Abstract
MicroRNAs are single-stranded 17–27 nucleotide RNA molecules that regulate gene expression by post-transcriptional silencing of target mRNAs. Here, we transformed rat 9L gliosarcoma cells to express cel-miR-67, a miRNA that lacks homology in rat. Co-culture of these cells with cells that expressed a luciferase reporter, which contained a complementary sequence to cel-miR-67, resulted in significant suppression of luciferase expression. This effect was also observed in the U87-MG human glioma cell line. Moreover, luciferase suppression was inhibited by the addition of carbenoxolone to co-cultures, suggesting that gap junction communication regulates intercellular transfer of microRNA. Finally, in situ hybridization revealed the presence of cel-miR-67 in cel-miR-67 null 9L cells after co-culture with cel-miR-67 expressing cells. Our data demonstrate that microRNA transcribed in glioma cells can be transferred to adjacent cells and induces targeted inhibition of protein expression in the acceptor cells. These findings reveal a novel mechanism of targeted intercellular protein regulation between brain tumor cells.
Keywords: microRNA, glioma, intercellular signaling
Introduction
MicroRNAs (miRNA) are single-stranded 17–27 nucleotide RNA molecules that regulate gene expression by post-transcriptional silencing of target mRNAs via complementary binding (1). Each miRNA can affect a number of mRNAs, and depending upon its targets, has the potential to function as an oncogene and/or tumor suppressor (2). Recent studies indicate that some tumors, including gliomas, can secrete microvesicles that contain RNA, including miRNA (3). Other cells treated with purified tumor-shed microvesicles uptake miRNAs contained within (3, 4). It has also been shown that cardiac myocytes exchange RNA in a process mediated by gap junctions (5). However, it is unknown whether miRNA secreted by tumors, either by microvesicles or otherwise, can regulate protein expression in neighboring cells by inherent mechanisms. Here, we show that miRNAs transcribed in rat gliosarcoma cells are transferred to adjacent cells, and that this transferred miRNA induces targeted inhibition of protein expression in the acceptor cells.
Materials and Methods
Cell culture
The 9L rat gliosarcoma, U87-MG human glioblastoma, and U251 human glioblastoma cell lines were obtained from American Type Culture Collection in 2003, 2007 and 2000, respectively. Cells were resuscitated and cultured in accordance with ATCC guidelines for less than 4 months before use in these experiments. ATCC performs authentication of all cell lines via short tandem repeat profiling (human cells), karyotyping, and cytochrome C oxidase I (COI) testing; we did not re-authenticate these lines in our laboratory. Cells were maintained at 37°C in Dulbecco’s Modified Eagle Medium containing 10% fetal bovine serum (FBS) as previously described (6).
Cell co-cultures
Cells were cultured for 24 hours in 96-well plates. 9L cells were added at a 2:1 ratio of C. elegans miRNA-expressing cells (1.4 × 104 cells) to luciferase-expressing cells (7 × 103 cells). For in situ hybridization experiments, 4 × 104 C. elegans miRNA-expressing cells were co-cultured for 24 hours with 2 × 104 eGFP-expressing cells in 8-well glass chamber slides.
Cell transfection
Cel-miR-67 pMIR-report luciferase and control plasmids (Signosis) and cel-miR-67 and cel-miR-239 expression plasmids (GenScript) were used. Transfection was carried out for 12 hours using Lipofectamine 2000 (Invitrogen) with 4 μg DNA per transfection. 24 hours after transfection, cells were washed 3 times in PBS, collected by trypsinization and re-suspended in culture medium prior to use in experiments. Stable transfected cells were isolated under puromycin selection, tested for C. elegans miRNA expression, and subsequently used in experiments. We verified that only 9L-mir67 cells expressed cel-miR-67 using a cel-miR-67 TaqMan® microRNA assay (CT value = 28.7, Applied Biosystems).
Luciferase assays
Luciferase activity was determined using a Luciferase Assay System kit (Promega). Luminescence was determined on a Fusion plate reader (PerkinElmer). For all wells, each experimental group (e.g. 9L-miR-67) was the result of one transfection per experiment. Each luciferase experiment was performed 4 times, with 6 or more replicates per group.
