Introduction
Juvenile idiopathic arthritis (JIA) is a childhood onset autoimmune disorder characterized by inflammation of joints and other tissues. The histopathology of JIA shares features with other autoimmune diseases including infiltration of the synovium by lymphocytes, plasma cells, macrophages, dendritic cells, and related mediators of inflammation (1). It is the most common chronic childhood rheumatic disease in the Western world (2). Like other autoimmune diseases, there is convincing evidence that JIA is one or more complex genetic traits (3). Unlike most autoimmune diseases, children of European ancestry may be at greatest risk (4). Autoimmune diseases have been shown to aggregate within JIA families (5). The clustering of multiple autoimmune disorders in families and the evidence for overlapping disease susceptibility loci between different autoimmune diseases (6–8) suggest that clinically different phenotypes may share common susceptibility loci which may function at different points in the mechanistic process. Examples encompass HLA loci and non-HLA loci which include CTLA4, STAT4, and PTPN22 (9).
The basis for susceptibility to common autoimmune/inflammatory diseases (AD), including JIA, is a complex interplay between multiple genetic and environmental risk factors. Both disease-specific mechanisms and common pathways across diseases have been identified by genome-wide association studies (GWAS). Here we test for association with JIA loci previously implicated in GWAS from the following AD: (10): Rheumatoid Arthritis (RA), Type 1 Diabetes (T1D), Ankylosing Spondylitis, Systemic Lupus Erythematosus (SLE), Inflammatory Bowel Disease (IBD), including Crohn’s Disease and Ulcerative Colitis, Celiac Disease, Multiple Sclerosis (MS), Psoriasis and Psoriatic Arthritis, Autoimmune Thyroid Disease, Kawasaki Disease and JIA. For this study, loci implicated as risk factors in other AD were comprehensively tested in JIA. The MHC was considered separately (11).). The identification of shared risk variants may aid the understanding of disease pathways, improve diagnosis and ultimately improve prognosis through targeted therapies.
Materials and Methods
Initial cohort
DNA samples for 823 JIA patients and 535 local controls of self-reported non-Hispanic European American (EA) ancestry were available for this study. Approximately 95% of the patients were recruited at the Cincinnati Children’s Hospital Medical Center (CCHMC) or as part of a NIAMS supported JIA affected sibpair registry. The remaining patients were contributed by collaborating centers which included Children’s Hospital of Wisconsin, Schneider Children’s Hospital and Children’s Hospital of Philadelphia. The medical and clinical data relating to samples were collected in standardized case report forms, or in the Pediatric Rheumatology Research Registry maintained within the Division of Rheumatology. This study was approved by the Institutional Review Board of CCHMC and collaborating centers.
The ILAR revised criteria for juvenile idiopathic arthritis (12) were the criteria of choice. The cohort was limited to the two most common subtypes, IgM rheumatoid factor (RF) negative polyarticular (polyRFneg) and oligoarticular JIA (both persistent and extended). Patients recruited before ILAR criteria were published were originally classified using the American College of Rheumatology criteria for Juvenile Rheumatoid Arthritis or the EULAR criteria for Juvenile Chronic arthritis and subsequently reclassified by ILAR criteria when possible. For this study, a patient was considered RF negative on the basis of a single test. In multiplex pedigrees, a single individual from each pedigree was randomly selected from among those diagnosed as either polyRFneg or oligoarticular.
The control cohort included healthy children evenly dispersed through 3–18 years of age and gender and without known major health conditions. This cohort was recruited from the general population to represent the geographical region served by CCHMC to reduce bias associated with recruitment from tertiary medical centers or physician practices. In addition, the publicly available “out-of-study” control dataset (Affymetrix GeneChip 500K Mapping Array Set) for the Wellcome Trust Case Control Consortium, which included genotypes for 3004 individuals from the United Kingdom (WTCCC-1), was used (13).
Fourteen cases and 4 controls as well as 14 WTCCC-1 individuals were found to be ethnically different than the rest of sample based on the principal component analysis (see Statistical Methods) and were removed yielding 809 JIA cases, 531 local controls and 2990 WTCCC-1 controls for statistical inference (Table 1).
Table 1.
Cohort Origin and Demographics*
| Initial cohort |
Replication Cohort |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CCHMC |
Total | Texas |
Germany |
Utah |
Total | ||||||
| Male | Female | Male | Female | Male | Female | Unknown | Male | Female | |||
| Case | |||||||||||
| PolyRFneg | 76 | 276 | 352 | 15 | 63 | 30 | 57 | 61 | 21 | 78 | 325 |
| Oligoarticular | 89 | 368 | 457 | 9 | 60 | 47 | 160 | 217 | 60 | 137 | 690 |
| Total | 165 | 644 | 809 | 24 | 123 | 77 | 217 | 278 | 81 | 215 | 1015 |
| Control | |||||||||||
| Local Controls | 276 | 255 | 531 | 100 | 183 | 501 | 316 | 469 | 1569 | ||
| WTCCC-1 | 1470 | 1520 | 2990 | ||||||||
WTCCC-1 = out of study controls provided by the Wellcome Trust Case-Control Consortium
Replication cohort
To attempt to replicate associations in the initial cohort, three independent JIA case and control sample collections of self-reported European ancestry were studied (Table 1) and included patients diagnosed with oligoarticular or polyRFneg JIA by ILAR criteria. The respective Institutional Review Boards approved collection of these samples and participation in this study.
The “Texas samples” collected at Texas Scottish Rite Hospital for Children (TSRHC) included 147 JIA patients and 283 local controls without history of inflammatory disease.
The “Utah samples” included 296 patients with JIA from the Intermountain States Database of Childhood Rheumatic Diseases meeting criteria for oligoarticular or polyRFneg JIA and reported previously (14). Controls included 785 healthy adults ascertained from the same geographic region. Controls that reported an autoimmune disorder, or had a first-degree relative with an autoimmune disorder were excluded.
The “German samples” consisted of 572 patients and 501 controls. A subset of these samples have been included in reports of the HLA associations in JIA (15). These patients were recruited from: German Center for Rheumatology in Children and Adolescents, Garmisch-Partenkirchen, Department of Pediatrics, University of Tübingen, Children’s Rheumatology Unit Sendenhorst, Germany and the Department of Pediatrics, University of Prague, Czech Republic. JIA was determined retrospectively by chart review. German population-based controls were available from the SNiP consortium and prepared from cord-blood samples of healthy newborns (16).
Genotyping
For the initial phase, genotyping was done at the Affymetrix Service Center using the Genome-Wide Human SNP Array 6.0 (SNP Array 6.0). The Birdseed (version 2) calling algorithm (Affymetrix, Inc.) yielded an overall call rate of 98.97%. The out-of-study control dataset employed the 500K Mapping Array (BRLMM calling algorithm). The SNP Array 6.0 and 500K Array share 469,874 SNPs. The genotypic data were used to test for cryptic relatedness (duplicates and first degree relatives), autosomal heterozygosity outliers (Fst-statistic > | 0.07|), and plate effects. Specifically, for systematic plate-to-plate variation in genotype calls for a particular SNP, the minor allele frequency (MAF) for each plate was computed and tested whether this was within three standard deviations from the median MAF across all plates. In addition, we completed plate-specific visual examination of the genotype calling cluster plots for all SNPs reported.
Despite robust wet-lab and informatics genotyping quality control procedures, some SNPs may remain problematic for statistical inference. Thus, we report the results for SNPs with less than 5% missing genotype calls, no evidence of differential missingness between cases and controls (p-value<0.05), no evidence of departure from expectation in Hardy-Weinberg equilibrium proportions (control p-value < 0.01), and MAF>0.05 in cases and controls.
Statistical Methods
Admixture and SNP statistical quality control
To account for the potential confounding influences of population substructure, a principal component analysis (PCA) was computed using all SNPs on both the 500K array and SNP Array 6.0 after removing genomic regions with long range linkage disequilibrium since these regions can potentially influence the choice and the number of principal components (PC) retained (17). Numerical algebraic techniques were employed to reduce computation time (18). Velicer’s algorithm (19) and the Tracy-Widom test were used to identify PCs (20). The association analyses (outlined below) were computed adjusting for 10 PC that minimize the inflation factor in the ongoing JIA case-control GWAS. A genome-wide association analysis was computed for each of these 10 PCs to assure that the PCs were not capturing a single or a few potentially important regions. Replication samples were not genotyped sufficiently to be included in admixture analysis.
