leads to an induction of cathelicidin via the conversion of 25-hydroxyvitamin D3 (25D3) to 1,25D3 through Cyp27b1 hydroxylase expression in monocytes/macrophages (Liu et al., 2006). Although previous studies have reported that interleukin (IL)-15 was required in the activation of Cyp27b1 expression (Krutzik et al., 2008), the molecular mechanisms that govern Cyp27b1 expression have not been characterized. Additionally, although activation with several microbial ligands can induce autophagy (Huang et al., 2009; Kumar and Rangarajan, 2009), it is not clear whether or how mycobacterial ligands and host innate immune receptor pairs activate autophagy, including the relationship of such activation to the vitamin D-dependent antimicrobial pathway.
Major questions remain as to whether the mycobacterial antigen can activate autophagy, and how autophagy is modulated in human monocytes and macrophages. Here we clearly demonstrate that mycobacterial lipoprotein LpqH, a TLR2/1 agonist, robustly activates autophagy in human monocytes and exerts antimycobacterial activities through autophagy induction. We found that the LpqH-induced autophagy pathway was dependent on the induction of cathelicidin by the conversion of 25D3 to 1,25D3 through CYP27b1-hydroxylase expression. In addition, the current study revealed that LpqH-induced CCAAT/enhancer-binding protein (C/EBP)-β activation plays a role in the expression of Cyp27b1 hydroxylase. The LpqH-mediated autophagy pathway was activated through a transient increase in intracellular calcium following AMP-activated protein kinase (AMPK) activation via Src kinase and phospholipase C (PLC)-γ. Moreover, AMPK-dependent p38 mitogen-activated protein kinase (MAPK) activation was essentially required for LpqH-induced Cyp27b1 expression and autophagy activation. Therefore, our findings clearly demonstrate that TLR2/1 signalling regulates antibacterial autophagy pathway through functional vitamin D3 receptor activation and cathelicidin expression in human primary monocytes.
Results
LpqH activates autophagy in human primary monocytes
Recent work has demonstrated that innate receptor recognition of different pattern recognition molecules, including LPS and single-stranded RNA, are involved in autophagy and induce effector mechanisms with direct antimicrobial actions against intracellular microbes (Sanjuan et al., 2007; Xu et al., 2007; Delgado et al., 2008). Since mycobacterial antigens or components are recognized by distinct TLRs (Quesniaux et al., 2004; Jo et al., 2007), the possibility exists that mycobacterial antigen(s) participate in autophagy activation or affect autophagy regulation. It is unknown, however, whether mycobacterial lipoproteins induce autophagy in primary monocytes or macrophages.
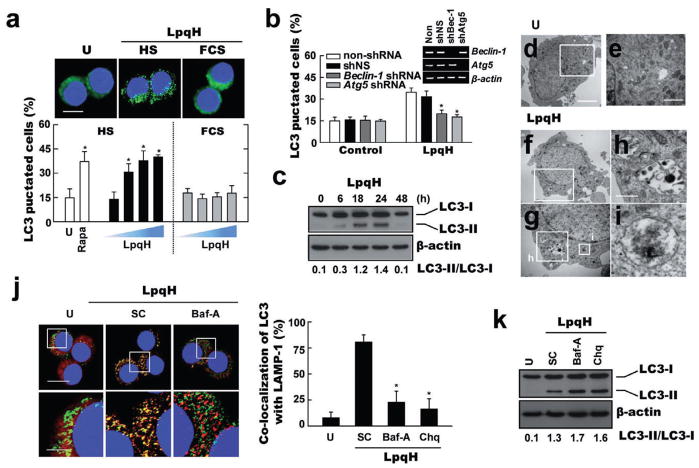
To investigate these questions, the number of microtubule-associated protein 1 light chain 3 (LC3) aggregates (Kabeya et al., 2000), which reflect the number of autophagosomes, was quantified in primary monocytes that were stimulated with LpqH under culture conditions with human sera containing physiological levels of provitamin D3 hormone (25D3, 62.6 ± 11.8 nM; Fig. S1A). As shown in Fig. 1A, LpqH stimulation caused a dose-dependent increase in endogenous LC3 aggregates in human primary monocytes cultured for 24 h with media containing human sera (Fig. 1A; quantification shown at bottom). Additionally, the percentage of LC3 vesicles induced by LpqH stimulation in human primary monocytes cultured in media containing human sera was comparable to those stimulated by 25D3 (100 nM; Fig. S1B). Based on the findings of Liu et al. (2006), these data suggest that LpqH-mediated autophagy induction and antimicrobial activation are dependent on the 25D3 levels in human sera, as this is required for the TLR-activated monocytes to convert 25D3 into sufficient levels of 1,25D leading to activation of vitamin D receptor (VDR) downstream genes. The cell viability after LpqH treatment in human primary monocytes or THP-1 cells using CCK-8 assay and Western analysis for caspase-3 activation (data not shown). The cell viability was not decreased in our system until 48 h of culture. In our previous study (Gehring et al., 2003), we also observed that there was no substantial induction of apoptosis after treatment of LpqH. Pre-treatment of cells with inhibitors of autophagy 3-methyladenine (3-MA) and Wortmannin (Seglen and Gordon, 1982) significantly decreased the formation of LC3 autophagic vesicles (Fig. S1C). Additionally, lentiviral shRNA specific to Beclin-1 (shBeclin-1) or Atg5 (shAtg5) transduction in human primary monocytes significantly inhibited the numbers of endogenous LC3 puncta expressing cells after LpqH stimulation, as compared with those transduced with non-specific control shRNA lentiviral particles (shNS) (Fig. 1B). The shRNA constructs knocked down either Beclin-1 or Atg5 mRNA expression by > 90% (Fig. 1B, inset).
Fig. 1. LpqH induces autophagy in human primary monocytes.
A. Human primary monocytes were incubated with LpqH (50, 100, 200, 500 ng ml−1; top, 100 ng ml−1) in the absence or presence of human serum (HS) for 24 h, fixed, stained with DAPI to visualize the nuclei (blue), and immunolabelled with the anti-LC3 Ab, followed by the addition of FITC-conjugated goat anti-rabbit IgG (green). Above, representative immunofluorescence images (scale bars, 5 μm); below, quantification of data; LC3 punctated cells were counted manually in DAPI-stained monocytes. *P < 0.006 (Rapa, 200 ng ml−1); *P < 0.002 (LpqH; 50 ng ml−1); *P < 0.0001 (LpqH; 100 ng ml−1); *P < 0.0004 (LpqH; 200 ng ml−1), versus control conditions. Scale bar, 10 μm.
B. Quantitative analysis of the LC3 fluorescence images. Human primary monocytes transduced with lentiviruses expressing non-specific shRNA (shNS) or specific shRNAs for Beclin-1 (shBec) or Atg5 (shAtg5) were incubated in the absence or presence of LpqH (100 ng ml−1) for 24 h; inset, RT-PCR for transfection efficiency. *P < 0.005, versus control conditions. Data shown (for A and B) represent the means ± SD of three independent samples, with each experiment including at least 250 cells scored in five random fields.
C. Immunoblot analyses with Abs to LC3 or β-actin for human monocytes. Representative gel images are shown (three experiments). The ratio of the intensities of the LC3-II and LC3-I bands is indicated below each lane.
D–I. Human THP-1 cells were treated with (for F–I) or without (for D, E) LpqH for 24 h and subjected to TEM analysis (representative image of two experiments). (E, G) Enlargement of the fields outlined in (D) and (F). Enlargement of autolysosome (H) and autophagosome (I) from (G). Scale bar, 2 μm (for D, F); 1 μm (for E, G); 200 nm (for H, I).
J. LpqH induces colocalization of autophagosomes (endogenous LC3, red) and lysosomes (LAMP-1, green) in human monocytes. Pre-treatment of bafilomycin A1 (Baf-A; 100 nM, 2 h) significantly reduces the extent of colocalization of autophagosomes and lysosomes (right). Representative immunofluorescence images of three representative experiments are shown. The bottom panels are enlargement of the corresponding fields outlined in the top panel. *P ≤ 0.01, versus control conditions. Scale bars, 20 μm (top); 5 μm (bottom). Right, the graph shows the number of profiles in the fields.
K. Immunoblot analyses with Abs to LC3 or β-actin for human monocytes. The cells were stimulated with LpqH in the absence or presence of bafilomycin A1 (Baf-A; 200 nM, 2 h) or chloroquine (Chq; 10 μM, 30 min). Representative gel images of three independent replicates are shown. The ratio of the intensities of the LC3-II and LC3-I bands is indicated below each lane.
U, untreated conditions containing 10% of human sera without LpqH. Rapa, rapamycin-treated; SC, treated with solvent control (0.1% DMSO, for E).
Moreover, Western blot analysis confirmed an elevation in LC3-II/LC3-I ratios, characteristics of autophagy induction (Kabeya et al., 2000), when human monocytes were stimulated with LpqH for 24 h (Fig. 1C). Transmission electron microscopy determined that the number of autophagosome-like vacuoles with double-membrane structures was increased in LpqH-treated cells (Fig. 1F–I). Furthermore, LC3 was found to colocalize with lysosomes (stained with LAMP-1) after LpqH stimulation (Fig. 1J). In addition, pre-treatment of cells with the vacuolar H+-ATPase inhibitor bafilomycin A1 (200 nM, 2 h) (Kabeya et al., 2000; Mizushima et al., 2001) or chloroquine (10 μM, 30 min) (Fedorko et al., 1968) significantly inhibited LpqH-induced colocalization of autophagosomes and lysosomes (Fig. 1J, P ≤ 0.01 for both treatments). To examine whether the LpqH induces a real autophagic flux, we further examined the degradation of LC3-II levels in cells pre-treated with bafilomycin A1 or chloroquine. As shown in Fig. 1K, treatment with either bafilomycin A1 or chloroquine produced an enhancement of LC3-II levels in human primary monocytes after stimulation with LpqH. These data show that LpqH induces autophagic flux and are in agreement with previous findings in cells treated with 1,25D3 (Yuk et al., 2009). Thus, LpqH actively induces both autophagy and autophagosome–lysosome fusion in human primary monocytes.
