Abstract
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized histopathologically by the presence of senile plaques (SP), neurofibrillary tangles, and synapse loss. The main component of SP is amyloid-β peptide (Aβ) that has been associated with increased oxidative stress, leading to oxidative modification of proteins and consequently to neurotoxicity and neurodegeneration. Low-density lipoprotein receptor-related protein 1 (LRP1) is the primary moiety responsible for the efflux of Aβ from the brain to the blood across the blood-brain barrier (BBB). Impaired brain-to-blood transport of Aβ by LRP1 has been hypothesized to contribute to increased levels of Aβ in AD brain. The cause of LRP1 dysfunction is unknown, but we have hypothesized that Aβ oxidizes LRP1, thus damaging its own transporter. Consistent with this notion, we report in the current study a significant increase in the levels of the lipid peroxidation product 4-hydroxy-2-nonenal (HNE) bound to transmembrane LRP1 in AD hippocampus. In contrast, the levels of LRP1-resident 3-nitrotyrosine (3NT) did not show a significant increase in AD hippocampus compared to age-matched controls. Based on this study, we propose that Aβ impairs its own efflux from the brain by oxidation of its transporter LRP1, leading to increased Aβ deposition in brain, thereby contributing to subsequent cognitive impairment in AD.
Keywords: Alzheimer’s disease, amyloid β-peptide, low-density lipoprotein receptor-related protein 1, oxidative stress, lipid peroxidation, 4-hydroxy-2-nonenal
Introduction
AD is characterized pathologically by the presence of senile plaques (SPs), neurofibrillary tangles (NFTs), and decreased synaptic density [1, 2]. The main component of SPs is amyloid-β peptide (Aβ) [3], comprised of 40–42 amino acids and generated by proteolytic cleavage of amyloid precursor protein (APP), a transmembrane protein, by β-secretase and γ-secretase. Aβ exists in various soluble and insoluble forms including aggregates, soluble monomers, oligomers, protofibrils, and fibrils [4, 5]. Recent studies have suggested that soluble oligomers are the most toxic form of Aβ [6]. Genetic mutations in APP and presenilin 1 (PS1) genes in familial AD cases show increased production of Aβ and consequently an early onset of AD, consistent with the notion that Aβ is central to the pathogenesis of AD [7]. Further, elevated levels of Aβ 1–40 and 1–42 have been found in AD hippocampus and cortex and have been associated with high levels of protein oxidation, lipid peroxidation, DNA and RNA damage [8]. Conversely, brain regions low in Aβ levels, such as the cerebellum, do not have extensive markers of oxidative stress [9–14]. Aβ has been shown to induce oxidative stress in vitro and in AD model systems in vivo, as evidenced by increased levels of protein oxidation (indexed by protein carbonyls and protein resident 3-nitrotyrosine 3NT) and lipid peroxidation (indexed by protein-bound 4-hydroxy-2-nonenal HNE) [15–19]. Studies by Liu et al show that the addition of HNE to tau protein, the primary component of NFTs, promote and contribute to conformations conducive to NFT formation further supporting the role of Aβ in the pathogenesis of AD [20].
The neurovascular hypothesis of AD states that impairment of the efflux of Aβ from the brain to the blood at the blood-brain barrier (BBB) is an important mechanism underlying Aβ accumulation in the brain and contributes to subsequent cognitive impairments in AD patients [21]. The major efflux pump for the clearance of Aβ from the brain to the periphery is the LDL-related receptor protein 1 (LRP1) [22, 23]. LRP1 is a membrane-associated protein initially synthesized as a 600 kDa precursor and further processed into two non-covalently linked α- and β-subunits [24]. The 515 kDa α-subunit is extracellular and non-covalently bound to the transmembrane 85 kDa β-subunit. The α-subunit is responsible for ligand binding, while the β-subunit cytoplasmic domain interacts with adapter proteins involved in cell signaling [22]. In the current study, we tested the hypothesis that LRP1 is oxidized in the hippocampus of subjects with AD. Such oxidative modifications to LRP1 would alter its structure, providing a mechanism by which LRP1’s ability to efflux Aβ would be affected. Aβ is hypothesized to lead to lipid peroxidation in AD brain [8, 25–29]. We reported elevated HNE bound to the glutamate transporter, GLT-1 (EAAT2) [30], which has decreased function in AD [31], and this elevation of HNE could be replicated by addition of Aβ (1–42) to synaptosomes [30]. Based on analogy to the case of GLT-1, we hypothesize that HNE bound to β-subunit of LRP1 would lead to increased Aβ accumulation in the brain with subsequent oxidative stress, plaque formation, and AD pathogenesis. Accordingly, in the present study, we measured levels of HNE-bound to and 3NT on the β-subunit of LRP1 in AD hippocampus to assess the level of oxidative post-translational modifications (PTMs) to LRP1. The β-subunit, as described above, contains the membrane-spanning portion of LRP1 and the subunit is rich overall in histidine, lysine, and cysteine residues (UniProt protein Database ID Q07954, Short name=LRP-85), likely providing potential targets in the β-subunit of LRP1 for HNE addition [28].
Materials and Methods
Materials
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) with the exceptions of nitrocellulose membranes (Bio-Rad, Hercules, CA). The anti-LRP1 antibody has been described in previously published research [23].
Subjects
Frozen hippocampus from AD and age-matched controls were obtained from the University of Kentucky Rapid Autopsy Program of the Alzheimer's Disease Clinical Center (UK ADC). All AD subjects displayed progressive intellectual decline. Control subjects underwent annual mental status testing as a part of the UK ADRC normal volunteer longitudinal aging study and did not have a history of dementia or other neurological disorders. Brains from subjects with neurodegeneration were collected after a short post mortem interval (PMI) that averaged less than 5 hours. AD brains had Braak stages ranging from 4–6. Braak staging indicates the severity of AD pathology [based largely on the number of neurofibrillary tangles and ranges from 1–6, with the most severe stage being 6 [32]]. All control subjects had test scores for dementia in the normal range and all the control brains had a short PMI average less than 3 hours and Braak stages of 2 or less. (Table I).
Table 1.
