Abstract
Rationale: Tissue injury and repair involve highly conserved processes governed by mechanisms that can be co-opted in tumors. We hypothesized that soluble factors released during the repair response to lung injury would promote orthotopic tumor growth.
Objectives: To determine whether lung injury promoted growth of orthotopic lung tumors and to study the molecular mechanisms.
Methods: We initiated lung injury in C57Bl6 mice using different stimuli, then injected Lewis lung carcinoma cells during the repair phase. We assessed tumor growth 14 days later. We measured tumor angiogenesis, cytokine expression, proliferation, and apoptosis.
Measurements and Main Results: Regardless of the mechanism, injured lungs contained more numerous and larger tumors than sham-injured lungs. Tumors from injured lungs were no more vascular, but had higher levels of proliferation and reduced rates of apoptosis. The cytokine macrophage migration inhibitory factor (MIF) was highly expressed in both models of tissue injury. We observed no increase in tumor growth after lung injury in MIF knockout mice. We induced lung-specific overexpression of MIF in a double-transgenic mouse, and observed that MIF overexpression by itself was sufficient to accelerate the growth of orthotopic Lewis lung carcinoma tumors.
Conclusions: Lung injury leads to increased expression of the cytokine MIF, which results in protection from apoptosis and increased proliferation in orthotopic tumors injected after the acute phase of injury.
Keywords: cytokines, angiogenesis, stroma
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Patients with chronic airway injury and repair, such as chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis, appear to have an increased risk of lung cancer.
What This Study Adds to the Field
This study demonstrates the role of macrophage migration inhibitory factor, expressed in response to tissue injury, in promoting tumor growth.
Dvorak characterized cancer using the paradigm that tumors are wounds that do not stop “healing” (1). This implies that tumors and tissue undergoing repair share common molecular pathways and mediators. The lung's response to injury includes a series of well-orchestrated events at the cellular and tissue level that lead to restoration of tissue homeostasis. Many of these processes are also common to the pathogenesis of tumor growth. Based on these commonalities, one would expect that the lung's response to injury could promote the growth of lung tumors. Although smoking remains the most identifiable risk factor for lung cancer, smokers who also develop chronic obstructive pulmonary disease (COPD), a disease characterized by abnormal lung inflammation (2–4), are fourfold more likely to develop lung cancer than smokers who do not develop COPD (5, 6). Even more striking, idiopathic pulmonary fibrosis, a condition characterized by evidence of persistent lung injury and disordered repair, confers a 14-fold increased risk of lung cancer in nonsmokers (7, 8). Lung cancer is a common cause of death in patients with idiopathic pulmonary fibrosis, independent of the risk associated with smoking (8, 9).
In this study we hypothesize that the microenvironment and the repair processes associated with lung injury result in accelerated growth of lung tumors. To test this hypothesis, we used models of lung epithelial injury using intratracheal administration of bleomycin, or systemic administration of naphthalene. After inducing injury, and in the midst of repair, we injected Lewis Lung carcinoma (LLC) cells, and removed the lungs 2 weeks later to enumerate the number of orthotopic tumors. We found that LLC cells formed tumors that were larger and more numerous in lungs recovering from injury compared with sham-injured lungs. The increased growth of LLC tumors was associated with increased rates of proliferation (Ki67), and reduced evidence of apoptosis (cleaved caspase 3, CC3) within the tumors. We found that the cytokine macrophage migration inhibitory factor (MIF) was expressed in the lung during the response to both bleomycin and naphthalene. Based on studies showing that that MIF is highly expressed in tissue repair (10–13) and in tumor growth (14–19) and inhibits apoptosis (20, 21), we hypothesized that MIF expression in response to tissue injury could in part account for accelerated tumor growth. We found that MIF was able to promote proliferation of LLC cells in vitro. We subjected MIF knockout and wild-type mice to lung injury, followed by injection of lung cancer cells. The increase in tumor growth after lung injury was completely abrogated in MIF knockout mice, whereas lung-directed transgenic overexpression of MIF was sufficient to promote tumor growth in this model. We conclude that the repair response in mice after different types of lung injury promotes tumor growth in part by increasing the local expression of the pro-tumor cytokine MIF.
