Abstract
Rationale: Persistent inflammation plays a major role in chronic obstructive pulmonary disease (COPD) pathogenesis, but its mechanisms are incompletely defined. Overproduction of the inflammatory mediator prostaglandin (PG) E2 by COPD fibroblasts contributes to reduced repair function.
Objectives: The present study determined if fibroblasts from subjects with COPD overproduce PGE2 after stimulation with the inflammatory cytokines IL-1β and tumor necrosis factor-α, and further defined the mechanism for overproduction.
Methods: Fibroblasts were isolated from parenchymal tissue obtained from smokers with and without COPD undergoing lung surgery. PGE2, cyclooxygenases (COX), and miR-146a in these cells were evaluated by in vitro studies.
Measurements and Main Results: After stimulation with inflammatory cytokines, COPD fibroblasts produced 2.7-fold more PGE2 compared with controls with similar smoking history. The increase in PGE2 depended on induction of COX-2, which increased to a greater degree in fibroblasts from subjects with COPD. Cytokines also induced microRNA miR-146a expression in both fibroblasts, but significantly less in COPD fibroblasts. miR-146a caused degradation of COX-2 mRNA; reduced expression prolonged COX-2 mRNA half-life in fibroblasts from subjects with COPD. Cytokine-stimulated PGE2 production and miR-146a expression in cultured fibroblasts correlated with clinical severity assessed by expiratory airflow and diffusion capacity.
Conclusions: miR-146a seems to play a pathogenetic role in the abnormal inflammatory response in COPD. Increased half-life of inflammatory mRNAs is a mechanism of abnormal inflammation in this disease.
Keywords: chronic obstructive pulmonary disease, miR-146a, prostaglandin E2, cyclooxygenase-2, fibroblasts
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Reduced expression of miR-146a is a pathogenetic mechanism that contributes to the abnormal inflammatory response in chronic obstructive pulmonary disease.
What This Study Adds to the Field
The reduced expression of miR-146a is a novel pathogenetic mechanism and suggests a new therapeutic target that can control the abnormal inflammatory response in chronic obstructive pulmonary disease.
Chronic obstructive pulmonary disease (COPD), currently the fourth leading cause of death in the United States, will become the third leading cause by 2020 in the United States and globally (1). Cigarette smoking is the most important cause, and smoking cessation early in the course of the disease can slow the rate at which lung function is lost (2). An abnormal inflammatory response of the lung, which persists despite cessation of smoking (3–6), is characteristic of COPD. This inflammation is believed to play a major role in the pathogenesis and progression of COPD (1, 7). Although this inflammation is believed to be “abnormal” (1), there is little information on how inflammation in COPD differs from normal.
The tissue alterations that underlie COPD result not only from tissue damage, but also from the inability of repair responses to restore tissue structure (1, 7, 8). In this context, cigarette smoke directly inhibits repair processes (9–12). Inflammatory mediators also modulate repair. Prostaglandin (PG) E2, an inflammatory mediator increased in the lungs of patients with COPD, is overproduced by COPD fibroblasts under basal culture conditions (13, 14). PGE2 is also a potent inhibitor of lung fibroblast repair functions (15–19). COPD fibroblasts cultured ex vivo are deficient in a number of repair functions in part because of overproduction of PGE2 by the cells under “baseline” culture conditions. However, the release of PGE2 increases greatly in response to a variety of inflammatory stimuli including IL-1β and tumor necrosis factor (TNF)-α, which are believed to play key roles in COPD. The current study, therefore, was designed to determine if COPD fibroblasts also overproduce PGE2 in response to inflammatory mediators.
We obtained lung tissue from smokers who did and did not have concurrent COPD who were undergoing surgery, generally for suspected lung cancer. Fibroblasts were cultured from cancer-free areas of the lung. This allowed us to compare cells from patients with COPD to a similarly aged group with similar smoking history and clinical diagnosis.
MicroRNAs (miRNAs) are a family of small, approximately 19- to 25-nucleotide, noncoding RNAs. They are transcribed from specific genes and generally undergo two cleavage steps that result in mature miRNAs. The mature miRNAs cause post-transcriptional gene repression by increasing mRNA degradation or by inhibiting translation (20). In the current study, we demonstrated that fibroblasts from patients with COPD produce increased amounts of PGE2 in response to the cytokines and that this is caused by the underexpression of miR-146a that, in turn, leads to overexpression of cyclooxygenase (COX)-2 and production of PGE2. Importantly, the level of expression of miR-146a correlated with both PGE2 production and two measures of COPD clinical severity. This represents a novel mechanism accounting for the altered inflammatory response that characterizes COPD.
METHODS
Human Subject Samples
Primary lung fibroblasts from 14 subjects with mild to very severe COPD and 16 subjects without clinical or functional signs of COPD (controls) were included (Table 1). All subjects were undergoing surgery for lung tumor resection except for two of the subjects with COPD undergoing volume reduction surgery. Acquisition of samples was approved by the Human Studies Committee of the Medical Board of the State of Schleswig-Holstein, and all subjects provided written informed consent for research.
TABLE 1.
CLINICAL FEATURES OF SUBJECTS
| Control | COPD | P Value | |
|---|---|---|---|
| n of subjects | 16 | 14 | |
| Age (yr) | 63.8 ± 2.2 | 60.2 ± 2.3 | 0.27 |
| Gender (M/F) | 13/3 | 8/6 | 0.27 |
| Histology of lung tumor | |||
| Adenocarcinoma | 7 | 7 | |
| Squamous cell carcinoma | 6 | 4 | |
| Large cell carcinoma | 2 | 1 | |
| Carcinoid | 1 | 0 | |
| No tumor* | 0 | 2 | |
| Height, cm | 169.6 ± 2.0 | 173.3 ± 2.5 | 0.29 |
| Weight, kg | 71.1 ± 3.3 | 71.6 ± 4.1 | 0.57 |
| Smoking (pack-years) | 43.2 ± 7.6 | 43.7 ± 4.3 | 0.63 |
| Current smoker† | 6 | 6 | 0.82 |
| GOLD stage (I/ II/ III/ IV) | 0/ 0/ 0/ 0 | 1/ 6/ 5/ 2 | |
| Pre-medications | |||
| LAMA | 0 | 7 | |
| LABA | 0 | 7 | |
| ICS | 0 | 8 | |
| Oral steroids | 0 | 5 | |
| Theophylline | 0 | 2 | |
| Statins | 4 | 3 | |
| VC (% of predicted value) | 103.9 ± 2.6 | 90.1 ± 4.8 | 0.02 |
| FEV1 (% of predicted value) | 93.5 ± 2.6 | 49.2 ± 4.6 | <0.0001 |
| DlCO (% of predicted value)‡ | 86.6 ± 3.4 | 63.1 ± 5.2 | 0.003 |
Definition of Abbreviations: COPD = chronic obstructive pulmonary disease; DlCO = diffusion capacity for carbon monoxide; GOLD = Global Initiative for Chronic Obstructive Lung Disease; ICS = inhaled corticosteroids; LABA = long acting β-agonist; LAMA = long-acting muscarinic antagonist.