Induction of autophagy by LpqH is dependent on TLR2, 1 and CD14
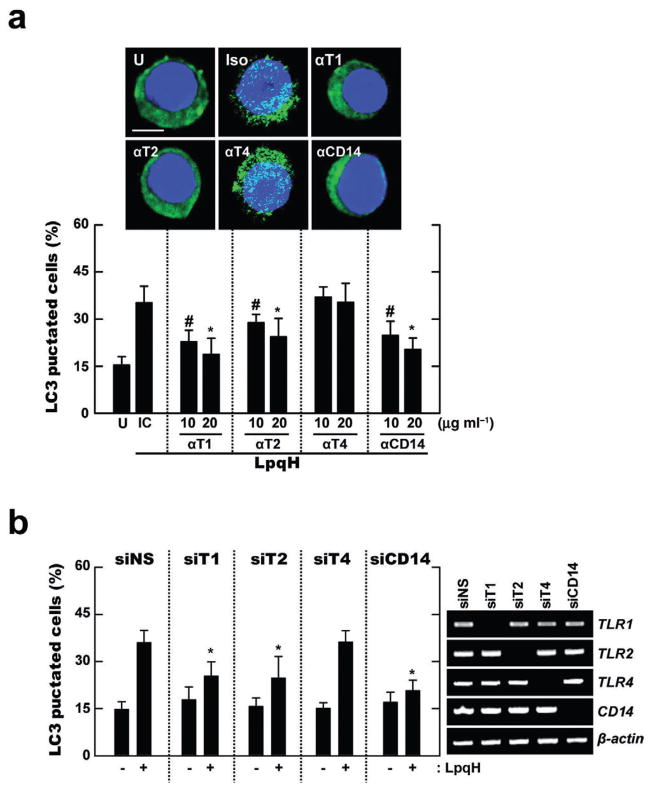
TLR2, TLR1 and CD14 all contribute to the recognition of the mycobacterial lipoprotein LpqH (Drage et al., 2009). Therefore, we first examined the roles of CD14, TLR2 and TLR1 in LpqH-mediated autophagy activation. Pre-treatment of human primary monocytes with neutralizing anti-CD14, anti-TLR2 or anti-TLR1 antibodies (Abs) significantly reduced LpqH-mediated autophagy activation (Fig. 2A). In contrast, pre-treatment of human primary monocytes with neutralizing Abs for TLR4 did not affect LC3 punctate formation after LpqH stimulation (Fig. 2A). Additionally, LpqH-mediated autophagy activation was markedly absent following siRNA-mediated suppression of CD14 (siCD14), TLR1 (siT1) and 2 (siT2) in THP-1 cells, but not transfection with siRNA for TLR4 (siTLR4) or siNS, as shown in Fig. 2B. These data indicate that LpqH recognition of TLR2, 1 and CD14 is required for autophagy activation in human monocytes.
Fig. 2. TLR2/1 and CD14, but not TLR4, are required for LpqH-induced autophagy.
A. Human primary monocytes were incubated with LpqH in the absence or presence of neutralizing Ab for hTLR1 (αT1), hTLR2 (αT2), hTLR4 (αT4), hCD14 (αCD14) or isotype control (IC; mIgG1 and mIgG2a; shown is the data for mIgG1 Ab), and subjected to confocal analysis as described in Fig. 1A. Top, a representative image with similar results is shown (three experiments); bottom, quantification of data; LC3 punctated cells were counted manually in DAPI-stained monocytes. #P < 0.02; *P < 0.001, versus control condition. Scale bars, 5 μm.
B. Human THP-1 cells transfected with non-specific siRNA (siNS) or specific siRNAs for hTLR1 (siT1), hTLR2 (siT2), hTLR4 (siT4) or hCD14 (siCD14) were incubated in the absence or presence of LpqH (100 ng ml−1) for 24 h, and subjected to confocal analysis as described in Fig. 1A. Graph is shown the percentage of LC3 punctated cells; right, RT-PCR for transfection efficiency. *P < 0.008, versus control condition. Data shown (for A and B) represent the means ± SD of three independent samples, with each experiment including at least 250 cells scored in five random fields. U, untreated and incubated.
Cathelicidin is required for LpqH-induced antibacterial autophagy in human monocytes
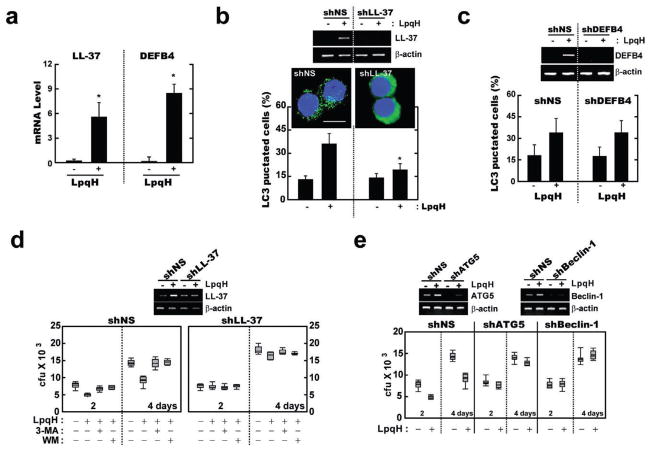
We recently showed that 1,25D3 treatment of human monocytes induces the formation of autophagosomes via human cathelicidin hCAP18/LL-37 (Yuk et al., 2009). Additionally, Liu et al. (2009) demonstrated that TLR2/1 activation triggers the induction of the defensin β4 gene (DEFB4), which is also required for TLR2/1-induced antimicrobial activity against intracellular mycobacteria (Liu et al., 2009). TLR2/1 stimulation of human primary monocytes using LpqH significantly increased the mRNA expression of DEFB4 and hCAP18/LL-37 (Fig. 3A), as consistent with previous results (Liu et al., 2009).
Fig. 3. Human cathelicidin, but not DEFB4, is required for LpqH-induced autophagy.
A. Human monocytes were incubated with LpqH (100 ng ml−1) for 12 h; results (means ± SD) are normalized to β-actin expression and are presented relative to expression in LpqH-stimulated cells. *P < 0.0001, versus control conditions.
B and C. Human primary monocytes transduced with lentiviruses expressing non-specific shRNA (shNS), specific shRNAs for LL-37 (shLL-37) or DEFB4 (shDEFB4) were incubated in the absence or presence of LpqH (100 ng ml−1) for 24 h, and subjected to confocal analysis, as described in Fig. 1A. Representative images (three independent experiments) are shown (for B); top, RT-PCR for transfection efficiency. Data shown (for B and C, bottom) represent the means ± SD of three independent samples, with each experiment including at least 250 cells scored in five random fields. *P < 0.001, versus control conditions. Scale bar, 10 μm.
D and E. Human primary monocytes were transduced with shLL-37 (for D), shBeclin-1, shAtg5 (for E) or shNS for 48 h. For (D), cells were then pre-treated with SC, 3-MA (10 μM, 2 h) or Wortmannin (WM; 100 nM, 45 min) before the addition of Mtb (moi = 1). After infection, the cells were treated with LpqH (100 ng ml−1) for 2 or 4 days, and then subjected to the cfu assay; inset, RT-PCR for transfection efficiency.
To assess the role of cathelicidin in LpqH-mediated autophagy, we transduced primary monocytes with lentiviral shRNA specific to hCAP18/LL-37 (shLL-37) or hDEFB4 (shDEFB4) and measured autophagy induction after LpqH stimulation. Silencing of hCAP18/LL-37 significantly inhibited the number of LC3-positive autophagic vesicles in human monocytes after LpqH stimulation (Fig. 3B), whereas transduction of cells with shNS or shDEFB4 did not (Fig. 3C). Consistent with these data, transduction of shLL-37 significantly blocked endogenous conversion of LC3-I to LC3-II in LpqH-treated primary monocytes, whereas shNS or shDEFB4 transduction did not (Fig. S2 and data not shown).
Based on the observation that hCAP18/LL-37 is important for 1,25D3-mediated antimycobacterial activity through autophagy (Yuk et al., 2009), we surmised that LpqH-induced cathelicidin may contribute to the efficient killing of intracellular mycobacteria through autophagy. To test this hypothesis, monocyte-derived macrophages (MDMs) were transduced with shNS or shLL-37, and then infected with Mtb at a multiplicity of infection (moi) of 5:1. After extensive washing to remove extracellular bacilli, the infected cells were further cultured with or without LpqH in human serum-containing medium. As shown in Fig. 3D, pre-treatment with 3-MA or Wortmannin significantly increased the viability of the intracellular Mtb in LpqH-treated MDMs that were transduced with shNS, whereas it did not affect the intracellular survival of bacteria in MDMs transduced with shLL-37. Moreover, Beclin-1 or Atg5 deficiency significantly inhibited the antimycobacterial activity in response to LpqH, when MDMs were transduced with shBeclin-1 or shAtg5 (Fig. 3E). It is noted that LpqH treatment reduced the viability of the intracellular bacteria by 35–42% in the control siRNA lentivirus-transduced cells as measured by cfu assay (Fig. 3D and E). Thus, hCAP18/LL-37, but not DEFB4, plays an essential role in the LpqH-mediated antibacterial autophagy induction, which is involved in the macrophage defences against Mtb.