METHODS
Cell Lines and Reagents
The LLC cell line was maintained in Dulbecco's modified Eagle medium supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 1% l-glutamine, and 2.5% N-2-hydroxyethylpiperazine-N′-ethane sulfonic acid buffer. ISO-1 was obtained from Dr. Yousef Al Abed (North Shore Hospital, Long Island, NY). Anti-CD74 monoclonal antibody was purchased from Abcam (Cambridge, MA) and anti-CXCR2 antibody was purchased from R&D Systems (Minneapolis, MN).
Animals
All animal studies were reviewed and approved by the University of Michigan Committee for the Use and Care of Animals. C57Bl6 mice were purchased from Jackson Laboratory (Bar Harbor, ME) and housed in a specific pathogen-free environment. MIF knockout mice (C57Bl6) were the kind gift of Dr. John David, and were maintained in a colony in a specific pathogen-free animal housing facility. For bleomycin experiments, mice were anesthetized with pentobarbital, and the trachea was exposed through a small incision on the anterior neck. Bleomycin (0.025 U in 50 μl saline), or saline control, were instilled into the trachea as previously described (22). For naphthalene experiments, mice received 200 mg/kg naphthalene or vehicle (corn oil) control intraperitoneally as described by Stripp and colleagues (23). Two weeks after initiation of injury, we injected 5 × 104 LLC cells suspended in 50 μl serum-free media via the intratracheal route. Two weeks later (28 d after lung injury) mice were killed, lungs were removed, and tumors were processed as previously described (24).
Generation of tetO-MIF Mice
The murine MIF cDNA was cloned from LLC cells using the primers (5′-TCT-AGA-CAT-GCC-TAT-GTT-CAT-CGT-G-3′) and (5′-GGG-CCC-AGG-ACT-CAA-GCG-AAG-GTG-3′). The cDNA was purified from the gel using Qiagen (Valencia, CA) kits and cloned into the PTarget (Promega, Madison, WI) vector. After confirming the sequence, the MIF cDNA was excised using the XbaI and ApaI sites nested in the primers, purified as before, and cloned in the tetO expression cassette. This expression cassette consisted of seven tetO repeats, a cytomegalovirus minimal promoter, and a bovine growth hormone polyadenylation sequence (a gift from Dr. J. Whitsett and Dr. J. Tischelaar, Cincinnati, OH). The resultant expression cassette was then excised from its plasmid backbone using the Asc I restriction enzyme and purified. Purified plasmid DNA from the pTet-MIF construct was microinjected into fertilized eggs obtained by mating (C57BL/6 × SJL) F1 or C57BL/6 female mice with (C57BL/6 × SJL) F1 male mice. Pronuclear microinjection was performed as described (25). The microinjected eggs were implanted in pseudopregnant mothers. Three founders possessing the transgenic construct were identified by polymerase chain reaction (PCR) of tail preps using the primers (sense 5′-CTC-CGT-GCC-AGA-GGG-GTT-TCT-GTC-GGA-GCT-CAC-3′ and antisense 5′-CGG-GGG-AGG-GGC-AAA-CAA-CAG-ATG-GCT-GGC-3′) and then bred with 6- to 8-week-old C57Bl/6 partners. The transgenic offspring (F1) from this cross were then bred with a second transgenic line containing the 2.3-kb rat Clara-cell secretory protein (CCSP) promoter, the 1.0-kb rtTA coding sequence, and a 2.0-kb fragment from the human growth hormone containing introns and a polyadenylation sequence (a gift from Dr. J. Whitsett and Dr. J Tischelaar). CCSP-rtTA mice were identified with PCR of tail preps using the primers (sense) 5′-ACT-GCC-CAT-TGC-CCA-AAC-AC-3′ and (antisense) 5′-AAA-ATC-TTG-CCA-GCT-TTC-CCC-3′. Littermates with either no or only a single transgenic construct were used as experimental control subjects. Overexpression of mRNA and protein in response to doxycycline administration in the drinking water was confirmed using Western blot on whole lung lysates, and quantitative real-time reverse transcription–PCR (see online supplement). SP-C–driven MIF was leaky in the absence of doxycycline, and CCSP–driven expression of MIF was used for the experiments described herein. The primer pair used to identify the presence of the CCSP-rtTA construct in tail samples are as follows: upper primer 5′-ACT-GCC-CAT-TGC-CCA-AAC-AC-3′, lower primer 5′-AAA-ATC-TTG-CCA-GCT-TTC-CCC-3′; product size 525 bp (see Figures E1–E5 in the online supplement).