Inhibition of gap junctions
To inhibit the function of gap junctions in 9L cells, we incubated the cells with 150 μM carbenoxolone for 7 hours. Carbenoxolone is a broad spectrum gap junction antagonist, and incubation for 7 hours in 150 μM has been demonstrated to disrupt the function of gap junctions (7).
In situ hybridization and immunostaining
Probes against rno-miR-21 and cel-miR-67 (Exiqon) were hybridized to miRNAs according to a published protocol that was modified for adherent cells (8). Cells were counterstained with a monoclonal antibody against eGFP (Aves Labs). The probe for rno-miR-21 was used as a positive hybridization control as miR-21 is highly expressed in gliomas (9).
Statistical analysis
Data are shown as mean ± s.d. P-values were calculated using one-way ANOVA or Student’s t-test.
Results
Cel-miR-67 is not expressed in rat, mouse or human, and for this reason is often employed as an inert miRNA negative control in experiments using mammalian cells (10). Using real-time RT-PCR with a cel-miR-67 specific primer, we confirmed that 9L rat gliosarcoma cells did not express cel-miR-67 miRNA.
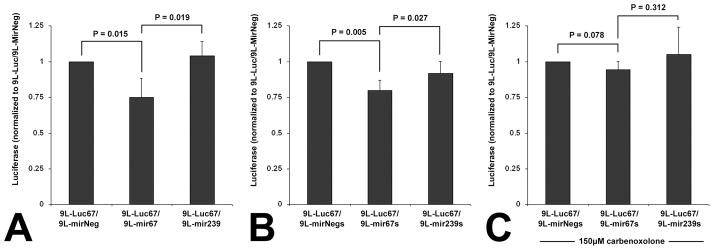
To test whether functional miRNA can be transferred from one tumor cell to another, we co-cultured 9L cells transfected with an expression vector for cel-miR-67 (9L-mir67) or cel-miR-239 (9L-mir239) or an empty expression vector (9L-mirNeg), with 9L cells transfected with a luciferase reporter encoding for mRNA with a 3′UTR containing a complementary sequence to cel-miR-67 (9L-Luc67). When 9L-mir67 cells were co-cultured 24 hours with 9L-Luc67 cells, we observed a 25% signal attenuation compared to the 9L-mirNeg control group. However, when 9L-mir239 cells were co-cultured with 9L-Luc67 cells, luminescence was not significantly altered compared to 9L-mirNeg/9L-Luc67 control. Luciferase activity detected in the 9L-mir67/9L-Luc67 co-culture cells was also significantly less than that detected in the 9L-mir239/9L-Luc67 group (Fig. 1A). These data demonstrate that the down-regulation of luciferase in the 9L-Luc67 cells was due to the presence of cel-miR-67, produced by the 9L-mir67 cells with which they were co-cultured.
Figure 1.
Cel-miR-67 expressing 9L gliosarcoma cells suppress luciferase expression in, and transfer cel-miR-67 to, neighboring cel-miR-67-negative cells by a gap junction-dependent mechanism. A, Luciferase detected in 9L-Luc cells co-cultured with 9L cells transfected with an empty expression vector (9L-mirNeg), or one encoding for cel-miR-67 (9L-mir67) or cel-miR-239 (9L-mir239). n = 4, ANOVA, P = 0.004. B, Luciferase detected in 9L-Luc67 cells co-cultured with 9L cells stably expressing either cel-miR-67 (9L-mir67s), cel-miR-239 (9L-mir239s) or the empty expression vector (9L-mirNegs). n = 4, ANOVA, P = 0.006. C, Luciferase detected in 9L-Luc67 cells co-cultured with 9L-mir67s, 9L-mir239s, or 9L-mirNegs, in the presence of 150 μM carbenoxolone, a gap junction antagonist. n = 4, ANOVA, P = 0.459. Error bars represent ± s.d. Post-hoc multiple comparisons are two-tailed t-tests.