Demographic characteristics of control and AD patients
| Samples | Age (yrs) | Gender (M/F) | Post Mortem interval (h) | Braak staging |
|---|---|---|---|---|
| Controls | 82 ± 6.2 | 6/3 | 2.6 ± 0.8 | 1–2 |
| AD | 85 ± 5.3 | 5/4 | 4.8 ± 1.6 | 4–6 |
Sample preparation
AD (n=9) and age-matched control (n=9) hippocampi were minced and homogenized separately in Media-I containing 10mM HEPES buffer (pH 7.4), 137 mM NaCl, 4.6 mM KCl, 1.1 mM KH2PO4, 0.1 mM EDTA, and 0.6 mM MgSO4 as well as protease inhibitors: leupeptin (0.5 mg/mL), pepstatin (0.7 µg/mL), type II S soybean trypsin inhibitor (0.5 µg/mL), and PMSF (40 µg/mL). These homogenates were centrifuged at 14,000 × g for 10 min to remove debris. Protein concentration in the supernatant was determined by the BCA assay using Pierce kit.
Immunoprecipitation of LRP1
Protein A/G-agarose beads (50 µL per sample, i.e., 900 µL for 18 samples) (Amersham Pharmacia Biotech, Piscataway, NJ, USA) were washed with immunoprecipitation (IP) buffer 3 times for 5 min using a vortex with shaker attachment. IP buffer contains phosphate buffered saline (PBS) with 0.05% NP-40 and protease inhibitors leupeptin (4 µg/mL final concentration), pepstatin (4 µg/mL final concentration), aprotinin (5 µg/mL final concentration) adjusted to pH 8. Hippocampal homogenates from AD and control subjects (300 µg) were first pre-cleared with washed protein A/G-agarose beads (50 µL) for 1 h at 4°C. Samples were then incubated overnight with anti-LRP1 antibody (5 µg) followed by 1 h incubation with protein A/G-agarose. The antigen-antibody-protein A/G complex was centrifuged at 1,000 × g for 5 min and the resultant pellet was washed 5 times with IP buffer (500 µL). The final pellet was suspended in deionized water. Proteins were resolved on SDS-PAGE, followed by immunoblotting on a nitrocellulose membrane (Bio-Rad, Hercules, CA, USA).
Immunodetection
For immunodetection of HNE-bound to and 3NT-resident LRP1 the nitrocellulose membranes were blocked with 3% bovine serum albumin (BSA) in phosphate-buffered saline containing 0.01% (w/v) sodium azide and 0.2% (v/v) Tween 20 (PBST) for 90 min at room temperature. The membranes were incubated with anti-LRP1 polyclonal antibody diluted 1:5000 in 3% BSA, anti-actin monoclonal antibody (Sigma-Aldrich, St. Louis, MO, USA) diluted 1:5000 in 3%BSA, anti-HNE polyclonal antibody (Alpha diagnostic, San Antonia, TX) diluted 1:5000 in 3% BSA, or anti-3 nitrotyrosine polyclonal antibody (3NT) (Sigma-Aldrich, St. Louis MO), diluted 1:2000 in 3% BSA, for 2 h at room temperature with rocking. Following completion of the primary antibody incubation, the membranes were washed three times in wash blot for 5 min each and incubated with anti-rabbit IgG alkaline phosphatase-linked (ALP) secondary antibody (Sigma, St. Louis, MO, USA) diluted 1:3000 in wash blot and incubated for 1 h at room temperature. The membranes were washed in wash blot three times for five min each and developed using Sigma Fast Tablets (BCIP/NBT substrate) (Sigma, St. Louis, MO, USA) The western blot measuring the levels of the β-subunit of LRP1 (Figure 1) was incubated with anti-LRP1 antibody (1:5000) as described above and following completion of the primary antibody incubation, the membrane were washed three times in wash blot for 5 min each and incubated with anti-rabbit IgG horse radish peroxidase-linked (HRP) secondary antibody (GE Healthcare, Piscataway, NJ, USA) diluted 1:3000 in wash blot and incubated for 1 h at room temperature and was visualized using ECL Plus western blotting detection reagents (GE Healthcare, Piscataway, NJ, USA). The blot was subsequently stripped using Reblot Plus Strong antibody Stripping Solution (Millipore, Billerica, MA, USA) as described by the manufacturer and redeveloped using anti-actin antibody (Sigma, St. Louis, MO, USA) as described above using anti-rabbit ALP secondary antibody. The western blot measuring the HNE-bound β-subunit of LRP1 normalized on the same blot with unmodified LRP1 (Figure 3) was visualized using anti-rabbit HRP antibody and stripped with prepared strong stripping solution (62.5 mM Tris-HCl (pH 6.8), SDS (2% wt/vol), and β-mercaptoethanol (10 mM)). The stripped western blot was washed three times in wash blot and blocked with BSA. The western blot was reprobed with anti-LRP1 antibody and anti-rabbit HRP linked secondary antibody as described above.
