Abstract
The effect of colchicine on the uptake of oxygen by human leukocytes during phagocytosis of live streptococci or of killed staphylococci was compared with the effect of colchicine on phagocytosis per se, measured in a sensitive bacterial system.
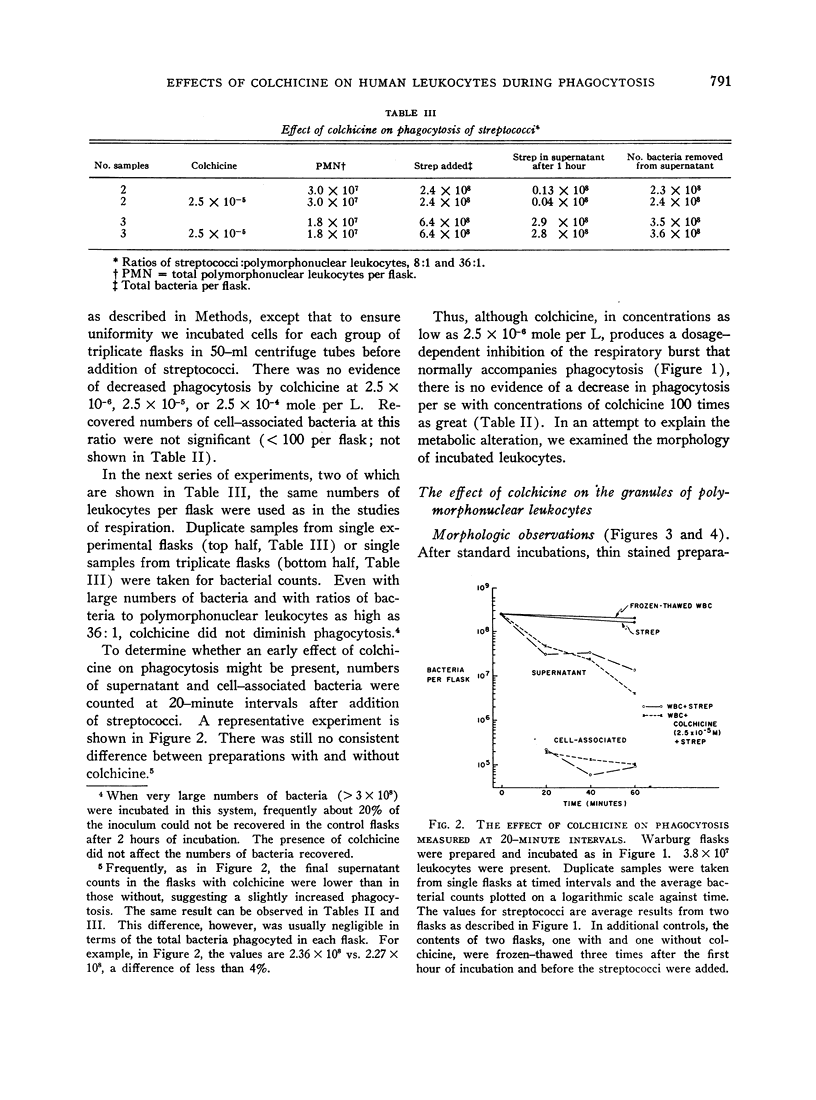
The increase in oxygen consumption that normally accompanies phagocytosis was consistently diminished in leukocytes incubated with colchicine in concentrations as low as 2.5 × 10-6 mole per L (1 μg per ml), and this inhibition was dosage dependent. Yet there was no evidence of decreased phagocytosis with concentrations of colchicine as high as 2.5 × 10-4 mole per L (100 μg per ml). Furthermore, with measurements at 20, 40, and 60 minutes, the rate of phagocytosis was comparable with and without colchicine.
