Abstract
Purpose
To determine the maximum tolerated dose (MTD), toxicity spectrum, clinical activity, and biological effects of the tropism-modified infectivity-enhanced CRAd, Ad5-Δ24-RGD, in patients with gynecologic malignancies.
Experimental Design
Cohorts of eligible patients were treated daily for 3 days via intraperitoneal catheter. Vector doses ranged from 1×109 to 1×1012 viral particles/day. Toxicity was evaluated utilizing CTCv3.0. CA-125 and RECIST criteria were utilized to determine clinical efficacy. Corollary biologic studies included assessment of CRAd replication, wild type virus generation, viral shedding, and neutralizing antibody response.
Results
Twenty-one patients were enrolled. Adverse clinical effects were limited to G1/2 fever, fatigue, or abdominal pain. No vector related grade 3/4 toxicities were noted. No clinically significant laboratory abnormalities were noted. The MTD was not reached. Over a 1 month follow up, 15 (71%) patients had stable disease and six (29%) had progressive disease. No partial or complete responses were noted. Seven patients had a decrease in CA-125; 4 had a >20% drop. RGD-specific-PCR demonstrated the presence of study vector in ascites of 16 patients. Seven revealed an increase in virus after day 3, suggesting replication of Ad5-Δ24-RGD. Minimal wild type virus generation was detected. Viral shedding studies demonstrated insignificant shedding in the serum, saliva, and urine. Anti-adenoviral neutralizing antibody effects were prevalent.
Conclusion
This study, the first to evaluate an infectivity enhanced CRAd in human cancer, demonstrates the feasibility, safety, potential antitumor response, and biologic activity of this approach in ovarian cancer. Further evaluation of infectivity enhanced virotherapy approaches for gynecologic malignancies is warranted.
Keywords: Ovarian cancer, Gene therapy, Virotherapy, Replicative Adenovirus, CRAd
Introduction
In recent years, advances in cancer therapy have brought about improvement in outcomes for patients with gynecologic malignancies. Although many patients with advanced ovarian, fallopian tube, or peritoneal carcinoma will initially respond to surgical debulking and cytotoxic chemotherapy, most will eventually recur and ultimately succumb to their disease. Likewise, only a few patients with advanced or recurrent endometrial will experience long term overall survival (1,2). Clearly, novel treatment strategies are needed for patients affected with these devastating gynecologic malignancies.
One such novel approach, oncolytic virotherapy, involves the development of replication competent viruses that specifically infect targeted cancer cells, proliferate in and induce cancer cell oncolysis, and subsequently release progeny viral particles that will infect and lyse surrounding cancer cells. Adenoviruses are well suited to development as a virotherapy agent due to excellent stability, unparalleled infectivity, efficient gene transfer, and biologic plasticity compared to other viruses (3). Conditionally replicating adenoviruses (CRAds) are rendered conditionally replicative by deleting viral genes that become extraneous in many tumor cells, such as genes involved in replication via p53 and Rb pathways (4). In normal cells, p53 and Rb serve as tumor suppressor genes modulating cell cycle and inducing apoptosis if cellular DNA damage is incurred. However, many cancers, such as ovarian cancer are known to have very low rates of functional p53 and Rb due to mutations in the aforementioned genes (5,6). As such, these tumors lend themselves well to potential CRAd therapy.
One such CRAd, ONYX 015, has been utilized previously in clinical trials of gynecologic and other malignancies, but with limited success (7,8). Limited efficacy has been, in part, attributed to inefficient gene transfer due to a relative paucity of the primary adenovirus receptor, known as CAR, on cancer cells (9). Our group has previously demonstrated that genetic manipulation of the adenoviral capsid proteins to incorporate an Arg-Gly-Asp (RGD) sequence in the HI loop of the fiber knob accomplishes enhanced infectivity of tumor cells via CAR independent pathways (10,11). RGD modification had been shown to exhibit a degree of specificity for ovarian cancer cells over normal tissue due to up-regulation of ανβ integrins in ovarian cancer cells and infectivity enhancement was shown to dramatically improve antitumor potency of various gene therapy approaches in vitro and in animal models of ovarian cancer (11).
In keeping with these data, we constructed a novel, infectivity enhanced CRAd, designated Ad5-Δ24-RGD that utilizes a 24 base pair deletion in the E1A gene known to be necessary for host cell Rb protein binding, thereby conferring conditional replication only in cells that are deficient in the Rb/p16 pathway. Incorporation of the RGD capsid modification also allows Ad5-Δ24-RGD to achieve enhanced tumor cell infectivity via integrin binding and relative increased infection specificity. Preclinical studies of Ad5-Δ24-RGD have demonstrated enhanced infectivity, oncolytic capacity, tumor specificity, and therapeutic efficacy in ovarian cancer cell lines, primary ovarian cancer cells, and in a well established murine model for ovarian cancer (12). In vivo biodistribution and toxicity studies noted appropriate viral clearance and no significant permanent pathologic or laboratory abnormalities associated with intraperitoneal administration to cotton rats, which are permissive to Ad serotype 5 replication (13).
These preclinical efficacy and safety studies provided justification for a phase I clinical trial designed to determine the maximum tolerated dose (MTD) and spectrum of toxicities encountered with intraperitoneal delivery of the tropism modified CRAd, Ad5-Δ24-RGD, in patients with recurrent ovarian and other select gynecological cancers. Secondary objectives included determination of potential clinical activity, biological effects of, and the immunological response to intraperitoneal administration of Ad5-Δ24-RGD. Importantly, this infectivity enhanced adenovirus represents the first ever tropism modified CRAd applied in the context of human cancer clinical trials.
Materials and Methods
Patient eligibility
This study was conducted by a 3 + 3 dose-escalation strategy at a single institution following IRB, IBC, RAC, and FDA approval. Participants were enrolled from July 2007 to April 2009. Eligible patients originally included histiologically documented persistent or recurrent epithelial ovarian or primary peritoneal adenocarcinoma and eventually was expanded to include fallopian tube and endometrial carcinoma. All patients were required to have previous treatment with conventional surgery and chemotherapy and have evidence of intra-abdominal disease. Patients were required to have adequate organ laboratory function defined as WBC > 3000 uL, granulocyte count > 1500 uL, platelets > 100,000, creatinine clearance > 80mg/dL, creatinine < 2.0, AST or ALT < 2.5× the upper limit of the normal range, bilirubin < 2.0, and PT/PTT/INR < 1.5× the upper limit of the normal range. Patients were required to have an ejection fraction > 55% on echocardiogram and an O2 saturation > 92%. Patients were required to be ≥ 19 years of age, have a GOG performance status of 0-2, have a life expectancy > 3 months, and signed an informed consent document. Patients with low malignant potential epithelial, stromal, or germ cell ovarian tumors were excluded. Patients with active heart disease, pulmonary disease, or coagulation disorders were excluded.
Ad5-Δ24-RGD manufacturing
The Ad5-Δ24 mutant adenovirus containing the 24 nucleotide deletion from Ad5 bp 923 to 946 was originally provided by Dr. Juan Fueyo (MD Anderson Cancer Center, Houston, TX). An E1 fragment containing the 24bp deletion from this plasmid was cloned via homologous recombination into a ClaI digested plasmid pVK503 containing the RGD fiber as previously described (14). Following PacI digestion the resulting genome was released from the plasmid backbone, transfected into A549 cells and rescued. RGD presence and Δ24 absence were verified via PCR.