Immunohistochemistry
We stained 5-μm–thick sections of paraffin-embedded lungs as described (24, 26) for the indicated markers. One exception is for staining vessels with factor VIII–related antigen (DAKO, Carpenteria, CA), for which we use 8-μm–thick sections. Antibodies used for immunohistochemistry were factor VIII–related antigen (DAKO), CC3 (cleaved caspase-3; Cell Signaling Technology, Inc., Danvers, MA), Ki67 (Abcam), with secondary antibodies from Vector (VectaStain Elite ABC kits; Burlingame, CA). Antigen retrieval was performed with citrate microwave bath (CD31) or with trypsin (0.1% for 30 min at 37°C, CC3, and Ki67). Each slide had two contiguous tissue slices, one of which was stained with control primary antibody. Results for immunohistochemistry were expressed as cells or vessels per high-power field (400×) for apoptosis (CC3) and vessel density, or percent of cells positive (out of 200 counted for Ki67 labeling). For each experimental group, all tumors from a minimum of five animals per group were stained and quantified to determine vessel density as described (18), proliferative index (% of Ki67-positive cells in 200 cells), or the rate of apoptosis (CC3-positive cells per 200× field).
Cell Proliferation
Cells were plated in triplicate wells of a 6-well dish at 5 × 104 cells/ml in Dulbecco's modified Eagle medium supplemented with antibiotics, and 10% fetal calf serum. MIF (20 ng/ml) was added with the indicated inhibitors (ISO-1, anti-CD74, or anti-CXCR2, or IgG) to a total of 1-ml serum-free medium after overnight adherence. After an additional 48 hours, cells were harvested by trypsin and counted in a hemacytometer. All experiments were repeated at least two times, and in each experiment, conditions were run in triplicate. Results were expressed in cells/ml. For time course and dose–response experiments, cells were plated at 2.5 × 103 cells/well in 96-well plates with MIF added in the indicated concentration (Figure E8). After 48 hours, cell proliferation was assessed by MTT assay (Promega, Madison, WI).
Statistical Analysis
Means were compared with Student t test for two experimental groups, or with analysis of variance when comparing with more than two experimental groups. Results were expressed as mean ± SEM. All statistical analysis was performed with GraphPad Prism 3.0 statistical software.