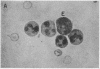
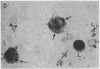
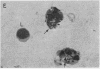
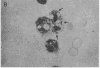
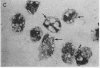
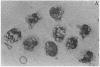
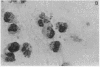
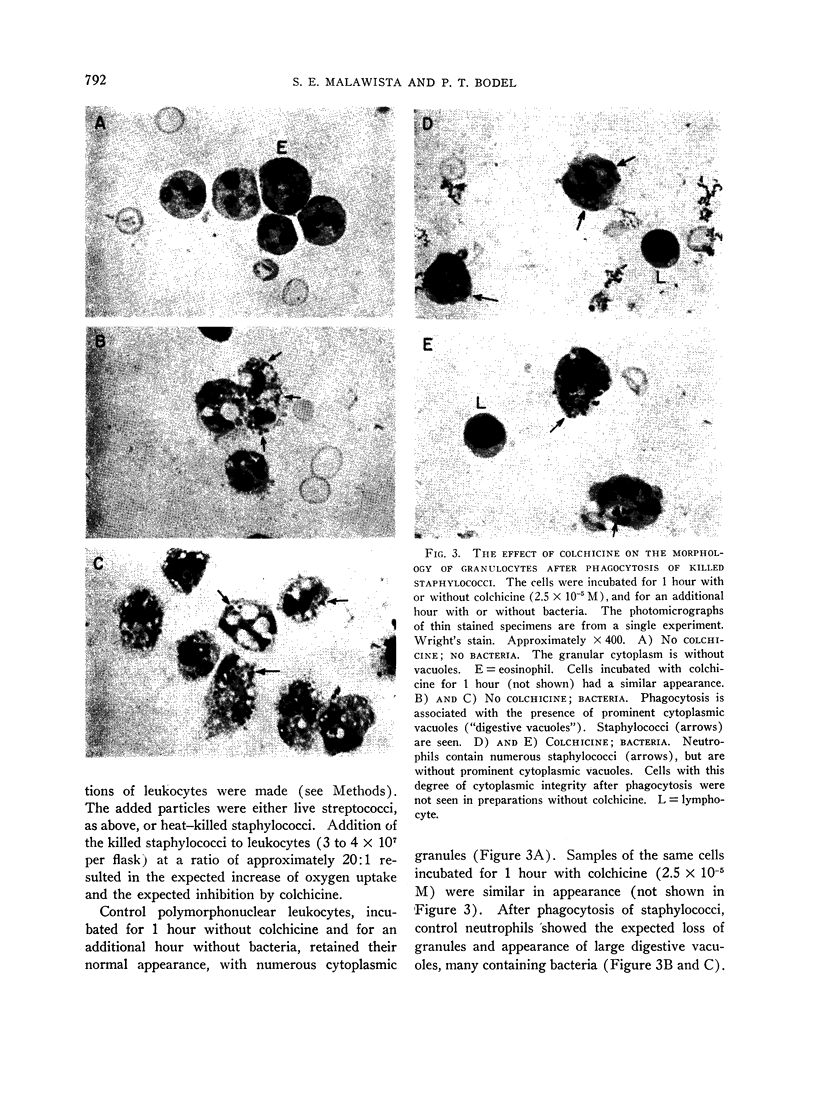
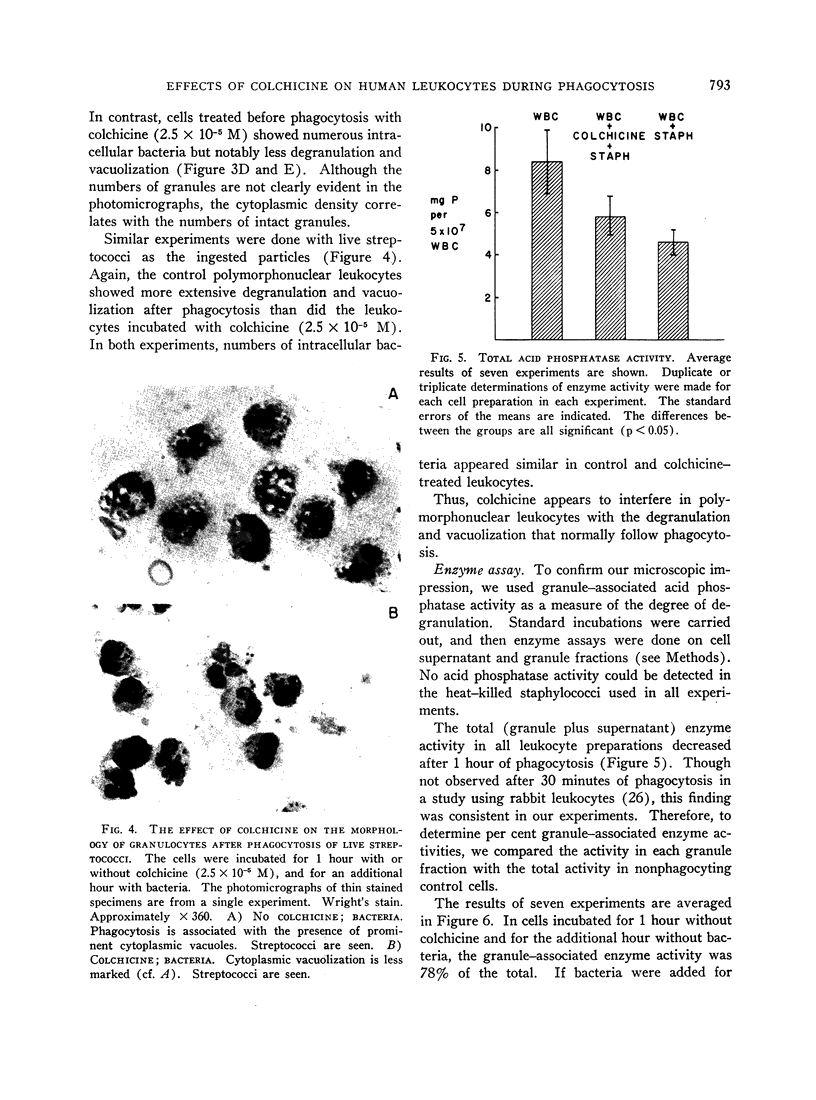
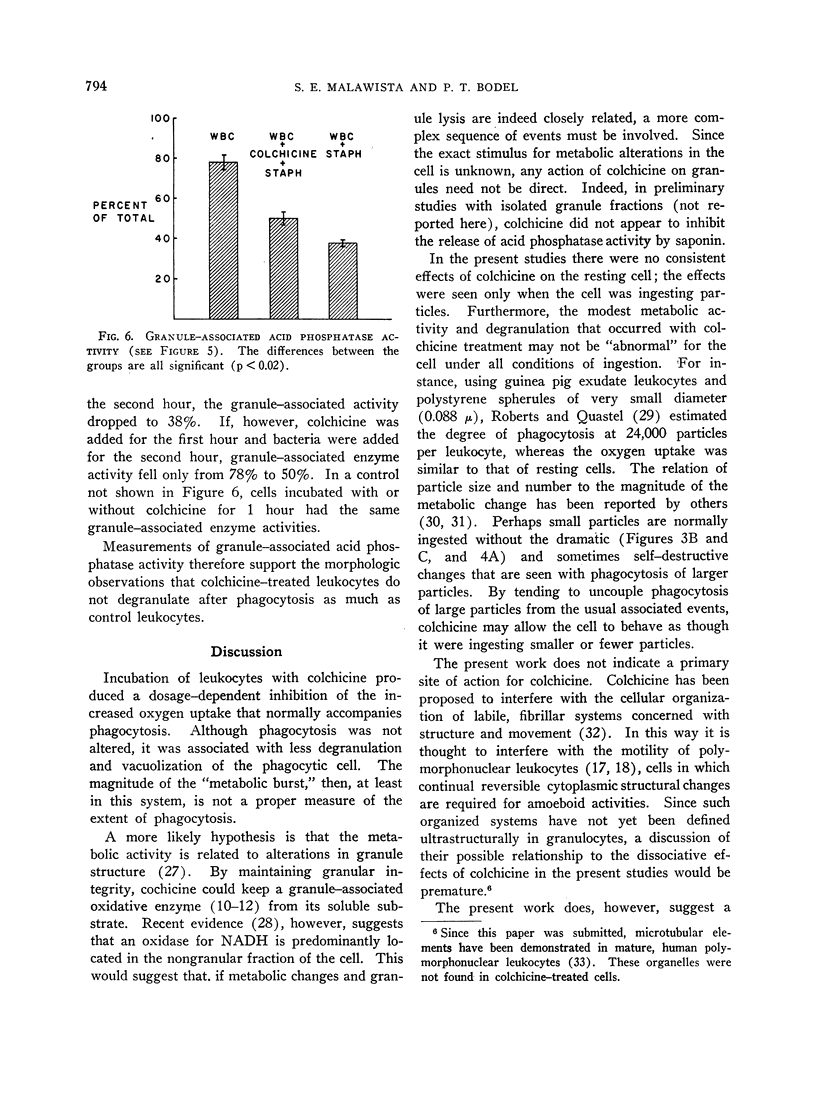
A clue to the dissociation between oxygen consumption and phagocytosis was found in rapidly dried preparations of the incubated leukocytes. Ingested bacteria were present in both control and colchicine-treated granulocytes. In addition, control cells showed normal loss of granules (lysosomal particles) and prominent cytoplasmic vacuoles (digestive vacuoles). Colchicine-treated cells, however, showed less such degranulation and vacuolization. Measurements of granule-associated acid phosphatase activity after phagocytosis support the morphologic observations of less degranulation in colchicine-treated leukocytes.