LpqH-induced autophagy requires C/EBP-β-mediated induction of Cyp27b1 hydroxylase and consequent cathelicidin expression
TLR2/1 activation of human monocytes/macrophages upregulates the expression of selected vitamin D receptor (VDR)-related genes including Cyp27b1 hydroxylase, which is the enzyme that catalyses the conversion of inactive provitamin D3 hormone (25D3) into the active form (1,25D3). In addition, C/EBP-β activation has been reported to be required for Cyp27b1 hydroxylase gene activation in response to IFN-γ and CD14/TLR4 activation by human monocytes (Stoffels et al., 2006).
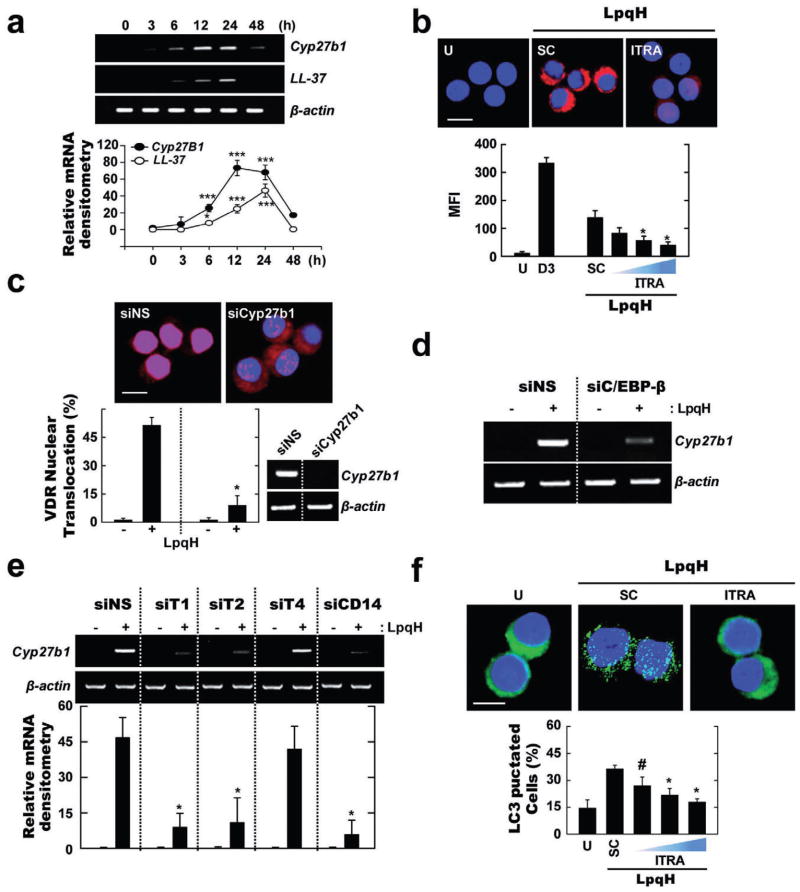
Given this background, we examined whether LpqH stimulation induces the expression of Cyp27b1 hydroxylase and cathelicidin in human monocytes. In monocytes stimulated with LpqH in culture medium containing human serum, LpqH stimulation induced a time-dependent increase in Cyp27b1 and cathelicidin mRNA expression (Fig. 4A). The expression of both Cyp27b1 hydroxylase and cathelicidin peaked at 12 h after LpqH stimulation (Fig. 4A). Additionally, inhibition of Cyp27b1 hydroxylase blocked LpqH-induced cathelicidin protein expression in monocytes (Fig. 4B). Based on our observation that the addition of 1,25D3 significantly activates nuclear translocation of VDR (data not shown), we sought to determine whether LpqH treatment causes nuclear translocation of VDR in human monocytes. We found that LpqH stimulation significantly activated nuclear translocation of VDR in a time-dependent manner, with the highest translocation of VDR occurring at 12 h (Fig. S3A). Moreover, blockade of Cyp27b1 hydroxylase by transfection of THP-1 cells with specific siRNA to Cyp27b1 (siCyp27b1) markedly inhibited LpqH-dependent VDR nuclear translocation at 12 h (Fig. 4C). However, VDR mRNA expression was not inhibited by silencing of Cyp27b1 (data not shown), consistent with the previous studies (Kemmis and Welsh, 2008).
Fig. 4. LpqH-induced autophagy is mediated through Cyp27B1 hydroxylase induction via TLR2/1/CD14-dependent functional VDR activation.
A. Semi-quantitative RT-PCR analysis for Cyp27b1 hydroxylase and LL-37. Human monocytes were incubated with LpqH for indicated times. Results (means ± SD, three independent experiments) are normalized to β-actin expression and are presented relative to expression in LpqH-stimulated cells.
B. LL-37 expression. Human monocytes were incubated in the absence or presence of Cyp27b1 antagonist itraconazole (ITRA; 50 nM, 100 nM, 200 nM), followed by LpqH treatment. After 24 h, the cells were stained with anti-LL-37-TRITC (1:400).
C–E. THP-1 cells were transfected with non-specific siRNA (siNS) or siRNAs specific for hCyp27b1 (siCyp27b1; for C), hC/EBP-β (siC/EBPβ; for D), hTLR1 (siT1), hTLR2 (siT2), hTLR4 (siT4) or hCD14 (siCD14; for E) followed by LpqH treatment for 24 h. (C) The cells were stained with anti-VDR-TRITC (1:400). Inset, RT-PCR for transfection efficiency. (D and E) Semi-quantitative RT-PCR analysis. (E, bottom) Results (means ± SD, three independent experiments) are normalized to β-actin expression and are presented relative to expression in LpqH-stimulated cells.
F. The experimental conditions were as outlined in (B). The cells were stained with anti-LC3-FITC (1:400).
Quantitative data shown represent the means ± SD of three independent samples (B, C, F), with each experiment including at least 250 cells scored in five random fields. #P ≤ 0.03; *P ≤ 0.05; ***P ≤ 0.001, versus control conditions (A, B, C, E, F). Scales bars, 10 μm (for B and C); 5 μm (for F). U, untreated and incubated; SC, treated with solvent control (0.1% DMSO, for B and F).
To assess the role of C/EBP-β in Cyp27b1 gene expression, THP-1 cells were transfected with siRNA specific to C/EBP-β (siC/EBP-β) or siNS, which was followed by LpqH stimulation. Figure 4D shows that C/EBP-β silencing significantly, but not completely, reduced the mRNA expression of Cyp27b1 hydroxylase in THP-1 cells after LpqH stimulation. As Cyp27b1 expression was partially modulated by C/EBP-β, other pathways could play a role for the LpqH-dependent Cyp27b1 hydroxylase expression. Additionally, Cyp27b1 hydroxylase expression in response to LpqH stimulation was profoundly affected by transfection of cells with siT1, siT2 or siCD14, but not siT4 (Fig. 4E), thus suggesting a role for TLR1, 2 and CD14 in LpqH-mediated Cyp27b1 expression. Furthermore, quantification of LC3 puncta formation showed that monocytes pre-treated with a Cyp27b1 antagonist followed by LpqH stimulation exhibited significantly reduced autophagosome formation as compared with those pre-treated with solvent controls (Fig. 4F). These results indicate that LpqH-induced autophagy activation requires the C/EBP-β-dependent expression of Cyp27b1-cathelicidin and the functional VDR activation via TLR1, 2 and CD14.