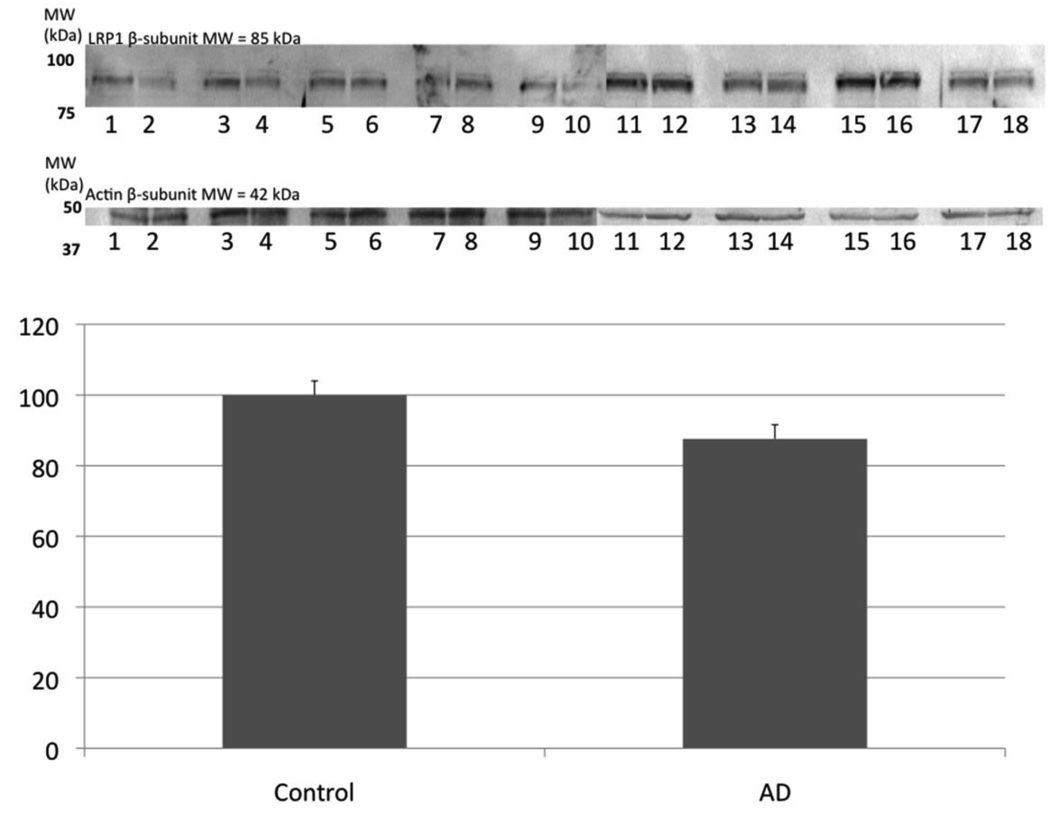
Figure 1.
Levels of LRP1 were determined using Western blotting and no significant difference in the protein level of LRP1 was found between AD hippocampus and age-matched control. Actin was used as a loading control as pictured. Data are shown as percent control (mean ± SEM). The images shown have odd numbers below the age-matched control hippocampal samples and even numbers below AD hippocampal samples, respectively.
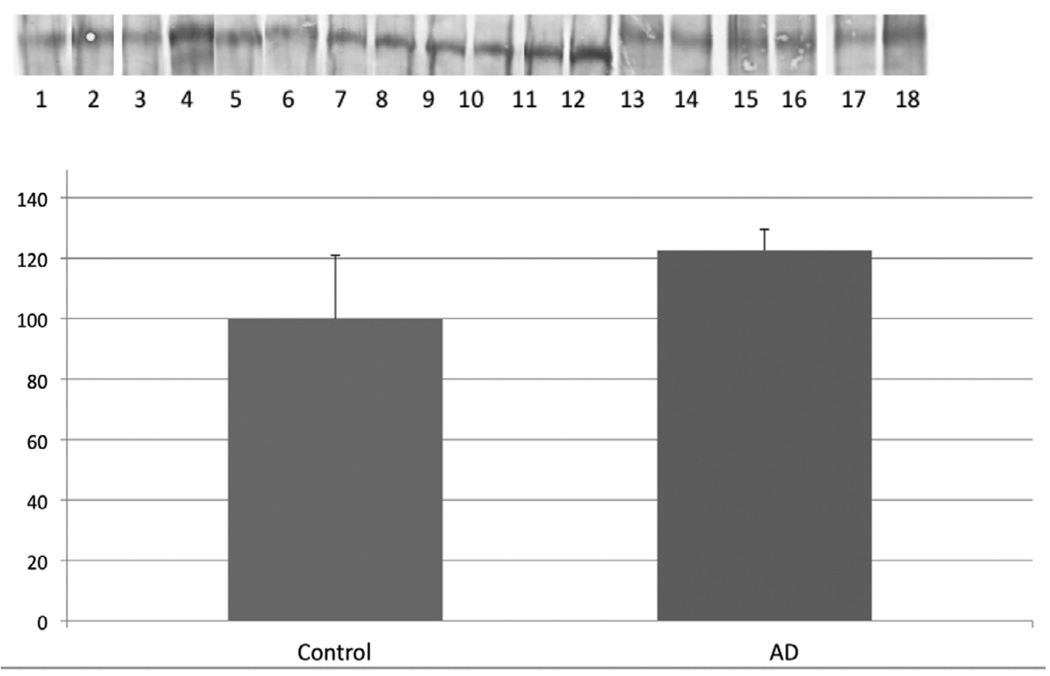
Figure 3.
Levels of LRP1-resident 3NT were determined by immunoprecipitation of LRP1 followed by immunochemical detection using anti-3NT antibody. The levels of LRP1-resident 3NT were not significantly different in AD hippocampus compared to age-matched controls. Data are shown as percent control (mean ± SEM). The images shown have odd numbers below age-matched control hippocampal samples and even numbers below AD hippocampal samples, respectively.
Image analysis
After immunodetection of oxidative modification of LRP1 the membranes were completely dried at room temperature and were then scanned using a Microtek Scanmaker 4900 scanner and a Storm860 phosphoimager (GE Healthcare, Piscataway, NJ, USA). Images were saved as tiff files in grayscale mode and the intensity of the LRP1 protein modification was quantified using ImageQuant (GE health science, Piscataway, NJ, USA) analysis software.
Statistical analysis
Raw values were exported to Microsoft Excel and normalized to percent control values. The resulting data were analyzed by Student's t tests. A value of p <0.05 was considered statistically significant.
Results
In the present study we measured the levels of LRP1 and the levels HNE-bound-as well as 3NT modification of LRP1 using immunoprecipitation techniques in AD and age-matched control hippocampus. Figure 1 is a Western blot showing levels of LRP1 β-subunit in AD hippocampus is not significantly different compared to age-matched controls. Figure 2 shows a 60% increase in levels of HNE-bound LRP1 β-subunit in AD hippocampus compared to age-matched controls. No significant increase of 3NT-modified LRP1 β-subunit was observed in AD hippocampus compared to age-matched control (Figure 3). The raw values for the intensity of the LRP1 β-subunit bands obtained in the immunoprecipitation studies represented in Figure 2 and Figure 3 were normalized against the LRP1 bands obtained from the Western blot represented in Figure 1. (e.g., The raw value for the HNE-bound LRP1 β-subunit band in Lane 1 (Figure 2) was divided by the raw value obtained from the LRP1 β-subunit band, corresponding with the same AD subject, from Lane 1 in the Western blot (Figure 1) probing for overall levels of the β-subunit and multiplied by 100 to obtain % control values.)