We next posited that intercellular regulation of luciferase might be dependent upon expression of cel-miR-67 DNA that had not incorporated into the genome. To test this hypothesis, we established, via puromycin selection, 9L cell lines that stably expressed either cel-miR-67 (9L-mir67s) or cel-miR-239 (9L-mir239s). We then co-cultured these puromycin-resistant 9L-mir67s- or 9L-mir239s-expressing cells with 9L-Luc67 cells. Similar to our results using cells that were transfected 24 hours prior, when 9L-mir67s cells were co-cultured with 9L-Luc67 cells, we observed a 13% signal attenuation compared to the 9L-mir239s/9L-Luc67 control group (Fig. 1B). These data demonstrate that cells that have incorporated cel-miR-67 into their genome down-regulate luciferase expression in 9L-Luc67 cells when cultured together.
As mentioned above, there is evidence that transfer of RNA between cardiac myocytes occurs, and that it is mediated by gap junctions (5). To determine if transfer of functional miRNA between brain tumor cells is regulated by gap junctions, we inhibited them by incubating the cells with carbenoxolone, a broad spectrum connexin channel antagonist. The addition of carbenoxolone to 9L co-cultures significantly blocked the effect of cel-miR-67 expressing cells upon luciferase expression in Luc67-expressing cells (Fig. 1C). These findings indicate that gap junction intercellular communication significantly mediates the transfer of functional miRNA between 9L cells.
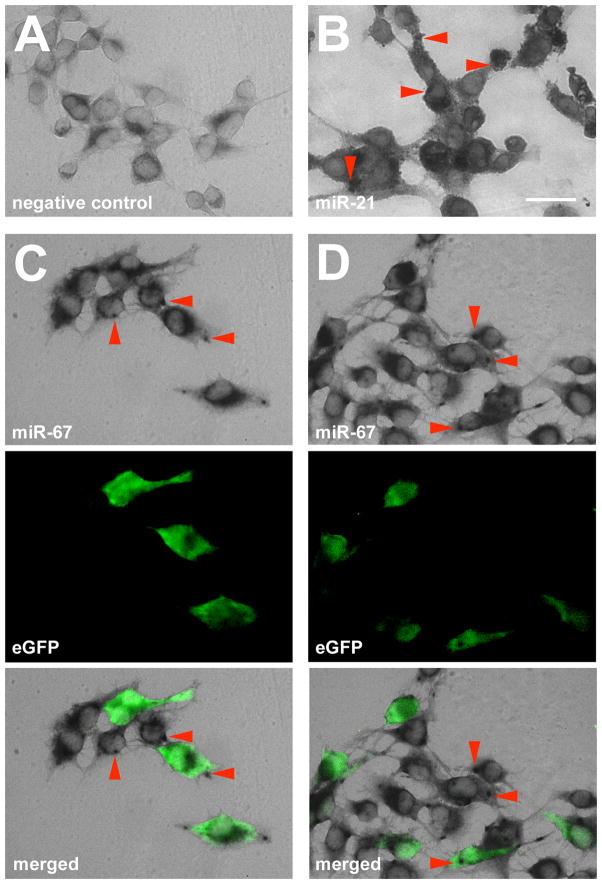
To confirm that cel-miR-67 was present in co-cultured 9L acceptor cells that did not express cel-mir-67, we co-cultured 9L-mir67s cells with 9L gliosarcoma cells that express enhanced green fluorescent protein (eGFP; 9L-GFP). We used in situ hybridization to visualize cel-miR-67. We observed co-localized GFP and cel-miR-67 signals in 9L-mir67/9L-GFP co-culture (~75% of GFP reactive cells were positive for cel-miR-67), but no cel-miR-67 in 9L-mir239/9L-GFP controls (Fig. 2A, B, C and D). These results confirmed the presence of cel-miR-67 in 9L-GFP acceptor cells. Thus, 9L gliosarcoma cells can transfer functional miRNA between cells, and this miRNA can regulate protein expression in the acceptor cells.
Figure 2.
Merged fluorescent and phase-contrast images reveal co-localization of cel-miR-67 in situ signal with eGFP 9L cells. A, No detection of cel-miR-67 in 9L-eGFP/9L-mir239 co-cultured cells (negative control). B, Detection of rno-miR-21 in 9L-eGFP/9L-mir67 co-cultured cells (positive control). C,D Detection of co-localized cel-miR-67 and eGFP in 9L-eGFP/9L-mir67 co-cultured cells. Red arrowheads indicate positive in situ signal. Bar = 25 μm.