AMPK activation via Src kinase-PLC-γ-calcium-dependent signalling is involved in LpqH-induced autophagy activation
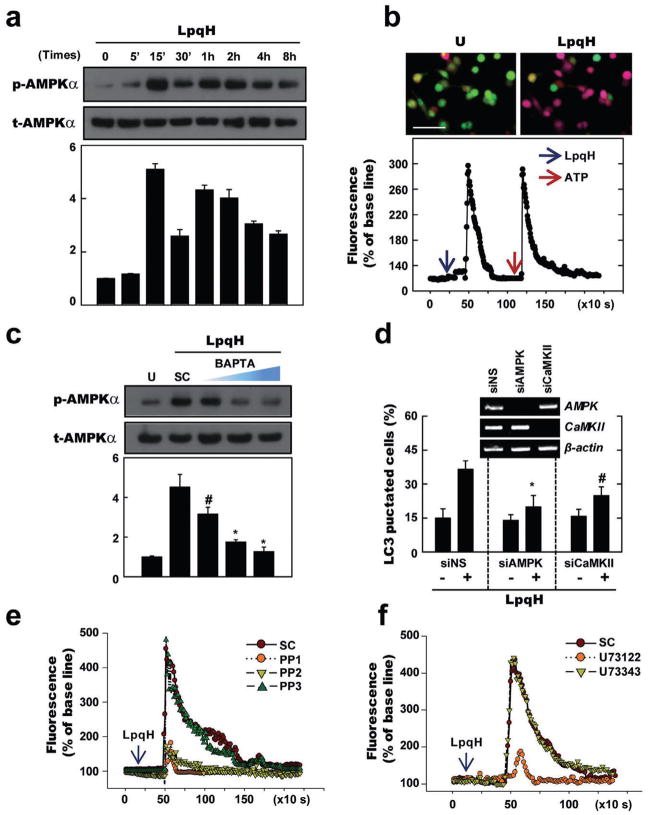
AMPK activation has been previously demonstrated to be essential for the induction of Ca2+-dependent autophagy (Høyer-Hansen et al., 2007). Therefore, we next determined whether LpqH stimulation induces AMPK phosphorylation at the Thr-172 residue of the α-subunit, which is crucial for AMPK activity in human monocytes (Hawley et al., 1996). As shown in Fig. 5A, Western blot analysis showed phosphorylation at the Thr-172 residue of the AMPKα catalytic domain after LpqH stimulation. When human monocytes were stimulated with LpqH, a rapid and marked increase in AMPKα phosphorylation was evident at 15 min post stimulation; these levels returned to baseline over an 8 h time period (Fig. 5A). In addition, LpqH stimulation resulted in a significant increase in intracellular Ca2+ release, which peaked at 3 min and returned to basal levels by the 15 min time point (Fig. 5B). We further examined whether the increase in AMPKα phosphorylation is modulated via intracellular Ca2+ release in response to LpqH stimulation. As shown in Fig. 5C, LpqH-stimulated AMPKα phosphorylation was reduced in a dose-dependent manner by pre-treatment with an intracellular calcium-specific chelator, BAPTA-AM. Furthermore, the number of endogenous LC3 puncta was significantly decreased in LpqH-treated monocytes after pre-treatment with BAPTA-AM, a Ca2+/calmodulin-dependent protein kinase kinase (CaMKK)-α/β inhibitor (STO-609), or an ATP-competitive inhibitor of AMPK (compound C; Fig. S4). SiRNA-mediated knockdown of CaMKK-β and AMPK also significantly abrogated LpqH-induced LC3 puncta formation in THP-1 cells (Fig. 5D), thus confirming the results using pharmacologic inhibitors. These data suggest that AMPK pathways are required for LpqH-induced autophagy activation in human monocytes through intracellular calcium release.
Fig. 5. LpqH-induced autophagy is mediated through AMPK activation via Src kinase-PLC-γ-Ca2+-dependent signal pathways.
A and C. Immunoblot analyses with Abs to p-AMPKα or t-AMPKα for human monocytes. Human primary monocytes were incubated with LpqH (100 ng ml−1) for indicated times (for A). Human monocytes were pre-treated with or without BAPTA-AM (5, 10, 25 μM; for C).
B. THP-1 cells were loaded with Fluo-2/AM and stimulated with LpqH, then imaged by Leica TCS SP2 confocal microscopy under a 10/0.40 CS objective lens at 5 s intervals. Top, representative images are shown (at least three independent experiments); bottom, quantification of data. Bottom data show the changes in mean fluorescence intensity from 15 cells per microscopic field over time.
D. Human THP-1 cells transfected with non-specific siRNA (siNS) or specific siRNA for hAMPK (siAMPK) or hCaMKK (siCaMKII) were incubated in the absence or presence of LpqH (100 ng ml−1) for 24 h, and subjected to confocal analysis as described in Fig. 1A. Graph is shown percentage of LC3 punctated cells; inset, RT-PCR for transfection efficiency. Data shown represent the means ± SD of three independent samples, with each experiment including at least 200 cells scored in five random fields.
E and F. Human THP-1 cells were loaded with Fluo-2/AM and stimulated with LpqH in the absence or presence of Src inhibitors PP1, PP2 or analogue PP3 (10 μM; for E) or PLC inhibitor U73122 or analogue U73343 (5 μM; for F), then analysed using live cell imaging by time-lapse microscope (Nikon Eclipse TE2000).
#P < 0.01; *P < 0.002, versus control conditions. Scales bars, 50 μm. U, untreated and incubated; SC, treated with solvent control (0.1% DMSO, for C).
In the next series of experiments, we sought to elucidate the molecular mechanisms responsible for signalling LpqH-induced calcium release and autophagy. Recent studies have shown that mouse dendritic cell stimulation with LPS induces calcium influx through activation of Src-family kinase and PLC-γ2 through CD14 (Zanoni et al., 2009). We also found that CD14, TLR2 and TLR1 were essentially involved in the LpqH-mediated intracellular calcium release, whereas TLR4 did not, in THP-1 cells by using specific siRNA transfection (Fig. S5). We then tested the roles of Src and PLC-γ in LpqH-induced calcium release and autophagy activation. Western blot analysis showed phosphorylation of c-Src at Tyr416 within 1 min of LpqH stimulation in human primary monocytes (Fig. S6A). Additionally, we found that pre-treatment of cells with the Src kinase inhibitors PP1 and PP2, but not the analogue PP3, selectively attenuated LpqH-induced intracellular calcium levels (Fig. 5E) and autophagy (Fig. S6B). Because PLC-γ is a downstream component of Src tyrosine kinase (Zanoni et al., 2009), we also explored the implications of PLC-γ activation on calcium release and autophagy activation due to LpqH stimulation. As shown in Fig. 5F, in human THP-1 cells, LpqH-mediated intracellular calcium release was attenuated by the inhibition of PLC-γ activity using the specific pharmacologic inhibitor U73122, but not the analogue U73343. Similarly, LpqH-induced autophagy was inhibited in a dose-dependent manner by pre-treatment with U73122, but not U73343 (Fig. S6C). These data link LpqH stimulation of Src-PLC-γ-calcium signalling to AMPK activation and indicate that LpqH-dependent calcium signalling is necessary for autophagy activation.
AMPK-dependent p38 MAPK activation is required for LpqH-induced autophagy activation and cathelicidin expression
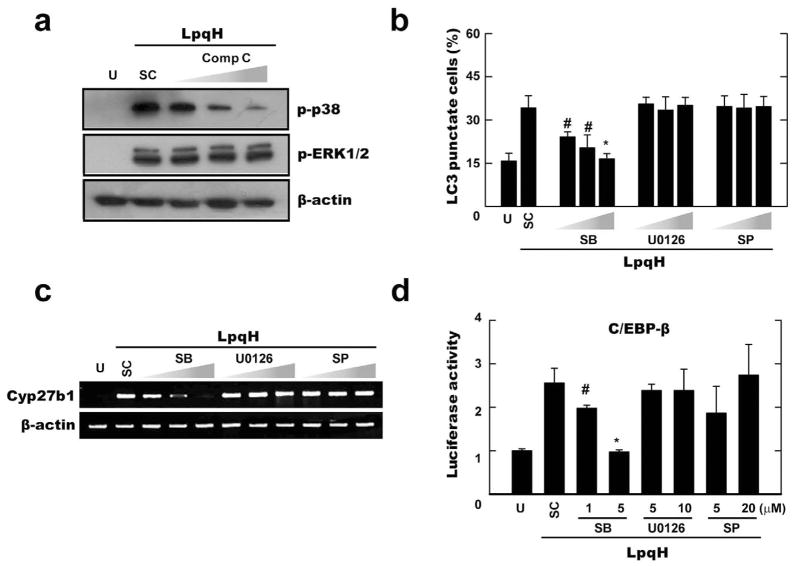
Previous studies have demonstrated that AMPK can facilitate the p38 MAPK signalling pathway in skeletal muscle cells (Lee et al., 2008); however, a key downstream target of AMPK that is necessary for autophagy activation has not been proven effective in monocytes. Our previous studies show that LpqH strongly activates ERK1/2 and p38 MAPK, but induces very little activation of JNK (Pennini et al., 2006). Therefore, we first examined p38 MAPK and ERK1/2 phosphorylation in human monocytes after LpqH stimulation. Western blot analysis confirmed the previous results (Pennini et al., 2006) that LpqH significantly activates p38 MAPK and ERK1/2 phosphorylation (Fig. S7). Activated levels of ERK1/2 and p38 MAPK phosphorylation peaked at 30 min after LpqH stimulation (Fig. S7). Notably, pre-treatment of cells with specific inhibitors for AMPK significantly inhibited LpqH-mediated phosphorylation of p38 MAPK, but not ERK1/2, in a dose-dependent manner (Fig. 6A). Moreover, LpqH-induced autophagosome formation was significantly inhibited in human monocytes by pre-treatment of specific inhibitors for p38 MAPK (SB203580), but not ERK1/2 (U0126) or c-Jun N-terminal kinase (SP600125) inhibitors (Fig. 6B).
Fig. 6. AMPK-p38 MAPK pathway plays a role for autophagy activation and C/EBP-β-dependent Cyp27b1 hydroxylase induction in response to LpqH stimulation.
A–C. Human monocytes were stimulated with LpqH (100 ng ml−1) in the absence or presence of various inhibitors (AMPK inhibitor compound C; Comp C, 5, 10, 25 μM; for A) or MAPK inhibitors [p38, SB203580 (SB), 1, 5, 10 μM; ERK, U0126, 5, 10, 20 μM; JNK, SP600125 (SP), 5, 10, 20 μM; for B and C], and then subjected to Western blot (for A), confocal analysis (for B), or semi-quantitative RT-PCR analysis (for C). (B) Data shown represent the means ± SD of three independent samples, with each experiment including at least 250 cells scored in five random fields. (A and C) Representative images are shown (at least three independent experiments).
D. THP-1 cells were transfected with a C/EBP-β luciferase reporter construct. After 36 h, cells were pre-treated with MAPK inhibitors (the same conditions as shown in C), followed by LpqH stimulation for 6 h. The luciferase activity was then measured and normalized to β-galactosidase activity.
