Abstract
Deterrent compounds are important in influencing the food selection of many phytophagous insects. Plants containing deterrents, such as alkaloids, are generally unfavored and typically avoided by many polyphagous lepidopteran species, including the gypsy moth, Lymantria dispar (L.) (Lepidoptera: Lymantriidae).
We tested the deterrent effects of eight alkaloids using two-choice feeding bioassays. Each alkaloid was applied at biologically relevant concentrations to glass fiber disks and leaf disks from red oak trees (Quercus rubra) (L.), a plant species highly favored by these larvae. All eight alkaloids tested on glass fiber disks were deterrent to varying degrees. When these alkaloids were applied to leaf disks, however only seven were still deterrent. Of these seven, five were less deterrent on leaf disks compared with glass fiber disks, indicating that their potency was dramatically reduced when they were applied to leaf disks. The reduction in deterrency may be attributed to the phagostimulatory effect of red oak leaves in suppressing the negative deterrent effect of these alkaloids, suggesting that individual alkaloids may confer context-dependent deterrent effects in plants in which they occur. This study provides novel insights into the feeding behavioral responses of insect larvae, such as L. dispar, to selected deterrent alkaloids when applied to natural versus artificial substrates and has the potential to suggest deterrent alkaloids as possible candidates for agricultural use.
INTRODUCTION
Plants contain a variety of allelochemicals, some of which are insect feeding deterrents. In different insect species, feeding deterrents, such as alkaloids, strongly influences plant selection, as reported for polyphagous gypsy moth larvae, Lymantria dispar (Barbosa and Krischik 1987; Barbosa et al. 1990). Unfavored plant hosts of L. dispar larvae contain alkaloids (Barbosa and Krischik, 1987; Barbosa et al., 1990).
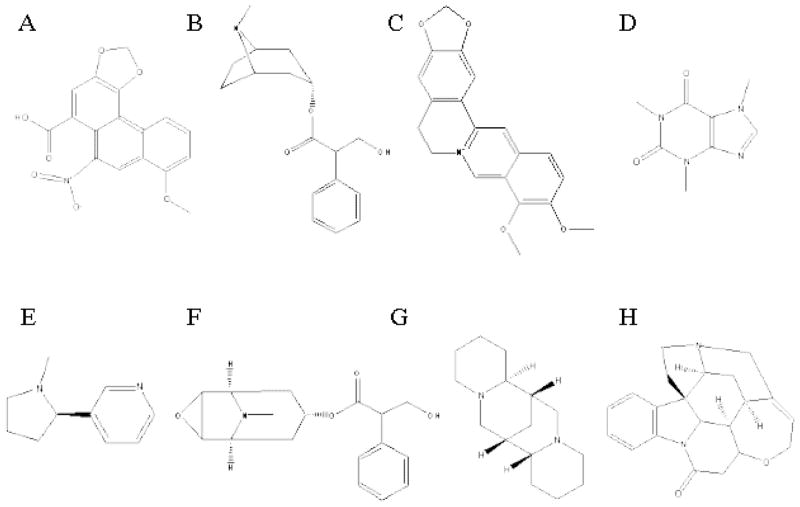
Alkaloids represent one of the largest chemically heterogenous groups of allelochemicals, occurring in 20–30% of higher plants with ca. 12,000 known structures (Wink 1993). The aim of this study was to test the deterrent effect of eight alkaloids, belonging to six alkaloid sub-classes, namely caffeine (purine), strychnine (indole), berberine and aristolochic acid (benzylisoquinoline), sparteine (quinolizidine), nicotine (pyridine), and scopolamine and atropine (tropane), on the feeding behavior of L. dispar (Fig. 1). These alkaloids are found in the families: Rubiaceae, Sterculiaceae, and Theaceae (caffeine), Apocynaceae and Loganiaceae (strychnine), Berberidaceae, Lauraceae, Annonaceae, Magnoliaceae, Papaveraceae, Ranunculaceae, Rutaceae (berberine), and Menispermaceae (berberine and aristolochic acid), Aristolochiaceae (aristolochic acid), Leguminosae (sparteine), and Solanaceae (nicotine, scopolamine, atropine) (Willaman and Schubert 1961). They were chosen since they act as potent feeding deterrents in other lepidopterous insects (e.g. Miller and Feeny, 1983; Wrubel and Bernays 1990).
Fig. 1.
Molecular structures of alkaloids. A, aristolochic acid; B, atropine; C, berberine; D, caffeine; E, nicotine; F, scopolamine; G, sparteine; H, strychnine. The structures were obtained from ChemFinder.com (http://chemfinder.cambridgesoft.com/).