Ad5-Δ24-RGD was manufactured with the support of the NCI RAID program at the Cell and Gene Therapy Center at Baylor College of Medicine and at the Biopharmaceutical Development Program/SAIC at NCI-Frederick. All viral doses were administered in 250 ml of 0.9% sodium chloride and kept refrigerated until administration.
General treatment plan and Ad5-Δ24-RGD dose cohorts
Pretreatment evaluation consisted of: history and physical, toxicity grading, performance status assignment, CBC, chemistry panel, liver profile, coagulation profile, CA-125, determination of ejection fraction by echocardiogram, O2 saturation, and CT of the abdomen and pelvis. Patients completing pretreatment evaluation and meeting all eligibility criteria were enrolled and had an intraperitoneal (IP) Quinton Curl, 22.4 inch, double cuffed, Tenchkhoff type catheters (Tyco Healthcare, Princeton, NJ) placed by interventional radiology at least one week prior to utilization.
Patients were then enrolled in successive escalating dose cohorts such that cohort 1 received 1×109 vp/d and each successive cohort dose increased by ½ log vp/d. The 7th and final cohort received 1×1012 vp/d. Assigned doses were instilled via the IP catheter daily for three consecutive days in an inpatient setting. On the 4th day, the patient was discharged. Dose escalation occurred 4 weeks after the final patient in the previous cohort was treated. No individual patient dose escalation was performed.
On days 0-3, 7, 14, and 28 patients were evaluated via history and physical, performance status assignment, toxicity grading, CBC, chemistry profile. Peritoneal aspirates for biologic ancillary studies and urine, saliva and serum specimens for viral shedding studies were obtained immediately preceding Ad5-Δ24-RGD administration on day 0, 3, 7, 14, and 28. Serum CA-125 and a CT of the abdomen and pelvis were repeated on day 28. All samples were processed to assure anonymity; individuals performing the biologic studies were blinded to patient identity.
Toxicity evaluation
Toxicity grading was performed utilizing NCI Common Toxicity Criteria v3.0. MTD was defined as the dose exceeded by the dose at which at least 2 patients experience dose-limiting toxicity (DLT). DLT was defined as any vector related grade 3 non-hematologic toxicity, not including nausea, vomiting, or fatigue. Dose limiting hematologic toxicities were defined as any admission for neutropenic fever, ANC < 500 for > 5 days, or platelet count < 20,000. Any patient experiencing vector related grade 3/4 toxicity prior to completion of scheduled treatment had subsequent days of treatment held until resolution of toxicity. If no resolution occurred within 72 hours or if a second dose interruption occurred, patients received no further study drug.
Evaluation of clinical efficacy
RECIST criteria version 1.0 was utilized to define patients' best radiographic response to treatment. Measurable disease was defined as at least one lesion that could be accurately measured in one dimension and greater than 1 cm. Up to 5 lesions per organ or 10 lesions total were identified as target lesions. Complete response (CR) required disappearance of all target lesions and normalization of CA-125. Partial response (PR) was defined as > 30% decrease in the sum of target lesions' recorded dimensions. Progressive disease (PD) was defined as > 20% increase in sum of target lesions recorded dimensions. Stable disease was a condition that did not qualify for PR or PD.
Evaluation of viral infection, CRAd replication, generation of wild type virus in peritoneal fluid cells
Genomic DNA of tumor cells in peritoneal fluid was isolated using a QIAamp DNA Mini Kit (QIAGEN, Valencia, CA) according to the manufacturer's protocols. Real-Time quantitatitve PCR (RTqPCR) was utilized to evaluate gene transfer and CRAd replication. RGD copies in genomic DNA were determined by amplification of the RGD gene with forward primer CACACTAAACGGTACACAGGAAACA, reverse primer ATGCAGATGGGCAGAAACAGT and probe: 6-FAM-AGACACAACTTGTGACTGCCGCGG-BHQ-1. Resultant RGD copies were normalized to a human cellular house-keeping gene (human β-Actin) which was amplified with forward primer CCAGCAGATGTGGATCAGCA, reverse primer CTAGAAGCATTT-GCGGTGGAC and probe 6-HEX-AGGAGTATGACGAG-TCCGGCCCCTC-BHQ-1.
To determine whether potential contaminating wild type adenovirus were replicating, the wild type E1 (WT-E1) gene was amplified from cells in the ascites fluid with forward primer TGCCAAACCTTGTACCGGA, reverse primer CGTCGTCACTGGGGTGGAAA and probe 6-FAM-ATCGATCTTACCTGCCACGAGGCTGG–BHQ. Resulted WT-E1 copies were normalized to house-keeping genes to allow for comparison between patients and at different time points and varying amount of cellular material.
All primers and probes were designed by the Primer Express 1.5 software and synthesized by Sigma-Aldrich (St. Louis, MO). FastStart TaqMan Probe Master (Roche Applied Science, Indianapolis, IN) was used for duplexing the PCR reaction on a LightCycler™ 480 (Roche Applied Science, Indianapolis, IN). Thermal cycling conditions began with 8 minutes at 95 °C followed by 45 cycles of 10 seconds at 95 °C and 40 seconds at 60 °C. Data were analyzed with LightCycler 480 1.5.0 SP1 software. WT and RGD specific primers were confirmed to only amplify the virus being tested (data not shown).
Immunohistochemistry
After being transported to the laboratory, DMSO (Dimethyl Sulfoxide,(Sigma Aldrich, St. Louis, MO) was added to ascites specimens containing tumor cells to reach 5% and immediately frozen at −80°C until assays and analysis commenced. Upon thawing, cells were suspended in cell culture media (Sigma Aldrich, St. Louis, MO). Cell suspensions of ascites were used to prepare at least two cytospin slides per sample using the ThermoShandon 3 Cytospin (Dreieich, Germany) at 2000rpm for 10 minutes at room temperature. After the cytospin, slides were removed and fixed in 70% ethanol overnight (Sigma Aldrich, St. Louis, MO). Slides were incubated with rabbit polyclonal anti-hexon antibody (Abcam, Cambridge, MA) at a dilution of 1:2000 and CC-49 anti-Tag72 antibody (provided by M. B. Khazaeli, PhD, University of Alabama at Birmingham) at a dilution of 5ug/ul for one hour. CC-49 anti-Tag72 antibody is an antibody to a tumor associated glycoprotein known to be expressed by ovarian cancer cells (15). Anti-hexon is an anti-adenovirus antibody. Negative controls were performed by omitting primary antibodies. Ascites cells from patients untreated with adenovirus were used to confirm that in the absence of adenovirus, staining with anti-hexon is negative. Ascites cells from patients not in this trial were treated ex vivo with adenovirus and stained with anti-hexon antibody to confirm that anti-hexon will detect present adenovirus. (Supplemental data, Figure 1). Secondary antibodies Alexafluor 488 (Invitrogen) and Alexafluor 594 (Invitrogen) were incubated at 1:100 for one hour in phosphate buffered saline. Slides were then mounted with 0.25% Propyl Gallate in a 9:1 (v/v) glycerol:PBS solution to prevent photobleaching (Sigma Aldrich, St. Louis, MO). Fluorescence microscopy was performed with an inverted IX-70 microscope (Olympus, Melville, NY) equipped with a Magnifire digital CCD camera (Optronics, Goleta, CA) or a DP71 digital camera (Olympus). All images were at 40× magnification. The images of fluorescent signals for tumor cells and adenovirus were merged using Adobe Photoshop CS (San Jose, CA).