Values are mean ± SEM, except for sex, histology of lung tumor, current smoker, GOLD stage, and premedications (number).
These subjects were undergoing volume reduction surgery.
Current smoker defined as someone who has smoked 100 cigarettes in his or her lifetime and who currently smokes cigarettes.
DlCO values are from 13 subjects of each group.
Human lung fibroblasts were cultured as described (14, 21) from normal-appearing areas of the pulmonary parenchyma in a region as far as possible from the tumor that was free of pleura or large airways.
Measurement of PGE2
PGE2 production from lung fibroblasts was determined by enzyme immunoassay (Cayman Chemical, Ann Arbor, MI) following the manufacturer's instructions.
Western Blot Analysis
Western blot analysis was performed as described (14).
COX Activity Assay
COX activity was determined using a commercially available kit (Cayman) following the manufacturer's instructions.
COX Silencing
Silencing of COX-1 and COX-2 by small interfering RNA (siRNA) was performed as described (22).
Real- Time Polymerase Chain Reaction
Total RNA was isolated from cells by using Trizol reagent (Invitrogen Life Technologies, Carlsbad, CA) and real-time polymerase chain reaction (PCR) was performed as described (23).
mRNA Stability Assay
Measurement of mRNA stability was accomplished as described (24, 25).
miRNA Preparation and Microarray Assay
Cells were pretreated either in the presence or absence of IL-1β and TNF-α. miRNAs were extracted and profiled as described (26).
Northern Blot Analysis
Northern blot analysis for the detection of miR-146a by DIG-labeled LNA probe (Exiqon, Vedbaek, Denmark) was performed as described with modifications (27).
Transfection of miRNA Mimic or Inhibitor
Cells were plated without antibiotic and antifungal agents and cultured. At 50% confluence, cells were treated with IL-1β and TNF-α for 24 hours and then transfected with miR-146a mimic, inhibitor, or negative control miRNA (final concentration = 50 nM each; Dharmacon, Lafayette, CO). After 6-hour transfection, cells were further cultured for 24 hours before assay.
Luciferase Reporter Assay
A luciferase (LUC) reporter construct containing 3′ untranslated region (UTR) of COX-2 mRNA (GeneCopoeia, Germantown, MD) was transformed following the manufacturer's instructions. LUC reporter assay was performed as described (28).
Statistical Analysis
Data are expressed as mean ± SEM. All data were analyzed using the GraphPad Prism 4 (GraphPad Software, San Diego, CA). Analysis of variance was performed with the use of the nonparametric Kruskal-Wallis test. When applicable, the Mann-Whitney U test was used for comparisons between groups. Correlation coefficients were calculated with the use of Spearman rank method. For the primary outcomes including PGE2 production, COX expression, and miR-146a expression, all 30 subjects were evaluated. For the mechanistic studies, cells cultured from selected subjects were evaluated. In these cases, each separate experiment, which included internal replicates, counted as a test. A P value of less than 0.05 was considered significant.
Additional details of all methods are available in the online supplement.
RESULTS
Clinical Features of Subjects
The clinical features of subjects without COPD (control) and subjects with COPD are presented in Table 1. These two groups were closely matched for age and smoking status. Using the Global Initiative for Chronic Obstructive Lung Disease classification based on FEV1, subjects with COPD were classified as stage I to IV (mild to very severe COPD). All controls lacked the criteria for COPD. Subjects with COPD showed the expected physiologic alterations including significantly lower FEV1 and lower diffusion capacity for carbon monoxide (DlCO). Also as expected, subjects with COPD received more medications than did controls. All control subjects and 12 of 14 subjects with COPD had lung tumors. Two subjects with COPD were operated on for volume reduction.
Effect of IL-1β and TNF-α on PGE2 Production in COPD and Control Fibroblasts
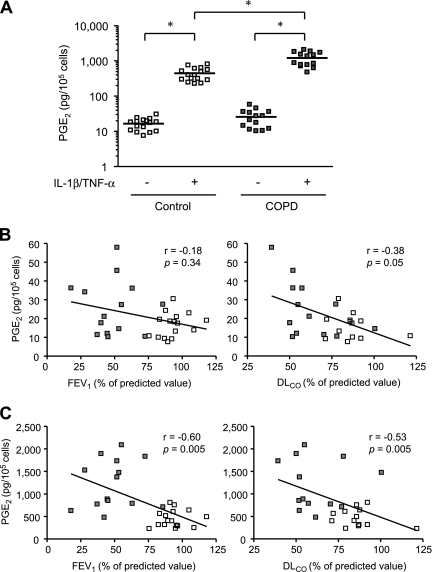
We first sought to evaluate PGE2 production by lung fibroblasts from COPD and control subjects in response to IL-1β and TNF-α. These cytokines are known to stimulate PGE2 release synergistically in lung fibroblasts (29). We first confirmed that IL-1β stimulated PGE2 production in control and COPD fibroblasts and that the combination of IL-1β and TNF-α stimulated more than the individual cytokines (see Figure E1A). In the absence of IL-1β and TNF-α (baseline condition), compared with control fibroblasts, COPD fibroblasts produced 1.6-fold more PGE2 (P = 0.07) (Figure 1A). Exogenous stimulation with IL-1β and TNF-α stimulated the production of PGE2 from fibroblasts from both controls and subjects with COPD (P < 0.0001). After exogenous stimulation, COPD fibroblasts produced 2.7-fold more PGE2 than did control fibroblasts (P < 0.0001) and the magnitude of the stimulation in the COPD fibroblasts was significantly greater, increasing approximately 63-fold compared with the 29-fold increase in control cells (P = 0.02).
Figure 1.
Effect of IL-1β and tumor necrosis factor (TNF)-α on prostaglandin (PG) E2 production and correlations between PGE2 production and lung function in chronic obstructive pulmonary disease (COPD) and control fibroblasts. (A) PGE2 production by 14 COPD and 16 control fibroblasts was measured over 24 hours. Each dot represents a separate subject. The bars show the mean values. Note that the vertical axis, which shows PGE2 production, is a log scale. * P < 0.0001. (B) Correlation of baseline (without cytokine treatment) PGE2 level with FEV1 and diffusing capacity of carbon monoxide (DlCO). (C) Correlation of IL-1β/TNF-α stimulated PGE2 level with FEV1 and DlCO. Subjects with COPD are indicated by the dark squares, and control subjects by the open squares. A solid line shows the regression line. FEV1 values are from all 30 subjects and DlCO were from 13 subjects (all available) of each group.