The muted metabolic and morpholgic response to phagocytosis in colchicine-treated cells may be important for the anti-inflammatory effect of colchicine in acute gouty arthritis. Colchicine may also find wider use in defining structure-function dependencies in metabolically stimulated cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BREWER D. B. ELECTRON MICROSCOPY OF PHAGOCYTOSIS OF STAPHYLOCOCCI. J Pathol Bacteriol. 1963 Oct;86:299–303. [PubMed] [Google Scholar]
- CAGAN R. H., KARNOVSKY M. L. ENZYMATIC BASIS OF THE RESPIRATORY STIMULATION DURING PHAGOCYTOSIS. Nature. 1964 Oct 17;204:255–257. doi: 10.1038/204255a0. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., HIRSCH J. G. The influence of phagocytosis on the intracellular distribution of granule-associated components of polymorphonuclear leucocytes. J Exp Med. 1960 Dec 1;112:1015–1022. doi: 10.1084/jem.112.6.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., HIRSCH J. G. The isolation and properties of the specific cytoplasmic granules of rabbit polymorphonuclear leucocytes. J Exp Med. 1960 Dec 1;112:983–1004. doi: 10.1084/jem.112.6.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., MORSE S. I. Functional and metabolic properties of polymorphonuclear leucocytes. I. Observations on the requirements and consequences of particle ingestion. J Exp Med. 1960 May 1;111:667–687. doi: 10.1084/jem.111.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., MORSE S. I. Functional and metabolic properties of polymorphonuclear leucocytes. II. The influence of a lipopolysaccharide endotoxin. J Exp Med. 1960 May 1;111:689–704. doi: 10.1084/jem.111.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., MORSE S. I. Interactions between rabbit polymorphonuclear leucocytes and staphylococci. J Exp Med. 1959 Sep 1;110:419–443. doi: 10.1084/jem.110.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caner J. E. Colchicine inhibition of chemotaxis. Arthritis Rheum. 1965 Oct;8(5):757–764. doi: 10.1002/art.1780080438. [DOI] [PubMed] [Google Scholar]
- EVANS W. H., KARNOVSKY M. L. The biochemical basis of phagocytosis. IV. Some aspects of carbohydrate metabolism during phagocytosis. Biochemistry. 1962 Jan;1:159–166. doi: 10.1021/bi00907a024. [DOI] [PubMed] [Google Scholar]
- FALLON H. J., FREI E., 3rd, DAVIDSON J. D., TRIER J. S., BURK D. Leukocyte preparations from human blood: evaluation of their morphologic and metabolic state. J Lab Clin Med. 1962 May;59:779–791. [PubMed] [Google Scholar]
- GOODMAN J. R., MOORE R. E. Electron microscopic study of phagocytosis of Staphylococcus by human leukocytes. J Bacteriol. 1956 May;71(5):547–556. doi: 10.1128/jb.71.5.547-556.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfinger S. E., Howell R. R., Seegmiller J. E. Suppression of metabolic accompaniments of phagocytosis by colchicine. Arthritis Rheum. 1965 Dec;8(6):1112–1122. doi: 10.1002/art.1780080610. [DOI] [PubMed] [Google Scholar]
- HIRSCH J. G., COHN Z. A. Degranulation of polymorphonuclear leucocytes following phagocytosis of microorganisms. J Exp Med. 1960 Dec 1;112:1005–1014. doi: 10.1084/jem.112.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRSCH J. G., STRAUSS B. STUDIES ON HEAT-LABILE OPSONIN IN RABBIT SERUM. J Immunol. 1964 Jan;92:145–154. [PubMed] [Google Scholar]
- IYER G. Y., QUESTEL J. H. NADPH and NADH oxidation by guinea pig polymorphonuclear leucocytes. Can J Biochem Physiol. 1963 Feb;41:427–434. [PubMed] [Google Scholar]
