Abstract
In studies by continuous flow electrophoresis the coumarin anticoagulant drug warfarin sodium was found to be bound solely to the albumin fraction of the plasma proteins. The interaction was studied in detail by equilibrium dialysis of solutions of crystalline human plasma albumin and warfarin sodium. Analysis of the data showed that albumin possesses a single strong binding site for warfarin with an association constant of 154,000 at 3° C and secondary classes of several sites with a much lower affinity. The free energy of binding for the first anion determined at 3° and 37° C was -6.54 and -7.01 kcal per mole, respectively. The standard enthalpy change for the interaction was -3.48 kcal per mole, and the entropy change was +11.2 U.
The negative enthalpy change was surprisingly large and the positive entropy change small for an anion-albumin interaction, suggesting significant nonionic binding. The inability to saturate the albumin binding sites, even when high concentrations of warfarin were used, is consistent with a reversible configurational alteration of the albumin molecule during the binding process. The thermodynamic data indicate that the albumin binding sites for warfarin sodium are formed during the process of binding, rather than being performed as in antigen-antibody reactions. The strength of the binding process suggests that many of the pharmacodynamic characteristics of warfarin sodium in man are determined by its strong interaction with plasma albumin. Such correlations of the physicochemical interactions and biologic effects of the coumarin anticoagulant drugs should lead to a better understanding of their mechanisms of action.
Full text
PDF

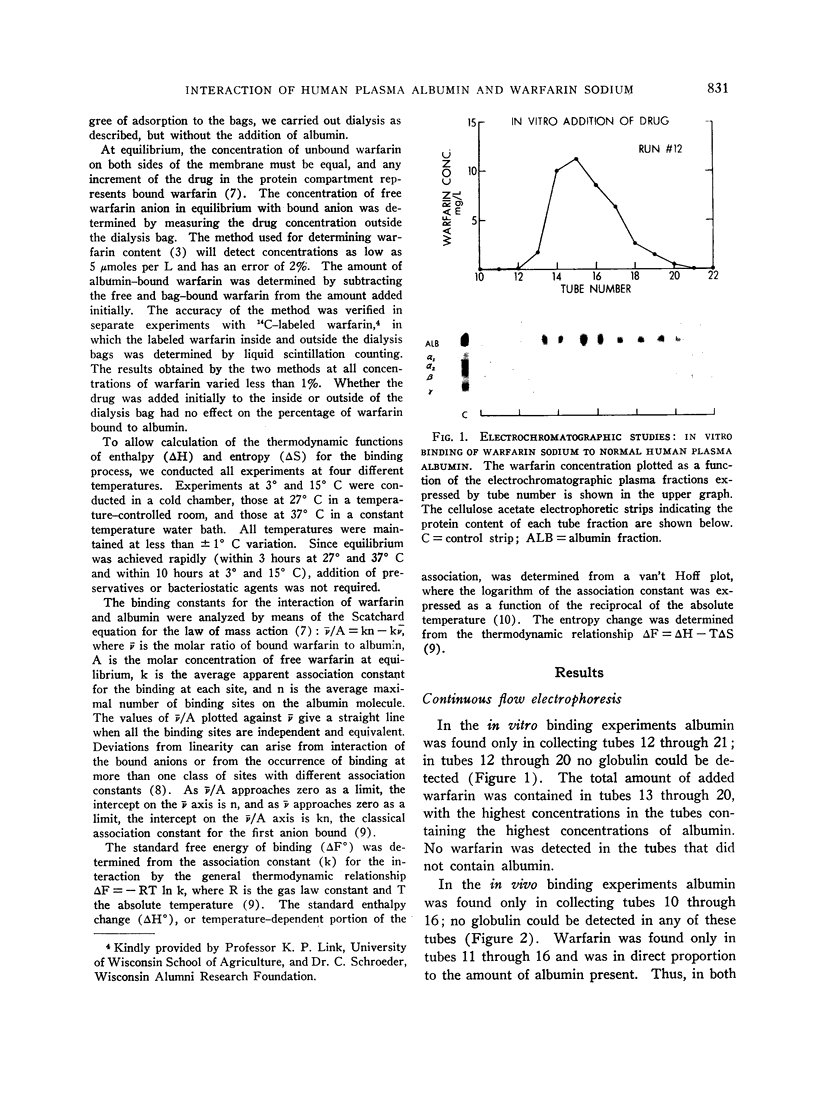
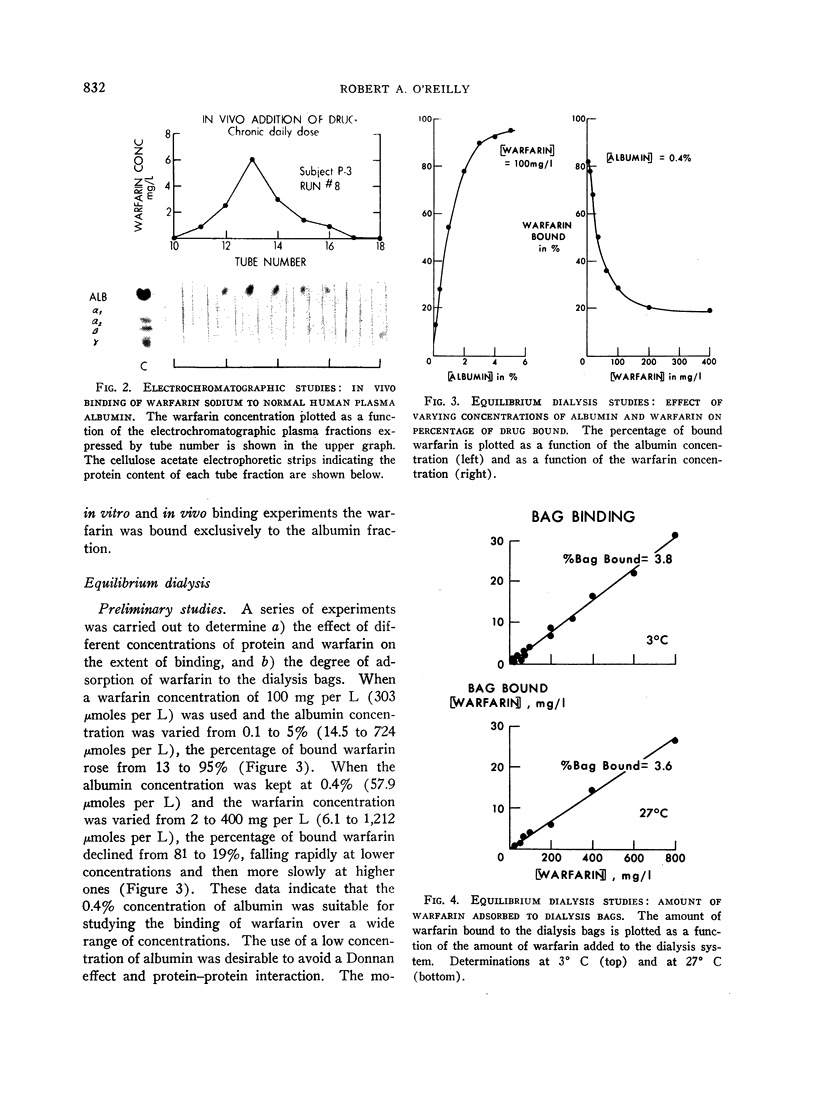



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERSON S. A., YALOW R. S. Quantitative aspects of the reaction between insulin and insulin-binding antibody. J Clin Invest. 1959 Nov;38:1996–2016. doi: 10.1172/JCI103979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRODIE B. B., HOGBEN C. A. Some physico-chemical factors in drug action. J Pharm Pharmacol. 1957 Jun;9(6):345–380. doi: 10.1111/j.2042-7158.1957.tb12289.x. [DOI] [PubMed] [Google Scholar]