We next evaluated the relationship between baseline and stimulated PGE2 production and two physiologic measures that characterize the severity of COPD and, in particular, emphysema: FEV1 and DlCO. The relationship between baseline PGE2 levels and both FEV1 and DlCO did not achieve statistical significance (Figure 1B). In contrast, there were significant correlations between PGE2 production after IL-1β and TNF-α stimulation and these physiologic measures (Figure 1C).
To explore the mechanism for PGE2 overproduction in COPD fibroblasts, we first defined the concentration dependence and time-course for PGE2 production in response to IL-1β and TNF-α stimulation in selected cell strains. Cytokines stimulated PGE2 production from both COPD and control fibroblasts in a concentration-dependent manner with a similar 50% effective dose, although the amount of PGE2 produced differed significantly (2.10 vs. 1.63 ng/ml) (see Figure E1B). The time-course of PGE2 production was also similar and showed that PGE2 levels from both COPD and control fibroblasts started to increase by 4 hours and reached a maximum around 24 hours after treatment (see Figure E1C).
Role of COX-2 in IL-1β– and TNF-α–i nduced PGE2 Production
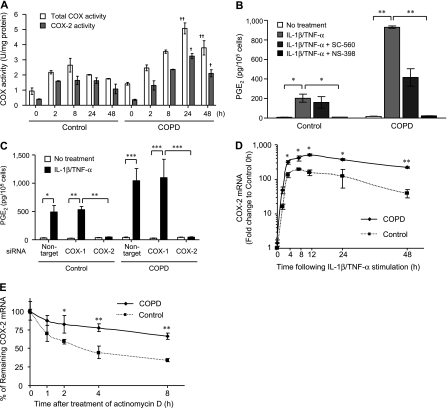
Because IL-1β and TNF-α induced PGE2 has been reported to induce increased COX-2 expression, but not COX-1 (30, 31), we evaluated COX-1 and COX-2 protein and mRNA expression. In the absence of stimulation, both protein and mRNA expression of COX-1 but not COX-2 were clearly demonstrated (Figures 2A and 2B). After 24 hours of cytokine stimulation, COX-2 protein and mRNA expression in both COPD and control fibroblasts increased to a great degree, whereas there was no significant change in COX-1 expression. Moreover, COPD fibroblasts showed higher protein and mRNA levels of COX-2 compared with controls after IL-1β and TNF-α stimulation (P = 0.005 and 0.02, respectively), whereas COX-1 expression in COPD fibroblasts did not differ from control.
Figure 2.
Effect of IL-1β and tumor necrosis factor (TNF)-α on cyclooxygenase (COX) protein and mRNA expression in chronic obstructive pulmonary disease (COPD) and control fibroblasts. (A) COX-1 protein and mRNA expression. (B) COX-2 protein and mRNA expression. Fourteen COPD and 16 control fibroblasts were stimulated with or without cytokines (2 ng/ml each) for 24 hours. Protein expression assessed by Western blot analysis is expressed relative amount to β-actin. COX mRNA expression quantified by real-time polymerase chain reaction is normalized to the amount of rRNA and expressed as 2-ΔΔCT values. Each dot represents a separate subject. * P < 0.05, ** P < 0.01, *** P < 0.0001.
We next confirmed the functional role of COX-2 in IL-1β and TNF-α induced PGE2 production. First, we assessed the COX enzyme activity. After stimulation with cytokines, there was an increase in total COX activity that was greater in COPD fibroblasts and was entirely accounted for by increased COX-2 activity (Figure 3A). We further demonstrated that pharmacologic inhibition with a COX-2 selective inhibitor, NS-398, completely blocked IL-1β– and TNF-α–induced PGE2 production. In contrast, use of a COX-1 selective inhibitor, SC-560, had no effect on cytokine-induced PGE2 production in control fibroblasts, but inhibited PGE2 release in COPD cells by about 50% (Figure 3B). Use of an siRNA to suppress COX-2 mRNA also resulted in complete inhibition of IL-1β– and TNF-α–induced PGE2 production, whereas a COX-1 targeting siRNA had no effect (Figure 3C).
Figure 3.
Role of cyclooxygenase (COX) in IL-1β and tumor necrosis factor (TNF)-α induced prostaglandin (PG) E2 production by chronic obstructive pulmonary disease (COPD) and control fibroblasts. (A) COX enzyme activity after IL-1β/TNF-α stimulation. Total COX (open bars) and COX-2 (dark bars) activity was determined by COX activity assay. + P < 0.05 and ++ P < 0.001, compared with control group. (B) Effect of pharmacologic inhibitors of COX on IL-1β/TNF-α induced PGE2 production. Cells were treated with an inhibitor of COX-1 (SC-560: 10−7 M) or COX-2 (NS-398: 10−6 M) for 1 hour and then stimulated with cytokines (2 ng/ml each) for 24 hours. (C) Effect of COX silencing on IL-1β/TNF-α induced PGE2 production. To inhibit specific cyclooxygeneses, cells were transfected with 100 nM of COX-1, COX-2, or nontarget siRNA for 6 hours after stimulation with cytokines. (D) IL-1β/TNF-α induction of COX-2 mRNA level, time-course. COX-2 mRNA expression expressed as fold change compared with the time 0 level of control fibroblasts. Note that the vertical axis is a log scale. (E) Stability of COX-2 mRNA. Cells were stimulated with cytokines for 24 hours, after which stimuli were removed and actinomycin D (2 μg/ml) was added. COX-2 mRNA is expressed as a percentage of mRNA level at the time of adding actinomycin D. Four strains each of COPD and control cells were evaluated on two separate occasions. Values are mean ± SEM. * P < 0.05, ** P < 0.01, *** P < 0.001. Comparison for D and E = COPD vs. control at indicated time point.
To evaluate the mechanism for the increased expression of COX-2 mRNA and protein, we first evaluated the time-course of the protein and mRNA response in selected strains. After stimulation with cytokines, there was no consistent change in COX-1 protein expression (see Figure E2). In contrast, COX-2 protein expression increased in a time- dependent manner and reached a maximum after 24 hours; the increase was approximately 52-fold in the control and 82-fold in the COPD strains tested (P = 0.04). Over the next 24 hours, the levels decreased approximately 54% in the control and 18% in the subjects with COPD (P = 0.02). Similarly, after cytokine stimulation, there was a time- dependent increase in COX-2 mRNA in both COPD and control fibroblasts (Figure 3D). The increase in COPD cells was of greater magnitude. The peak mRNA level was observed after 8 hours for the control and after 12 hours in the COPD cells. Moreover, the mRNA levels seemed to be more persistent in COPD fibroblasts, decreasing by 57% in COPD fibroblasts after 48 hours compared with an 80% decrease in the control fibroblasts (P = 0.01). Because this persistence might reflect a difference in mRNA stability, we next explicitly evaluated COX-2 mRNA stability in selected IL-1β and TNF-α treated COPD and control fibroblasts. The half-life of COX-2 mRNA was significantly longer in COPD fibroblasts compared with controls (11.9 vs. 4.61 h; P = 0.009) (Figure 3E).