#P < 0.01; *P < 0.0004, versus control conditions. U, untreated and incubated; SC, treated with solvent control (0.1% DMSO).
Based on these results, we next determined whether p38 MAPK activity is required for Cyp27b1 expression and C/EBP-β activation in response to LpqH stimulation. As shown in Fig. 6C and D, blockade of p38, but not ERK1/2 or JNK1/2, activities markedly inhibited LpqH-induced Cyp27b1 mRNA expression (Fig. 6C) and C/EBP-β activation (Fig. 6D) in human monocytes. Taken together, these data suggest that the AMPK-dependent p38 MAPK pathway is essentially involved in LpqH-induced autophagy activation through C/EBP-β-mediated cathelicidin expression.
Discussion
Autophagy is a cellular degradation system that contributes many different physiological processes, including the turnover of cellular components, differentiation, development and death (Levine and Kroemer, 2008; Mizushima et al., 2008). Recently, autophagy was shown to be associated with TLR signalling. Specifically, innate sensing pathways via certain TLR ligands play an important role in the initiation and regulation of autophagy activation (Delgado et al., 2008; Shi and Kehrl, 2008; Orvedahl and Levine, 2009). Importantly, a subset of TLR ligands including poly I:C, a TLR3 ligand, and single-stranded RNA, a TLR7 ligand, are robust activators of autophagy induction (Delgado et al., 2008; Shi and Kehrl, 2008). Moreover, expression of the TRIF dominant-negative form inhibited poly I:C-activated TLR3-induced autophagosome formation in murine macrophage RAW264.7 cells (Shi and Kehrl, 2008). Since mycobacterial antigens and components can act as agonists for TLRs (Quesniaux et al., 2004; Jo et al., 2007), they may be able to activate autophagy through TLR signalling in mononuclear phagocytes. However, little is known regarding the molecular mechanisms by which mycobacterial antigen(s) induce autophagy activation in human cells.
We found that LpqH stimulation robustly activates autophagy in human monocytes in cultures containing physiological levels of 25D3. Previous studies have demonstrated that physiological levels of 1,25D3 can activate autophagy in human monocytes/macrophages (Yuk et al., 2009). The present results significantly extend our previous findings on vitamin D3-induced autophagy activation (Yuk et al., 2009) to TLR-dependent autophagy by demonstrating direct activation of innate immune system via TLRs. Not only is the relationship between the TLRs and autophagy, but it provides also a strong evidence that adjoins vitamin D metabolism with TLR2/1-dependent autophagy through Cyp27b1 activation. The effects of cathelicidin on LpqH-mediated autophagy are likely mediated by Cyp27b1 hydroxylase activation (see Fig. 4F), which functionally modulates VDR signalling activation downstream (Liu et al., 2006). Previously, IL-15 was found to be required for the TLR2/1-induced upregulation of Cyp27b1 leading to bioconversion of 25D3 to 1,25D3 and subsequent induction of cathelicidin in human monocytes (Krutzik et al., 2008). Although TLR2/1 ligands activate monocytes production of IL-1β, this cytokine had no effect on Cyp27b1 expression, but instead was required for induction of DEFB4 (Liu et al., 2009). Consistent with previous findings (Liu et al., 2009), we found that LpqH stimulation markedly upregulates gene expression of DEFB4 in human monocytes. Therefore, LpqH-induced IL-15 or IL-1β would affect the indirect activation of Cyp27b1 hydroxylase and DEFB4 expression.
In recent years, great emphasis has been placed on the role of antimicrobial peptides including defensins in the host defence against pathogens (Klotman and Chang, 2006; Liu et al., 2009; Salzman et al., 2010). The current data show that cathelicidin, but not DEFB4, expression is important for LpqH-induced autophagy induction. In addition, we found that LpqH-induced cathelicidin is necessary for autophagy-dependent antimicrobial responses against mycobacteria. Although DEFB4 expression is also required for TLR2/1-mediated antimicrobial activity (Liu et al., 2009), the greater dependence on cathelicidin expression indicates that vitamin D3-activated (Yuk et al., 2009), cathelicidin-dependent autophagy is a central aspect of the antimicrobial pathway. The ability of cathelicidin to induce autophagolysomal fusion (Yuk et al., 2009) may facilitate the delivery of several antimicrobial mediators to the compartment where the bacilli reside. Recently, the adaptor molecule p62/SQSTM1 has been postulated to deliver ribosomal and ubiquitinated cytosolic proteins to autolysosomes, resulting in the antimycobacterial responses (Ponpuak et al., 2010). Together, these data strongly suggest that several distinct mechanisms contribute to TLR-induced antimicrobial pathways, separately or linked together, i.e. cathelicidin-dependent autophagy, IL-1β-dependent DEFB4 expression and p62-mediated delivery of antimycobacterial components into autolysosomes.
In the current study, manipulation of Cyp27b1 hydroxylase expression impacted LpqH-dependent autophagy induction through TLR2/1 and CD14. The present data also emphasize the role of CD14, TLR2 and TLR1 in autophagy activation as an upstream signal for intracellular calcium release and the expression of Cyp27b1 hydroxylase and cathelicidin in response to LpqH stimulation. Previously, Stoffels et al. demonstrated that Cyp27b1 hydroxylase gene expression is synergistically induced by interferon-γ and CD14/TLR4 ligation, and paralleled by 1,25D3 in human monocytes (Stoffels et al., 2006). Recent studies also show that CD14, a glycosylphosphatidylinositol-anchored receptor, is responsible for Ca2+ influxes after LPS activation (Zanoni et al., 2009). In addition, glycosylphosphatidylinositol-anchored receptors, including CD59 molecules, activate intracellular inositol-1,4,5-trisphosphate-dependent Ca2+ mobilization through the recruitment of Lyn to CD59 clusters (Suzuki et al., 2007a; Suzuki et al., 2007b). Thus, together with our previous results (Shin et al., 2008), TLR2 localization in lipid rafts appear to be required for LpqH-induced Ca2+ signalling through TLR2, TLR1 and CD14. Moreover, blockade of TLR2, TLR1 and CD14 significantly inhibited autophagy activation (see Fig. 2), thus indicating direct involvement of these PRRs in LpqH-induced autophagy.
Accumulating evidence suggests that autophagy is a coordinated biological response and that several signalling pathways can act in concert to bring about directed antibacterial autophagy. Of note, AMPK, a serine/threonine kinase, acts as a cellular energy sensor and plays a crucial role in autophagy activation through the regulation of intracellular protein degradation (Meley et al., 2006; Liang et al., 2007; Matsui et al., 2007). The current data show that AMPK activation is critical for LpqH-dependent autophagy activation through Ca2+-dependent signalling. These data partly agree with previous reports that AMPK-dependent autophagy activity is regulated through Ca2+-CaMKK signalling (Høyer-Hansen et al., 2007; Hoyer-Hansen and Jaattela, 2007). The present data along with our previous findings (Yuk et al., 2009) indicate that the Ca2+-CaMKK-AMPK pathway is involved in two key steps in the TLR2/1 ligand, vitamin D-dependent induction of autophagy. We have found that the CaMKK-AMPK pathway is required for the TLR2/1-mediated activation of Cyp27b1 hydroxylase, which, in turn, induces the cathelicidin gene expression through a functional vitamin D-receptor signalling. However, the inhibition of AMPK was not involved in the 1,25D3 induction of cathelicidin mRNA (Yuk et al., 2009), likely because 1,25D3 can directly activate the cathelicidin gene promoter which contains three VDREs (Liu et al., 2009). The Ca2+-CaMKK-AMPK pathway was required for both steps in 1,25D3-induced autophagy and colocalization between cathelicidin and LC3-autophagosomes (Yuk et al., 2009). Therefore we conclude that the Ca2+-CaMKK-AMPK signalling plays a crucial role in both the TLR2/1-mediated upregulation of CYP27b1 hydroxylase and the ability of 1,25D3 to induce authophagy via cathelicidin (see Fig. 7). Thus, the effects of AMPK pathway on the cathelicidin expression would differ depending on the stimulatory ligands.
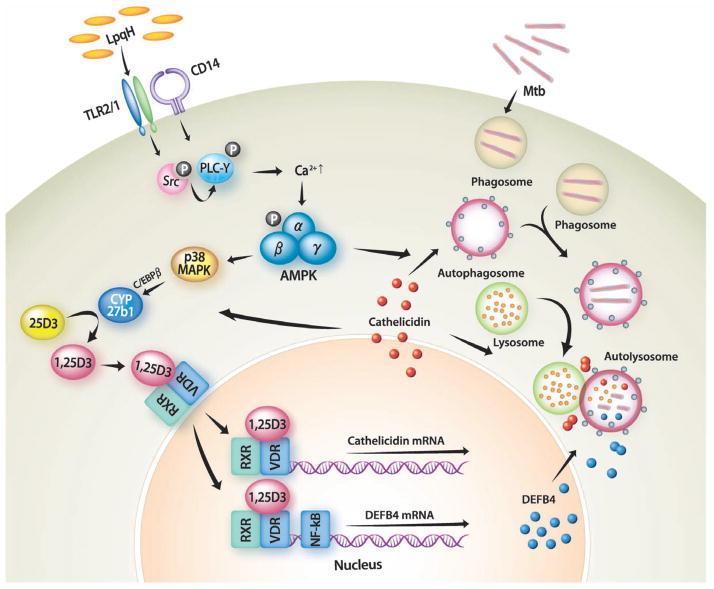
Fig. 7.