RESULTS
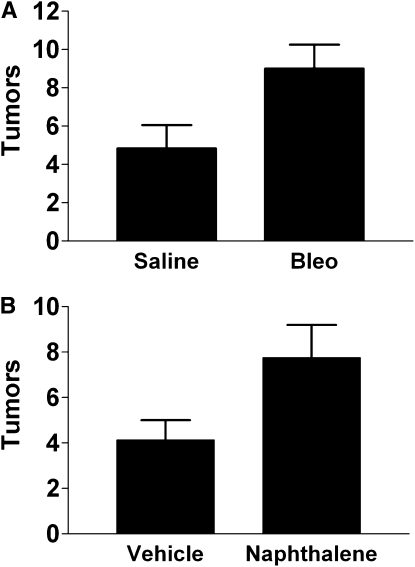
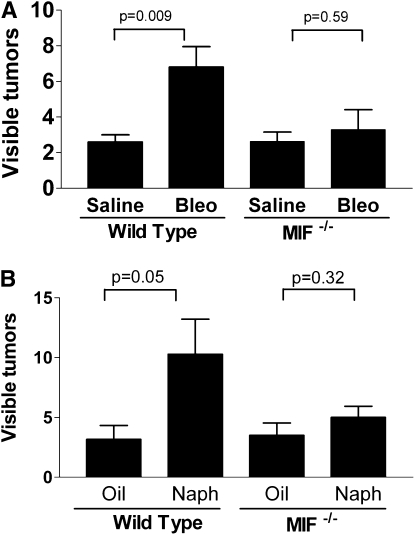
The response to lung injury increases tumor growth. To determine the effect of a wound repair response on tumor growth, we selected two models of lung injury: intratracheal bleomycin and systemic naphthalene. We chose these models because they are widely used and have an extensively characterized stereotypical response in which injury, inflammation, and repair follow a predictable course. We gave intratracheal bleomycin (low dose, 0.025 U) or saline (as a control) to C57Bl6 mice. In a second model, we gave 200 mg/kg naphthalene or vehicle (corn oil) via the intraperitoneal route. At Day 14 after injury we delivered 5 × 104 LLC cells to the lung, choosing this as a time point at which maximal inflammation is resolved (23, 27, 28). Fourteen days after intratracheal delivery of LLC cells, we killed the mice and removed the lungs to enumerate surface tumors. Tumors from bleomycin-treated mice were more numerous and larger than tumors from control (saline-exposed) mice (Figures 1A and 2). Similar increases in tumor size and number were seen in mice recovering from naphthalene-induced lung injury (Figures 1B and 2).
Figure 1.
Visible tumors are increased in the lungs of mice undergoing repair after (A) bleomycin (bleo)- or (B) naphthalene-induced lung injury as compared with control (sham) injury. P < 0.04 for both models. Each bar represents the mean ± SEM of six to eight mice per experimental group. Each experiment was repeated two times.
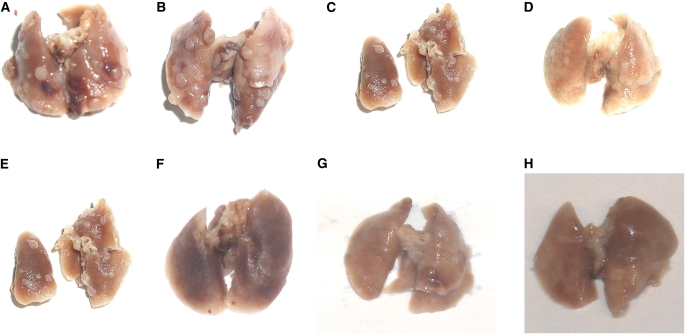
Figure 2.
Gross appearance of excised lungs at Day 28, showing increased tumor numbers in lungs undergoing repair from (A, B) bleomycin, or (C, D) naphthalene, as compared with sham (E, F, saline; G, H, corn oil)-injured lungs.
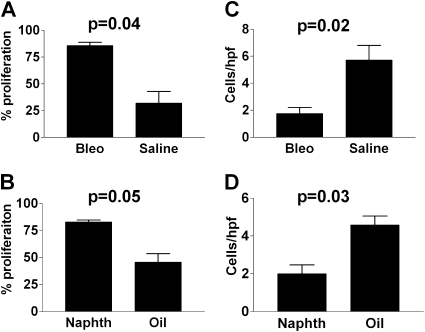
We hypothesized initially that the increase in tumor growth was due to an increased angiogenic response in the context of an injured lung. We stained the resulting lung tumors for vessels with factor VIII–related antigen as previously described (29) to determined the vessel density, but contrary to our expectations, these tumors were poorly vascularized and there were no differences between tumors from mice undergoing lung repair as compared with tumors from sham-injured mice (data not shown). To further investigate the mechanism by which lung repair resulted in greater tumor growth, we measured the level of proliferation and apoptosis in tumors using Ki67 (% Ki67-positive cells) and CC3 (positive cells per 400× power field), respectively. Tumors from lungs undergoing repair displayed greater Ki67 labeling and lower rates of apoptosis as compared with tumors from sham-injured mice (Figure 3).