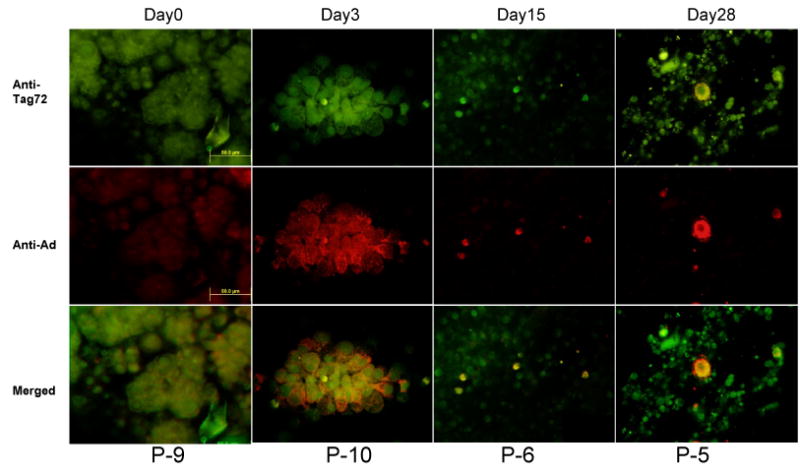
Figure 1. Quantification of Ad5-Δ24-RGD in ascites/peritoneal lavage samples (A) and immunohistochemical evidence of colocalization of Ad5-Δ24-RGD and ovarian cancer cells (B).
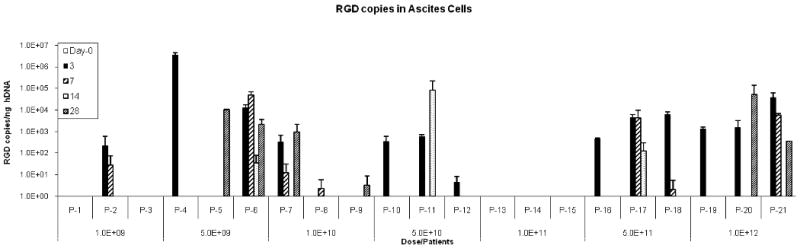
A. Using real time quantitative PCR, Ad5-Δ24-RGD copy numbers were quantified in each patient's lavage samples (in triplicate) on Day 0,3,7,14, and 28.
B. Representative immunohistochemical stains of ascites from selected patients are depicted. All images are 40× magnification. The top panels depict localization of Tag-72, an antibody to a tumor associated glycoprotein known to be expressed by ovarian cancer cells. The second row depicts localization of anti-hexon antibody, an anti-adenovirus antibody. The bottom row shows a digital overlay of the two previous images. On day 0, green fluorescence demonstrates the presence of ovarian cancer cells while the anti-adenovirus staining panel demonstrates only background red fluorescence. Some red background fluorescence is present in adenovirus negative cells. Representative images shown after treatment contain cells that stain for both the tumor marker and adenovirus.
Evaluation of viral shedding
Viral DNA from urine specimens was isolated with a QIAamp Viral RNA Mini Kit (QIAGEN, Valencia, CA) after being concentrated with Millipore* Amicon* Ultra-4 and Amicon Ultra-15 Centrifugal Filter Units (Billerica, MA). Viral DNA from saliva specimens was isolated using a QIAampMinElute Virus Spin Kit (QIAGEN, Valencia, CA) following the manufacturer's instructions. Viral DNA from sera specimens was isolated with the DNeasy Tissue Kit's blood protocol (QIAGEN, Valencia, CA). One microliter from resultant samples were used as a template for RTqPCR since many of these samples lacked genomic DNA for normalization of results. We amplified a fragment of RGD with forward primer CACACTAAACGGTACACAGGAAACA, reverse primer ATGCAGATGGGCAGAAACAGT and probe: 6-FAM-AGACACAACTTGTGACTGCCGCGG-BHQ-1 (Sigma Aldrich, St. Louis, MO). RTqPCR conditions were similar to those previously described to evaluate viral infection, CRAd replication, and generation of wild type virus.
Evaluation of an anti-adenovirus neutralizing antibody (Nabs) response
For evaluation of induced anti-adenovirus Nabs response in serum and ascites specimens after treatment, a nonreplicative luciferase expressing virus, Ad5-RGD-Luc1, was neutralized by either serum or ascites prior to infection of SKOV3.ip1 cells. SKOV3.ip1 cells are a cell line derived from the implantation of SKOV3 cells (ATCC, Manassas, VA) in nude mice. These cells were last tested and authenticated for epithelial staining via pooled AE1/AE3 antibody in December, 2009. These antibodies stain normal and neoplastic cells of epithelial origin.
Following neutralization in respective samples, Ad5-RGD-Luc1 transduction efficacy was determined by a luciferase assay. Triplicates of SKOV3.ip1 cells were plated into 96-well plates (10,000/well) and allowed to grow overnight before infection. A 1:2 dilution of serum or ascites of each day point specimen was prepared in Opti-MEM (Media Preparation Shared Facility, UAB), in a normalized volume. Non-replicative Ad5-RGD-Luc1 at 100 PFU/cell was mixed with each dilution for 30 minutes at room temperature before adding to appropriate wells. This infection was allowed to proceed for 48 hours. A luciferase assay was carried out using a luciferase assay system (Promega, Madison, WI) on an Orion microplate luminometer (Berthold, Pforzheim, Germany) reading Culturplate-96 (Research Parkway, Meriden, CT) according to manufacturer's protocols.
Statistical Analysis
Demographic and baseline characteristics of the treated patients are summarized descriptively. The incidence of adverse events and laboratory tests are also briefly summarized respectively. For the analysis of biological effects, a repeated measures analysis of variance was used to compare baseline values to the other study day values. The raw data were transformed into logarithms to the base 10 (log10) to meet the normality assumption before statistical testing.
Results
Patient demographics
From 2007 to 2009, 26 patients were consented to participate. Five of these patients were not treated with Ad5-Δ24-RGD: three patients were ineligible due to leukopenia, thrombocytopenia, or abnormal liver function tests on screening, respectively. Two additional patients had intraperitoneal catheter placement complications that did not allow for intraperitoneal administration of the study CRAd. A total of 21 patients were successfully treated in seven dose levels per study guidelines.
Study demographics for treated patients are provided in Table 1. In summary, the median and mean age of the treated patients were 66 and 65.2 years, respectively (range 47-83). Ninety percent were Caucasian and 86% had recurrent ovarian cancer. The median and mean number of prior chemotherapy treatments was 3 and 3.4, respectively (range 1-7).