Because several miRNAs have been suggested to contribute to COX-2 gene regulation and could account for a difference in COX-2 mRNA stability (28, 32, 33), we suspected that altered miRNA expression may contribute to altered COX-2 regulation and PGE2 overproduction in COPD fibroblasts after stimulation with IL-1β and TNF-α. Therefore, we used a microarray- based approach to assess miRNA expression with and without cytokine stimulation using two selected strains each of COPD and control fibroblasts. Two potential miRNA candidates were underexpressed in COPD fibroblasts compared with control after cytokine stimulation, miR-146a and miR-138 (see Figure E3A), although neither had been previously described to regulate COX-2. In contrast, the miRNAs that were previously reported to regulate COX-2 were expressed to the same extent in COPD and control fibroblasts and were not altered in expression by cytokine stimulation. Moreover, miR-138 expression was not affected by cytokine stimulation through the current microarray analyses (see Figure E3B). Nevertheless, next, we evaluated candidate miRNA target sites in the COX-2 3′ UTR by bioinformatic analysis using online databases (www.targetscan.org; www.mirbase.org). MiR-146a was found to have a homology with the COX-2 3′ UTR (see Figure E3C), whereas miR-138 had no homology revealed. Based on the microarray and in silico assessments, we focused on miR-146a for further analysis.
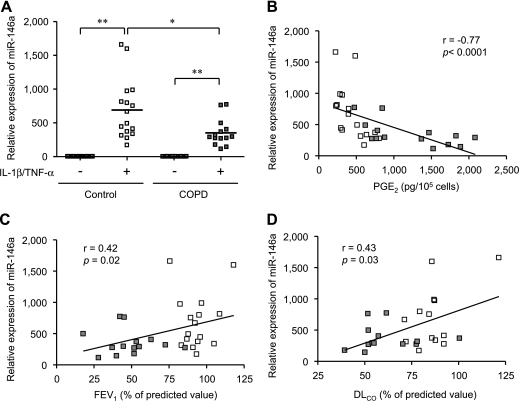
We first quantified miR-146a expression both at baseline and after IL-1β and TNF-α stimulation in all 30 strains of COPD and control fibroblasts by real-time PCR. Consistent with a previous report (34), IL-1β and TNF-α induced the expression of miR-146a. Although there was overlap among the miR-146a expression levels between COPD and control fibroblasts, the magnitude of the induction was significantly less in the COPD fibroblasts than in the control cells (P = 0.008) (Figure 4A). Importantly, the expression of miR-146a assessed showed significant negative correlation with PGE2 production following cytokine stimulation (Figure 4B). Expression of miR-146a after cytokine stimulation was also significantly correlated with COPD severity assessed by FEV1 and DlCO (Figures 4C and 4D). Because real-time PCR can detect both mature and precursor forms of miRNAs, we confirmed reduced expression of the mature form by Northern blot analysis. In all six strains of control and COPD fibroblasts assessed, IL-1β and TNF-α induced the expression of both precursor and mature forms of miR-146a (see Figure E4A). Densitometric quantification confirmed reduced expression of mature miR-146a in COPD cells and reduced conversion of pre–miR-146a to mature miR-146a in COPD fibroblasts compared with control cells (3.42 vs. 5.09; P = 0.04) (see Figure E4B).
Figure 4.
miR-146a expression after IL-1β and tumor necrosis factor (TNF)-α stimulation by chronic obstructive pulmonary disease (COPD) and control fibroblasts and in vivo correlation. (A) Effect of IL-1β/TNF-α on miR-146a expression by real time polymerase chain reaction. Fourteen COPD and 16 control cell strains were stimulated with or without cytokines (2 ng/ml each) for 24 hours. miR-146a expression is normalized to the amount of rRNA and expressed as 2-ΔΔCT values. Each dot represents a separate subject. * P < 0.01, ** P < 0.0001. (B) Correlation between miR-146a expression and prostaglandin (PG) E2 production after IL-1β/TNF-α stimulation. (C) Correlation between miR-146a expression after IL-1β/TNF-α stimulation and FEV1. (D) Correlation between miR-146a expression after IL-1β/TNF-α stimulation and diffusing capacity of carbon monoxide (DlCO). Subjects with COPD are indicated by the dark squares, and control subjects by the open squares. A solid line shows the regression line. FEV1 values are from all 30 subjects and diffusing capacity of carbon monoxide (all available) were from 13 subjects of each group.
Role for miR-146a in Modulation of COX-2 and PGE2 Production in COPD Fibroblasts
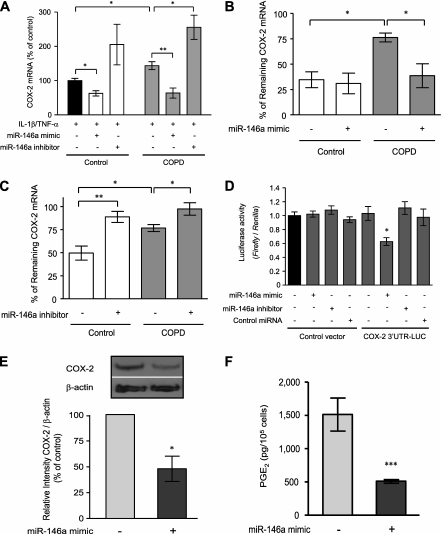
To define a mechanistic role for miR-146a in modulation of COX-2 and PGE2 production, we transfected COPD and control fibroblasts with miR-146a mimics or inhibitors. After stimulation with IL-1β and TNF-α, an miR-146a mimic reduced COX-2 mRNA levels when transfected into both COPD and control fibroblasts and an miR-146a inhibitor had the opposite effect (Figure 5A). Moreover, we confirmed this mechanism of action by demonstrating that the miR-146a mimic reduced the prolonged COX-2 mRNA half-life present in cytokine-treated COPD fibroblasts (Figure 5B) and that the miR-146a inhibitor enhanced the stability of COX-2 mRNA (Figure 5C). We then demonstrated binding of miR-146a to COX-2 3′ UTR using a LUC reporter assay. The LUC activity significantly decreased only after the cotransfection with both the LUC reporter construct containing COX-2 mRNA 3′ UTR and miR-146a mimic (Figure 5D), and this effect was clearly observed in both COPD and control cells. Finally, the functional role of miR-146a in COPD fibroblasts was further established by demonstrating significantly reduced COX-2 protein expression (P = 0.02) (Figure 5E) and PGE2 production (P = 0.0003) (Figure 5F) after transfection of the miR-146a mimic into cytokine-treated COPD fibroblasts.