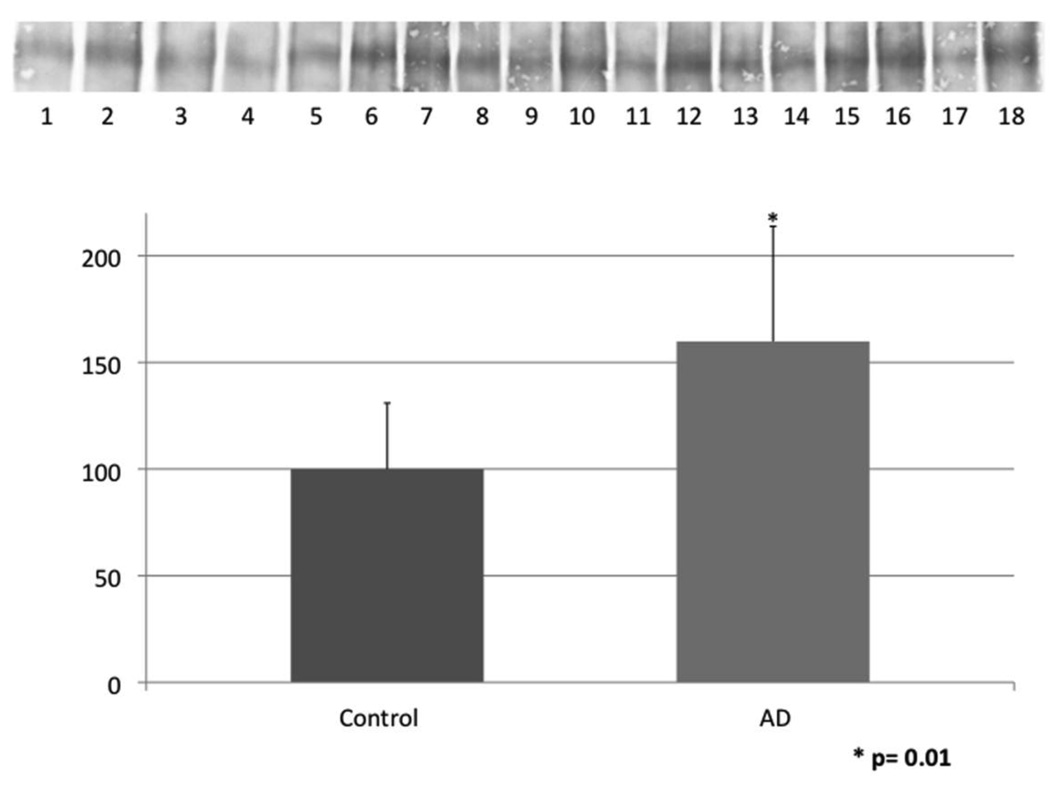
Figure 2.
Levels of HNE-bound LRP1 were determined by immunoprecipitation of LRP1 followed by immunochemical detection with anti-HNE antibody. The level of HNE-bound LRP1 was significantly increased by 60% in AD hippocampus compared to age-matched controls. Data are shown as percent control (mean ± SEM) and * signifies p<0.05. The images shown have odd numbers below age-matched control hippocampal samples and even numbers below AD hippocampal samples, respectively.
To confirm our results of the increased levels of HNE-bound LRP1 in AD hippocampus, immunoprecipitated LRP1 was probed on a western blot with anti-HNE antibody, stripped, and reprobed with anti-LRP1 antibody. The HNE-bound LRP1 bands were normalized with the unmodified LRP1 bands obtained from the same blot. The results show a 67% in AD hippocampus compared to age-matched controls (Figure 4).
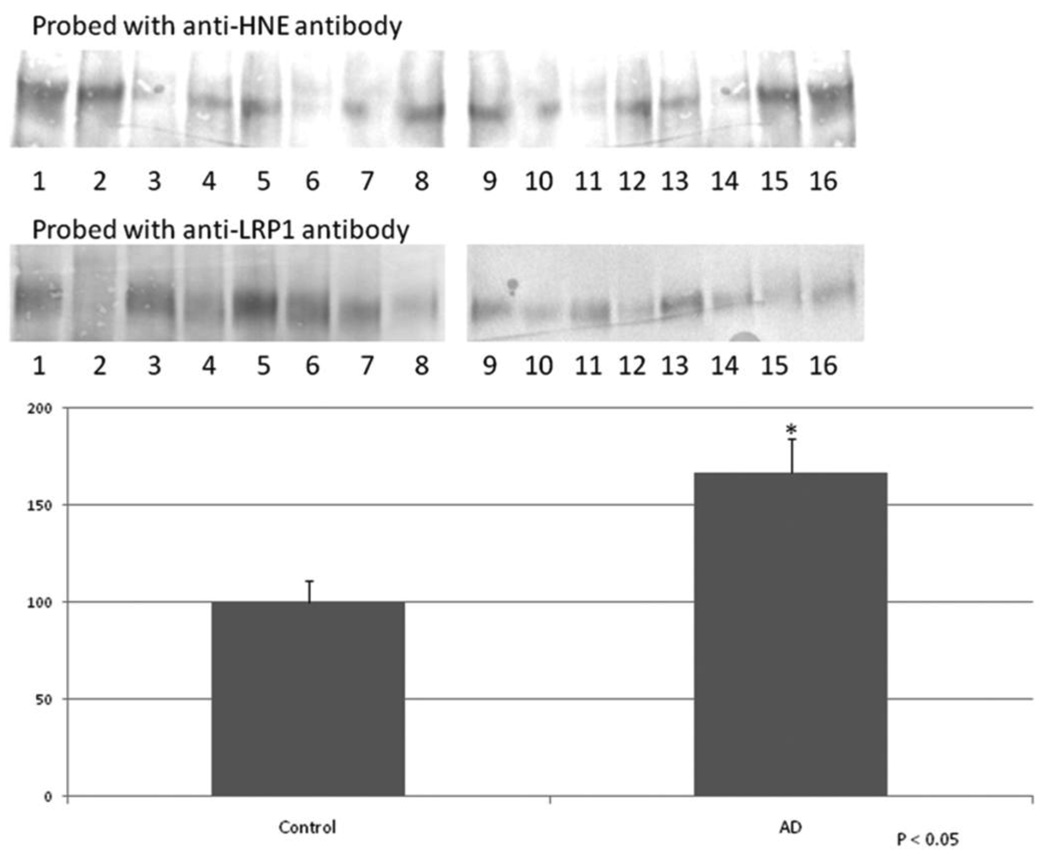
Figure 4.