Table 1. Selected demographics of treated patients.
| Pt ID | Age | Race | GOG score | Cancer | Original Stage | Histology | Prior regimens |
|---|---|---|---|---|---|---|---|
| 1 | 65 | C | 0 | ovarian | 3c | serous | 2 |
| 2 | 77 | C | 0 | ovarian | 3c | serous | 3 |
| 3 | 54 | C | 0 | ovarian | 3b | endometriod | 3 |
| 4 | 52 | C | 0 | ovarian | 3c | serous | 7 |
| 5 | 54 | C | 0 | ovarian | 3c | serous | 5 |
| 6 | 70 | C | 1 | ovarian | 3c | serous | 4 |
| 7 | 47 | C | 0 | ovarian | 3c | serous | 2 |
| 8 | 71 | C | 1 | ovarian | 3c | serous | 5 |
| 9 | 67 | C | 2 | ovarian | 3c | serous | 7 |
| 10 | 72 | C | 0 | ovarian | 3c | serous | 1 |
| 11 | 83 | C | 1 | ovarian | 3c | serous | 3 |
| 12 | 75 | A | 2 | endometrial | 3a | endometriod | 2 |
| 13 | 64 | C | 1 | ovarian | 3b | clear cell | 3 |
| 14 | 57 | C | 0 | ovarian | 2c | endometriod | 2 |
| 15 | 65 | C | 1 | ovarian | 3c | serous | 3 |
| 16 | 77 | A | 1 | ovarian | 3c | serous | 6 |
| 17 | 68 | C | 0 | ovarian | 3b | serous | 4 |
| 18 | 61 | C | 0 | ovarian | 4 | serous | 2 |
| 19 | 66 | C | 0 | ovarian | 3c | serous | 4 |
| 20 | 53 | C | 0 | endometrial | 4 | endometriod | 2 |
| 21 | 72 | C | 1 | peritoneal | 3c | serous | 2 |
GOG Score = Performance score system
C=Caucasian, A=African American.
Toxicity associated with intraperitoneal delivery of Ad5-Δ24-RGD
One consented patient experienced grade 3 abdominal pain and grade 3 infection related to her intraperitoneal catheter but was not treated with Ad5-Δ24-RGD and not included in final analysis of reagent specific toxicities. Table 2 provides a summary of clinical and laboratory adverse events by severity as reported on scheduled study visits for patients treated with Ad5-Δ24-RGD. No Ad5-Δ24-RGD related grade 3 or 4 clinical or laboratory toxicities were observed. The most common clinical toxicities listed as “possible”, “probable” or “definitely” attributable to the Ad5-Δ24-RGD were limited to grade 1 or 2 constitutional symptoms (fever or fatigue) and gastrointestinal/pain symptoms (abdominal pain). The most common laboratory abnormalities included anemia and abnormalities of glucose not thought to be associated with viral administration. Four treated patients experienced a total of ten grade 3 toxicities. One patient had grade 3 shortness of breath and grade 3 chest pain due to a disease related pleural effusion. Two patients experienced grade 3 nausea and vomiting, dehydration, and bowel obstruction symptoms related to their underlying disease. One patient experienced grade 3 hypokalemia related to nausea and vomiting. Listed grade 3 toxicities were “not” or “unlikely” to be attributed to the study vector. There were no grade 4 or 5 toxicities encountered. No vector-associated DLT's were noted and the MTD of Ad5-Δ24-RGD was not identified.
Table 2. Clinical (A.) and laboratory (B.) toxicity in patients treated with Ad5-Δ24-RGD by category and grade.
| A. | |||||
|---|---|---|---|---|---|
| Body System | Toxicity Grades (NCI CTC V.3) | Total Occurrences | |||
| 1 | 2 | 3 | 4 | ||
| Gastrointestinal | 61 | 34 | 6 | 0 | 101 |
| Constitutional | 18 | 6 | 0 | 0 | 24 |
| Pain | 10 | 8 | 1 | 0 | 19 |
| Neurology | 4 | 4 | 0 | 0 | 8 |
| Infection | 1 | 6 | 0 | 0 | 7 |
| Dermatology/Skin | 0 | 4 | 1 | 0 | 5 |
| Cardiac General | 2 | 2 | 0 | 0 | 4 |
| Lymphatics | 3 | 1 | 0 | 0 | 4 |
| Musculoskeletal | 3 | 1 | 0 | 0 | 4 |
| Allergy/Immunology | 3 | 0 | 0 | 0 | 3 |
| Syndromes | 0 | 2 | 0 | 0 | 2 |
| Respiratory | 0 | 0 | 1 | 0 | 1 |
| Renal/Genitourinary | 0 | 1 | 0 | 0 | 1 |
| Blood/Bone Marrow | 1 | 0 | 0 | 0 | 1 |
| Cardiac Arrhythmia | 1 | 0 | 0 | 0 | 1 |
| B. | |||||
|---|---|---|---|---|---|
| Lab Tests | Toxicity Grades (NCI CTC V.3) | Total Occurrences | |||
| 1 | 2 | 3 | 4 | ||
| Hemoglobin | 42 | 7 | 0 | 0 | 49 |
| Hematocrit | 34 | 12 | 0 | 0 | 46 |
| Glucose | 24 | 5 | 0 | 0 | 29 |
| Potassium | 11 | 1 | 1 | 0 | 14 |
| LDH | 8 | 2 | 0 | 0 | 10 |
| Creatinine | 6 | 0 | 0 | 0 | 6 |
| GGT | 4 | 1 | 0 | 0 | 5 |
| WBC | 5 | 0 | 0 | 0 | 5 |
| Sodium | 2 | 3 | 0 | 0 | 5 |
| Alkaline Phosphatate | 5 | 0 | 0 | 0 | 5 |
| Calcium | 2 | 2 | 0 | 0 | 4 |
| Phosphorous | 2 | 2 | 0 | 0 | 4 |
| Bicarbonate | 4 | 0 | 0 | 0 | 4 |
| Chloride | 0 | 2 | 0 | 0 | 2 |
| BUN | 2 | 0 | 0 | 0 | 2 |
| Total Bilirubin | 1 | 0 | 0 | 0 | 1 |
| Uric Acid | 0 | 0 | 0 | 0 | 0 |
| Platelet | 0 | 0 | 0 | 0 | 0 |
Clinical efficacy associated with intraperitoneal delivery of Ad5-Δ24-RGD
Of the 21 treated patients, 19 had measureable disease and were evaluable for best response using RECIST criteria one month following Ad5-Δ24-RGD treatment. Fourteen patients had stable disease and 5 patients had progressive disease. There were no partial or complete responses noted. Two additional patients had evaluable but nonmeasurable disease; one had stable disease and one had progressive disease one month following Ad5-Δ24-RGD treatment (Table 3).