We used gypsy moth larvae since they generally avoid plants containing alkaloids (Barbosa and Krischik 1987; Barbosa et al. 1990). This allowed us to determine (i) if all alkaloids tested were equally deterrent and (ii) if deterrency of a particular alkaloid changed depending on the context in which it was presented, namely application of an individual alkaloid to a glass fiber disk versus a natural feeding substrate, such as a highly favored tree species (i.e. leaf disk). The latter question addressed the difficulty in discerning deterrent alkaloids in the presence of other allelochemicals.
MATERIALS AND METHODS
L. dispar larvae (New Jersey strain) were obtained from USDA-APHIS, Otis Air National Guard Base (Falmouth, Massachusetts) and reared on a high wheat germ artificial diet (Bio-Serv, Frenchtown, NJ) (Shields et al., 2003). The feeding responses of gypsy moth larvae were evaluated using a two-choice bioassay (Shields, 2003, after Jermy et al. 1968). Fifth instar larvae, 12–18 hours postmolt, were randomly selected for all tests. We chose late instars since they carry out the majority of feeding. The insects were deprived of food 10–14 h prior to the experiments. Experiments were conducted June-July, 2002–2004.
Glass fiber disks (2.1 cm2 GF/A) (Whatman Inc., Clifton, NJ) or disks (9 mm diameter cork borer) punched out from highly favored red oak (Quercus rubra (L.)) leaves were arranged circularly in an ABABABAB fashion (A=control; B=treatment) in a Petri dish (100 mm diameter, 15 mm deep) (Fisher Scientific, Pittsburgh, PA) lined with a moistened filter paper to reduce desiccation of the disks. A pin was pushed through the center of each disk and into a piece of dental wax (Electron Microscopy Sciences, Hatfield, PA).
Eight alkaloids were chosen to determine their effect in feeding behavioral biossays: (−)-nicotine, aristolochic acid sodium salt, berberine hemisulfate salt, scopolamine hydrobromide, atropine, caffeine, (−) sparteine, strychnine hemisulfate, (MP Biomedicals, Aurora, OH, and Fisher Scientific).
Each test was replicated with 12–34 larvae and run at 24 °C (± 1 °C), for a maximum of 18 h (typically 5–15h) or until ca. 50% of the total area (estimated visually) of either control or test disks had been consumed. The test compounds were dissolved in appropriate solvents (deionized ultra filtered water, ethanol, methanol, or chloroform) (Fisher Scientific) and applied so that the chemical amounted to 1% of the dry weight of the disk. While the concentration of an allelochemical in a particular plant species can vary among individuals, leaves, and anatomical parts (e.g. leaf surface versus interior, or tip versus petiole), we chose a 1% concentration, since this level falls well within the range that has been reported to occur in nature for the alkaloids used in this study (Willaman and Schubert 1961; Wrubel and Bernays 1990; Wink 1993). This corresponds to the following molarities of the applied aliquots: nicotine, 12 mM; caffeine, 10 mM; sparteine, 9 mM; atropine, 7 mM; aristolochic acid, 6 mM, and strychnine, scopolamine, and berberine, 5 mM. Aliquots, enough to thoroughly wet the disks (108 μl for glass fiber disks or 20 μl for red oak leaf disks), were applied to all disks. An equal amount of solvent, without test compound, was applied to control disks. After the solvent had evaporated, a similar aliquot of an aqueous 0.06 M sucrose solution was applied to both test and control disks only for experiments using glass fiber disks. Test compounds that were soluble in water were dissolved directly in the sucrose solution and applied to all glass fiber disks. Sucrose acted to enhance palatability and induce feeding of glass fiber disks.
Each larva was placed in the center of a Petri dish (Shields et al., 2003). For each test, a set of control disks was held in the absence of the larvae (called control disks II). After each experiment, the disks (control, control II, and test) were air dried at room temperature (glass fiber disks) or oven-dried at 80°C (red oak leaf disks) for 24–48 h. Mean consumption (mg, for red oak leaf disks and mean area, mm2, for glass fiber disks) was calculated by subtracting the remaining mass (red oak leaf disks) or area (glass fiber disks) of a test or control disk from the mass or area of a control disk II. The areas of test and control disks consumed and the area of control disks II were measured using SigmaScan Pro 5 (Systat Software, Inc., Point Richmond, CA). Leaf disks were weighed (±0.01 mg) (Sartorius BP 211 D). Values were reported as percent relative mean consumption of control consumption. The difference between control consumption (100%) and relative mean consumption of disks (glass fiber or red oak leaf disks) was tested using a paired Wilcoxon signed rank test; the unpaired Mann-Whitney U-test was used for comparisons between consumption of glass fiber versus red oak leaf disks (significance level alpha=0.05). Data were analyzed using Statmost 3.5 (Dataxiom Software Inc., Los Angeles, CA) and Excel (Microsoft Corp.)