Figure 5.
miR-146a regulates cyclooxygenase (COX)-2 expression and prostaglandin (PG) E2 production by a direct binding to 3′ untranslated region (UTR) of COX-2 mRNA. (A) Effect of miR-146a mimic or inhibitor on COX-2 mRNA expression. COX-2 mRNA expression represented as a percentage of control (indicated as a black bar). (B) Effect of miR-146a mimic on COX-2 mRNA stability. (C) Effect of miR-146a inhibitor on COX-2 mRNA stability. COX-2 mRNA was assessed after 6 hours treatment of actinomycin D (2 μg/ml) after IL-1β and TNF-α stimulation and is expressed as a percentage of mRNA level at the time of adding actinomycin D. (D) Direct binding of miR-146a to COX-2 3′UTR-LUC construct. Cells were cotransfected with a COX-2 3′ UTR-LUC construct, control vector, miR-146a mimic, miR-146a inhibitor, and control microRNA. Cell layers were harvested 48 hours after transfection, and luciferase (LUC) activities were determined by dual LUC assay. Data are normalized to Renilla LUC activity and expressed as a relative value to control (indicated as a black bar). (E) Modulation of COX-2 protein in cytokine-treated chronic obstructive pulmonary disease (COPD) fibroblasts by miR-146a mimic. The inset shows an example of the Western blot for COX-2 with and without exogenous miR-146a mimic for a single cell strain. Densitometric quantification of COX-2 is expressed relative to control after normalization to β-actin. The statistics were calculated from two strains of cells each evaluated on three separate occasions. (F) Effect of miR-146a mimic on PGE2 production by cytokine-treated COPD fibroblasts. Two strains each of COPD or control cells were evaluated on three separate occasions in all experiments. Values are mean ± SEM. * P < 0.05, ** P < 0.01, *** P < 0.001.
DISCUSSION
The present study demonstrates that, after stimulation with IL-1β and TNF-α, fibroblasts from subjects with COPD produce more PGE2 than do fibroblasts from control subjects. This increased production of PGE2 was caused by an increase in the expression of COX-2 and was associated with increased expression of COX-2 mRNA. The increased COX-2 mRNA expression was caused, at least in part, by prolonged COX-2 mRNA half-life. IL-1β and TNF-α stimulation also induced the expression of miR-146a in both COPD and control fibroblasts, but the induction was less in COPD fibroblasts. Because miR-146a has homology with the 3′ UTR of COX-2 mRNA, this suggests a mechanism whereby reduced expression of miR-146a in COPD fibroblasts leads to reduced feedback degradation of COX-2 mRNA and subsequent prolonged half-life and overexpression of COX-2 and consequent overproduction of PGE2. This mechanism was confirmed by demonstrating that transfection with an miR-146a mimic reduced COX-2 mRNA stability, COX-2 protein expression, and PGE2 production in COPD fibroblasts. Conversely, an miR-146a inhibitor prolonged the stability of COX-2 mRNA in control fibroblasts. The functional role of miR-146a in modulating COX-2 was further confirmed by a demonstration of binding of miR-146a to the 3′ UTR of COX-2 mRNA. Finally, the statistically significant correlations between miR-146a expression and PGE2 production after cytokine stimulation and functional measures of COPD severity suggests that miR-146a expression could play a key mechanistic role in the pathogenesis of COPD.
The COPD and control subjects from whom samples were obtained had similar smoking histories and were of similar ages. As a result, it is unlikely that cigarette smoking or age accounts for the differences observed. Similarly, the control subjects and all but two of the subjects with COPD were operated on for suspected lung cancer, suggesting that underlying diagnosis was not a reason for the differences observed. Although every effort was made to culture cells from tissue free of involvement with cancer, we cannot exclude the possibility that our observations are specific for the population of subjects with COPD and cancer. The subjects with COPD were taking more medications. Thus, we cannot exclude an effect of the COPD medications causing the differences observed. However, because these differences persisted with passage in culture, it is unlikely that the differences are caused by concurrent exposure to COPD-related medications.
In a prior study, our group demonstrated that fibroblasts from patients with COPD produce more PGE2 than do fibroblasts from control subjects (14). The current study is consistent with this prior study, demonstrating a trend toward increased production of PGE2 under baseline conditions. Importantly, the current study extends our prior results by assessing PGE2 production after stimulation with the inflammatory cytokines, IL-1β and TNF-α. PGE2 is produced not only by cells under baseline conditions, but, perhaps more importantly, in response to stimulation by proinflammatory mediators. IL-1β and TNF-α were used in the current study because they are prominent inflammatory mediators believed to play a pathogenic role in COPD, and they are known to stimulate potently, and potentially synergistically, the production of PGE2 by fibroblasts (29). The current study, therefore, extends prior results by demonstrating that PGE2 production is increased in COPD fibroblasts after exogenous stimulation. The magnitude of the stimulation in COPD fibroblasts also showed a greater relative increase, despite the COPD cells having a greater baseline PGE2 production.
The current study also suggests that increased PGE2 production in response to IL-1β and TNF-α likely results from different mechanisms that account for increased baseline production. Specifically, after cytokine stimulation, there was an increase in the expression of COX-2 in both COPD and control fibroblasts, but the increase was much greater in the COPD cells. This contrasts with the lack of change in COX-1 expression after stimulation with IL-1β and TNF-α. Measurement of COX enzymatic activity demonstrated a trend toward an increase in basal conditions that was entirely caused by COX-1. After cytokine stimulation, the significant increase in COX activity, which was greater in the COPD fibroblasts, was caused by COX-2. This suggests that COX-1 may account for the increased basal production of PGE2 but that increased PGE2 production in response to IL-1β and TNF-α is caused by COX-2. The increase in COX-2 seems to be essential in mediating the increased PGE2 production by COPD fibroblasts after IL-1β and TNF-α stimulation because inhibition of COX-2 by either a pharmacologic inhibitor or by siRNA suppression blocks the stimulatory effect of these cytokines. A role for COX-1 in COPD cells is not excluded: the lack of effect of the siRNA could be caused by incomplete suppression; alternatively, the partial inhibition by SC-560 could have been caused by a nonspecific effect of the inhibitor, although this was not seen in control cells.