Table 3. Best response by RECIST criteria and CA-125 values.
| Pt ID | Dose Cohort (Vp/d) | Best Response | CA-125 (units/mL) Pre/Post | CA-125 |
|---|---|---|---|---|
| 1 | 1×109 | SD | 1390 / 1060 | Decrease* |
| 2 | 1×109 | SD | 164 / 278 | Increase |
| 3 | 1×109 | SD | 52 / 56 | Increase |
| 4 | 5×109 | PD | 505 / 1148 | Increase |
| 5 | 5×109 | SD | 1540 / 2261 | Increase |
| 6 | 5×109 | SD | 914 / 424 | Decrease* |
| 7 | 1×1010 | PD | 618 / 854 | Increase |
| 8 | 1×1010 | SD | 112 / 231 | Increase |
| 9 | 1×1010 | PD | 429 / 286 | Decrease* |
| 10 | 5×1010 | SD | 11 / 24 | Increase |
| 11 | 5×1010 | PD† | 1103 / 1662 | Increase |
| 12 | 5×1010 | SD | 1092 / 967 | Decrease |
| 13 | 1×1011 | PD | 1339 / 1128 | Decrease |
| 14 | 1×1011 | PD | 47 / 79 | Increase |
| 15 | 1×1011 | SD | 187 / 131 | Decrease* |
| 16 | 5×1011 | SD | 166 / 247 | Increase |
| 17 | 5×1011 | PD | 3044 / 5774 | Increase |
| 18 | 5×1011 | SD | 46 / 90 | Increase |
| 19 | 1×1012 | SD | 64 / 153 | Increase |
| 20 | 1×1012 | SD | 158 / 129 | Decrease |
| 21 | 1×1012 | SD† | 992 / NA | N/A |
SD = Stable disease
PD = Progressive disease
= Nonmeasurable disease by RECIST criteria
= > 20% decrease in CA-125
Seven of 20 evaluable patients had decrease in CA-125 from pretreatment values to day 29 values; one patient did not have a post treatment CA-125 level drawn (Table 3). Four of these patients had a > 20% drop in CA-125 levels. Five of these seven patients had stable disease by RECIST criteria; the other two patients were noted to have progressive disease.
Viral infection and replication
At time points specified in the methods, both pre and post Ad5-Δ24-RGD administration, peritoneal lavage samples were obtained from each patient and evaluated for the presence of Ad5-Δ24-RGD via RTqPCR. All patient specimens were positive for cellular DNA. Any value greater than 1 RGD copy number /ng of cellular DNA was considered positive. By day 3 (after treatment), 16 of the 21 patients had RGD specific DNA detected (Figure 1A) after Ad5-Δ24-RGD treatment was completed. In 7 patients (patients 5, 6, 7, 8, 9, 11, and 20), the detected copy number of RGD specific virus increased at time points after Ad5-Δ24RGD treatment. Although rigorous statistical evaluation was not feasible given the limited sample numbers, these data suggest that replication of Ad5-Δ24-RGD may have occurred in these patients. Qualitative evidence of viral localization within cancer cells is depicted in representative ascites samples using immunohistocehmistry. Images of select patient samples depict the presence of Ad5-Δ24-RGD even at day 28 (Figure 1B).
Generation of wild type virus
In general, generation of replication competent wild type adenovirus (RCA) was minimal. Wild type E1 (WT-E1), was detected in ascites specimens of 8 patients, 4 of which had detectable WT-E1 prior to Ad5-Δ24-RGD administration. Only 1 patient had more than 25 copies of WT- E1 DNA per ng of DNA following administration of Ad5-Δ24-RGD. Specifically, patient 20, in the highest dose cohort, had an elevated level of WT-E1 DNA (62 copies/ng DNA) detected prior to administration of Ad5-Δ24-RGD and had 549 copies/ng DNA detected on day 28. Interestingly, this patient also reported upper respiratory symptoms prior to vector administration.
Viral shedding
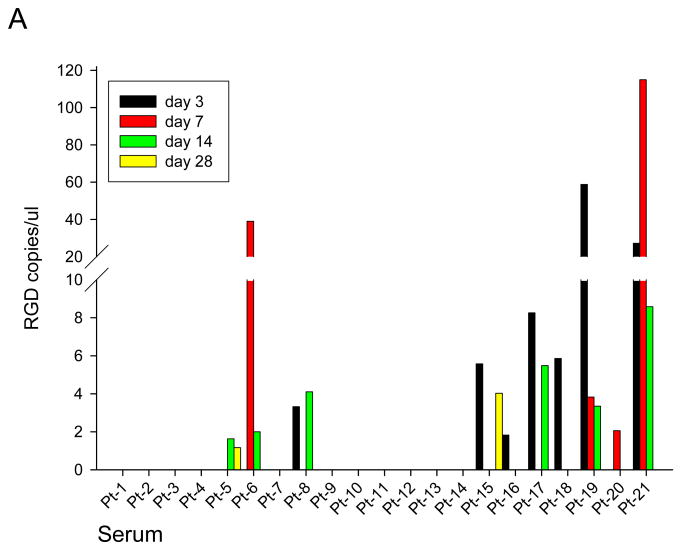
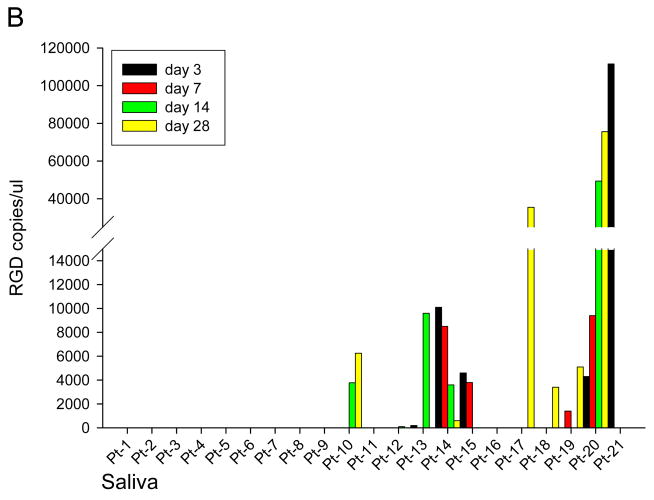
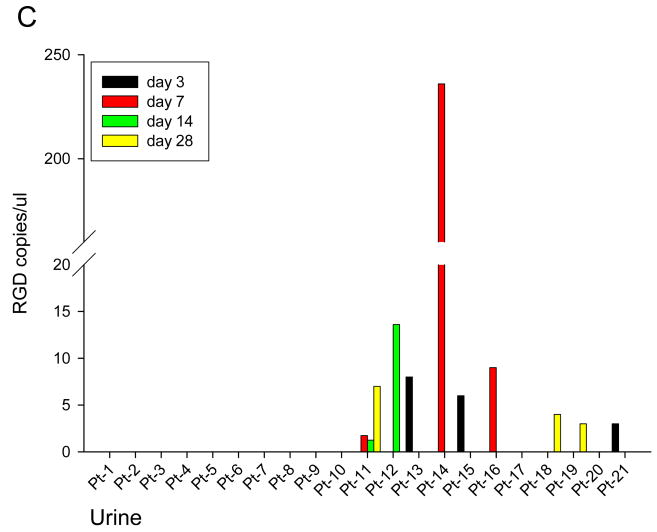
At specified time points pre and post Ad5-Δ24-RGD administration, serum, saliva, and urine were obtained from each patient to assess for viral shedding (Figure 2). This data is normalized to day 0 RGD copies and any value less than 1 copy RGD / uL was considered negative. RGD specific virus was detected in serum samples from 10 patients as shown in Figure 2A. Figure 2B shows the presence of RGD copies in saliva samples from 10 patients. Viral shedding was detected in urine samples from 9 patients (Figure 2C). In general, viral shedding was noted more frequently and to a greater extent in patients in higher dose cohorts and in the saliva.