RESULTS
Of the eight alkaloids tested (Fig. 1) on glass fiber disks, caffeine was the most potent feeding deterrent (−3.1%) (n=12, P=0.005) (Fig. 2). The remaining alkaloids were also deterrent: strychnine (−0.5%) (n=12, P=0.012), berberine (1.4%) (n=12, P=0.018), nicotine (3.6%) (n=12, P=0.008), aristolochic acid (5.2%) (n=13, P=0.004), sparteine (5.3%) (n=14, P=0.002), atropine (6.9%) (n=14, P=0.003) and scopolamine (57.6%) (n=12, P=0.012) (Fig. 2). Negative mean consumption values signify that test disks weighed slightly heavier than control II disks, indicating that no feeding had occurred.
Fig. 2.
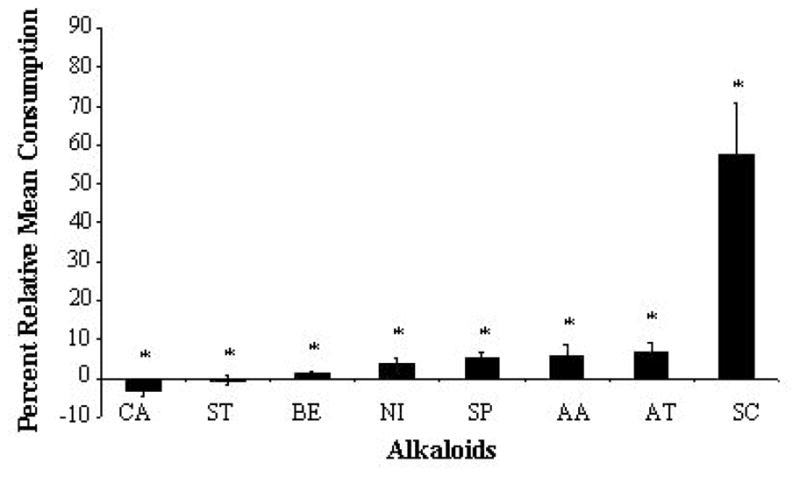
Effect of eight selected alkaloids applied to glass fiber disks on feeding consumption by fifth instar Lymantria dispar larvae using two-choice feeding bioassays. Consumption was normalized with respect to control disks (100%). Bars represent the alkaloids tested. AA, aristolochic acid; AT, atropine; BE, berberine, CA, caffeine; NI, nicotine, SC, scopolamine; SP, sparteine, and ST, strychnine. Results are derived from 13, 14, 12, 12, 12, 12, 14, and 12 larvae (number of replicates), respectively. Asterisks indicate alkaloids that significantly deterred feeding (P < 0.05). Negative values indicate that no feeding had occurred. Error bars represent S.E.
Seven alkaloids deterred feeding when applied to red oak leaf disks (caffeine, 39.9%, n = 34; P<0.001); strychnine, (33.8%, n=15; P<0.001), berberine, (31.5%, n=15; P=0.005), nicotine, (41.0%, n=50; P<0.001), sparteine, (37.4%, n=21; P<0.001), aristolochic acid, (19.8%, n=23; P=0.017), atropine, (28.4%, n=25; P=0.005) (Fig. 3). Scopolamine (70.8%) displayed no deterrent effect (n=34; P= 0.054) (Fig. 3). The concentration of all alkaloids tested was the same (1%) and the calculated molarities fell within a narrow range (5–12 mM). The differences in the molarity between alkaloids did not account for observed differences in feeding deterrency. For example, nicotine (12 mM, high concentration) was only the fourth and seventh most potent feeding deterrents on disks and leaves, respectively, while strychnine and berberine (both 5 mM, low concentration) were the second and third most potent feeding deterrents on glass fiber disks, respectively, and fourth and third highest on leaf disks, respectively (Figs. 2, 3).
Fig. 3.