A schematic model for molecular mechanisms of LpqH-induced antibacterial autophagy against mycobacteria. Engagement of TLR2/1 and CD14 results in a rapid phosphorylation of Src kinase at Tyr416, which leads to intracellular Ca2+ influx through PLC-γ activation. Increased Ca2+ release induces a phosphorylation of AMPKα at Thr172, leading to a downstream activation of p38 MAPK. Activation of intracellular signalling pathways can contribute to upregulate Cyp27b1 hydroxylase induction, which can convert the vitamin D prohormone 25D3 into 1,25D3. The ligation of VDR leads to its increased translocation into nucleus and hormone-dependent transcriptional activation (Liu et al., 2009), resulting in an upregulation of antimicrobial peptide cathelicidin. As in case of 1,25D3-dependent autophagy (Yuk et al., 2009), cathelicidin plays an essential role for antibacterial autophagy, resulting in a fusion of autophagosomes with lysosomes, and finally eliminates intracellular mycobacteria.
The current data are unique in that we demonstrate a role for AMPK/p38 as a powerful activator of autophagy and antimycobacterial effects on monocytes/macrophages. These results warrant further investigation of AMPK/p38 as a promising alternative for antimycobacterial therapy. In addition, the current data demonstrate a role for the p38 MAPK pathway in C/EBP-β activation and Cyp27b1 gene expression (see Fig. 6C and D). These data are partially in agreement with previous results showing that the p38 MAPK pathway is involved in the phosphorylation of C/EBP-β (Stoffels et al., 2006). It seems to be controversial to our previous findings (Yuk et al., 2009) that C/EBP-β expression and p38 MAPK activation was inhibited in cathelicidin-knocked-down cells. Our previous findings (Yuk et al., 2009) can be explained that the basal constitutive or early induced cathelicidin is required for the activation of C/EBP-β expression and p38 MAPK signalling. Therefore these data indicate that C/EBP-β is required for TLR-induced Cyp27b1 expression and that cathelicidin is also required for C/EBP-β activation and phosphorylation of p38. These signalling pathways seem to connect together and make a positive circuit in the amplification of TLR2/1-mediated antimicrobial activities through upregulation of cathelicidin expression. Our proposed model for the mechanism by which Ca2+/CaMKK/AMPK/p38 pathways mediate the stimulatory effects of LpqH on autophagy activation through C/EBP-β-dependent activation of Cyp27b1 expression is summarized in Fig. 7.
However, because the LpqH is only one of a number of molecules that mediate the immune modulation by Mtb, in the context of the whole bacillus, the autophagy-inducing effect of LpqH could be masked by the whole set of mycobacterial components that might inhibit autophagy and antimicrobial responses. Future studies are needed to clarify the essential effect of LpqH using the bacteria lacking LpqH expression as well as the in vivo evaluations. Collectively, our data reveal a previously uncharacterized interaction between autophagy and TLR2/1-induced antimicrobial responses against mycobacteria.
Experimental procedures
Cell preparation
This study was approved by the Bioethics Committee of Chungnam University Hospital, which oversees studies that use samples from human subjects. Human primary monocytes, MDMs, THP-1 cells were prepared as described previously (Song et al., 2003; Yuk et al., 2009). For monocyte isolation, peripheral blood mononuclear cells from healthy donors were isolated from buffy coats obtained by density sedimentation over Histopaque-1077 (Sigma, St Louis, MO, USA). After cells were allowed to adhere to culture flasks (Nunc, NY, Rochester, USA) for 1 h at 37°C, the non-adherent cells were removed by vigorous washing with PBS. The recovered cells were > 95% monocytes, determined by flow cytometric analysis. Human MDMs were prepared by culturing peripheral blood monocytes for 5 days in the presence of 4 ng ml−1 human macrophage colony-stimulating factor (Sigma). The human monocytic THP-1 cells were purchased (American Type Culture Collection TIB-202) and maintained in RPMI 1640 (Invitrogen Life Technologies, Carlsbad, CA, USA) with 10% of vitamin D-sufficient human sera. The cells were treated with 5 nM phorbol myristate acetate (Sigma) for 48 h to induce differentiation into macrophage-like cells and then washed three times with PBS.
Preparation of lipoprotein
The LpqH lipoprotein was prepared from Mtb H37Ra, as previously described (Drage et al., 2009). Preparations of the LpqH lipoprotein used in experiments were tested for LPS contamination by a Limulus amebocyte lysate assay (BioWhittaker) and contained less than 1.6 pg μg−1 at the concentrations of the LpqH used in experiments.
Reagents, DNA and Abs
3-Methyladenine, bafilomycin A1 and DAPI were from Sigma. Specific inhibitors of MEK1 (PD98059), c-Jun N-terminal kinases (SP600125), p38 MAPK (SB203580), ROS (NAC), NADPH oxidase (DPI) and xanthine oxidease (allopurinol), PP1, PP2, the inactive PP3, U73122 and inactive U73343 were purchased from Calbiochem (San Diego, CA, USA). DMSO (Sigma) was added to the cultures at 0.1% (v/v) as a solvent control.
The hCyp27b1 targeting siRNA (L-009457; a pool of four target-specific 22 nt siRNAs), hC/EBPβ targeting siRNA (L-006423; a pool of four target-specific 24 nt siRNAs) or control non-targeting siRNA (D-001810) were purchased from Dharmacon (Lafayette, CO, USA). SiRNAs specific to hCD14 (sc-29248; a pool of four target-specific 20–25 nt siRNAs), hTLR1 (sc-40254; a pool of three target-specific 20–25 nt siRNAs), hAMPK (sc-29673; a pool of three target-specific 19–25 nt siRNAs) and hCaMKKIIβ (sc-38951; a pool of three target-specific 19–25 nt siRNAs) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The psiRNA-h7SKGFPzeo plasmids for hTLR2 shRNA were purchased from InvivoGen. The cells were transfected using LipofectAMINE 2000 as indicated by the manufacturer (Invitrogen). Predesigned siRNA oligos were resuspended and stored as recommended by the manufacturer. The human pLKO.1 lentiviral hCAP18/LL-37 shRNA (RHS4533; GenBank Accession NM_004345), Beclin-1 shRNA (RHS4533; GenBank Accession NM_003766) and Atg5 shRNA (RHS4531; GenBank Accession NM_004849) constructs were from Open Biosystems; the non-target pLKO.1-scrambled shRNA (designated shNS) was from Sigma.
Anti-LC3 Ab used for Western blotting was from Novus Biologicals; anti-LC3 Ab used for immunofluorescence analysis was from MBL International; anti-rabbit IgG-FITC, anti-rabbit IgG-TRITC, anti-mouse IgG-PE, anti-mouse IgG-Cy2 Abs were from Jackson Immunoresearch (West Grove, PA, USA); anti-β-actin was from Santa Cruz Biotechnology; anti-VDR was from Abcam (Cambridge, MA, USA); Abs against ERK1/2, phospho-(Thr202/Tyr204)-ERK1/2, p38, phospho-(Thr180/Tyr182)-p38, were from Cell Signaling (Danvers, MA, USA). The Abs to β-actin, phospho-c-Src (N-16) and Lamp-1 (H5G11) were purchased from Santa Cruz. Specific mAbs against hTLR2 (2392, IgG2a) and hTLR4 (HTA 125, IgG2a) were obtained from eBio-science (San Diego, CA, USA); hCD14 (IgG1) from R&D systems (Minneapolis, MN, USA); hTLR1 (GD2.F4, IgG1) from Biolegend (San Diego, CA, USA). Mouse IgG1 isotype control Abs (16-4714) and IgG2a isotype control Abs (16-4724) were obtained from eBioscience. Materials not listed were purchased from Sigma. All primers used in this study are shown in Table S1.
Mycobacteria culture, infection and cfu assay
Mycobacterium tuberculosis H37Rv (kindly provided by Dr Richard L. Friedman, University of Arizona, Tucson, AZ) were cultured and the single cell suspensions of mycobacteria were obtained as described previously (Zhang et al., 1998; Yang et al., 2009). Human MDMs were infected with Mtb at a moi of 1. The cells were incubated for 4 h with Mtb and then washed with PBS three times, and the majority of extracellular bacteria (> 99%) were removed as determined by auramine-rhodamine stain (Merck, Darmstadt, Germany). After washing, the fresh media were added and the incubation continued for the additional time. The efficiency of infection was determined by auramine rhodamine stain and was in a range of 30–35%, with an average of 1.4 bacteria per infected cell. More than 70% of the infected cells contained one intracellular bacterium. The intracellular bacteria were harvested and assayed for viability based on the numbers of cfu, as previously described (Liu et al., 2007; Yuk et al., 2009). In brief, Mtb-infected cells were treated with LpqH for 2 or 4 days in triplicate wells, pelleted by centrifugation, washed twice with 1× PBS, and then lysed by resuspension in an autoclave distilled water and vigorous pipetting. The lysate was centrifuged to pellet the harvested bacteria and then the pellets were resuspended in 7H9 broth. Bacterial viability (relative cfu) was calculated as the bacterial count from the Day 2 or 4 culture divided by the Day 0 bacterial count, multiplied by 20. Inoculated 7H10 plates were incubated at 37°C and then the number of cfu in each plate was counted.