Figure 2. Quantification of Ad5-Δ24-RGD in Serum (A), Saliva (B) and Urine (C).
Using RT-PCR, Ad5-Δ24-RGD copy numbers were quantified in each patient's serum (A), saliva (B) and urine (C) samples on Day 0,3,7,14, and 28.
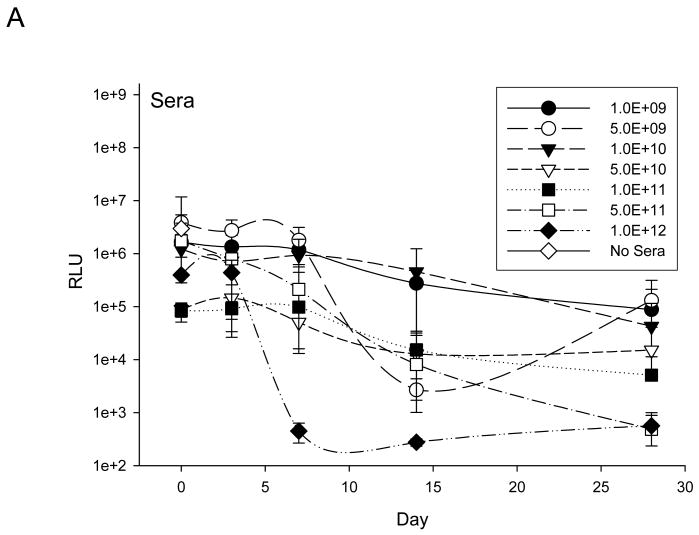
Anti-adenovirus neutralizing antibody (Nabs)
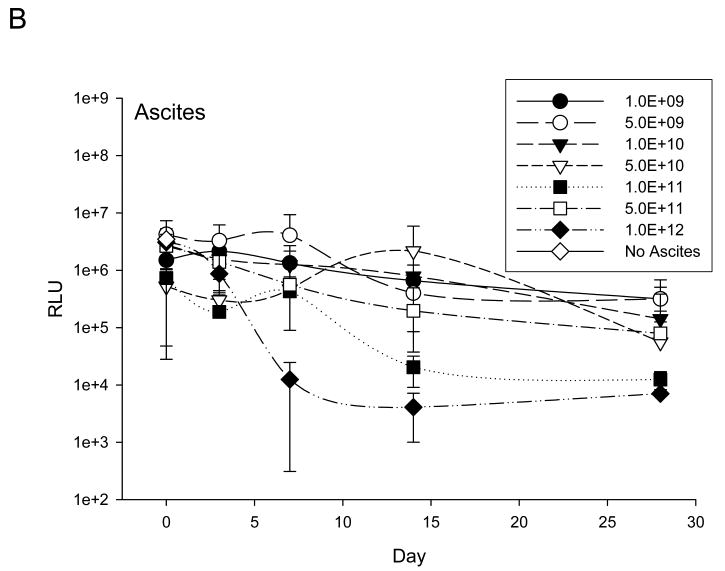
At specified time points pre and post Ad5-Δ24-RGD administration, an anti-adenoviral neutralizing antibody (Nabs) response was determined in serum and ascites (Figure 3). Overall, neutralizing antibody effects in both serum and ascites were consistent and dose dependent. Following exposure to day 14 sera and ascites samples, infection of Ad5-RGD-luc was, in general, significantly limited, and, by day 28, evidence of transduction was minimal. Neutralizing antibody effects were present in all patients and in when all data points from all patients for Day 0 and Day 3 were compared to all data points for all patients for Days 14 and 28 effects of neutralizing antibody were significantly higher by day 14 and beyond (p<0.0001).
Figure 3. Assessment of anti-adenoviral neutralizing antibody (Nabs) response in serum (A) and ascites (B).
Assessment of induced anti-adenovirus Nabs response after treatment was carried by exposing a nonreplicative luciferase expressing virus, Ad5-RGD-Luc1, to either patient serum (A) or ascites (B) prior to infection of SKOV3.ip1 cells. Following neutralization in respective samples, Ad5-RGD-Luc1 transduction efficacy was determined by a luciferase assay. Each treatment cohort's mean response is documented here in addition to a negative control represented by No Sera or No Ascites.
Discussion
This report serves as one of the first published reports evaluating an infectivity enhanced virotherapy approach for the treatment of patients with cancer. Specifically, this study evaluated intraperitoneal administration of the infectivity enhanced CRAd Ad5-Δ24-RGD in a previously heavily treated cohort of patients with recurrent ovarian or endometrial cancer. A single three day cycle of Ad5-Δ24-RGD in dosages ranging from 1×109 to 1×1012 vp/D was administered to treated patients. The most commonly noted Ad5-Δ24-RGD related toxicity consisted of primarily grade 1 and 2 constitutional and gastrointestinal symptoms. Most grade 3 toxicities noted were in general disease related and not associated with Ad5-Δ24-RGD treatment. No grade 4 toxicities were reported. Manufacturing constraints limited the ability to administer higher dosages of Ad5-Δ24-RGD. Thus the MTD was not identified and the maximum feasible dose in this study was identified to be 1×1012 vp daily for three days.
While not a primary aim of this study, there was some suggestion of potential antitumor activity associated with Ad5-Δ24-RGD treatment. Specifically, 14 of the 21 patients were noted to have stable disease by RECIST criteria over the month of observation following administration of Ad5-Δ24-RGD. Of note, 4 patients experienced a > 20% decline in CA-125 levels, a common marker of overall disease burden for advanced ovarian and endometrial cancers. There did not appear to be a dose related response.
Our ancillary studies provided potential evidence of Ad5-Δ24-RGD replication and other important insights regarding the biologic effects of intraperitoneal administration of Ad5-Δ24-RGD. Specifically, Ad5-Δ24-RGD specific DNA was detected at various time points after treatment in 16 of the 21 patients. In seven of these patients, higher quantities of Ad5-Δ24-RGD specific DNA was noted after day 3 of Ad5-Δ24-RGD treatment and could potentially be attributed to replication of the virus within the abdominal cavity. Ad5-Δ24-RGD specific viral shedding was noted in serum, urine and saliva. Shedding was highest in saliva and appeared to be dose dependant. This noted level of shedding and tropism for the upper respiratory tract is not inconsistent with what has been documented in other adenoviral based gene therapy based trials (15, 16,17). In addition, the generation of wild type adenovirus (>25 copies/ng DNA) was minimal and was not detected in Ad5-Δ24-RGD treated patients at a dose of less than 5 × 1010 Vp/D. One patient had elevated levels of wild type adenovirus (>50 copies prior to administration and on day 28); this patient was noted to have clinical evidence of an upper respiratory tract infection at the time. Lastly, anti-adenovirus neutralizing antibodies were uniformly present in both serum and ascites samples from most patients and noted to be significant 14 days after Ad5-Δ24-RGD treatment. This neutralizing anti-adenoviral antibody response is similar to what has been noted in other adenoviral based trials (18,19). However, evidence of potential Ad5-Δ24-RGD replication noted in this study and in prior preclinical studies, may point towards diminished interaction of neutralizing antibodies with RGD modified adenoviruses. These findings may also demonstrate the potential ability of this infectivity enhanced modified CRAd to abrogate immunologic interactions that would mitigate potential anti-tumor activity (20). Additional studies evaluating the effect of Ad5-Δ24-RGD on other immunologic parameters are planned and a clinical trial evaluating intracranial administration of Ad5-Δ24-RGD in patients with malignant glioblastoma is currently in progress.