With regard to PGE2 biosynthesis, several miRNAs have been reported to regulate COX-2 including miR-16 (32), miR-101 (28, 33), and miR-199a* (33). The current study extends these studies by demonstrating that miR-146a can also modulate COX-2. Interestingly, miR-16, which has been reported to regulate COX-2 mRNA (32), lacks a defined homology with COX-2 through the in silico assessment, suggesting that miRNA regulation has as yet undefined complexities. The current study, however, supports a direct effect of miR-146a on COX-2 mRNA levels, rather than an indirect effect mediated by altered transcription factor expression. In the current study, miR-101, miR-199a, and miR-16 were not evaluated, but their expression indicated by the microarray analyses was not noted to be altered after IL-1β and TNF-α stimulation in COPD or control fibroblasts. In addition, miR-146a has homologies with two other enzymes that could contribute to PGE2 biosynthesis: phospholipase A2 (type IV) and microsomal PGE2 synthase-1 (TargetScan). Whether these enzymes are altered in COPD fibroblasts or altered by miR-146a remains undefined.
In the current study, LUC reporter assays were performed to confirm interactions between miR-146a and COX-2 mRNA. The type of assay is often performed to assess the miRNA interactions with target mRNAs (28, 33). The data generated confirm that miR-146a modulates the expression of a reporter driven by the COX-2 promoter to which it has homology. Although this is consistent with direct binding, a role for an intermediate is not excluded.
In addition, inflammation in COPD has been described as being abnormal, particularly in its persistence long after removal of the inciting stimulus, most commonly cigarette smoking. The current study suggests that underproduction of an miRNA, specifically miR-146a, may be a mechanism for abnormal inflammation in COPD. In this context, miR-146a is known to be induced by IL-1β and TNF-α (34) and is thought to be a feedback control mechanism that limits the intensity and duration of the inflammatory response by inducing degradation of key mRNAs. Based on homologies, miR-146a has the potential to regulate several thousand potential mRNAs. The current study focused on miR-146a regulation of COX-2 and the production of PGE2. In this context, PGE2 is a mediator released during the inflammatory response that has pleiotropic effects on inflammation and repair. It was the focus of the current study because our previous work demonstrated that increased PGE2 production by COPD fibroblasts contributes to their altered repair function (14). Other effects on the inflammatory response are likely. For example, miR-146a has reported homologies with IL-1 receptor-associated kinase and TNF receptor-associated factor 6 and negatively regulates the IL-1β–induced inflammatory response in human lung alveolar epithelial cells (34, 35). The reduced production of miR-146a, therefore, could also contribute to other aspects of the abnormal inflammatory response in COPD.
In addition to advancing the understanding of COPD pathogenesis, identifying altered miR-146a expression in COPD fibroblasts raises several novel strategies for developing therapies for COPD. miRNA mimics are being developed for therapeutic use. By targeting and reducing the augmented PGE2 production, miRNA- based therapy could restore normal tissue repair and remodeling. In addition, miR-146a may target other aspects of the inflammatory response. An miRNA- based therapy could, therefore, address several aspects of COPD pathogenesis. Finally, because miRNAs generally modulate, but do not completely inhibit their target pathways, these therapeutic strategies may be able to restore “balance” without severe untoward side effects.
The current study has several limitations. We focused on cultured fibroblasts. Whether miR-146a is altered in other cell types in the lung, or in the lung as a whole, and whether this results in altered inflammation remains undefined. In addition, the current study did not define the mechanisms for reduced expression of miR-146a in COPD fibroblasts. However, a common G/C polymorphism (rs2910164) within miR-146a precursor has been reported to be associated with reduced processing and expression of mature miR-146a and the genetic predisposition to papillary thyroid carcinoma (36).
Conclusion
The current study demonstrates that fibroblasts from patients with COPD underexpress miR-146a after stimulation with IL-1β and TNF-α. This leads to reduced degradation of COX-2 mRNA and overproduction of PGE2. The reduced expression of miR-146a offers a novel pathogenetic mechanism that can contribute to the abnormal inflammatory response in COPD and links the inflammatory response to altered tissue repair in this disease.
Supplementary Material
Acknowledgments
The authors thank Lillian Richards for excellent secretarial support.
Supported by the National Heart Lung Blood Institute grant RO1–064088, by the Pulmonary Section, Department of Internal Medicine, the Chancellor's office, and the Larson Endowment of the University of Nebraska Medical Center (S.I. R.); the Uehara Memorial Foundation and the Japanese Respiratory Research Foundation, Pfizer Fellowship (T. S). The University of Nebraska Medical Center Microarray Core receives partial support from National Institutes of Health grant number P20 RR016469 from the INBRE Program of the National Center for Research Resources.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201001-0055OC on July 16, 2010