Association Analysis
Five tests of genotypic association were computed: two degrees of freedom overall test for 2×3 tables, dominant model, additive model (Cochran-Armitage trend test), recessive model, and lack-of-fit to an additive model using the program SNPGWA (www.phs.wfubmc.edu). The recessive model required at least 10 homozygotes for the minor allele. The genetic model and odds ratios were defined relative to the minor allele. The primary inference for this study was based on the additive genetic model unless the lack-of-fit to an additive model was statistically significant (p-value<0.05). If the lack-of-fit test was significant, then the minimum p-value from the dominant, additive or recessive models was used. Odds ratios (ORs), 95% confidence intervals (CIs), and p-values were calculated. SNPs not represented on the respective arrays were imputed using the program IMPUTE (21) based on the HapMap Phase II and our JIA GWAS data, using only those SNPs that met quality control criteria (mentioned above). To account for genotypic uncertainty, tests of association using imputed data were limited to SNPs with an imputation confidence score > 0.90 and information score ≥ 0.5 (21). To account for the number of tests, a Bonferroni corrected p-value of 1.2×10−4 provides a conservative threshold of significance.
Disease loci
This study included largely SNPs identified as risk factors for autoimmune and autoinflammatory diseases (supplemental table 1). The primary source for this list was the National Human Genome Research Institute’s Catalog of Published Genome-Wide Association Studies (http://www.genome.gov/gwastudies). Studies that focused on candidate genes or haplotype-based association analyses were not considered. SNP selection was generally limited to SNPs achieving p-value < 1.0 × 10−5 levels of significance for association in another disease. If the individual study had alternate criteria that did not meet this threshold, the top three SNPs were considered. Exceptions to these criteria were made for SNPs that represented proteins of considerable functional or mechanistic interest. Most SNPs studied have reported association findings in more than one disease.
Two approaches were used to identify common loci between JIA and other autoimmune diseases. First, SNPs were identified from the listings that were identical to those available in the SNP Array 6.0 JIA dataset. Second, genotypes were imputed for the remaining SNPs that were present in the Human HapMap Phase II data.
Results
There were 809 cases and 531 controls genotyped on the SNP Array 6.0 that passed all individual level quality control criteria (Table 1). Of these, 352 were polyRFneg and 457 were oligoarticular JIA patients. There were 165 males (76 polyRFneg and 89 oligoarticular) and 644 females (276 polyRFneg and 368 oligoarticular). The mean (± standard deviation) age of onset for JIA was 6.95 ± 4.75 for female polyRFneg, 4.49 ± 3.69 for female oligoarticular, 7.36 ± 3.97 for male polyRFneg, and 6.24 ± 3.74 male oligoarticular. The potential for HLA-B27 enthesitis-related JIA in the initial cohort is low, since only 6 JIA males in this study had both disease onset >8 years of age and an HLA-B27 allele (11). In addition, 2990 WTCCC out-of-study controls (13) were genetically well-matched to this study’s cases and controls and were integrated into the initial cohort, yielding a total sample of 809 cases and 3521 controls for analysis.
A review of the literature identified ~233 autoimmune loci represented by 519 SNPs, (non-MHC). Of these 519 SNPs, 257 passed genotyping quality control and were included in the 500K array, while another 168 were imputed with high quality. JIA association results for these 425 SNPs that met quality control criteria are provided (Supplemental Table 1). This suggests a conservative Bonferroni corrected p-value of 1.2×10−4 would be declared statistically significant.
Twenty-one SNPs were selected for further testing based on statistical significance levels of 1.0 × 10−3 in the initial cohort, on scope of reported findings or interest in loci (Table 2). The selected SNPs included markers defining the PTPN22 locus at chromosome 1p13 (rs6679677, p=8.66 × 10−8, OR=1.58; rs2476601, p=6.99 × 10−8, OR=1.65 and rs2488457, p=5.05 × 10−5, OR=1.32). PTPN22 has been previously associated with JIA in candidate gene/SNP studies (22–24) in addition to RA, T1D, SLE and Crohn’s disease (10). For JIA, the minor allele (A) of rs6679677 and rs2476601 conferred increased risk, consistent with findings in T1D, SLE and RA, while for Crohn’s disease the major allele (G) of rs2476601, a missense variant, is the reported susceptibility allele.
Table 2.
Initial cohort findings of Association for non-MHC autoimmune disease variants in JIA*
| Marker | chr | kbp | locus | MA | MAF |
Reported finding | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial | WTCCC | Initial | Including WTCCC Controls | |||||||||
| JIA | Control | Control | P-value§ | OR [95%CI] | P-value | OR [95%CI] | ||||||
| Intracellular signaling | ||||||||||||
| rs6679677 | 1 | 114105 | PTPN22 | A | 0.142 | 0.095 | 0.097 | 3.38 × 10−4a | 1.57 [1.23–2.02] | 8.66 × 10−8a | 1.58 [1.34–1.86] | RA, T1D: (10) |
| rs2476601† | 1 | 114179 | PTPN22 | A | 0.143 | 0.094 | 0.095 | 3.29 × 10−4a | 1.54 [1.22–1.94] | 6.99 × 10−8a | 1.65 [1.38–1.98] | RA, T1D, CD (10) |
| rs2488457 | 1 | 114216 | PTPN22 | C | 0.249 | 0.193 | 0.203 | 9.13 × 10−4a | 1.39 [1.14–1.57] | 5.05 × 10−5a | 1.32 [1.15–1.50] | Personal communication |
| rs2542151 | 18 | 12770 | PTPN2 | G | 0.213 | 0.164 | 0.164 | 4.93 × 10−4a | 1.45 [1.18–1.78] | 3.05 × 10−7a | 1.45 [1.26–1.67] | RA (13), T1D, CD (10) |
| rs2847297 | 18 | 12787 | PTPN2 | G | 0.399 | 0.334 | 0.345 | 3.95 × 10−4a | 1.34 [1.14–1.57] | 1.29 × 10−6a | 1.33 [1.19–1.49] | T1D (49) |
| rs1893217† | 18 | 12799 | PTPN2 | C | 0.218 | 0.165 | 0.165 | 2.13 × 10−4a | 1.45 [1.19–1.77] | 3.48 × 10−8a | 1.52 [1.31–1.76] | T1D (10) |
| rs7234029 | 18 | 12867 | PTPN2 | C | 0.226 | 0.144 | 0.16 | 2.29 × 10−7a | 1.74 [1.41–2.14] | 7.19 × 10−11a | 1.59 [1.38–1.82] | RA (50) |
| Cytokines and cytokine receptors | ||||||||||||
| rs11465804† | 1 | 67475 | IL23R | G | 0.054 | 0.077 | 0.062 | 3.64 × 10−3d | 0.60 [0.43–0.85] | 7.99 × 10−3d | 0.72 [0.56–0.92] | CD, UC: (10) |
| rs2104286 | 10 | 6139 | IL2RA | C | 0.231 | 0.292 | 0.285 | 7.13 × 10−4a | 0.74 [0.62–0.88] | 3.83 × 10−5a | 0.76 [0.66–0.86] | RA, T1D: (13), MS (10) |
| rs12251307‡ | 10 | 6164 | IL2RA | T | 0.087 | 0.122 | 0.123 | 3.72 × 10−3a | 0.74 [0.62–0.88] | 9.18 × 10−5a | 0.68 [0.56–0.82] | T1D (10) |