Two other virotherapy approaches have been evaluated in the context of recurrent ovarian cancer. ONYX-015, the first CRAd to be evaluated in this disease context, has a E1B gene deletion that allows for conditional replication in p53 deficient tumor cells but does not have any modification that allow for improved ovarian cancer cell transfection. (7) In this phase I study, patients were treated via IP ONYX-015 in dose cohorts up to 1× 1011 pfu daily for five days every four weeks. A total of 35 cycles was administered to 16 treated patients with a median of 2 cycles per patient. Only one dose limiting toxicity (grade 3 abdominal pain and diarrhea) was noted and the MTD was not identified. Four of the 16 patients exhibited stable disease as a best response and one patient exhibited a significant drop in her serum CA-125. Five of 8 evaluable patients had PCR detectable ONYX-015 DNA in peritoneal lavage specimens 10 days following the last administration. Interestingly, one patient had specific DNA detectable 354 days following her last treatment. Despite this unique finding, the capacity to document any evidence of replication was limited due to the inability to quantitatively demonstrate increased ONYX-015 specific DNA remote from administration. All 8 tested patients had undetectable levels of ONYX-015 DNA in their serum. As noted in the current study, nearly all patients had very high levels of neutralizing antibodies develop over the course of the evaluation period (7).
In a similar approach, Galanis et al. evaluated a replicative–competent Edmonston B measles vaccine strain, MV-CEA, in a phase I trial for patients with recurrent ovarian cancer (20). MV-CEA capitalizes on the over-expression of the measles virus receptor CD46 in tumor cells and is modified to express the soluble marker peptide carcinoembryonic antigen (CEA). Eligible ovarian cancer patients were treated intraperitoneally with MV-CEA monthly for up to 6 doses in dosages ranging from 103-109 TCID50. A total of 126 cycles were administered to a total of 21 patients with a median number of 6 cycles per patient. Minimal toxicities were noted and the MTD was not identified. Fourteen of the 21 patients experienced durable SD as a best response. An apparent dose dependant response was noted with higher dose cohorts having higher rates of SD. CA-125 levels were noted to have decreased > 30% in 5 of 21 patients. CEA elevation was detected in the peritoneal fluid of one patient and in the serum of three patients in the highest dose cohort. Evidence of MV-CEA viral DNA in the serum was detected in four patients by quantitative RT-PCR. No MV-CEA specific shedding was noted in urine or saliva. No development of anti-measles or anti-CEA antibodies was detected (21).
The results of these prior studies demonstrate the feasibility to deliver relatively high dosages of conditionally replicative viruses intraperitoneally in patients with recurrent ovarian cancer with good tolerance. The current study demonstrates for the first time the ability to deliver an infectivity enhanced CRAd (Ad5-Δ24-RGD) at high dosages in a similar cohort. Clinical trials evaluating Ad5-Δ24-RGD in the context of multiple cycles of Ad5-Δ24-RGD at the maximum feasible dose (1×1012 vp/D × 3 days) and evaluating Ad5-Δ24-RGD in combination with chemotherapy in patients with recurrent ovarian cancer are in development. Preclinical studies (22) and ongoing clinical trials support the feasibility of a combination virotherapy and chemotherapy approach (23).
The importance of the rationale for utilization of infectivity enhancement and the consequent improvement in overall antitumor potential cannot be understated as we strive to forward the utility of adenoviral virotherapy. The culmination of rigorous preclinical work (9-12) in a RAC-guided preclinical safety trial (13) led to a fully vetted tropism modified and conditionally replicative virus for utilization in this unique clinical trial. Hopefully, this previous research, in concert with the findings of the current trial, serves to establish a precedent for the safety of tropism modified adenoviral vectors and will open the door for clinical utilization of future enhancements in CRAd therapy.
Other strategies designed to improve the potential therapeutic index of adenoviral based virotherapy have been evaluated in vitro and in vivo. These include, for example, the arming of CRAds (24), incorporation of tissue specific promoters (25), novel means to assess virus trafficking (26), the development of new serotype chimeric modifications to further enhance infectivity (27, 28), and assessment of stem cell delivery mechanisms for CRAds (29). Clinical translation of several of these approaches into early phase clinical trials is ongoing or in development.
In summary, this study serves as the first clinical trial to evaluate an infectivity enhanced CRAd in patients with recurrent ovarian and other selected gynecologic cancers and provides guidance for the future development of Ad5-Δ24-RGD and other infectivity enhanced virotherapeutics intended for the treatment of cancer.
Acknowledgments
The authors acknowledge the work of Jolane Gable, RN, Michael Ann Markiewicz, PharmD, and the staff of the Participant and Clinical Interactions Resources unit of the Center for Clinical and Translational Science at UAB for their care of patients with gynecologic malignancies and support of this research.
Financial Support: The authors' work in this study was supported, in part, by the following:
R.D. Harris:
Federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government;
The Developmental Therapeutics Program in the Division of Cancer Treatment and Diagnosis of the National Cancer Institute.
D.T.Curiel:
NIH grant 5R01CA121187;
NIH grant NCI R21 CA128222;
NIH NCI P50-CA83591.
R.D. Alvarez:
NIH grant NCI R21 CA128222;
NIH NCI P50-CA83591;
Footnotes
Statement of Translational Relevance: Novel treatment strategies are needed for patients with advanced or recurrent gynecologic cancers. One such novel therapeutic approach, oncolytic virotherapy, is a promising therapy for these malignancies. This study is the first to evaluate an infectivity-enhanced conditionally replicative adenovirus (CRAd) in the context of human cancer and demonstrates the feasibility, safety, potential antitumor response, and biologic activity of this virotherapy approach in gynecologic malignancies.