Autophagy evaluation
Autophagy was evaluated in cells by immunostaining of LC3, TEM or WB analysis. Fluorescence microscopy and TEM analysis were performed as described previously (Yuk et al., 2009). For immunostaining, human monocytes and THP-1 cells were fixed with 4% paraformaldehyde in PBS for 10 min, and permeabilized with 0.01% Triton X-100 in PBS for 5 min. The cultures were then stained with LC3-FITC or -TRITC or Lamp-1-Cy2 Abs. Cells were imaged with a Zeiss confocal microscope (LSM510 META; Zeiss). In some experiments, human monocytes were loaded with DAPI (Sigma) for visualization of nuclei. Quantification of autophagy was performed based on the percentage of LC3-positive autophagic vacuoles per cell or cells with LC3 punctate dots. For positive control of autophagy activation, cells were incubated in complete medium that contained 500 nM rapamycin (Sigma) for 12 h.
For TEM analysis, THP-1 cells were fixed, and embedded in straight resin, and then cured at 80°C for 24 h. Ultrathin sections (70–80 nm) were obtained using an ultramicrotome (RMC MT6000-XL). Sections were stained with uranyl acetate and lead citrate and examined under a Tecnai G2 Spirit Twin transmission electron microscope (FEI Company, USA) and a JEM ARM 1300S high-voltage electron microscope (JEOL, Japan). Autophagosome formation was determined by observation of typical double-membrane vesicles. In TEM analysis, autophagy was quantified by determining the number of autophagic vacuoles per cross-sectioned cell. In all experiments, a minimum of 150 cells per sample were counted and duplicate or triplicate samples were counted per experimental condition. For TEM experiments, data obtained from a minimum of 50 independent sectioned cells were used.
Autophagy was also evaluated using Western analysis by examination of the cleaved lapidated forms (LC3-II) of the autophagy marker LC3, which was detected by anti-LC3 Ab (1:2000, Novus Biologicals). The LC3-I to LC3-II ratio was calculated by densitometric analysis.
Fluorescence microscopy
For immunostaining of VDR translocation or LL-37 expression, human monocytes and THP-1 cells were fixed with 4% paraformaldehyde in PBS for 10 min, and permeabilized with 0.25% Triton X-100 in PBS for 5 min. The cultures were then stained with VDR-TRITC or hCAP18/LL-37-TRITC Abs. Cells were imaged with a Zeiss confocal microscope (LSM510 META; Zeiss). Quantification of VDR translocation was performed based on the percentage of cells with VDR translocation. The fluorescence intensity of each field was measured by the ImageJ analysis software or Adobe Photoshop CS4™ software. In all experiments, a minimum of 150 cells per sample were counted and duplicate or triplicate samples were counted per experimental condition.
Western blot analysis
Western blot analysis was performed as described previously (Yuk et al., 2009). In brief, the equal amounts (20 mg) of protein/cell lysate in sample buffer (62.5 mmol l−1 Tris-HCl, pH 6.8, 10% glycerol, 1% SDS, 1% β-mercaptoethanol, 0.1% bromophenol blue) were loaded on a 10–15% SDS-polyacrylamide gel. Proteins were blotted on a PVDF membrane (Bio-Rad; 0.45 mm pore size). Non-specific binding was blocked in 5% blocking buffer (5% non-fat dry milk, 150 mmol l−1 Tris-HCl, pH 7.4, 50 mmol l−1 NaCl, 0.05% Tween 20) for 1 h, and membranes were incubated overnight at 4°C with indicated Abs at a 1:1000 dilution to detect the presence of MAPKs, Src, AMPK, hCAP/LL-37, LC3 or β-actin. The membranes were then incubated with horseradish peroxidase-coupled secondary Abs (1:5000; Santacruz). The blots were developed with enhanced chemiluminescence reagents (PerkinElmer Life Sciences, Boston, MA).
RNA Extraction, real-time and semi-quantitative RT-PCR
RNA extraction, real-time quantitative PCR and semi-quantitative RT-PCR were performed as described previously (Lee et al., 2009). RNA was isolated using an RNeasy Mini Kit (Qiagen, Hilden) and treated with RNase-Free DNase as per the manufacturer’s instructions. Total RNA (2 μg each) was used to generate first-strand cDNA in reverse-transcription reactions (Invitrogen, Carlsbad, CA). PCR amplifications were conducted using the PCR kit (Qiagen, Valencia, CA, USA). The reaction conditions used for quantitative real-time RT-PCR were as described previously (Lee et al., 2009). Each reaction was performed in triplicate, and the optical data were analysed using the default and variable parameters available in the iCycler iQ™ Optical System (ver. 3.0a; Bio-Rad). The samples were normalized to the reference reporter β-actin.
Transfections and reporter gene assay
Transfection of siRNAs into the indicated cells were performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. The reporter gene assay was performed as previously described (Yuk et al., 2009). The luciferase reporter vector controlled by a synthetic promoter containing direct repeats of the transcription recognition sequences for the C/EBP was from Stratagene. At 36 h following transfection, cell extracts were prepared by adding 100 μl of 1× Passive Reporter Lysis Buffer (Promega, Madison, WI). Luciferase activity was measured using the Luciferase Assay System (Promega), according to the manufacturer’s protocol. Each transfection was performed in triplicate and three independent experiments were conducted.
Lentiviral shRNA generation and transduction to primary cells
For silencing of hCAP18/LL-37, hBeclin-1 or hAtg5 in primary cells, lentiviral shRNAs were produced, as described previously (Yuk et al., 2009). Briefly, lentiviruses were produced by transient transfection using packaging plasmids (pMDLg/pRRE, pRSV-Rev, pMD2.VSV-G, purchased from Addgene) with Lipofectamine 2000-mediated transient transfection into HEK293T cells. Viral containing media were collected 72 h post transfection and filtered. Supernatant was used to infect cells in presence of 8 μg ml−1 Polybrene. For lentivirus infection, human monocytes were infected with lentiviral vectors at a multiplicity of infection of 10 in a presence of 8 μg ml−1 Polybrene. After 4–6 h, the medium was replaced with fresh medium, and the cells were incubated for 2 or 3 days and then analysed for gene specific knockdown.
Intracellular Ca2+ measurements
THP-1 cells grown on coverslips were loaded with the Ca2+ indicator, fluo-4/AM (10 μM, 30 min for confocal measurements), or fluo-2 AM (10 μM, 30 min for kinetic measurements) in HBSS buffer according to the manufacturer’s instructions (Molecular Probes). The cells were washed twice subsequently with HBSS buffer and then followed by treatment with LpqH for indicated times. For confocal measurements, images were obtained with LSM510 confocal microscope (Zeiss). Fluo-4/AM was excited with the 488 nm line of an argon laser, and its fluorescence emission was recorded between 500 and 550 nm. For Kinetic measurements, images of cells were taken both before and after LpqH stimulation, at every 5 s for 20 min at 37°C. Images were obtained with Leica SP-2 AOBS confocal microscope (Leica-Microsystems) or Nikon Eclipse microscope (Nikon). Fluo-2/AM was excited with the 380 nm (calcium free) and 340 nm (calcium complex), and its fluorescence emission was recorded between 510 nm.
Statistical analysis
All data were analysed using Student’s t-test with a Bonferroni adjustment or ANOVA for multiple comparisons and are presented as the mean ± SD. Differences were considered significant at P < 0.05.
Acknowledgments
This work was supported by the Korea Science & Engineering Foundation through the Infection Signaling Network Research Center (R13-2007-020-01000-0) at Chungnam National University and by NIH grants AI073539 and AI047868. We are grateful to M. Fabri (University of California, Los Angeles, CA) for helpful discussion; K.K. Kim for helpful discussion and reagents; J.B. Park (Chungnam National University, Daejeon, Korea) for technical support; Z. Lee (Korea Basic Science Institute, Daejeon, Korea) for contribution of tools.