| rs17388568 | 4 | 123549 | ADAD1-IL2-IL21 | A | 0.313 | 0.286 | 0.26 | 9.09 × 10−2a | 1.16 [0.98–1.37] | 1.59 × 10−4a | 1.27 [1.12–1.43] | T1D (10) |
| rs13143866 | 4 | 123760 | ADAD1-IL2-IL21 | T | 0.249 | 0.279 | 0.288 | 1.22 × 10−1a | 0.87 [0.73–1.04] | 8.87 × 10−3a | 0.84 [0.74–0.96] | Personal communication |
| Transcriptional regulators | ||||||||||||
| rs3821236 | 2 | 191611 | STAT4 | A | 0.227 | 0.203 | 0.19 | 1.10 × 10−1a | 1.17 [0.96–1.42] | 4.75 × 10−4a | 1.28 [1.11–1.47] | SLE (10) |
| rs7574865† | 2 | 191673 | STAT4 | T | 0.249 | 0.227 | 0.21 | 3.17 × 10−2d | 1.28 [1.02–1.60] | 2.98 × 10−4d | 1.36 [1.15–1.60] | SLE (10) |
| rs7746082 | 6 | 106542 | BLIMP1 | C | 0.324 | 0.282 | 0.282 | 2.96 × 10−2a | 1.21 [1.02–1.44] | 3.87 × 10−4a | 1.25 [1.11–1.42] | CD (10) |
| Unknown | ||||||||||||
| rs17696736 | 12 | 110971 | C12orf30 | G | 0.481 | 0.433 | 0.424 | 2.32 × 10−2a | 1.20 [1.03–1.40] | 3.26 × 10−3a | 1.19 [1.06–1.33] | T1D (10) |
| rs7151781 | 14 | 42557 | Intergenic | C | 0.386 | 0.334 | 0.342 | 4.87 × 10−3a | 1.27 [1.07–1.49] | 1.55 × 10−4a | 1.25 [1.11–1.41] | Kawasaki (31) |
| Other | ||||||||||||
| rs1010824 | 8 | 108321 | ANGPT1 | T | 0.136 | 0.16 | 0.168 | 4.68 × 10−2d | 0.78 [0.61–1.00] | 4.93 × 10−3d | 0.77 [0.61–0.85] | Kawasaki (31) |
| rs1132200 | 3 | 120634 | TMEM39A | T | 0.132 | 0.169 | 0.176 | 6.18 × 10−3d | 0.71 [0.56–0.91] | 1.13 × 10−4a | 0.72 [0.61–0.85] | MS (13) |
| rs10259085† | 7 | 7235 | C1GALT1/COL28A1 | T | 0.464 | 0.393 | 0.423 | 2.05 × 10−3a | 1.28 [1.09–1.49] | 4.50 × 10−2r | 1.23 [1.00–1.51] | MS (10) |
| rs7993214† | 13 | 39249 | COG6 | T | 0.299 | 0.347 | 0.342 | 1.11 × 10−3d | 0.69 [0.55–0.86] | 3.98 × 10−3d | 0.79 [0.67–0.93] | PS (10) |
All SNPs passed HWE testing
Genotypes were imputed in the initial cohort
rs1221307 failed genotyping in the replication cohort and is excluded from subsequent analyses/tables
The genetic model is indicated for each p-value as follows; a = additive, d = dominant, r = recessive
There were also strong associations between JIA and multiple SNPs within the PTPN2 region located at chromosome 18p11. The strongest associations in the PTPN2 region were rs7234029 (p= 7.19×10−11, OR=1.59), rs1893217 (p=3.48 × 10−8, OR=1.52) and rs2542151 (p=3.05 × 10−7, OR=1.45). The risk allele, which was reported for rs2542151 in Crohn’s disease and T1D, was the same as JIA.
The third most strongly associated finding was within the 10p15 region including IL2RA. Here the minor alleles for rs2104286 (p=3.83 × 10−5, OR=0.76) and rs12251307 (p=9.18 × 10−5, OR=0.68) were associated with reduced risk to JIA. These variants have been previously implicated in RA, T1D, and MS as well as reported in JIA including preliminary findings of the Cincinnati JIA dataset (25). The risk allele identified in JIA was consistent with that of rs2104286 when were reported for RA, T1D and MS.
The ADAD1-IL2-IL21 region at 4q27 has been associated with an increasing number of autoimmune diseases. This region has been strongly associated with Celiac disease and T1D (10), and was recently reported in JIA (26). Two SNPs previously implicated in other diseases had data available and met quality criteria.. Findings for both SNPs, rs17388568 (p=1.59 × 10−4, OR=1.27) and rs13143866 (p=8.87 × 10−3, OR=0.84) support an association with JIA. For rs17388568, the risk allele (A) was the same for T1D and JIA.
The STAT4 gene is located in the region at 2q32.3. SNPs for the STAT4 loci reported in Table 2 include rs3821236 (p=4.75 × 10−4, OR=1.28) and rs7574865 (p=2.98 × 10−4, OR=1.36). Associations for these SNPs were reported in GWAS for SLE (10) and in candidate gene studies in the literature including RA (27) as well as JIA (14, 28). In both JIA and SLE, T was the reported risk allele.
Although the association between rs11465804 within IL23R (1p31) and JIA failed to reach the preset significance level of 1 × 10−3, it may nonetheless be an interesting association since this SNP has been widely reported in GWAS including Crohn’s disease and ulcerative colitis (10, 29). Interestingly these diseases have only limited HLA class II associations. Like these other diseases, the minor allele for rs11465804 (G) was associated with reduced risk for JIA (p=7.99 × 10−3, OR=0.72).
Reported for multiple T1D GWAS (10) and consistent with a recent report in JIA (14), an association of the G allele of rs17696736, located at 12q24 in the C12orf30 loci (p=3.26 × 10−3, OR=1.19) was found. In addition, there were a number of modest associations between JIA and SNPs implicated in a single autoimmune GWAS. These include rs7746082 (p=3.87 × 10−4, OR=1.25) within the transcriptional regulator BLIMP1 which was previously implicated for Crohn’s disease (10) rs1132200 (p=1.13 × 10−4, OR=0.72), a missense variant in the membrane protein TMEM39A implicated in MS (30), and rs7993214 on chromosome 13q13 (p=3.98×10−3, OR= 0.79), near the conserved oligomeric Golgi complex component 6 (COG6) gene, implicated in psoriasis (10).
Finally, JIA associations were found for 2 SNPs reported in Kawasaki disease (31), an inflammatory vasculitis predominantly affecting young children. These SNPs include rs1010824 on chromosome 8 near ANGPT1 (p=4.93 × 10−3, OR=0.77) and rs7151781 (p=1.55 × 10−4, OR=1.25) found in an intergenic region on chromosome 14 (risk alleles not available).
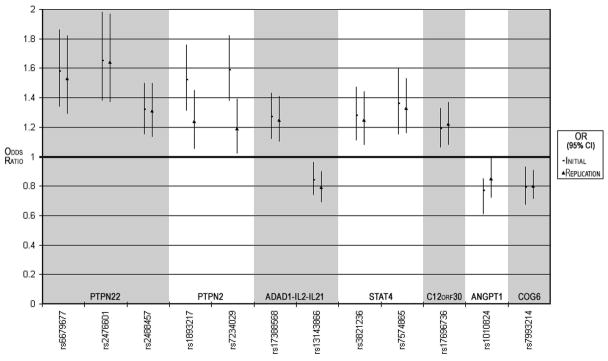
Replication of these JIA association findings was sought using a second cohort comprised of nearly 1000 additional JIA patients and about 1250 local controls. This cohort included samples from 3 separate pediatric centers located in the US or Germany. Like the initial cohort, the replication cohort was limited to polyRFneg and oligoarticular JIA and included about 3 times as many females as males (Table 1). Association testing was performed for each of the 3 centers separately (Supplemental Figure 1) as well as in joint- and meta-analyses. Of the 21 SNPs tested (Table 2), 12 reached significance levels of <0.05 in the replication cohort when analyzed jointly (Table 3). SNP rs12251307 failed typing in the replication cohorts. The twelve SNPs represent PTPN22, PTPN2, IL2-IL21, STAT4, ANGPT1, C12orf30 and COG6 loci and showed consistency of the associated allele and effect size (i.e., odds ratio) across the initial and replication cohorts (Figure 1). The odds ratios observed for the susceptibility alleles ranged from about 1.20 to 1.65 in the meta-analysis of the initial and replication cohorts and are consistent with the autoimmune disease literature (Table 4).
Table 3.