- Baker K. J., Bradley S. E. Binding of sulfobromophthalein (BSP) sodium by plasma albumin. Its role in hepatic BSP extraction. J Clin Invest. 1966 Feb;45(2):281–287. doi: 10.1172/JCI105341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansch C., Kiehs K., Lawrence G. L. The role of substituents in the hydrophobic bonding of phenols by serum and mitochondrial proteins. J Am Chem Soc. 1965 Dec 20;87(24):5770–5773. doi: 10.1021/ja00952a044. [DOI] [PubMed] [Google Scholar]
- Hiskey C. F., Melnitchenko V. Clathrates of sodium warfarin. J Pharm Sci. 1965 Sep;54(9):1298–1302. doi: 10.1002/jps.2600540916. [DOI] [PubMed] [Google Scholar]
- KLOTZ I. M. The nature of some ion-protein complexes. Cold Spring Harb Symp Quant Biol. 1950;14:97–112. doi: 10.1101/sqb.1950.014.01.014. [DOI] [PubMed] [Google Scholar]
- O'REILLY R. A., AGGELER P. M., HOAG M. S., LEONG L. S., KROPATKIN M. L. HEREDITARY TRANSMISSION OF EXCEPTIONAL RESISTANCE TO COUMARIN ANTICOAGULANT DRUGS. THE FIRST REPORTED KINDRED. N Engl J Med. 1964 Oct 15;271:809–815. doi: 10.1056/NEJM196410152711602. [DOI] [PubMed] [Google Scholar]
- O'REILLY R. A., AGGELER P. M., LEONG L. S. STUDIES ON THE COUMARIN ANTICOAGULANT DRUGS: A COMPARISON OF THE PHARMACODYNAMICS OF DICUMAROL AND WARFARIN IN MAN. Thromb Diath Haemorrh. 1964 Apr 15;11:1–22. [PubMed] [Google Scholar]
- O'REILLY R. A., AGGELER P. M., LEONG L. S. STUDIES ON THE COUMARIN ANTICOAGULANT DRUGS: THE PHARMACODYNAMICS OF WARFARIN IN MAN. J Clin Invest. 1963 Oct;42:1542–1551. doi: 10.1172/JCI104839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly R. A., Aggeler P. M. Coumarin anticoagulant drugs: hereditary resistance in man. Fed Proc. 1965 Nov-Dec;24(6):1266–1273. [PubMed] [Google Scholar]
- SCHOLTAN W. BESTIMMUNGSMETHODEN UND GESETZMAESSIGKEITEN DER EIWEISSBINDUNG VON SULFONAMIDEN UND PENICILLINEN. Antibiot Chemother. 1964;12:103–134. [PubMed] [Google Scholar]
- STERLING K. MOLECULAR STRUCTURE OF THYROXINE IN RELATION TO ITS BINDING BY HUMAN SERUM ALBUMIN. J Clin Invest. 1964 Sep;43:1721–1729. doi: 10.1172/JCI105047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STERLING K., ROSEN P., TABACHNICK M. Equilibrium dialysis studies of the binding of thyroxine by human serum albumin. J Clin Invest. 1962 May;41:1021–1030. doi: 10.1172/JCI104552. [DOI] [PMC free article] [PubMed] [Google Scholar]