Author Disclosure: T.S. has a pending patent through the University of Nebraska Medical Center covering microRNA applications for the treatment of diseases, which is currently marketing to various commercial entities. X.L. has a pending patent through the University of Nebraska Medical Center covering microRNA applications for the treatment of diseases, which is currently marketing to various commercial entities. A.N. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.N. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. N.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. X.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. Y.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. H.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. O.H. served as a consultant for Aerocrine and Roche ($1,001– $5,000). He received lecture fees from Aerocrine (up to $1,000) and received grant support from Roche ($10,001– $50,000). He receives funds through the German Retirement Fund for basic funding of hospital Grosshandorf Research (more than $100,001). H.M. serves on the Board or Advisory Board for Altana, Boehringer Ingelheim, and AstraZeneca ($1,001– $5,000). He received lecture fees from Boehringer Ingelheim, AstraZeneca, Chiesi, and Altana ($1,001– $5,000). He received grant support (institutional) from Boehringer Ingelheim ($50,001– $100,000), Almiral, and AstraZeneca ($10,001– $50,000). S.I.R. has had or currently has a number of relationships with companies who provide products or services relevant to outpatient management of chronic obstructive pulmonary disease. These relationships include serving as a consultant, advising regarding clinical trials, speaking at continuing medical education programs, and performing funded research both at basic and clinical levels. He does not own any stock in any pharmaceutical companies. S.I.R. has received compensation for consultancies for Abbott ($1,001–$5,000), KOL Connection (Up to $1,000), Able Associates ($1,001–$5,000), Leerink Swan (Up to $1,000), Almirall ($10,001– $50,000), MedaCorp (Up to $1,000), Almirall/Forest (Up to $1,000), Mpex (Up to $1,000), Altana ($1,001–5,000), Novartis ($10,001–$50,000), Anthera (Up to $1,000), Otuska ($1,001–$5,000), APT Pharma/Britnall ($1,001–$5,000), Pfizer ($1,001–$5,000), Aradigm ($1,001–$5,000), Propagate (Up to $1,000), AstraZeneca ($1,001–$5,000), Pulmatrix (Up to $1,000), Boehringer Ingelheim ($5,000–$10,000), Quintiles (Up to $1,000), Britnall and Nicolini (Up to $1,000), Roche ($1,001–$5,000), Defined Health (Up to $1,000), Scimed (Up to $1,000), Dunn Group (Up to $1,000), TargeGen ($1,001–$5,000), Eaton Associates (Up to $1,000), Theravance ($1,001–$5,000), Gerson (Up to $1,000), UBS ($1,001–$5,000), GlaxoSmithKline ($10,001–$50,000), VantagePoint (Up to $1,000), Infomed (Up to $1,000), VantagePoint Mgmt ($5,000– $10,000), and Johnson & Johnson ($1,001–$5,000). He has served on Advisory Boards for Abbott ($1,001–$5,000), Nycomed ($10,001–$50,000), Almirall ($10,001–$50,000), Nycomed/Strategicare ($1,001–$5,000), Boehringer Ingelheim ($1,001–$5,000), Pfizer ($5,000–$10,000), COPDForum ($1,001–$5,000), Pharmaxis ($1,001–$5,000), Dey ($1,001–$5,000), Schering-Plough ($1,001–$5,000), GlaxoSmithKline ($10,001–$50,000), TargeGen ($5,001–$10,000), and Novartis ($10,001–50,000). He has received compensation for lectures from the American Thoracic Society, ACCP (Up to $1,000), AstraZeneca ($10,001–$50,000), Boehringer Ingelheim ($5,001–$10,000), the California Allergy Society ($1,001–$5,000), Creative Educational Concept ($10,001–$50,000), France Foundation ($5,000–$10,000), Information TV, Network for Continuing Ed ($1,000–$5,000), Novartis ($10,001–$50,000), Pfizer, and SOMA ($1,001–$5,000). He has received compensation for industry-sponsored grants from AstraZeneca, Biomarck, Centocor, Mpex, Nabi, Novartis, Otsuka ($50,001– $100,000), Almirall, Lorillard ($10,001–50,000), Pfizer, Philip Morris, GlaxoSmithKline, Roche (more than $100,001), and IFSH ($10,001–50,000). He has a pending patent through the University of Nebraska Medical Center covering microRNA applications for the treatment of diseases, which is currently marketing to various commercial entities. S.I.R. has received funds from RJ Reynolds for consultancies (1996–2007 more than $100,001) and for other matters (1999– 2007 more than $100,001), Lorilard (2005–2007 more than $100,001), Institute for Science and Health (2005–2007 more than $100,001), and Philip Morris (2002, 2005–2007 more than $100,001). Please note that S.I.R. has had tobacco industry funding. Specifically, he has received funding from the tobacco industry for studies relating to harm reduction and to the impact of tobacco smoke on stem cells. He has also consulted with RJ Reynolds without personal fee on the topic of harm reduction. He received funding from RJ Reynolds to evaluate the effect of a harm reduction product in a normal smoker (1996) and in subjects with chronic bronchitis (1999), and to assess the effect of smoking cessation on lower respiratory tract inflammation (2000). He participated in a Philip Morris multicenter study to assess biomarkers of smoke exposure (2002). He received funding for a clinical trial from the Institute for Science and Health (2005), which receives support form the tobacco industry to evaluate biomarkers in exhaled breath associated with smoking cessation and reduction. This study was supplemented with funding from Lorillard and RJ Reynolds. He has received a grant from the Philip Morris External Research Program (2005) to assess the impact of cigarette smoking on circulating stem cells in the mouse. He has consulted with RJ Reynolds on the topic of harm reduction until 2007, but did not receive personal remuneration for this. There are no active tobacco-industry funded projects. All ties with tobacco industry companies and entities supported by tobacco companies were terminated in 2007.
References
- 1.Global strategy for diagnosis. Management and prevention of COPD. 2008. (Accessed May 8, 2009). Available from: www.goldcopd.com.
- 2.Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of lung health study participants after 11 years. Am J Respir Crit Care Med 2002;166:675–679. [DOI] [PubMed] [Google Scholar]
- 3.Turato G, Di Stefano A, Maestrelli P, Mapp CE, Ruggieri MP, Roggeri A, Fabbri LM, Saetta M. Effect of smoking cessation on airway inflammation in chronic bronchitis. Am J Respir Crit Care Med 1995;152:1262–1267. [DOI] [PubMed] [Google Scholar]
- 4.Rutgers SR, Postma DS, ten Hacken NH, Kauffman HF, van Der Mark TW, Koeter GH, Timens W. Ongoing airway inflammation in patients with COPD who do not currently smoke. Thorax 2000;55:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004;350:2645–2653. [DOI] [PubMed] [Google Scholar]
- 6.Willemse BW, ten Hacken NH, Rutgers B, Lesman-Leegte IG, Postma DS, Timens W. Effect of 1-year smoking cessation on airway inflammation in COPD and asymptomatic smokers. Eur Respir J 2005;26:835–845. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro SD, Snider GL, Rennard SI. Chronic bronchitis and emphysema. In: Mason RJ, Broadus VC, Murray JF, Nadel JA, editors. Textbook of respiratory medicine, 4th ed. Philadelphia, Pennsylvania: Elsevier; 2005. Pp. 1115–1167.
- 8.Rennard SI. Repair. In: Calverley PMA, MacNee W, Pride NB, Rennard SI, editors. Chronic obstructive pulmonary disease. London: Arnold; 2003. Pp. 139–150.