Association results for the replication cohort (Germany+Texas+Utah) *
| Marker | chr | kbp | locus | MA | Germany +Texas +Utah |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| MAF |
Joint Analysis |
Meta Analysis |
||||||||
| JIA | Control | P-value§ | OR [95%CI] | P-value | OR [95%CI] | |||||
| Intracellular signaling | ||||||||||
| rs6679677 | 1 | 114105 | PTPN22 | A | 0.151 | 0.102 | 1.24 × 10−6 | 1.53 [1.29–1.82] | 4.19 × 10−6 | 1.52 [1.27–1.83] |
| rs2476601† | 1 | 114179 | PTPN22 | A | 0.149 | 0.095 | 9.07 × 10−8 | 1.64 [1.37–1.97] | 3.12 × 10−7 | 1.63 [1.35–1.97] |
| rs2488457 | 1 | 114216 | PTPN22 | C | 0.252 | 0.204 | 1.96 × 10−4 | 1.31 [1.13–1.50] | 3.07 × 10−4 | 1.30 [1.12–1.51] |
| rs2542151 | 18 | 12770 | PTPN2 | G | 0.180 | 0.174 | 1.62 × 10−1 | 0.67 [0.38–1.18] | 2.48 × 10−1 | 0.43 [0.17–1.09] |
| rs2847297 | 18 | 12787 | PTPN2 | G | 0.358 | 0.349 | 4.99 × 10−1 | 1.04 [0.92–1.18] | 5.79 × 10−1 | 1.02 [0.90–1.17] |
| rs1893217† | 18 | 12799 | PTPN2 | C | 0.191 | 0.161 | 8.87 × 10−3 | 1.24 [1.05–1.45] | 7.93 × 10−3 | 1.20 [1.01–1.41] |
| rs7234029 | 18 | 12867 | PTPN2 | C | 0.183 | 0.158 | 2.90 × 10−2 | 1.19 [1.02–1.39] | 6.39 × 10−2 | 1.15 [0.97–1.35] |
| Cytokines and cytokine receptors | ||||||||||
| rs11465804† | 1 | 67475 | IL23R | G | 0.064 | 0.062 | 7.96 × 10−1 | 1.03 [0.81–1.32] | 7.02 × 10−1 | 1.01 [0.79–1.30] |
| rs2104286 | 10 | 6139 | IL2RA | C | 0.258 | 0.266 | 5.33 × 10−1 | 0.96 [0.84–1.10] | 8.45 × 10−1 | 0.99 [0.86–1.14] |
| rs17388568 | 4 | 123549 | ADAD1-IL2-IL21 | A | 0.342 | 0.292 | 5.54 × 10−4 | 1.25 [1.10–1.41] | 2.02 × 10−3 | 1.21 [1.06–1.38] |
| rs13143866 | 4 | 123760 | ADAD1-IL2-IL21 | T | 0.227 | 0.271 | 6.45 × 10−4 | 0.79 [0.69–0.90] | 6.09 × 10−3 | 0.83 [0.72–0.96] |
| Transcriptional regulators | ||||||||||
| rs3821236 | 2 | 191611 | STAT4 | A | 0.241 | 0.203 | 2.42 × 10−3 | 1.25 [1.08–1.44] | 1.24 × 10−3 | 1.26 [1.08–1.46] |
| rs7574865† | 2 | 191673 | STAT4 | T | 0.275 | 0.221 | 4.14 × 10−5 | 1.33 [1.16–1.53] | 4.75 × 10−5 | 1.31 [1.14–1.51] |
| rs7746082 | 6 | 106542 | BLIMP1 | C | 0.291 | 0.288 | 8.17 × 10−1 | 1.02 [0.89–1.16] | 6.81 × 10−1 | 1.00 [0.88–1.15] |
| Unknown | ||||||||||
| rs17696736 | 12 | 110971 | C12orf30 | G | 0.478 | 0.428 | 9.52 × 10−4 | 1.22 [1.08–1.37] | 1.90 × 10−3 | 1.19 [1.06–1.35] |
| rs7151781 | 14 | 42557 | Intergenic | C | 0.361 | 0.351 | 4.83 × 10−1 | 1.05 [0.92–1.18] | 5.08 × 10−1 | 1.02 [0.90–1.17] |
| Other | ||||||||||
| rs1010824 | 8 | 108321 | ANGPT1 | T | 0.155 | 0.178 | 4.21 × 10−2 | 0.85 [0.72–0.99] | 2.37 × 10−2 | 0.83 [0.70–0.98] |
| rs1132200 | 3 | 120634 | TMEM39A | T | 0.148 | 0.161 | 2.19 × 10−1 | 0.90 [0.77–1.06] | 1.75 × 10−1 | 0.86 [0.73–1.02] |
| rs10259085† | 7 | 7235 | C1GALT1/COL28A1 | T | 0.450 | 0.468 | 2.33 × 10−1 | 0.93 [0.82–1.05] | 1.33 × 10−1 | 0.90 [0.79–1.02] |
| rs7993214† | 13 | 39249 | COG6 | T | 0.311 | 0.359 | 8.29 × 10−4 | 0.80 [0.71–0.91] | 1.92 × 10−3 | 0.81 [0.71–0.93] |
For the “Replication cohort, the association findings of individual sample collections (Texas, Utah, Germany) are not shown, but rather combined in “joint” and “meta”-analyses All SNPs passed HWE testing
Genotypes were imputed in the initial cohort
The additive genetic model was used for all SNPs except rs2542151; recessive model used for both Joint and Meta analyses
Figure 1.
Consistent findings for case-control association results of variants from initial and replication JIA cohorts are illustrated. Odds ratios (OR) correspond to data from the initial and replication cohorts are indicated by the symbols “-” and “Δ”, respectively; 95% confidence intervals (95% CIs) for these OR are indicated by vertical lines. Individual SNP variants are grouped by genetic loci.
Table 4.
Meta-analysis for SNPs with evidence of genetic association in Initial and Replication cohorts
| Marker | chr | kbp | locus | MA | Initial Cohort (including WTCCC controls) |
Replication cohort |
Meta analysis |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N |
MAF |
N |
MAF |
Initial + Replication |
||||||||||
| JIA | Control | JIA | Control | JIA | Control | JIA | Control | P-value | OR [95%CI] | |||||
| Intracellular signaling | ||||||||||||||
| rs6679677 | 1 | 114105 | PTPN22 | A | 809 | 3515 | 0.142 | 0.096 | 955 | 1337 | 0.151 | 0.102 | 1.98 × 10−12 | 1.58 [1.39–1.80] |
| rs2476601† | 1 | 114179 | PTPN22 | A | 800 | 3501 | 0.143 | 0.096 | 949 | 1214 | 0.149 | 0.095 | 1.90 × 10−13 | 1.64 [1.44–1.87] |
| rs2488457 | 1 | 114216 | PTPN22 | C | 809 | 3519 | 0.249 | 0.202 | 976 | 1260 | 0.252 | 0.204 | 6.74 × 10−8 | 1.32 [1.19–1.46] |
| rs1893217† | 18 | 12799 | PTPN2 | C | 807 | 3516 | 0.218 | 0.165 | 934 | 1248 | 0.191 | 0.161 | 1.60 × 10−9 | 1.33 [1.19–1.49] |
| rs7234029 | 18 | 12867 | PTPN2 | C | 809 | 3514 | 0.226 | 0.157 | 978 | 1257 | 0.183 | 0.158 | 1.86 × 10−10 | 1.35 [1.20–1.51] |
| Cytokines and cytokine receptors | ||||||||||||||
| rs17388568 | 4 | 123549 | ADAD1-IL2-IL21 | A | 808 | 3517 | 0.313 | 0.264 | 962 | 1250 | 0.342 | 0.292 | 1.13 × 10−6 | 1.24 [1.13–1.36] |
| rs13143866 | 4 | 123760 | ADAD1-IL2-IL21 | T | 807 | 3490 | 0.249 | 0.287 | 982 | 1240 | 0.227 | 0.271 | 1.95 × 10−4 | 0.83 [0.76–0.92] |
| Transcriptional regulators | ||||||||||||||
| rs3821236 | 2 | 191611 | STAT4 | A | 808 | 3519 | 0.227 | 0.192 | 940 | 1259 | 0.241 | 0.203 | 2.36 × 10−6 | 1.27 [1.15–1.41] |
| rs7574865† | 2 | 191673 | STAT4 | T | 780 | 3418 | 0.249 | 0.218 | 946 | 1240 | 0.275 | 0.221 | 2.21 × 10−6 | 1.31 [1.16–1.49] |
| Unknown | ||||||||||||||
| rs17696736 | 12 | 110971 | C12orf30 | G | 808 | 3519 | 0.481 | 0.426 | 980 | 1320 | 0.478 | 0.428 | 2.59 × 10−5 | 1.19 [1.09–1.30] |
| Other | ||||||||||||||
| rs1010824 | 8 | 108321 | ANGPT1 | T | 809 | 3520 | 0.136 | 0.167 | 988 | 1246 | 0.155 | 0.178 | 2.91 × 10−4 | 0.79 [0.69–0.90] |
| rs7993214† | 13 | 39249 | COG6 | T | 803 | 3470 | 0.300 | 0.348 | 950 | 1252 | 0.311 | 0.359 | 1.10 × 10−5 | 0.76 [0.67–0.86] |
Genotypes were imputed in the initial cohort
The meta analysis includes the WTCCC controls for the initial cohort
Of special interest were SNPs with previously reported associations for JIA that have been identified in GWAS (32, 33) or in candidate gene studies of JIA based on reports of association in RA (14, 25, 26, 28, 34, 35). Reported p-values and odds ratios from the literature as well as from the initial JIA cohort/local control comparison are provided in table 5. The meta-analyses include only initial cohort findings, since not all SNPs were genotyped in the replication cohort and/or in some cases the WTCCC-1 cohort was also used in the referenced comparison. Notable in this data are the lack of support for all SNPs related to VCTN1, a region reported in JIA by genome-wide association (32). Conversely, while rs6897932, a marker for IL7R, did not meet criteria for testing in the replication cohort, the findings of the initial cohort are consistent with a recent report in the UK JIA cohort (table 5, pmeta=0.0043) and as well as with reported GWAS findings in T1D and MS (10) and statistically suggestive findings in RA (36). IL7R, the receptor for IL7, plays a role in T cell development and is also a critical anti-apoptotic survival factor. Finally, the initial cohort findings also support a role for PRKCQ (rs4750316; pmeta= 0.015) consistent with another JIA study (28) and supported by GWAS findings in RA (10).