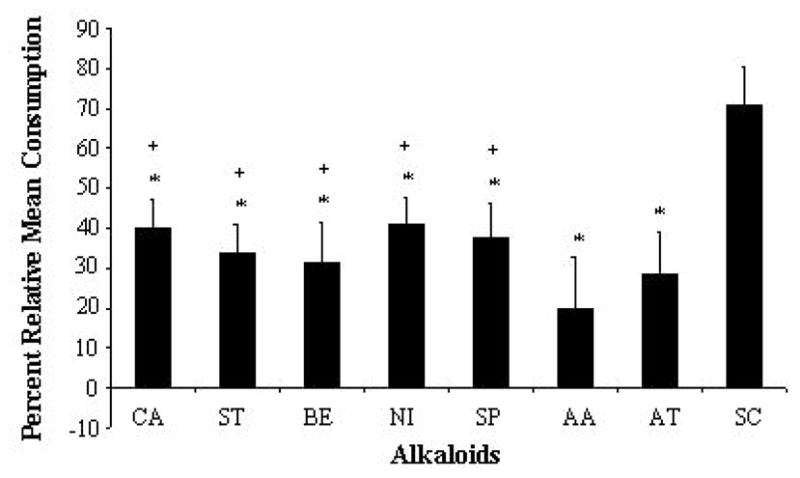
Effect of eight selected alkaloids applied to red oak leaf disks on feeding consumption by fifth instar Lymantria dispar larvae using two-choice feeding bioassays. Consumption was normalized with respect to control disks (100%). Bars represent the alkaloids tested. AA, aristolochic acid; AT, atropine; BE, berberine, CA, caffeine; NI, nicotine, SC, scopolamine; SP, sparteine, and ST, strychnine. Results are derived from 23, 25, 15, 34, 30, 34, 21, and 15 larvae (number of replicates). Asterisks indicate alkaloids that significantly deterred feeding (P < 0.05). Plus symbols indicate alkaloids that were significantly less deterrent on red oak leaves compared with glass fiber disks (see also Figure 1). Negative values indicate that no feeding had occurred. Error bars represent S.E.
Subsequently, we compared the deterrent effect of each alkaloid on glass fiber disks versus red oak leaf disks. The concentration of alkaloids applied to glass fiber disks and leaf disks was the same (1%); the only difference was in the amount applied to the larger glass fiber disks. Overall, consumption was higher when alkaloids were applied to red oak leaves versus glass fiber disks. Of the seven alkaloids found to be deterrent, five were less deterrent on red oak leaf disks (glass fiber disk versus leaf disk; caffeine: −3.1% (n=12) vs 39.9% (n=34) (P = 0.004); strychnine: −0.5% (n=12) vs 33.8% (n=15) (P=0.006); berberine: 1.4% (n=12) vs 31.5% (n=15) (P=0.017); nicotine: 3.6% (n=12) vs 41.0%; (n=50) (P<0.001), sparteine: 5.3% (n=14) vs 37.4% (n=21) (P=0.027) (Fig. 3). No difference in deterrency was observed for aristolochic acid (5.2%, n=13) vs 19.8% (n=23, P=0.385), atropine (6.9%, n=14) vs 28.4% (n=25, P=0.622), and scopolamine (57.6%, n=12) vs 70.8% (n=34, P=0.483) (Fig. 3).
DISCUSSION
We examined the effects of eight alkaloids on the feeding behavior of fifth instar gypsy moth larvae. When each alkaloid was tested on glass fiber disks treated with the phagostimulant, sucrose, all eight alkaloids were deterrent. When these eight alkaloids were presented to larvae on red oak leaf disks, only seven alkaloids were deterrent and scopolamine was not deterrent.
We attributed the reduction in deterrency to the phagostimulatory effect of red oak leaves, which suppressed the negative deterrent effect of these alkaloids. This suggested that (i) red oak leaves may provide the larvae with positive feedback, resulting in increased consumption and reduced potency of these alkaloids, and (ii) individual alkaloids may confer context-dependent deterrent effects in plants in which they occur. For aristolochic acid and atropine, we detected no difference in deterrency between glass fiber and red oak leaf disks. Similarly, Wrubel and Bernays (1990) found that some alkaloids, when applied to glass fiber disks, deterred Manduca sexta larvae but were ineffective when applied to natural leaves. They attributed this finding to the phagostimulatory effect and positive cues from tobacco leaves in overwhelming the negative feeding cues attributed to the deterrent alkaloids. This would also suggest that the neural code that signals deterrency to the brain of an insect changes, depending on the context in which the allelochemical is presented. Context dependency of alkaloid deterrency is not limited to the environment in which alkaloids are presented to the insect (i.e. leaf species on which the insects feeds), but can also be influenced by such factors as prior feeding experience (Wrubel and Bernays 1990; Akhtar and Isman 2004).