Footnotes
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Crotzer VL, Blum JS. Autophagy and its role in MHC-mediated antigen presentation. J Immunol. 2009;182:3335–3341. doi: 10.4049/jimmunol.0803458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MA, Elmaoued RA, Davis AS, Kyei G, Deretic V. Toll-like receptors control autophagy. EMBO J. 2008;27:1110–1121. doi: 10.1038/emboj.2008.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V, Singh S, Master S, Harris J, Roberts E, Kyei G, et al. Mycobacterium tuberculosis inhibition of phagolysosome biogenesis and autophagy as a host defence mechanism. Cell Microbiol. 2006;8:719–727. doi: 10.1111/j.1462-5822.2006.00705.x. [DOI] [PubMed] [Google Scholar]
- Deuretzbacher A, Czymmeck N, Reimer R, Trulzsch K, Gaus K, Hohenberg H, et al. Beta(1) integrin-dependent engulfment of Yersinia enterocolitica by macrophages is coupled to the activation of autophagy and suppressed by type III protein secretion. J Immunol. 2009;183:5847–5860. doi: 10.4049/jimmunol.0804242. [DOI] [PubMed] [Google Scholar]
- Drage MG, Pecora ND, Hise AG, Febbraio M, Silverstein RL, Golenbock DT, et al. TLR2 and its co-receptors determine responses of macrophages and dendritic cells to lipoproteins of Mycobacterium tuberculosis. Cell Immunol. 2009;258:29–37. doi: 10.1016/j.cellimm.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskelinen EL, Saftig P. Autophagy: a lysosomal degradation pathway with a central role in health and disease. Biochim Biophys Acta. 2009;1793:664–673. doi: 10.1016/j.bbamcr.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Fedorko ME, Hirsch JG, Cohn ZA. Autophagic vacuoles produced in vitro. I. Studies on cultured macrophages exposed to chloroquine. J Cell Biol. 1968;38:377–391. doi: 10.1083/jcb.38.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring AJ, Rojas RE, Canaday DH, Lakey DL, Harding CV, Boom WH. The Mycobacterium tuberculosis 19-kilodalton lipoprotein inhibits gamma interferon-regulated HLA-DR and Fc gamma R1 on human macrophages through Toll-like receptor 2. Infect Immun. 2003;71:4487–4497. doi: 10.1128/IAI.71.8.4487-4497.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- Hoyer-Hansen M, Jaattela M. AMP-activated protein kinase: a universal regulator of autophagy? Autophagy. 2007;3:381–383. doi: 10.4161/auto.4240. [DOI] [PubMed] [Google Scholar]
- Høyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell. 2007;25:193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Huang J, Canadien V, Lam GY, Steinberg BE, Dinauer MC, Magalhaes MA, et al. Activation of antibacterial autophagy by NADPH oxidases. Proc Natl Acad Sci USA. 2009;106:6226–6231. doi: 10.1073/pnas.0811045106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo EK, Yang CS, Choi CH, Harding CV. Intracellular signalling cascades regulating innate immune responses to mycobacteria: branching out from Toll-like receptors. Cell Microbiol. 2007;9:1087–1098. doi: 10.1111/j.1462-5822.2007.00914.x. [DOI] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmis CM, Welsh J. Mammary epithelial cell transformation is associated with deregulation of the vitamin D pathway. J Cell Biochem. 2008;105:980–988. doi: 10.1002/jcb.21896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotman ME, Chang TL. Defensins in innate antiviral immunity. Nat Rev Immunol. 2006;6:447–456. doi: 10.1038/nri1860. [DOI] [PubMed] [Google Scholar]
- Krutzik SR, Hewison M, Liu PT, Robles JA, Stenger S, Adams JS, Modlin RL. IL-15 links TLR2/1-induced macrophage differentiation to the vitamin D-dependent antimicrobial pathway. J Immunol. 2008;181:7115–7120. doi: 10.4049/jimmunol.181.10.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SH, Rangarajan A. Simian virus 40 small T antigen activates AMPK and triggers autophagy to protect cancer cells from nutrient deprivation. J Virol. 2009;83:8565–8574. doi: 10.1128/JVI.00603-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YM, Lee JO, Jung JH, Kim JH, Park SH, Park JM, et al. Retinoic acid leads to cytoskeletal rearrangement through AMPK-Rac1 and stimulates glucose uptake through AMPK-p38 MAPK in skeletal muscle cells. J Biol Chem. 2008;283:33969–33974. doi: 10.1074/jbc.M804469200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HM, Yuk JM, Shin DM, Yang CS, Kim KK, Choi DK, et al. Apurinic/apyrimidinic endonuclease 1 is a key modulator of keratinocyte inflammatory responses. J Immunol. 2009;183:6839–6848. doi: 10.4049/jimmunol.0901856. [DOI] [PubMed] [Google Scholar]
- Levine B, Deretic V. Unveiling the roles of autophagy in innate and adaptive immunity. Nat Rev Immunol. 2007;7:767–777. doi: 10.1038/nri2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Shao SH, Xu ZX, Hennessy B, Ding Z, Larrea M, et al. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol. 2007;9:218–224. doi: 10.1038/ncb1537. [DOI] [PubMed] [Google Scholar]
- Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179:2060–2063. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- Liu PT, Schenk M, Walker VP, Dempsey PW, Kanchanapoomi M, Wheelwright M, et al. Convergence of IL-1beta and VDR activation pathways in human TLR2/1-induced antimicrobial responses. PLoS ONE. 2009;4:e5810. doi: 10.1371/journal.pone.0005810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- Meley D, Bauvy C, Houben-Weerts JH, Dubbelhuis PF, Helmond MT, Codogno P, Meijer AJ. AMP-activated protein kinase and the regulation of autophagic proteolysis. J Biol Chem. 2006;281:34870–34879. doi: 10.1074/jbc.M605488200. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Yamamoto A, Hatano M, Kobayashi Y, Kabeya Y, Suzuki K, et al. Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J Cell Biol. 2001;152:657–668. doi: 10.1083/jcb.152.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvedahl A, Levine B. Autophagy in mammalian antiviral immunity. Curr Top Microbiol Immunol. 2009;335:267–285. doi: 10.1007/978-3-642-00302-8_13. [DOI] [PubMed] [Google Scholar]
- Pennini ME, Pai RK, Schultz DC, Boom WH, Harding CV. Mycobacterium tuberculosis 19-kDa lipoprotein inhibits IFN-gamma-induced chromatin remodeling of MHC2TA by TLR2 and MAPK signaling. J Immunol. 2006;176:4323–4330. doi: 10.4049/jimmunol.176.7.4323. [DOI] [PubMed] [Google Scholar]
- Ponpuak M, Davis AS, Roberts EA, Delgado MA, Dinkins C, Zhao Z, et al. Delivery of cytosolic components by autophagic adaptor protein p62 endows autophagosomes with unique antimicrobial properties. Immunity. 2010;32:329–341. doi: 10.1016/j.immuni.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesniaux V, Fremond C, Jacobs M, Parida S, Nicolle D, Yeremeev V, et al. Toll-like receptor pathways in the immune responses to mycobacteria. Microbes Infect. 2004;6:946–959. doi: 10.1016/j.micinf.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Salzman NH, Hung K, Haribhai D, Chu H, Karlsson-Sjoberg J, Amir E, et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol. 2010;11:76–83. doi: 10.1038/ni.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjuan MA, Dillon CP, Tait SW, Moshiach S, Dorsey F, Connell S, et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–1257. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- Seglen PO, Gordon PB. 3-Methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc Natl Acad Sci USA. 1982;79:1889–1892. doi: 10.1073/pnas.79.6.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi CS, Kehrl JH. MyD88 and Trif target Beclin 1 to trigger autophagy in macrophages. J Biol Chem. 2008;283:33175–33182. doi: 10.1074/jbc.M804478200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DM, Yang CS, Lee JY, Lee SJ, Choi HH, Lee HM, et al. Mycobacterium tuberculosis lipoprotein-induced association of TLR2 with protein kinase C zeta in lipid rafts contributes to reactive oxygen species-dependent inflammatory signalling in macrophages. Cell Microbiol. 2008;10:1893–1905. doi: 10.1111/j.1462-5822.2008.01179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song CH, Lee JS, Lee SH, Lim K, Kim HJ, Park JK, et al. Role of mitogen-activated protein kinase pathways in the production of tumor necrosis factor-alpha, interleukin-10, and monocyte chemotactic protein-1 by Mycobacterium tuberculosis H37Rv-infected human monocytes. J Clin Immunol. 2003;23:194–201. doi: 10.1023/a:1023309928879. [DOI] [PubMed] [Google Scholar]
- Stoffels K, Overbergh L, Giulietti A, Verlinden L, Bouillon R, Mathieu C. Immune regulation of 25-hydroxyvitamin-D3-1alpha-hydroxylase in human monocytes. J Bone Miner Res. 2006;21:37–47. doi: 10.1359/JBMR.050908. [DOI] [PubMed] [Google Scholar]
- Suzuki KG, Fujiwara TK, Edidin M, Kusumi A. Dynamic recruitment of phospholipase C gamma at transiently immobilized GPI-anchored receptor clusters induces IP3-Ca2+ signaling: single-molecule tracking study 2. J Cell Biol. 2007a;177:731–742. doi: 10.1083/jcb.200609175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki KG, Fujiwara TK, Sanematsu F, Iino R, Edidin M, Kusumi A. GPI-anchored receptor clusters transiently recruit Lyn and G alpha for temporary cluster immobilization and Lyn activation: single-molecule tracking study 1. J Cell Biol. 2007b;177:717–730. doi: 10.1083/jcb.200609174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson MS. Autophagy: eating for good health. J Immunol. 2006;177:4945–4951. doi: 10.4049/jimmunol.177.8.4945. [DOI] [PubMed] [Google Scholar]
- Xu Y, Jagannath C, Liu XD, Sharafkhaneh A, Kolodziejska KE, Eissa NT. Toll-like receptor 4 is a sensor for autophagy associated with innate immunity. Immunity. 2007;27:135–144. doi: 10.1016/j.immuni.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CS, Shin DM, Kim KH, Lee ZW, Lee CH, Park SG, et al. NADPH oxidase 2 interaction with TLR2 is required for efficient innate immune responses to mycobacteria via cathelicidin expression. J Immunol. 2009;182:3696–3705. doi: 10.4049/jimmunol.0802217. [DOI] [PubMed] [Google Scholar]
- Yuk JM, Shin DM, Lee HM, Yang CS, Jin HS, Kim KK, et al. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. 2009;6:231–243. doi: 10.1016/j.chom.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Zanoni I, Ostuni R, Capuano G, Collini M, Caccia M, Ronchi AE, et al. CD14 regulates the dendritic cell life cycle after LPS exposure through NFAT activation. Nature. 2009;460:264–268. doi: 10.1038/nature08118. [DOI] [PubMed] [Google Scholar]
- Zhang M, Gong J, Lin Y, Barnes PF. Growth of virulent and avirulent Mycobacterium tuberculosis strains in human macrophages. Infect Immun. 1998;66:794–799. doi: 10.1128/iai.66.2.794-799.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]