Confirmation of increased HNE-bound LRP1 levels in AD hippocampus. Immunoprecipitated LRP1 was probed on a western blot with anti-HNE antibody, stripped, and reprobed with anti-LRP1 antibody. The HNE-bound LRP1 bands were normalized with the unmodified LRP1 bands on the same blot. The results show a 67% in AD hippocampus compared to age-matched controls. Data are shown as percent control (mean ± SEM) and * signifies p<0.05. The images shown have odd numbers below age-matched control hippocampal samples and even numbers below AD hippocampal samples, respectively.
Discussion
LRP1 is a multifunctional protein that scavenges, serves as a signaling receptor, and transports multiple binding partners, including apoE, α-2- macroglobulin, tissue plasminogen activator, plasminogen activator inhibitor-1, factor VIII, lactoferrin, and Aβ [33–35]. Recent studies show that LRP1 interacts with APP, BACE1 and PS1, proteins involved in Aβ production [36, 37] and that LRP1 activity is diminished at the BBB of AD patients [38]. In addition, LRP1 has been shown to mediate both apoE and cholesterol levels in the CNS through APP and regulate the influence of apoE on microglial inflammation in cell culture systems [23, 39, 40].
However, it is unclear what causes LRP1 dysfunction in AD. The current study shows that HNE-bound LRP1 β-subunit, containing the transmembrane portion of the protein, is significantly increased in AD hippocampus compared to age-matched controls, consistent with the hypothesis that oxidative modification to LRP1 contributes to increased Aβ load in AD brain. LRP1 in other tissues is readily oxidized with resulting loss of function [41, 42]. As noted, previous studies show that oxidative modifications to biomolecules occur in AD brain [8, 30, 43–45]. Oligomeric Aβ has been shown to induce oxidative stress under in vitro and in vivo conditions, and the Aβ-induced oxidative changes are believed to contribute to neuronal loss and AD pathogenesis [16, 19, 46]. The numbers of senile plaques are elevated in the hippocampus compared to the cerebellum in AD brain [14]. In addition, histopathological studies show extensive cell loss in the hippocampus from AD subjects [37, 47–49]. Previous studies show impaired Aβ efflux at the blood brain barrier (BBB) in transgenic animal models of AD, and as noted there is evidence that LRP1 activity is reduced at the BBB of AD subjects [38, 50]. As noted above, studies from peripheral tissues suggest that the oxidation of LRP1 may reduce the activity of this receptor to its other ligands such as α-2-macroglobulin [41, 42]. Epidemiologic studies propose that oxidation of LRP1 in the blood is one of the risk factors for AD [51, 52]. Further, previous studies reported altered levels of LRP1 in AD brain, possibly leading to increased senile plaque formation, cell death, cognitive impairment and AD pathogenesis [53, 54]. Since LRP1 serves as the main efflux pump of Aβ from the brain to the blood, oxidation of LRP1 by its substrate Aβ may be a mechanism of increased accumulation of Aβ in AD brain.
Protein oxidation often leads to loss of function and cell death via necrotic or apoptotic processes [13]. In the current study we tested the hypothesis that oxidatively modified LRP1 is increased in AD hippocampus compared to age-matched controls. LRP1 β-subunit was immunoprecipitated and probed for protein-bound HNE and 3NT as indices of lipid peroxidation and protein nitration, respectively, in age-matched control and AD hippocampus. We show, for the first time, that the LRP-1 β-subunit is oxidatively modified by HNE in AD hippocampus, a region of the brain with high levels of Aβ and senile plaques. The observed increase in HNE-bound to LRP1 can be explained based on the notion that Aβ, as small oligomers, can insert in the lipid bilayer of brain membranes including brain endothelial cells [27, 30, 55, 56]. The membrane is composed of high levels of polyunsaturated fatty acids, and the incorporation of Aβ into the lipid bilayer alters membrane fluidity and initiates a lipid peroxidation chain reaction, subsequent production of HNE [8, 44, 57], and as shown in the current study, a resulting Michael addition-mediated binding of HNE to LRP1. As presented in the Introduction, we previously demonstrated Aβ-induced lipid peroxidation to another membrane-bound protein, the excitatory amino acid transporter 2 (EAAT2) in rat synaptosomes, and we found elevated HNE bound to EAAT2 in AD brain [30]. This transporter has decreased activity in AD [31] and we speculate that LRP1 activity will decrease with HNE modification. However, further studies are needed to confirm this hypothesis.
Aβ is a neurotoxic peptide that contributes to oxidative stress in AD brain [15–19], and this neurotoxic peptide is removed from the brain by LRP1 [33–35]. Our results from the current study support the notion that Aβ–induced lipid peroxidation inhibits its own efflux mechanism from the brain by increasing the levels of HNE-bound LRP1. The results of this study are consistent with the concept that oxidative modification of LRP1 and not the reduction in levels of LRP1 may be responsible for the increased level of Aβ accumulation in the hippocampus of subjects with AD. Further research is in progress in our laboratories to understand the role of LRP1 oxidation in AD pathogenesis and progression.