Table 5.
Comparison of association findings and meta analyses for the Initial cohort with previously reported non-MHC associations in JIA*
| Marker† | chr | kbp | locus | Initial Cohort |
Literature |
Meta analysis |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case MAF | Control MAF | P-value | Model | OR [95%CI] | Case MAF | Control MAF | P-value | Reference | P-value | ||||
| rs2476601†# | 1 | 114179 | PTPN22 | 0.14 | 0.09 | 3.28 × 10−4 | add | 1.54 [1.22–1.94] | 0.15 | 0.10 | 5.00 × 10−4 | (22) | 5.68 × 10−7 |
| rs2358820 | 1 | 117514 | VTCN1 | 0.08 | 0.06 | 2.77 × 10−1 | add | 1.19 [0.87–1.61] | 0.04 | 0.01 | 4.00 × 10−4 | (32) | 5.71 × 10−1 |
| rs12046117† | 1 | 117552 | VTCN1 | 0.13 | 0.13 | 6.86 × 10−1 | add | 0.95 [0.75–1.21] | 0.13 | 0.09 | 1.00 × 10−6 | (32) | 2.09 × 10−4 |
| rs10923223† | 1 | 117548 | VTCN1 | 0.14 | 0.15 | 3.83 × 10−1 | add | 0.9 [0.72–1.13] | 0.16 | 0.12 | 1.00 × 10−4 | (32) | 8.69 × 10−3 |
| rs2051047† | 1 | 117497 | VTCN1 | 0.08 | 0.06 | 2.66 × 10−1 | dom | 1.2 [0.87–1.65] | 0.06 | 0.08 | 1.00 × 10−2 | (32) | 1.55 × 10−1 |
| rs6669320† | 1 | 117532 | VCTN1 | 0.14 | 0.14 | 8.11 × 10−1 | dom | 0.97 [0.75–1.25] | 0.14 | 0.17 | 2.00 × 10−2 | (32) | 4.36 × 10−2 |
| rs6673837 | 1 | 117488 | VCTN1 | 0.19 | 0.20 | 5.55 × 10−1 | add | 0.94 [0.77 –1.15] | 0.22 | 0.20 | 5.00 × 10−2 | (32) | 2.18 × 10−1 |
| rs2358817 | 1 | 117492 | VCTN1 | 0.09 | 0.07 | 2.23 × 10−1 | add | 1.2 [0.9–1.6] | 0.06 | 0.09 | 5.00 × 10−3 | (32) | 1.22 × 10−1 |
| rs4376721 | 1 | 117524 | VCTN1 | 0.30 | 0.31 | 5.95 × 10−1 | add | 0.96 [0.81–1.13] | 0.31 | 0.28 | 4.00 × 10−2 | (32) | 1.79 × 10−1 |
| rs7574865†# | 2 | 191673 | STAT4 | 0.25 | 0.23 | 3.17 × 10−2 | dom | 1.28 [1.02–1.6] | 0.26 | 0.22 | 4.00 × 10−4 | (28) | |
| 0.28 | 0.24 | 2.90 × 10−2 | (14) | 3.14 × 10−6 | |||||||||
| rs10181656† | 2 | 191678 | STAT4 | 0.25 | 0.23 | 3.27 × 10−2 | dom | 1.28 [1.02–1.61] | 0.26 | 0.22 | 2.00 × 10−4 | (28) | 1.81 × 10−5 |
| rs8179673† | 2 | 191677 | STAT4 | 0.25 | 0.23 | 3.30 × 10−2 | dom | 1.28 [1.02–1.61] | 0.26 | 0.22 | 2.00 × 10−4 | (28) | 1.82 × 10−5 |
| rs3087243 | 2 | 204447 | CTLA4 | 0.44 | 0.46 | 3.43 × 10−1 | add | 0.93 [0.79–1.08] | 0.43 | 0.46 | 5.00 × 10−2 | (26) | 2.96 × 10−2 |
| rs1160542 | 2 | 100198 | AFF3 | 0.48 | 0.46 | 5.81 × 10−1 | add | 1.05 [0.89–1.23] | 0.50 | 0.45 | 2.05 × 10−5 | (26) | 6.09 × 10−5 |
| rs6897932 | 5 | 3591 | IL7R | 0.25 | 0.29 | 1.15 × 10−2 | add | 0.8 [0.68–0.95] | 0.27 | 0.29 | 6.00 × 10−2 | (26) | 4.29 × 10−3 |
| rs13207033 | 6 | 138007 | TNFAIP3 | 0.24 | 0.25 | 5.22 × 10−1 | add | 0.94 [0.79–1.13] | 0.25 | 0.28 | 2.00 × 10−2 | (28) | 1.87 × 10−2 |
| rs10499194† | 6 | 138044 | TNFAIP3 | 0.24 | 0.26 | 3.20 × 10−1 | dom | 0.89 [0.71–1.12] | 0.23 | 0.29 | <4.00 × 10−2 | (14) | 7.96 × 10−3 |
| rs6920220 | 6 | 138048 | TNFAIP3 | 0.22 | 0.24 | 5.12 × 10−1 | add | 0.94 [0.78–1.13] | 0.24 | 0.21 | 2.00 × 10−2 | (28) | |
| 0.23 | 0.19 | 1.50 × 10−2 | (14) | 1.09 × 10−2 | |||||||||
| rs3761847† | 9 | 12273 | TRAF1-C5 | 0.43 | 0.43 | 7.31 × 10−1 | dom | 1.04 [0.82–1.32] | 0.49 | 0.40 | 3.51 × 10−2 | (33) | 6.42 × 10−2 |
| rs10818488†‡ | 9 | 122744 | TRAF1-C5 | 0.43 | 0.44 | 8.06 × 10−1 | rec | 0.97 [0.73–1.28] | 0.44 | 0.41 | 2.81 × 10−1 | (35) | 1.70 × 10−1 |
| rs2900180† | 9 | 122746 | TRAF1-C5 | 0.36 | 0.35 | 5.53 × 10−1 | add | 1.05 [0.89–1.24] | 0.39 | 0.35 | 3.00 × 10−4 | (28) | 5.35 × 10−4 |
| rs2104286# | 10 | 6139 | IL2RA | 0.23 | 0.29 | 7.13 × 10−4 | add | 0.74 [0.62–0.88] | 0.22 | 0.27 | 2.00 × 10−4 | (25) | 1.03 × 10−6 |
| rs4750316 | 10 | 6433 | PRKCQ | 0.17 | 0.19 | 1.42 × 10−1 | add | 0.86 [0.71–1.05] | 0.18 | 0.20 | 5.00 × 10−2 | (28) | 1.54 × 10−2 |
| rs939898 | 12 | 90192 | Intergenic | 0.20 | 0.20 | 7.15 × 10−1 | add | 0.96 [0.79–1.18] | 0.16 | 0.25 | 4.00 × 10−4 | (32) | 3.50 × 10−2 |
| rs17696736 | 12 | 110971 | C12orf30 | 0.48 | 0.43 | 2.32 × 10−2 | add | 1.2 [1.03–1.4] | 0.45 | 0.41 | 4.10 × 10−2 | (14) | 2.26 × 10−3 |
| rs1074044† | 13 | 87841 | Intergenic | 0.48 | 0.48 | 7.04 × 10−1 | dom | 1.05 [0.82–1.35] | 0.56 | 0.43 | 1.00 × 10−4 | (32) | 2.15 × 10−2 |
Initial cohort results do not include the WTCCC out-of-study controls; Meta analysis combines datasets from the literature for rs7574865 and rs6920220; Data was not available for rs6822844 (Chr 4, IL2-21 region) for comparison with published data (35).