This study provides novel insights into the variability of alkaloids as effective feeding deterrents when they are applied to natural versus artificial substrates. We found that alkaloids, originally thought to be potent feeding deterrents, are highly variable, depending on the substrate on which they were presented. This study enhances our understanding of insect-plant interactions and provides insights into the feeding behavioral responses of larvae, such as L. dispar. While we hypothesize that taste information is pivotal in determining the effect of alkaloids on feeding, we cannot ascertain from our study the actual neural mechanisms mediating the effects of these compounds. Specifically, gustatory, olfactory, tactile, somatosensory, habituation, or toxicity mechanisms may be playing roles in determining the effect of these alkaloids in deterring feeding (e.g. Szentesi and Bernays 1984; Raffa and Frazier 1988; Wruble and Bernays 1990; Glendinning 1996). It is possible that each alkaloid may be activating one or several of these response mechanisms. The results of our study have the potential to suggest deterrent alkaloids as possible novel candidates in designing suitable new strategies for crop protection from insect pests.
Acknowledgments
We thank Elizabeth Bernays and John Glendinning for critically reviewing this manuscript. We thank Martin Shapiro, Jill Discordia, Howard Kaplon, and Raymond Cardillo for assistance during this study. We also thank students, Mikaela Walker, Lukas Goodmuth, Leila Dodson, Kismet-Gerald Agbasi, and Katherine Magruder. Supported by NSF (DBI-0097478), NIH (GM-58384), Towson University (FCSM) and NIH (1 R15 DC007609-01) and Franklin (Amer. Philos. Soc.) research grants to V.D.S.
References
- Akhtar Y, Isman MB. Generalization of a habituated feeding deterrent response to unrelated antifeedants following prolonged exposure in a generalist herbivore, Trichoplusia ni. J Chem Ecol. 2004;30:1349–1362. doi: 10.1023/b:joec.0000037744.73291.b6. [DOI] [PubMed] [Google Scholar]
- Barbosa P, Krischik VA. Influence of alkaloids on feeding preference of eastern deciduous forest trees by the gypsy moth Lymantria dispar. Amer Naturalist. 1987;130:53–69. [Google Scholar]
- Barbosa P, Gross P, Provan GJ, Pacheco DY, Stermitz FR. Allelochemicals in foliage of unfavored tree hosts of the gypsy moth, Lymantria dispar L. 1. Alkaloids and other components of Liriodendron tulipifera L. (Magnoliaceae), Acer rubrum L. (Aceraceae), and Cornus florida L. (Cornaceae) J Chem Ecol. 1990;16:1719–1730. doi: 10.1007/BF01014103. [DOI] [PubMed] [Google Scholar]
- deBoer G, Hanson FE. Feeding responses to solanaceous allelochemicals by larvae of the tobacco hornworm, Manduca sexta. Entomol Exp Appl. 1987;45:123–131. [Google Scholar]
- Glendinning JI. Is chemosensory input essential for the rapid rejection of toxic foods? J Exp Biol. 1996;199:1523–1534. doi: 10.1242/jeb.199.7.1523. [DOI] [PubMed] [Google Scholar]
- Jermy T, Hanson FE, Dethier VG. Induction of specific food preference in lepidopterous larvae. Entomol Exp Appl. 1968;11:211–230. [Google Scholar]
- Miller JS, Feeny P. Effects of benzylisoquinoline alkaloids on the larvae of polyphagous Lepidoptera. Oecologia. 1983;58:332–339. doi: 10.1007/BF00385232. [DOI] [PubMed] [Google Scholar]
- Raffa KF, Frazier JL. A general model for quantifying behavioral desensitization to antifeedants. Ent Exp Appl. 1988;46:93–100. [Google Scholar]
- Shields VDC, Broomell BP, Salako JOB. Host selection and acceptability of selected tree species by gypsy moth larvae, Lymantria dispar (L.) Ann Entomol Soc Amer. 2003;96:920–926. [Google Scholar]
- SigmaScan Pro Version 5. Systat Software, Inc; Point Richond, CA: [Google Scholar]
- Statmost Version 3.2. DataMost Corp; Los Angeles, CA: [Google Scholar]
- Szentesi A, Bernays EA. A study of behavioral habituation to a feeding deterrent in nymphs of Schistocerca gregaria. Physiol Entomol. 1984;9:329–340. [Google Scholar]
- Willaman JJ, Schubert BG. Alkaloid-bearing plants and their contained alkaloids. USDA Tech Bull. 1961:1234. [Google Scholar]
- Wink M. Allelochemical properties or the raison d’être of alkaloids. In: Cordell GA, editor. The alkaloids: chemistry and pharmacology. Vol. 43. Academic Press, Inc; Boston, MA: 1993. pp. 1–118. [Google Scholar]
- Wrubel RP, Bernays EA. The relative insensitivity of Manduca sexta larvae to non-host plant secondary compounds. Entomol Exp Appl. 1990;54:117–124. [Google Scholar]