Acknowledgements
The authors thank the University of Kentucky ADRC Clinical Neuropathology Core faculty for providing the brain tissue used for this study. This research was supported by a NIH grant to D.A.B. [AG-029839], NIH [AG-029839] and VA Merit Review grants to W.A.B., and a NIH grant to G.B. [R01-027924].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Markesbery WR. Neuropathological criteria for the diagnosis of Alzheimer's disease. Neurobiol. Aging. 1997;18:S13–S19. doi: 10.1016/s0197-4580(97)00064-x. [DOI] [PubMed] [Google Scholar]
- 2.Nelson PT, Jicha GA, Schmitt FA, Liu H, Davis DG, Mendiondo MS, Abner EL, Markesbery WR. Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles "do count" when staging disease severity. J. Neuropathol. Exp. Neurol. 2007;66:1136–1146. doi: 10.1097/nen.0b013e31815c5efb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glenner GG, Wong CW, Quaranta V, Eanes ED. The amyloid deposits in Alzheimer's disease: their nature and pathogenesis. Appl. Pathol. 1984;2:357–369. [PubMed] [Google Scholar]
- 4.Ferreira ST, Vieira MN, De Felice FG. Soluble protein oligomers as emerging toxins in Alzheimer's and other amyloid diseases. IUBMB Life. 2007;59:332–345. doi: 10.1080/15216540701283882. [DOI] [PubMed] [Google Scholar]
- 5.Ward RV, Jennings KH, Jepras R, Neville W, Owen DE, Hawkins J, Christie G, Davis JB, George A, Karran EH, Howlett DR. Fractionation and characterization of oligomeric, protofibrillar and fibrillar forms of beta-amyloid peptide. Biochem J. 2000;348(Pt 1):137–144. [PMC free article] [PubMed] [Google Scholar]
- 6.Resende R, Ferreiro E, Pereira C, Resende de Oliveira C. Neurotoxic effect of oligomeric and fibrillar species of amyloid-beta peptide 1–42: involvement of endoplasmic reticulum calcium release in oligomer-induced cell death. Neuroscience. 2008;155:725–737. doi: 10.1016/j.neuroscience.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 7.Murphy MP, Beckett TL, Ding Q, Patel E, Markesbery WR, St Clair DK, LeVine H, 3rd, Keller JN. Abeta solubility and deposition during AD progression and in APPxPS-1 knock-in mice. Neurobiol. Dis. 2007;27:301–311. doi: 10.1016/j.nbd.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Butterfield DA, Lauderback CM. Lipid peroxidation and protein oxidation in Alzheimer's disease brain: potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radic. Biol. Med. 2002;32:1050–1060. doi: 10.1016/s0891-5849(02)00794-3. [DOI] [PubMed] [Google Scholar]
- 9.Arai H, Kashiwagi S, Nagasaka Y, Uchida K, Hoshii Y, Nakamura K. Oxidative modification of apolipoprotein E in human very-low-density lipoprotein and its inhibition by glycosaminoglycans. Arch. Biochem. Biophys. 1999;367:1–8. doi: 10.1006/abbi.1999.1222. [DOI] [PubMed] [Google Scholar]
- 10.Lovell MA, Markesbery WR. Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer's disease. Nucleic Acids Res. 2007;35:7497–7504. doi: 10.1093/nar/gkm821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sultana R, Boyd-Kimball D, Poon HF, Cai J, Pierce WM, Klein JB, Merchant M, Markesbery WR, Butterfield DA. Redox proteomics identification of oxidized proteins in Alzheimer's disease hippocampus and cerebellum: an approach to understand pathological and biochemical alterations in AD. Neurobiol. Aging. 2006;27:1564–1576. doi: 10.1016/j.neurobiolaging.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 12.Butterfield DA, Yatin SM, Varadarajan S, Koppal T. Amyloid beta-peptide-associated free radical oxidative stress, neurotoxicity, and Alzheimer's disease. Methods Enzymol. 1999;309:746–768. doi: 10.1016/s0076-6879(99)09050-3. [DOI] [PubMed] [Google Scholar]
- 13.Butterfield DA, Stadtman ER. Protein Oxidation processes in aging brain. Adv. Cell Aging Gerontol. 1997;2:161–191. [Google Scholar]
- 14.Hensley K, Hall N, Subramaniam R, Cole P, Harris M, Aksenov M, Aksenova M, Gabbita SP, Wu JF, Carney JM, et al. Brain regional correspondence between Alzheimer's disease histopathology and biomarkers of protein oxidation. J. Neurochem. 1995;65:2146–2156. doi: 10.1046/j.1471-4159.1995.65052146.x. [DOI] [PubMed] [Google Scholar]
- 15.Boyd-Kimball D, Sultana R, Mohmmad-Abdul H, Butterfield DA. Neurotoxicity and oxidative stress in D1M-substituted Alzheimer's A beta(1–42): relevance to N-terminal methionine chemistry in small model peptides. Peptides. 2005;26:665–673. doi: 10.1016/j.peptides.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Boyd-Kimball D, Sultana R, Poon HF, Lynn BC, Casamenti F, Pepeu G, Klein JB, Butterfield DA. Proteomic identification of proteins specifically oxidized by intracerebral injection of amyloid beta-peptide (1–42) into rat brain: implications for Alzheimer's disease. Neuroscience. 2005;132:313–324. doi: 10.1016/j.neuroscience.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 17.Resende R, Moreira PI, Proenca T, Deshpande A, Busciglio J, Pereira C, Oliveira CR. Brain oxidative stress in a triple-transgenic mouse model of Alzheimer disease. Free Radic. Biol. Med. 2008;44:2051–2057. doi: 10.1016/j.freeradbiomed.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Opii WO, Joshi G, Head E, Milgram NW, Muggenburg BA, Klein JB, Pierce WM, Cotman CW, Butterfield DA. Proteomic identification of brain proteins in the canine model of human aging following a long-term treatment with antioxidants and a program of behavioral enrichment: relevance to Alzheimer's disease. Neurobiol. Aging. 2008;29:51–70. doi: 10.1016/j.neurobiolaging.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drake J, Link CD, Butterfield DA. Oxidative stress precedes fibrillar deposition of Alzheimer's disease amyloid beta-peptide (1–42) in a transgenic Caenorhabditis elegans model. Neurobiol. Aging. 2003;24:415–420. doi: 10.1016/s0197-4580(02)00225-7. [DOI] [PubMed] [Google Scholar]