- 9.Laurent P, Janoff A, Kagan HM. Cigarette smoke blocks cross-linking of elastin in vitro. Am Rev Respir Dis 1983;127:189–192. [DOI] [PubMed] [Google Scholar]
- 10.Osman M. Cigarette smoke impairs elastin resynthesis in lungs of hamsters with elastase-induced emphysema. Am Rev Respir Dis 1985;132:640–643. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Liu X, Umino R, Skold CM, Zhu Y, Kohyama T, Spurzem JR, Romberger DJ, Rennard SI. Cigarette smoke inhibits human bronchial epithelial cell repair processes. Am J Respir Cell Mol Biol 2001;25:772–779. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura Y, Romberger DJ, Tate L, Ertl RF, Kawamoto M, Adachi Y, Mio T, Sisson JH, Spurzem JR, Rennard SI. Cigarette smoke inhibits lung fibroblast proliferation and chemotaxis. Am J Respir Crit Care Med 1995;151:1497–1503. [DOI] [PubMed] [Google Scholar]
- 13.Montuschi P, Kharitonov SA, Carpagnano E, Culpitt S, Russell R, Collins JV, Barnes PJ. Exhaled prostaglandin E2: a new biomarker of airway inflammation in COPD. Am J Respir Crit Care Med 2000;161:A821. [Google Scholar]
- 14.Togo S, Holz O, Liu X, Sugiura H, Kamio K, Wang X, Kawasaki S, Ahn Y, Fredriksson K, Skold CM, et al. Lung fibroblast repair functions in COPD patients are altered by multiple mechanisms. Am J Respir Crit Care Med 2008;178:248–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohyama T, Ertl RF, Valenti V, Spurzem J, Kawamoto M, Nakamura Y, Veys T, Allegra L, Romberger D, Rennard SI. Prostaglandin E2 inhibits fibroblast chemotaxis. Am J Physiol 2001;281:L1257–L1263. [DOI] [PubMed] [Google Scholar]
- 16.Bitterman P, Rennard S, Ozaki T, Adelberg S, Crystal RG. PGE2: a potential regulator of fibroblast replication in the normal alveolar structures. Am Rev Respir Dis 1983;127:A271. [Google Scholar]
- 17.Lama V, Moore BB, Christensen P, Toews GB, Peters-Golden M. Prostaglandin E2 synthesis and suppression of fibroblast proliferation by alveolar epithelial cells is cyclooxygenase-2-dependent. Am J Respir Cell Mol Biol 2002;27:752–758. [DOI] [PubMed] [Google Scholar]
- 18.Huang S, Wettlaufer SH, Hogaboam C, Aronoff DM, Peters-Golden M. Prostaglandin E(2) inhibits collagen expression and proliferation in patient-derived normal lung fibroblasts via E prostanoid 2 receptor and cAMP signaling. Am J Physiol Lung Cell Mol Physiol 2007;292:L405–L413. [DOI] [PubMed] [Google Scholar]
- 19.Huang SK, Wettlaufer SH, Chung J, Peters-Golden M. Prostaglandin E2 inhibits specific lung fibroblast functions via selective actions of PKA and Epac-1. Am J Respir Cell Mol Biol 2008;39:482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nana-Sinkam SP, Hunter MG, Nuovo GJ, Schmittgen TD, Gelinas R, Galas D, Marsh CB. Integrating the MicroRNome into the study of lung disease. Am J Respir Crit Care Med 2009;179:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holz O, Zuhlke I, Jaksztat E, Muller KC, Welker L, Nakashima M, Diemel KD, Branscheid D, Magnussen H, Jorres RA. Lung fibroblasts from patients with emphysema show a reduced proliferation rate in culture. Eur Respir J 2004;24:575–579. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi T, Liu X, Wen FQ, Fang Q, Abe S, Wang XQ, Hashimoto M, Shen L, Kawasaki S, Kim HJ, et al. Smad3 mediates TGF-beta1 induction of VEGF production in lung fibroblasts. Biochem Biophys Res Commun 2005;327:393–398. [DOI] [PubMed] [Google Scholar]
- 23.Liu X, Kohyama T, Wang H, Zhu YK, Wen FQ, Kim HJ, Romberger DJ, Rennard SI. Th2 cytokine regulation of type I collagen gel contraction mediated by human lung mesenchymal cells. Am J Physiol 2002;282:L1049–L1056. [DOI] [PubMed] [Google Scholar]
- 24.Faour WH, He Y, He QW, de Ladurantaye M, Quintero M, Mancini A, Di Battista JA. Prostaglandin E(2) regulates the level and stability of cyclooxygenase-2 mRNA through activation of p38 mitogen-activated protein kinase in interleukin-1β-treated human synovial fibroblasts. J Biol Chem 2001;276:31720–31731. [DOI] [PubMed] [Google Scholar]
- 25.Tamura M, Sebastian S, Yang S, Gurates B, Fang Z, Bulun SE. Interleukin-1β elevates cyclooxygenase-2 protein level and enzyme activity via increasing its mRNA stability in human endometrial stromal cells: an effect mediated by extracellularly regulated kinases 1 and 2. J Clin Endocrinol Metab 2002;87:3263–3273. [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Nelson A, Wang X, Kanaji N, Kim M, Sato T, Nakanishi M, Li Y, Sun J, Michalski J, et al. MicroRNA-146a modulates human bronchial epithelial cell survival in response to the cytokine-induced apoptosis. Biochem Biophys Res Commun 2009;380:177–182. [DOI] [PubMed] [Google Scholar]
- 27.Weston MD, Pierce ML, Rocha-Sanchez S, Beisel KW, Soukup GA. MicroRNA gene expression in the mouse inner ear. Brain Res 2006;1111:95–104. [DOI] [PubMed] [Google Scholar]
- 28.Strillacci A, Griffoni C, Sansone P, Paterini P, Piazzi G, Lazzarini G, Spisni E, Pantaleo MA, Biasco G, Tomasi V. MiR-101 downregulation is involved in cyclooxygenase-2 overexpression in human colon cancer cells. Exp Cell Res 2009;315:1439–1447. [DOI] [PubMed] [Google Scholar]
- 29.Elias JA, Gustilo K, Baeder W, Freundlich B. Synergistic stimulation of fibroblast prostaglandin production by recombinant interleukin 1 and tumor necrosis factor. J Immunol 1987;138:3812–3816. [PubMed] [Google Scholar]
- 30.Skold CM, Liu X, Umino T, Ertl R, Romberger D, Rennard SI. Blood monocytes attenuate lung fibroblast contraction of three dimensional collagen gels co-culture. Am J Physiol Lung Cell Mol Physiol 2000;279:L667–L674. [DOI] [PubMed] [Google Scholar]
- 31.Zhu YK, Liu X-D, Skold CM, Umino T, Wang H, Romberger DJ, Spurzem JR, Kohyama T, Wen FQ, Rennard SI. Cytokine inhibition of fibroblast-induced gel contraction is mediated by PGE2 and NO acting through separate parallel pathways. Am J Respir Cell Mol Biol 2001;25:245–253. [DOI] [PubMed] [Google Scholar]
- 32.Shanmugam N, Reddy MA, Natarajan R. Distinct roles of heterogeneous nuclear ribonuclear protein K and microRNA-16 in cyclooxygenase-2 RNA stability induced by S100b, a ligand of the receptor for advanced glycation end products. J Biol Chem 2008;283:36221–36233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chakrabarty A, Tranguch S, Daikoku T, Jensen K, Furneaux H, Dey SK. MicroRNA regulation of cyclooxygenase-2 during embryo implantation. Proc Natl Acad Sci USA 2007;104:15144–15149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA 2006;103:12481–12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perry MM, Moschos SA, Williams AE, Shepherd NJ, Larner-Svensson HM, Lindsay MA. Rapid changes in microRNA-146a expression negatively regulate the IL-1β-induced inflammatory response in human lung alveolar epithelial cells. J Immunol 2008;180:5689–5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jazdzewski K, Murray EL, Franssila K, Jarzab B, Schoenberg DR, de la Chapelle A. Common SNP in pre-miR-146a decreases mature miR expression and predisposes to papillary thyroid carcinoma. Proc Natl Acad Sci USA 2008;105:7269–7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.