Genotypes were imputed in the initial cohort
Detailed results are included in Table 2
Significant findings specific to polyRFneg subtype were reported in the literature; Case MAF 0.52, p-value = 0.012.
Discussion
This study reports the evidence of association with JIA of 425 non-MHC region SNPs previously implicated in other autoimmune diseases using a large JIA case-control cohort and replicates multiple associations in an independent set of JIA cases and controls. These data provide compelling evidence for 7 JIA susceptibility loci. Four of these loci have been previously reported in JIA and include SNPs representing the PTPN22 (22–24), STAT4 (14, 28), C12orf30 (14) and the ADAD1-IL2-IL21 (26) regions. With the exception of C12orf30, which may play a role in cell cycle progression, these confirmed JIA risk loci have clear roles in immune regulation and function. PTPN22 is an intracellular phosphatase, which modulates cytokine signal transduction through the Jak/Stat signaling pathways. STAT4 is a transcription factor expressed in lymphocytes, macrophages and dendritic cells and is essential for mediating responses to IL12 in lymphocytes, and regulating the Th1 differentiation. Th1 cells produce IFN-γ, an inflammatory cytokine reported in JIA. IL2 plays an established role in T cell regulation through binding the to the high affinity IL2 receptor, which includes IL2Rα (also a risk factor in JIA).
Three novel loci for JIA were identified and include PTPN2, COG6 and ANGPT1. PTPN2 is a classical, non-receptor protein tyrosine phosphatase and similar to PTPN22. This enzyme is a key regulator in immune cell signaling. Furthermore particularly high expression of PTPN2 is found in hematopoietic tissues and appears to influence most, if not all, cells involved in the development of the immune system. It is interesting that mice lacking the PTPN2 gene have high levels of TNF-α, an important target in the treatment of both adult and juvenile arthritis, although joint pathology has not been studied in these mice. The role of PTPN2 as a negative regulator of T cell activation is consistent with a common role across multiple autoimmune diseases.
COG6 is a component of the conserved oligomeric golgi (COG) complex which is involved in processes such as protein sorting, glycosylation, and Golgi integrity (37). The large variety of glycans produced by the golgi apparatus are critical in basic cellular functions and development and have been associated with a number of inherited diseases including diabetes and cancer (reviewed in (38)). An association for the COG6 region was recently reported in psoriasis and the following was proposed as a plausible functional relationship (39). The glycosylation pathways in the golgi apparatus must be intact for protein secretion to continue unabated. In C. elegans, a COG complex is required to glycosylate an ADAM protease (a disintegrin and metalloprotease) (40). In humans, ADAM play roles in inflammatory diseases, including ADAM33 as a susceptibility gene in both asthma (41) and psoriasis (42), lending support to a functional role for COG6 in JIA.
Angiopoietin-1 (ANGPT1) is secreted by endothelial and other cell types as oligomers into the extracellular matrix, where it can bind to and activate the receptor tyrosine kinase (TIE2). This in turn leads to expression of matrix metalloproteinases and plasmin, alteration of adhesion molecule function, smooth muscle cell and neutrophil chemotaxis, and smooth muscle cell differentiation to endothelial cells, generally resulting in angiogenesis. TIE2 and ANGPT1 are reported to be elevated in human RA synovium (43). Using SCID mouse-human JIA synovium chimeras, Scola et al. studied the in vivo regulation of angiogenesis in JIA and found high angiogenic activity that could be correlated with the presence of ANGPT1 mRNA (44). Furthermore, TNFα up-regulates ANGPT1 in synoviocytes and TIE2 in endothelial cells through nuclear factor κB. Thus ANGPT11 is a biologically plausible candidate gene in JIA.
The IL2RA locus has been implicated in GWAS for many autoimmune diseases and is functionally important for IL2 signaling in T cell growth and differentiation. While statistical evidence of an association in JIA with IL2RA polymorphisms (rs12251307 and rs2104286) was found for the initial cohort, these associations were not corroborated in our replication cohort. It should be noted that genotyping for rs12251307 failed for the replication cohort and could not be considered, and two recent reports measuring rs2104286 provide conflicting results (14, 28). Hinks et al. reported an association where replication that included preliminary data from the Cincinnati-based initial case cohort and considered JIA subtype (Table 5). Prahalad et al. failed to find an association with this same SNP using samples overlapping in part with the Utah collection presented here. It should be noted that the Texas and German collections support association for the C allele of rs2104286 when limited to polyRFneg subtype (German, p=0.014, OR=0.66; Texas, p=0.08, OR=0.69) which is in contrast to findings in the initial cohort where there was no distinction between oligoarticular and polyRFneg subtypes. Therefore an association with the IL2RA region in JIA cannot be excluded.
Finally, strong support for a role for STAT4 as a susceptibility locus in JIA is consistent across sample collections and with previous reports in JIA (Table 5 and supplemental figure 1). It should be noted that there is overlap of samples in this report and a previous report including rs7574865 (14).
Well-powered genetic association studies in adult RA have revealed the potential importance of the CD40 signaling pathway in disease pathogenesis and include associations with CD40 (45), REL (46), TRAF1 (47), TNFAIP3 (48) and CTLA4. For JIA, there are reports of associations for SNPs related to TRAF1, TNFAIP3 and CTLA4, which were not supported in the current study (Table 5). The variability in findings between different JIA sample collections highlights the need for further work which will likely benefit from collaborative efforts to attain clinically homogeneous cohorts with sufficient numbers of samples, allowing subtypes to be considered independently and include replication. One of the difficulties presented to the clinical investigator in pediatric diseases, especially in pediatric rheumatology, is the limited phenotypes available to make the diagnosis, the overlapping nature of these phenotypes and the absence of agreed upon biomarkers in separating the disease subtypes. This presents challenges to the development of the uniform populations necessary for these studies.
This study explored a set of 425 polymorphisms previously implicated in autoimmune disease GWAS. No effort was made to declare experiment-wise significance in the initial cohort for these a priori SNPs, and resources were not available to exhaustively rule out SNPs with smaller effect sizes. Rather, the initial cohort was used to suggest a modest list of polymorphisms for replication. The initial cohort was well-powered to detect SNPs with effect sizes similar to those reported in other autoimmune diseases. Specifically, assuming an additive genetic model, a minor allele frequency of 0.20, and type 1 error rates of 0.001 and 0.0001 (0.05 divided by 425 tests), the initial cohort had ~0.80 power to detect odds ratios of 1.31 and 1.36, respectively. Under the same assumptions (α=0.0001), the combined initial and replication cohorts (1824 cases, 5104 controls) has ~0.80 power to detect an OR=1.24 and ~0.50 power to detect OR=1.20. This study is limited to SNPs on the SNP Array 6.0 and published p-value < 1.0×10−5(10). Allowing this threshold for SNP candidacy admits some loci that are not considered “established”. However, this strategy is consistent with many follow-up genotyping studies and should 1) improve the statistical power, and 2) potentially adds to the general autoimmune disease literature by “confirming” in another autoimmune disease variants not yet established. Thus, although not a definitive examination of previously implicated autoimmune disease polymorphisms or surrounding regions, this comprehensive study has identified novel JIA loci in common with AD findings as well as provided confirmation for several reported JIA associations.