Finally, we tested whether miRNA could be transferred between other glioma cells. To this end, we transfected the human glioma cell lines U87-MG and U251 with the cel-mir-67, cel-mir-239, or Luc67 plasmids and performed the same co-culture experiment. Here, we found that luciferase expression in the U87-MG cells was significantly reduced by co-culture with cel-miR-67-expressing U87-MG cells (U87-mir67), however no effect was observed in U251 co-cultures (Fig 3A, B). These results indicate that miRNA transfer may occur in human as well as rat glioma cells; however a lack of luciferase suppression in U251 co-cultures suggests that miRNA transfer may not occur, or may be limited in some glioma cell types.
Figure 3.

U87-MG human glioma cells expressing cel-miR-67 suppress luciferase expression in neighboring cel-miR-67-negative cells, but U251 cells expressing cel-miR-67 do not. A, Luciferase detected in U87-Luc cells co-cultured with U87-MG cells transfected with an empty expression vector (U87-mirNeg), or one encoding for cel-miR-67 (U87-mir67), or cel-miR-239 (U87-mir239). n = 5, ANOVA, P = 0.003. B, Luciferase detected in U251-Luc cells co-cultured with U251 cells transfected with an empty expression vector (U251-mirNeg), or one encoding for cel-miR-67 (U251-mir67) or cel-miR-239 (U251-mir239). n = 5, ANOVA, P = 0.903. Error bars represent ± s.d. Post-hoc multiple comparisons are two-tailed t-tests.
Discussion
Alterations in the expression of miRNA contribute to the pathogenesis of human cancers (11). Dysregulation of miRNAs promotes malignancy of glioblastoma, and contributes to cell proliferation, invasion and angiogenesis, and glioma stem cell multi-potency and survival (12, 13). Intercellular transfer of RNA was hypothesized as early as 1971 (14). Recent evidence indicates that gliomas can shed microvesicles that contain functional miRNAs, mRNAs and receptors (3, 15). These microvesicles have been detected in biological fluids including blood, urine and cerebral spinal fluid (3, 16). Proteins, RNAs and miRNAs transported by microvesicles are now believed to play a critical role in tumor invasion and metastases (17). Previous studies demonstrate that cells exposed to tumor-shed microvesicles uptake and incorporate proteins and nucleic acids contained within (3, 4, 15). These prior experiments provided compelling evidence that intercellular protein regulation via transferred miRNA is possible, if not likely. However, employing a vector encoding for alien miRNA, we provide direct evidence that gliosarcomas exchange functional miRNA, and importantly, that this transferred miRNA leads to significant alterations in protein expression in the acceptor cells.
Previously, Valiunas et al. demonstrated that oligonucleotides the size of siRNA are permeable to gap junctions (18). More recently, it was demonstrated that cardiac myocytes exchange small RNAs via a gap junction-dependent mechanism (5). These studies are important as they suggest a route of intercellular RNA transfer independent of microvesicles. Our findings indicate that gap junctions mediate the transfer of miRNA between 9L cells. However, it remains to be determined whether miRNAs are transported through these intercellular channels directly, or if gap junctions influence processes such as microvesicle release. It is also possible that carbenoxolone affects mechanisms other than gap junctions, and future experiments targeting specific connexins with siRNA could test for this.
An obvious next step will be to determine if tumors can manipulate protein expression in neighboring non-tumor cells, which if true, may contribute to recruitment or transformation of non-tumor cells. Interestingly, miRNA transfer either does not occur in U251 cells, or elicits an effect below the sensitivity of our assay. This finding raises the possibility that intercellular miRNA transfer varies between glioma cell types. Of note, it has been reported that connexin43 is highly expressed in cultured U87-MG cells, but is not in U251 cells (19). As gap junctions contribute to miRNA transfer, it would be interesting to test if the difference we observed was due to the differential connexin43 expression between the two cell lines. Furthermore, we have not established whether cell-to-cell contact is necessary for miRNA transfer or for effective suppression of protein expression in the acceptor cells. Nevertheless, this study demonstrates direct and targeted regulation of protein expression between brain tumor cells. These findings have wide-ranging implications for our understanding of tumorgenesis and progression, as well as for development of miRNA-based anti-tumor therapies.