- KARNOVSKY M. L. Metabolic basis of phagocytic activity. Physiol Rev. 1962 Jan;42:143–168. doi: 10.1152/physrev.1962.42.1.143. [DOI] [PubMed] [Google Scholar]
- KRENIS L. J., STRAUSS B. Effect of size and concentration of latex particles on respiration of human blood leucocytes. Proc Soc Exp Biol Med. 1961 Aug-Sep;107:748–750. doi: 10.3181/00379727-107-26743. [DOI] [PubMed] [Google Scholar]
- LOCKWOOD W. R., ALLISON F. ELECTRON MICROGRAPHIC STUDIES OF PHAGOCYTIC CELLS. I. MORPHOLOGICAL CHANGES OF THE CYTOPLASM AND GRANULES OF RABBIT GRANULOCYTES ASSOCIATED WITH INGESTION OF ROUGH PNEUMOCOCCUS. Br J Exp Pathol. 1963 Dec;44:593–600. [PMC free article] [PubMed] [Google Scholar]
- MALAWISTA S. E. ON THE ACTION OF COLCHICINE, THE MELANOCYTE MODEL. J Exp Med. 1965 Aug 1;122:361–384. doi: 10.1084/jem.122.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALAWISTA S. E., SEEGMILLER J. E. THE EFFECT OF PRETREATMENT WITH COLCHICINE ON THE INFLAMMATORY RESPONSE TO MICROCRYSTALLINE URATE: A MODEL FOR GOUTY INFLAMMATION. Ann Intern Med. 1965 Apr;62:648–657. doi: 10.7326/0003-4819-62-4-648. [DOI] [PubMed] [Google Scholar]
- MCCARTY D. J., HOLLANDER J. L. Identification of urate crystals in gouty synovial fluid. Ann Intern Med. 1961 Mar;54:452–460. doi: 10.7326/0003-4819-54-3-452. [DOI] [PubMed] [Google Scholar]
- Malawista S. E. The action of colchicine in acute gout. Arthritis Rheum. 1965 Oct;8(5):752–756. doi: 10.1002/art.1780080437. [DOI] [PubMed] [Google Scholar]
- OREN R., FARNHAM A. E., SAITO K., MILOFSKY E., KARNOVSKY M. L. Metabolic patterns in three types of phagocytizing cells. J Cell Biol. 1963 Jun;17:487–501. doi: 10.1083/jcb.17.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS J., QUASTEL J. H. PARTICLE UPTAKE BY POLYMORPHONUCLEAR LEUCOCYTES AND EHRLICH ASCITES-CARCINOMA CELLS. Biochem J. 1963 Oct;89:150–156. doi: 10.1042/bj0890150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSSI F., ZATTI M. CHANGES IN THE METABOLIC PATTERN OF POLYMORPHO-NUCLEAR LEUCOCYTES DURING PHAGOCYTOSIS. Br J Exp Pathol. 1964 Oct;45:548–559. [PMC free article] [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- SEEGMILLER J. E., HOWELL R. R. The old and new concepts of acute gouty arthritis. Arthritis Rheum. 1962 Dec;5:616–623. doi: 10.1002/art.1780050610. [DOI] [PubMed] [Google Scholar]
- STETSON C. A., Jr Studies on the mechanism of the Shwartzman phenomenon; certain factors involved in the production of the local hemorrhagic necrosis. J Exp Med. 1951 May;93(5):489–504. doi: 10.1084/jem.93.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS L. POSSIBLE ROLE OF LEUCOCYTE GRANULES IN THE SHWARTZMAN AND ARTHUS REACTIONS. Proc Soc Exp Biol Med. 1964 Jan;115:235–240. doi: 10.3181/00379727-115-28879. [DOI] [PubMed] [Google Scholar]
- Wallace S. L. Mechanism of action of colchicine. Arthritis Rheum. 1965 Oct;8(5):744–748. doi: 10.1002/art.1780080435. [DOI] [PubMed] [Google Scholar]
- ZATTI M., ROSSI F., MENEGHELLI V. METABOLIC AND MORPHOLOGICAL CHANGES OF POLYMORPHONUCLEAR LEUCOCYTES DURING PHAGOCYTOSIS. Br J Exp Pathol. 1965 Apr;46:227–233. [PMC free article] [PubMed] [Google Scholar]
- ZUCKER-FRANKLIN D., HIRSCH J. G. ELECTRON MICROSCOPE STUDIES ON THE DEGRANULATION OF RABBIT PERITONEAL LEUKOCYTES DURING PHAGOCYTOSIS. J Exp Med. 1964 Oct 1;120:569–576. doi: 10.1084/jem.120.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]