There is still much to be discovered in JIA which will require well powered studies and comprehensive SNP panels. The strong genetic correlation of the SNPs across most regions adds complexity to analysis; therefore genetic studies alone are unlikely to identify the causal variant, thus necessitating functional studies. The identification of common autoimmune loci will further the knowledge about shared and disease-specific pathways that may ultimately become the target of therapeutic intervention.
Supplementary Material
Supplemental Figure 1. Forest plots and case-control association results for variants for initial (CCHMC-WTCCC) and individual replication samples (Germany, Texas, and Utah) are provided. 95% confidence intervals (95% CIs) for these ORs are indicated by horizontal lines. For comparison, results for the combined replication samples and a meta analysis using both the initial and replication cohorts are provided. Individual SNP variants are grouped by genetic loci.
Acknowledgments
Grant support RC1AR058587 (SDT), P30AR473639 (SDT); R01AR057106 (SDT and CDL); N01AR42272 (DNG), U01 AI067150 (DNG). TSRHC 0305756 (CAW and MP); BMBF-Germany 01GM0907, 01 ZZ 0403 (JPH); K23 AR50177, Arthritis Foundation (SP); Val A. Browning Foundation (JB).
The out-of-study control data was kindly provided by the Wellcome Trust Case Control Consortium. We thank Chris Cotsapas for discussions concerning risk factors common in autoimmune disease and the selection of additional SNP markers not available in public databases and Sandy Kramer for her efforts in recruiting JIA patients for these genetic studies. The Cincinnati control cohort including SNP typings was funded by the Cincinnati Children’s Hospital Medical Center and directed by Dr. Michael Spigarelli. Wake Forest University Health Sciences Center for Public Health Genomics provided resources for computing.
References
- 1.Grom AA, Hirsch R. T-cell and T-cell receptor abnormalities in the immunopathogenesis of juvenile rheumatoid arthritis. Curr Opin Rheumatol. 2000;12(5):420–4. doi: 10.1097/00002281-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Bowyer S, Roettcher P. Pediatric rheumatology clinic populations in the United States: results of a 3 year survey. Pediatric Rheumatology Database Research Group. J Rheumatol. 1996;23(11):1968–74. [PubMed] [Google Scholar]
- 3.Glass DN, Giannini EH. Juvenile rheumatoid arthritis as a complex genetic trait. Arthritis Rheum. 1999;42(11):2261–8. doi: 10.1002/1529-0131(199911)42:11<2261::AID-ANR1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 4.Saurenmann RK, Rose JB, Tyrrell P, Feldman BM, Laxer RM, Schneider R, et al. Epidemiology of juvenile idiopathic arthritis in a multiethnic cohort: ethnicity as a risk factor. Arthritis Rheum. 2007;56(6):1974–84. doi: 10.1002/art.22709. [DOI] [PubMed] [Google Scholar]
- 5.Prahalad S, Shear ES, Thompson SD, Giannini EH, Glass DN. Increased prevalence of familial autoimmunity in simplex and multiplex families with juvenile rheumatoid arthritis. Arthritis Rheum. 2002;46(7):1851–6. doi: 10.1002/art.10370. [DOI] [PubMed] [Google Scholar]
- 6.Maas K, Chan S, Parker J, Slater A, Moore J, Olsen N, et al. Cutting edge: molecular portrait of human autoimmune disease. J Immunol. 2002;169(1):5–9. doi: 10.4049/jimmunol.169.1.5. [DOI] [PubMed] [Google Scholar]
- 7.Myerscough A, John S, Barrett JH, Ollier WE, Worthington J. Linkage of rheumatoid arthritis to insulin-dependent diabetes mellitus loci: evidence supporting a hypothesis for the existence of common autoimmune susceptibility loci. Arthritis Rheum. 2000;43(12):2771–5. doi: 10.1002/1529-0131(200012)43:12<2771::AID-ANR17>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 8.Hemminki K, Li X, Sundquist J, Sundquist K. Familial associations of rheumatoid arthritis with autoimmune diseases and related conditions. Arthritis Rheum. 2009;60(3):661–8. doi: 10.1002/art.24328. [DOI] [PubMed] [Google Scholar]
- 9.Gregersen PK, Olsson LM. Recent Advances in the Genetics of Autoimmune Disease. Annual Review of Immunology. 2009;27(1):363–391. doi: 10.1146/annurev.immunol.021908.132653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hindorff LA, Junkins HA, Mehta JP, Manolio TA. A Catalog of Published Genome-Wide Association Studies. [Accessed: (December 23, 2009)]; Available at: < http://www.genome.gov/gwastudies>.
- 11.Hollenbach J, Thompson SD, Bugawan T, Ryan M, Sudman M, Marion M, et al. Juvenile idiopathic arthritis and HLA class I and class II interaction and age-at-onset effects. Arthritis Rheum. 2010;42(6):1781–1791. doi: 10.1002/art.27424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31(2):390–2. [PubMed] [Google Scholar]
- 13.The Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447(7145):661–78. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prahalad S, Hansen S, Whiting A, Guthery SL, Clifford B, McNally B, et al. Variants in TNFAIP3, STAT4, and C12orf30 loci associated with multiple autoimmune diseases are also associated with juvenile idiopathic arthritis. Arthritis Rheum. 2009;60(7):2124–30. doi: 10.1002/art.24618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haas JP, Nevinny-Stickel C, Schoenwald U, Truckenbrodt H, Suschke J, Albert ED. Susceptible and Protective MHC-class II Haplotypes in Early Onset Pauciarticular Juvenile Chronic Arthritis. Human Immunol. 1994;41:225–233. doi: 10.1016/0198-8859(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 16.Beyersdorff A, Hoffmann W, Lingnau ML, Ebner A, Fusch C, Haas JP. Survey of Neonates in Pomerania (SNiP): A population based analysis of the motherśquality of life after delivery with spezial relations to their social integration. Int J Public Health. 2008;53:87–93. doi: 10.1007/s00038-008-6114-5. [DOI] [PubMed] [Google Scholar]
- 17.Fellay J, Shianna KV, Ge D, Colombo S, Ledergerber B, Weale M, et al. A whole-genome association study of major determinants for host control of HIV-1. Science. 2007;317(5840):944–7. doi: 10.1126/science.1143767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38(8):904–9. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 19.Velicer W. Determining the number of components from the matrix of partial correlations. Psychometrika. 1976;41(3):321–327. [Google Scholar]
- 20.Patterson N, Price AL, Reich D. Population structure and eigenanalysis. PLoS Genet. 2006;2(12):e190. doi: 10.1371/journal.pgen.0020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marchini J, Howie B, Myers S, McVean G, Donnelly P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nat Genet. 2007;39(7):906–13. doi: 10.1038/ng2088. [DOI] [PubMed] [Google Scholar]
- 22.Hinks A, Barton A, John S, Bruce I, Hawkins C, Griffiths CE, et al. Association between the PTPN22 gene and rheumatoid arthritis and juvenile idiopathic arthritis in a UK population: further support that PTPN22 is an autoimmunity gene. Arthritis Rheum. 2005;52(6):1694–9. doi: 10.1002/art.21049. [DOI] [PubMed] [Google Scholar]
- 23.Seldin MF, Shigeta R, Laiho K, Li H, Saila H, Savolainen A, et al. Finnish case-control and family studies support PTPN22 R620W polymorphism as a risk factor in rheumatoid arthritis, but suggest only minimal or no effect in juvenile idiopathic arthritis. Genes Immun. 2005;6(8):720–2. doi: 10.1038/sj.gene.6364255. [DOI] [PubMed] [Google Scholar]
- 24.Viken MK, Amundsen SS, Kvien TK, Boberg KM, Gilboe IM, Lilleby V, et al. Association analysis of the 1858C>T polymorphism in the PTPN22 gene in juvenile idiopathic arthritis and other autoimmune diseases. Genes Immun. 2005;6(3):271–3. doi: 10.1038/sj.gene.6364178. [DOI] [PubMed] [Google Scholar]