Acknowledgments
We thank Zheng Gang Zhang and Feng Jiang for comments and reagents, and Ann Hozeska-Solgot for image analysis.
Grant Support
This work was supported by National Institutes of Health grant RO1 CA129446, and a Henry Ford Hospital Research Proposal Development Program Grant (M.K.).
References
- 1.Visone R, Croce CM. MiRNAs and Cancer. Am J Pathol. 2009 doi: 10.2353/ajpath.2009.080794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fabbri M, Croce CM, Calin GA. MicroRNAs. Cancer J. 2008;14:1–6. doi: 10.1097/PPO.0b013e318164145e. [DOI] [PubMed] [Google Scholar]
- 3.Skog J, Wurdinger T, van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–6. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong BS, Cho JH, Kim H, et al. Colorectal cancer cell-derived microvesicles are enriched in cell cycle-related mRNAs that promote proliferation of endothelial cells. BMC Genomics. 2009;10:556. doi: 10.1186/1471-2164-10-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kizana E, Cingolani E, Marban E. Non-cell-autonomous effects of vector-expressed regulatory RNAs in mammalian heart cells. Gene Ther. 2009;16:1163–8. doi: 10.1038/gt.2009.64. [DOI] [PubMed] [Google Scholar]
- 6.Zheng X, Jiang F, Katakowski M, Kalkanis SN, Hong X, Zhang X, et al. Inhibition of ADAM17 reduces hypoxia-induced brain tumor cell invasiveness. Cancer Sci. 2007;98:674–84. doi: 10.1111/j.1349-7006.2007.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pollmann MA, Shao Q, Laird DW, Sandig M. Connexin 43 mediated gap junctional communication enhances breast tumor cell diapedesis in culture. Breast Cancer Res. 2005;7:R522–34. doi: 10.1186/bcr1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pena JT, Sohn-Lee C, Rouhanifard SH, et al. miRNA in situ hybridization in formaldehyde and EDC-fixed tissues. Nat Methods. 2009;6:139–41. doi: 10.1038/nmeth.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–33. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 10.Sharma A, Kumar M, Aich J, et al. Posttranscriptional regulation of interleukin-10 expression by hsa-miR-106a. Proc Natl Acad Sci U S A. 2009;106:5761–6. doi: 10.1073/pnas.0808743106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10:704–14. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Novakova J, Slaby O, Vyzula R, Michalek J. MicroRNA involvement in glioblastoma pathogenesis. Biochem Biophys Res Commun. 2009;386:1–5. doi: 10.1016/j.bbrc.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 13.Lawler S, Chiocca EA. Emerging functions of microRNAs in glioblastoma. J Neurooncol. 2009;92:297–306. doi: 10.1007/s11060-009-9843-2. [DOI] [PubMed] [Google Scholar]
- 14.Kolodny GM. Evidence for transfer of macromolecular RNA between mammalian cells in culture. Exp Cell Res. 1971;65:313–24. doi: 10.1016/0014-4827(71)90007-3. [DOI] [PubMed] [Google Scholar]
- 15.Al-Nedawi K, Meehan B, Micallef J, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619–24. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 16.Huttner HB, Janich P, Kohrmann M, et al. The stem cell marker prominin-1/CD133 on membrane particles in human cerebrospinal fluid offers novel approaches for studying central nervous system disease. Stem Cells. 2008;26:698–705. doi: 10.1634/stemcells.2007-0639. [DOI] [PubMed] [Google Scholar]
- 17.Muralidharan-Chari V, Clancy JW, Sedgwick A, D’Souza-Schorey C. Microvesicles: mediators of extracellular communication during cancer progression. J Cell Sci. 123:1603–11. doi: 10.1242/jcs.064386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valiunas V, Polosina YY, Miller H, et al. Connexin-specific cell-to-cell transfer of short interfering RNA by gap junctions. J Physiol. 2005;568:459–68. doi: 10.1113/jphysiol.2005.090985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang RP, Fan Y, Hossain MZ, Peng A, Zeng ZL, Boynton AL. Reversion of the neoplastic phenotype of human glioblastoma cells by connexin 43 (cx43) Cancer Res. 1998;58:5089–96. [PubMed] [Google Scholar]