- 20.Liu Q, Smith MA, Avila J, DeBernardis J, Kansal M, Takeda A, Zhu X, Nunomura A, Honda K, Moreira PI, Oliveira CR, Santos MS, Shimohama S, Aliev G, de la Torre J, Ghanbari HA, Siedlak SL, Harris PL, Sayre LM, Perry G. Alzheimer-specific epitopes of tau represent lipid peroxidation-induced conformations. Free Radic. Biol. Med. 2005;38:746–754. doi: 10.1016/j.freeradbiomed.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Zlokovic BV, Deane R, Sallstrom J, Chow N, Miano JM. Neurovascular pathways and Alzheimer amyloid beta-peptide. Brain Pathol. 2005;15:78–83. doi: 10.1111/j.1750-3639.2005.tb00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deane R, Bell RD, Sagare A, Zlokovic BV. Clearance of amyloid-beta peptide across the blood-brain barrier: implication for therapies in Alzheimer's disease. CNS Neurol. Disord. Drug Targets. 2009;8:16–30. doi: 10.2174/187152709787601867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Q, Zerbinatti CV, Zhang J, Hoe HS, Wang B, Cole SL, Herz J, Muglia L, Bu G. Amyloid precursor protein regulates brain apolipoprotein E and cholesterol metabolism through lipoprotein receptor LRP1. Neuron. 2007;56:66–78. doi: 10.1016/j.neuron.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willnow TE, Rohlmann A, Horton J, Otani H, Braun JR, Hammer RE, Herz J. RAP, a specialized chaperone, prevents ligand-induced ER retention and degradation of LDL receptor-related endocytic receptors. EMBO J. 1996;15:2632–2639. [PMC free article] [PubMed] [Google Scholar]
- 25.Markesbery WR, Lovell MA. Damage to lipids, proteins, DNA, and RNA in mild cognitive impairment. Arch. Neurol. 2007;64:954–956. doi: 10.1001/archneur.64.7.954. [DOI] [PubMed] [Google Scholar]
- 26.Montine TJ, Neely MD, Quinn JF, Beal MF, Markesbery WR, Roberts LJ, Morrow JD. Lipid peroxidation in aging brain and Alzheimer's disease. Free Radic. Biol. Med. 2002;33:620–626. doi: 10.1016/s0891-5849(02)00807-9. [DOI] [PubMed] [Google Scholar]
- 27.Reed T, Perluigi M, Sultana R, Pierce WM, Klein JB, Turner DM, Coccia R, Markesbery WR, Butterfield DA. Redox proteomic identification of 4-hydroxy-2-nonenal-modified brain proteins in amnestic mild cognitive impairment: insight into the role of lipid peroxidation in the progression and pathogenesis of Alzheimer's disease. Neurobiol. Dis. 2008;30:107–120. doi: 10.1016/j.nbd.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Sayre LM, Lin D, Yuan Q, Zhu X, Tang X. Protein adducts generated from products of lipid oxidation: focus on HNE and one. Drug Metab. Rev. 2006;38:651–675. doi: 10.1080/03602530600959508. [DOI] [PubMed] [Google Scholar]
- 29.Williams TI, Lynn BC, Markesbery WR, Lovell MA. Increased levels of 4-hydroxynonenal and acrolein, neurotoxic markers of lipid peroxidation, in the brain in Mild Cognitive Impairment and early Alzheimer's disease. Neurobiol. Aging. 2006;27:1094–1099. doi: 10.1016/j.neurobiolaging.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Lauderback CM, Hackett JM, Huang FF, Keller JN, Szweda LI, Markesbery WR, Butterfield DA. The glial glutamate transporter, GLT-1, is oxidatively modified by 4-hydroxy-2-nonenal in the Alzheimer's disease brain: the role of Abeta1–42. J. Neurochem. 2001;78:413–416. doi: 10.1046/j.1471-4159.2001.00451.x. [DOI] [PubMed] [Google Scholar]
- 31.Masliah E, Alford M, DeTeresa R, Mallory M, Hansen L. Deficient glutamate transport is associated with neurodegeneration in Alzheimer's disease. Ann. Neurol. 1996;40:759–766. doi: 10.1002/ana.410400512. [DOI] [PubMed] [Google Scholar]
- 32.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta. Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 33.Ito S, Ohtsuki S, Kamiie J, Nezu Y, Terasaki T. Cerebral clearance of human amyloid-beta peptide (1–40) across the blood-brain barrier is reduced by self-aggregation and formation of low-density lipoprotein receptor-related protein-1 ligand complexes. J. Neurochem. 2007;103:2482–2490. doi: 10.1111/j.1471-4159.2007.04938.x. [DOI] [PubMed] [Google Scholar]
- 34.Goretzki L, Mueller BM. Low-density-lipoprotein-receptor-related protein (LRP) interacts with a GTP-binding protein. Biochem J. 1998;336(Pt 2):381–386. doi: 10.1042/bj3360381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sagare A, Deane R, Bell RD, Johnson B, Hamm K, Pendu R, Marky A, Lenting PJ, Wu Z, Zarcone T, Goate A, Mayo K, Perlmutter D, Coma M, Zhong Z, Zlokovic BV. Clearance of amyloid-beta by circulating lipoprotein receptors. Nat. Med. 2007;13:1029–1031. doi: 10.1038/nm1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waldron E, Heilig C, Schweitzer A, Nadella N, Jaeger S, Martin AM, Weggen S, Brix K, Pietrzik CU. LRP1 modulates APP trafficking along early compartments of the secretory pathway. Neurobiol. Dis. 2008;31:188–197. doi: 10.1016/j.nbd.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 37.May P, Bock HH, Nimpf J, Herz J. Differential glycosylation regulates processing of lipoprotein receptors by gamma-secretase. J. Biol. Chem. 2003;278:37386–37392. doi: 10.1074/jbc.M305858200. [DOI] [PubMed] [Google Scholar]