- 25.Hinks A, Ke X, Barton A, Eyre S, Bowes J, Worthington J, et al. Association of the IL2RA/CD25 gene with juvenile idiopathic arthritis. Arthritis Rheum. 2009;60(1):251–7. doi: 10.1002/art.24187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hinks A, Eyre S, Ke X, Barton A, Martin P, Flynn E, et al. Association of the AFF3 gene and IL2/IL21 gene region with juvenile idiopathic arthritis. Genes Immun. 2010:1–5. doi: 10.1038/gene.2009.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Remmers EF, Plenge RM, Lee AT, Graham RR, Hom G, Behrens TW, et al. STAT4 and the risk of rheumatoid arthritis and systemic lupus erythematosus. N Engl J Med. 2007;357(10):977–86. doi: 10.1056/NEJMoa073003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hinks A, Eyre S, Ke X, Barton A, Martin P, Flynn E, et al. Overlap of disease susceptibility loci for rheumatoid arthritis (RA) and juvenile idiopathic arthritis (JIA) Ann Rheum Dis. 2009 doi: 10.1136/ard.2009.110650. ard.2009.110650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silverberg MS, Cho JH, Rioux JD, McGovern DP, Wu J, Annese V, et al. Ulcerative colitis-risk loci on chromosomes 1p36 and 12q15 found by genome-wide association study. Nat Genet. 2009;41(2):216–20. doi: 10.1038/ng.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burton PR, Clayton DG, Cardon LR, Craddock N, Deloukas P, Duncanson A, et al. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat Genet. 2007;39(11):1329–1337. doi: 10.1038/ng.2007.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burgner D, Davila S, Breunis WB, Ng SB, Li Y, Bonnard C, et al. A genome-wide association study identifies novel and functionally related susceptibility Loci for Kawasaki disease. PLoS Genet. 2009;5(1):e1000319. doi: 10.1371/journal.pgen.1000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hinks A, Barton A, Shephard N, Eyre S, Bowes J, Cargill M, et al. Identification of a novel susceptibility locus for juvenile idiopathic arthritis by genome-wide association analysis. Arthritis Rheum. 2009;60(1):258–63. doi: 10.1002/art.24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Behrens EM, Finkel TH, Bradfield JP, Kim CE, Linton L, Casalunovo T, et al. Association of the TRAF1-C5 locus on chromosome 9 with juvenile idiopathic arthritis. Arthritis Rheum. 2008;58(7):2206–7. doi: 10.1002/art.23603. [DOI] [PubMed] [Google Scholar]
- 34.Albers HM, Kurreeman FA, Houwing-Duistermaat JJ, Brinkman DM, Kamphuis SS, Girschick HJ, et al. The TRAF1/C5 region is a risk factor for polyarthritis in juvenile idiopathic arthritis. Ann Rheum Dis. 2008;67(11):1578–80. doi: 10.1136/ard.2008.089060. [DOI] [PubMed] [Google Scholar]
- 35.Albers HM, Kurreeman FA, Stoeken-Rijsbergen G, Brinkman DM, Kamphuis SS, van Rossum MA, et al. Association of the autoimmunity locus 4q27 with juvenile idiopathic arthritis. Arthritis Rheum. 2009;60(3):901–4. doi: 10.1002/art.24296. [DOI] [PubMed] [Google Scholar]
- 36.Barton A, Thomson W, Ke X, Eyre S, Hinks A, Bowes J, et al. Re-evaluation of putative rheumatoid arthritis susceptibility genes in the post-genome wide association study era and hypothesis of a key pathway underlying susceptibility. Hum Mol Genet. 2008;17(15):2274–9. doi: 10.1093/hmg/ddn128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith RD, Lupashin VV. Role of the conserved oligomeric Golgi (COG) complex in protein glycosylation. Carbohydr Res. 2008;343(12):2024–31. doi: 10.1016/j.carres.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ungar D. Golgi linked protein glycosylation and associated diseases. Semin Cell Dev Biol. 2009;20(7):762–9. doi: 10.1016/j.semcdb.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, Helms C, Liao W, Zaba LC, Duan S, Gardner J, et al. A genome-wide association study of psoriasis and psoriatic arthritis identifies new disease loci. PLoS Genet. 2008;4(3):e1000041. doi: 10.1371/journal.pgen.1000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kubota Y, Sano M, Goda S, Suzuki N, Nishiwaki K. The conserved oligomeric Golgi complex acts in organ morphogenesis via glycosylation of an ADAM protease in C. elegans. Development. 2006;133(2):263–73. doi: 10.1242/dev.02195. [DOI] [PubMed] [Google Scholar]
- 41.Van Eerdewegh P, Little RD, Dupuis J, Del Mastro RG, Falls K, Simon J, et al. Association of the ADAM33 gene with asthma and bronchial hyperresponsiveness. Nature. 2002;418(6896):426–30. doi: 10.1038/nature00878. [DOI] [PubMed] [Google Scholar]
- 42.Lesueur F, Oudot T, Heath S, Foglio M, Lathrop M, Prud’homme JF, et al. ADAM33, a new candidate for psoriasis susceptibility. PLoS ONE. 2007;2(9):e906. doi: 10.1371/journal.pone.0000906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeBusk LM, Chen Y, Nishishita T, Chen J, Thomas JW, Lin PC. Tie2 receptor tyrosine kinase, a major mediator of tumor necrosis factor alpha-induced angiogenesis in rheumatoid arthritis. Arthritis Rheum. 2003;48(9):2461–71. doi: 10.1002/art.11213. [DOI] [PubMed] [Google Scholar]
- 44.Scola MP, Imagawa T, Boivin GP, Giannini EH, Glass DN, Hirsch R, et al. Expression of angiogenic factors in juvenile rheumatoid arthritis: correlation with revascularization of human synovium engrafted into SCID mice. Arthritis Rheum. 2001;44(4):794–801. doi: 10.1002/1529-0131(200104)44:4<794::AID-ANR135>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 45.Raychaudhuri S, Remmers EF, Lee AT, Hackett R, Guiducci C, Burtt NP, et al. Common variants at CD40 and other loci confer risk of rheumatoid arthritis. Nat Genet. 2008;40(10):1216–23. doi: 10.1038/ng.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gregersen PK, Amos CI, Lee AT, Lu Y, Remmers EF, Kastner DL, et al. REL, encoding a member of the NF-kappaB family of transcription factors, is a newly defined risk locus for rheumatoid arthritis. Nat Genet. 2009;41(7):820–3. doi: 10.1038/ng.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Plenge RM, Seielstad M, Padyukov L, Lee AT, Remmers EF, Ding B, et al. TRAF1-C5 as a Risk Locus for Rheumatoid Arthritis -- A Genomewide Study. N Engl J Med. 2007;357(12):1199–1209. doi: 10.1056/NEJMoa073491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Plenge RM, Cotsapas C, Davies L, Price AL, de Bakker PI, Maller J, et al. Two independent alleles at 6q23 associated with risk of rheumatoid arthritis. Nat Genet. 2007;39(12):1477–82. doi: 10.1038/ng.2007.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Todd JA, Walker NM, Cooper JD, Smyth DJ, Downes K, Plagnol V, et al. Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet. 2007;39(7):857–64. doi: 10.1038/ng2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raychaudhuri S, Thomson BP, Remmers EF, Eyre S, Hinks A, Guiducci C, et al. Genetic variants at CD28, PRDM1 and CD2/CD58 are associated with rheumatoid arthritis risk. Nat Genet. 2009;41(12):1313–8. doi: 10.1038/ng.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Forest plots and case-control association results for variants for initial (CCHMC-WTCCC) and individual replication samples (Germany, Texas, and Utah) are provided. 95% confidence intervals (95% CIs) for these ORs are indicated by horizontal lines. For comparison, results for the combined replication samples and a meta analysis using both the initial and replication cohorts are provided. Individual SNP variants are grouped by genetic loci.