References
- 1.Brinton LA, Lacey JV, Sherman ME. Epidemiology of Gynecologic Cancers. In: Hoskins WJ, Perez CJ, Young RC, et al., editors. Principles and practice of gynecologic oncology. 4th. Philadelphia, PA: Lippincott-Raven; 2005. pp. 3–32. [Google Scholar]
- 2.Liu TC, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract. 2007;4:101–17. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- 3.Kimball KJ, Numnum TM, Rocconi RP, Alvarez RD. Gene therapy for ovarian cancer. Curr Oncol Rep. 2006;6:441–7. doi: 10.1007/s11912-006-0073-x. [DOI] [PubMed] [Google Scholar]
- 4.Alemany R, Balague C, Curiel D. Replicative adenovirus for cancer therapy. Nature Biotechnol. 2000;18:723–7. doi: 10.1038/77283. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal R, Linch M, Kaye SB. Novel therapeutic agents in ovarian cancer. Eur J Surg Oncol. 2006;32:875–86. doi: 10.1016/j.ejso.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 6.Niederacher D, Yan HY, An HX, Bender HG, Beckman MW. CDKN2 gene inactivation in epithelial sporadic ovarian cancer. Br J Cancer. 1999;80:1920–6. doi: 10.1038/sj.bjc.6690621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasey PA, Schulman LN, Campos S, et al. Phase I trial of intraperitoneal injection of the E1B-55kd-gene deleted adenovirus ONYX-015 (dl1520) given on days 1 through 5 every 3 weeks in patients with recurrent/refractory epithelial ovarian cancer. J Clin Oncol. 2002;20:1562–9. doi: 10.1200/JCO.2002.20.6.1562. [DOI] [PubMed] [Google Scholar]
- 8.Neumunaitis J, Cunningham C, Buchanan A, et al. Intravenous infusion of a replication-selective adenovirus (ONYX-015) in cancer patients: safety, feasibility, and biological activity. Gene Therapy. 2001;8:746–59. doi: 10.1038/sj.gt.3301424. [DOI] [PubMed] [Google Scholar]
- 9.Douglas JT, Kim M, Sumerel LA, Carey DE, Curiel DT. Efficient oncolysis by a replicating adenovirus (ad) in vivo is critically dependant on tumor expression of primary Ad receptors. Ca Res. 2001;61:813–7. [PubMed] [Google Scholar]
- 10.Dmitriev I, Krasnykh V, Miller CR, et al. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. Virol. 1999;72:9706–13. doi: 10.1128/jvi.72.12.9706-9713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanderkwaak T, Wang M, Navarro J, et al. An advanced generation of adenoviral vectors selectively enhances gene transfer for ovarian cancer gene therapy approaches. Gynecol Oncol. 1999;74:227–34. doi: 10.1006/gyno.1999.5432. [DOI] [PubMed] [Google Scholar]
- 12.Bauerschmitz G, Lam J, Kanerva A, et al. Treatment of ovarian cancer with a tropism modified oncolytic adenovirus. Ca Research. 2002;62:1266–9. [PubMed] [Google Scholar]
- 13.Page JG, Tian B, Schweikart K, et al. Identifying the safety profile of a novel infectivity-enhanced conditionally replicative adenovirus, Ad5-delta24-RGD, in anticipation of a phase I trial for recurrent ovarian cancer. Am J Obstet Gynecol. 2007;196:389e1–10. doi: 10.1016/j.ajog.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki K, Alemany R, Yamamoto M, Curiel DT. The presence of the adenovirus E3 region improves the oncolytic potency of conditionally replicative adenoviruses. Clin Cancer Res. 2002;8:3348–59. [PubMed] [Google Scholar]
- 15.Ponnusamy MP, Venkatraman G, Singh AP, et al. Expression of TAG-72 in ovarian cancer and its correlation with tumor stage and patient prognosis. Cancer Lett. 2007;251:247–57. doi: 10.1016/j.canlet.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 16.Schenk-Braat EA, van Mierlo MM, Wagemaker G, Bangma CH, Kaptein LC. An inventory of shedding data from clinical gene therapy trials. J Gene Med. 2007;9:910–21. doi: 10.1002/jgm.1096. [DOI] [PubMed] [Google Scholar]
- 17.Kawahira H, Matsushita K, Shiratori T, et al. Viral shedding after p53 adenoviral gene therapy in 10 cases of esophageal cancer. Cancer Science. 2010;101:289–91. doi: 10.1111/j.1349-7006.2009.01381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alvarez RD, Barnes MN, Gomez-Navarro J, et al. A cancer gene therapy approach utilizing an anti-erbB-2 single-chain antibody-encoding adenovirus (AD21): a phase I trial. Clin Can Res. 2000;6:3081–7. [PubMed] [Google Scholar]
- 19.Barnett BG, Crews CJ, Douglas JT. Targeted adenoviral vectors. Biochim Biophys Acta. 2002;1575:1–14. doi: 10.1016/s0167-4781(02)00249-x. [DOI] [PubMed] [Google Scholar]
- 20.Blackwell J, Li H, Navarro J, et al. Circumventing inhibitory factors in ascites fluid using a tropism modified adenoviral vector. Hum Gene Ther. 2000;11:1657–69. doi: 10.1089/10430340050111313. [DOI] [PubMed] [Google Scholar]
- 21.Galanis E, Hartmann LC, Cliby WA, et al. Phase I Trial of Intraperitoneal Administration of an Engineered Strain of Measles Virus, Modified to Express Carcinoembryonic Antigen for Recurrent Ovarian Cancer. Can Res. 2010;70:875–82. doi: 10.1158/0008-5472.CAN-09-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hemminki A, Belousova N, Zinn K, et al. An adenovirus with enhanced infectivity mediates molecular chemotherapy of ovarian cancer cells and allows imaging of gene expression. Mol Ther. 2001;4:223–31. doi: 10.1006/mthe.2001.0446. [DOI] [PubMed] [Google Scholar]
- 23.Opyrchal M, Aderca I, Galanis E. Phase I clinical trial of locoregional administration of the oncolytic adenovirus ONYX-015 in combination with mitomycin-C, doxorubicin, and cisplatin chemotherapy in patients with advanced sarcomas. Methods Mol Biol. 2009;542:705–17. doi: 10.1007/978-1-59745-561-9_35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cody JJ, Douglas JT. Armed replicating adenoviruses for cancer virotherapy. Cancer Gene Ther. 2009;16:473–88. doi: 10.1038/cgt.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rocconi RP, Zhu ZB, Stoff-Khalili M, et al. Gynecol Oncol. 2007;105:113–21. doi: 10.1016/j.ygyno.2006.10.057. [DOI] [PubMed] [Google Scholar]
- 26.Kimball KJ, Rivera AA, Zinn KR, et al. Novel infectivity-enhanced oncolytic adenovirus with a capsid-incorporated dual-imaging moiety for monitoring virotherapy in ovarian cancer. Mol Imaging. 2009;8:264–77. [PMC free article] [PubMed] [Google Scholar]
- 27.Bauerschmitz GJ, Guse K, Kanerva A, et al. Triple-Targeted Oncolytic Adenoviruses Featuring the Cox2 Promoter, E1A Transcomplementation, and Serotype Chimerism for Enhanced Selectivity for Ovarian Cancer Cells. Mol Ther. 2006;14:164–74. doi: 10.1016/j.ymthe.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Stoff-Khalili MA, Rivera AA, Glascow JN, et al. A human adenoviral vector with a chimeric fiber from a canine adenovirus type 1 results in novel expanded tropism for cancer therapy. Gene Ther. 2005;12:1696–706. doi: 10.1038/sj.gt.3302588. [DOI] [PubMed] [Google Scholar]
- 29.Yong RL, Shinojima N, Fueyo J, et al. Human bone marrow-derived mesenchymal stem cells for intravascular delivery of oncolytic adenovirus Delta24-RGD to human gliomas. Cancer Res. 2009;69:8932–40. doi: 10.1158/0008-5472.CAN-08-3873. [DOI] [PMC free article] [PubMed] [Google Scholar]