- 38.Jeynes B, Provias J. Evidence for altered LRP/RAGE expression in Alzheimer lesion pathogenesis. Curr. Alzheimer Res. 2008;5:432–437. doi: 10.2174/156720508785908937. [DOI] [PubMed] [Google Scholar]
- 39.Pocivavsek A, Mikhailenko I, Strickland DK, Rebeck GW. Microglial low-density lipoprotein receptor-related protein 1 modulates c-Jun N-terminal kinase activation. J. Neuroimmunol. 2009;214:25–32. doi: 10.1016/j.jneuroim.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.LaDu MJ, Shah JA, Reardon CA, Getz GS, Bu G, Hu J, Guo L, Van Eldik LJ. Apolipoprotein E and apolipoprotein E receptors modulate A beta-induced glial neuroinflammatory responses. Neurochem. Int. 2001;39:427–434. doi: 10.1016/s0197-0186(01)00050-x. [DOI] [PubMed] [Google Scholar]
- 41.Bullido MJ, Guallar-Castillon P, Artiga MJ, Ramos MC, Sastre I, Aldudo J, Frank A, Coria F, Rodriguez-Artalejo F, Valdivieso F. Alzheimer's risk associated with human apolipoprotein E, alpha-2 macroglobulin and lipoprotein receptor related protein polymorphisms: absence of genetic interactions, and modulation by gender. Neurosci. Lett. 2000;289:213–216. doi: 10.1016/s0304-3940(00)01304-5. [DOI] [PubMed] [Google Scholar]
- 42.Carter CJ. Convergence of genes implicated in Alzheimer's disease on the cerebral cholesterol shuttle: APP, cholesterol, lipoproteins, and atherosclerosis. Neurochem. Int. 2007;50:12–38. doi: 10.1016/j.neuint.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 43.Smith MA, Richey Harris PL, Sayre LM, Beckman JS, Perry G. Widespread peroxynitrite-mediated damage in Alzheimer's disease. J. Neurosci. 1997;17:2653–2657. doi: 10.1523/JNEUROSCI.17-08-02653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Butterfield DA, Drake J, Pocernich C, Castegna A. Evidence of oxidative damage in Alzheimer's disease brain: central role for amyloid beta-peptide. Trends Mol. Med. 2001;7:548–554. doi: 10.1016/s1471-4914(01)02173-6. [DOI] [PubMed] [Google Scholar]
- 45.Markesbery WR. Oxidative stress hypothesis in Alzheimer's disease. Free Radic. Biol. Med. 1997;23:134–147. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- 46.Clementi ME, Pezzotti M, Orsini F, Sampaolese B, Mezzogori D, Grassi C, Giardina B, Misiti F. Alzheimer's amyloid beta-peptide (1–42) induces cell death in human neuroblastoma via bax/bcl-2 ratio increase: an intriguing role for methionine 35. Biochem. Biophys. Res. Commun. 2006;342:206–213. doi: 10.1016/j.bbrc.2006.01.137. [DOI] [PubMed] [Google Scholar]
- 47.Armstrong RA. Quantitative differences in beta/A4 protein subtypes in the parahippocampal gyrus and frontal cortex in Alzheimer's disease. Dementia. 1994;5:1–5. doi: 10.1159/000106686. [DOI] [PubMed] [Google Scholar]
- 48.Clinton J, Blackman SE, Royston MC, Roberts GW. Differential synaptic loss in the cortex in Alzheimer's disease: a study using archival material. Neuroreport. 1994;5:497–500. doi: 10.1097/00001756-199401120-00032. [DOI] [PubMed] [Google Scholar]
- 49.Jacob CP, Koutsilieri E, Bartl J, Neuen-Jacob E, Arzberger T, Zander N, Ravid R, Roggendorf W, Riederer P, Grunblatt E. Alterations in expression of glutamatergic transporters and receptors in sporadic Alzheimer's disease. J. Alzheimers Dis. 2007;11:97–116. doi: 10.3233/jad-2007-11113. [DOI] [PubMed] [Google Scholar]
- 50.Jaeger S, Pietrzik CU. Functional role of lipoprotein receptors in Alzheimer's disease. Curr. Alzheimer Res. 2008;5:15–25. doi: 10.2174/156720508783884675. [DOI] [PubMed] [Google Scholar]
- 51.Solfrizzi V, Panza F, D'Introno A, Colacicco AM, Capurso C, Basile AM, Capurso A. Lipoprotein(a), apolipoprotein E genotype, and risk of Alzheimer's disease. J. Neurol. Neurosurg. Psychiatry. 2002;72:732–736. doi: 10.1136/jnnp.72.6.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laatsch A, Merkel M, Talmud PJ, Grewal T, Beisiegel U, Heeren J. Insulin stimulates hepatic low density lipoprotein receptor-related protein 1 (LRP1) to increase postprandial lipoprotein clearance. Atherosclerosis. 2008 doi: 10.1016/j.atherosclerosis.2008.07.046. [DOI] [PubMed] [Google Scholar]
- 53.Goto JJ, Tanzi RE. The role of the low-density lipoprotein receptor-related protein (LRP1) in Alzheimer's A beta generation: development of a cell-based model system. J. Mol. Neurosci. 2002;19:37–41. doi: 10.1007/s12031-002-0008-4. [DOI] [PubMed] [Google Scholar]
- 54.Bu G, Cam J, Zerbinatti C. LRP in amyloid-beta production and metabolism. Ann. N. Y. Acad. Sci. 2006;1086:35–53. doi: 10.1196/annals.1377.005. [DOI] [PubMed] [Google Scholar]
- 55.Small DH, Maksel D, Kerr ML, Ng J, Hou X, Chu C, Mehrani H, Unabia S, Azari MF, Loiacono R, Aguilar MI, Chebib M. The beta-amyloid protein of Alzheimer's disease binds to membrane lipids but does not bind to the alpha7 nicotinic acetylcholine receptor. J. Neurochem. 2007;101:1527–1538. doi: 10.1111/j.1471-4159.2006.04444.x. [DOI] [PubMed] [Google Scholar]
- 56.Banks WA, Kastin AJ, Maness LM, Banks MF, Shayo M, McLay RN. Interactions of beta-amyloids with the blood-brain barrier. Ann. N. Y. Acad. Sci. 1997;826:190–199. doi: 10.1111/j.1749-6632.1997.tb48470.x. [DOI] [PubMed] [Google Scholar]
- 57.Subramaniam R, Roediger F, Jordan B, Mattson MP, Keller JN, Waeg G, Butterfield DA. The lipid peroxidation product, 4-hydroxy-2-trans-nonenal, alters the conformation of cortical synaptosomal membrane proteins. J. Neurochem. 1997;69:1161–1169. doi: 10.1046/j.1471-4159.1997.69031161.x. [DOI] [PubMed] [Google Scholar]