Figure 3.
Quantitative assessment of (A, B) the percentage of proliferating cells, or (C, D) the number of apoptotic cells per high-power field in tumors from mice recovering from lung injury or control. (A) P = 0.04, (B) P = 0.05, (C) P = 0.02, and (D) P = 0.03. Each bar represents the analysis of tumors from the lungs of at least five mice per group. Bleo = bleomycin; naphth = naphthalene.
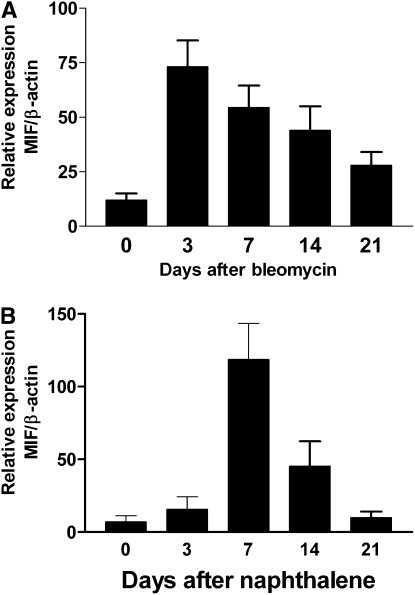
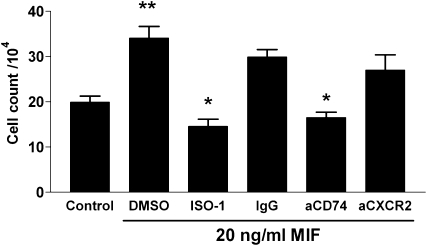
These data demonstrate that tumor cells in a lung microenvironment undergoing repair enjoy a growth advantage characterized by enhanced proliferation and protection from apoptosis. We have previously shown that levels of the cytokine MIF is present in lung cancer (18, 30), as is its receptor (31). MIF expression is associated with tissue repair (10, 32–34), cell proliferation (35), and protection from apoptotic stimuli (21, 36–38), as well as increased angiogenesis (17, 30, 39, 40). Collectively, these properties suggest the potential of MIF involvement in several cancer-related pathways, particularly in the context of tissue repair. We therefore measured the expression of MIF in the lungs of mice subjected to bleomycin- or naphthalene-induced lung injury. We found that MIF mRNA expression in response to lung injury peaked 3 days after bleomycin injury and remained elevated above the levels of saline-treated mice up to 21 days after bleomycin (Figures 4A and E7). Immunohistochemistry showed MIF expression associated with bronchial, alveolar, and endothelial lining cells, whereas baseline MIF stain of uninjured lung shows MIF mostly in alveolar macrophages (Figures E3 and E7). MIF expression after naphthalene similarly increased to a peak at 14 days, and remained above baseline at Day 21 (Figure 4B). Although MIF is known to inhibit apoptosis (20, 21, 41), we assessed in vitro proliferation of LLC cells in the presence of MIF, with or without inhibitors of MIF. We found that MIF promoted cell proliferation in vitro, and that this was inhibited in the presence of a pharmacologic inhibitor of MIF (ISO-1), and antibody to the putative MIF receptor (CD74), but not to CXCR2 (which some have postulated as a MIF receptor [42]) (Figures 5 and E8).
Figure 4.
Expression of macrophage migration inhibitory factor (MIF) after lung injury with (A) bleomycin, or (B) naphthalene using real-time semi-quantitative reverse transcription–polymerase chain reaction. Each bar represents three mice per time point.
Figure 5.
Macrophage migration inhibitory factor (MIF; 20 ng/ml) promotes proliferation of Lewis lung carcinoma cells (**P = 0.02) relative to control. The proliferative response to MIF is inhibited in the presence of ISO-1 (pharmacologic MIF inhibitor), or antibody to the MIF cell surface receptor (CD74), but not CXCR2 or control IgG.
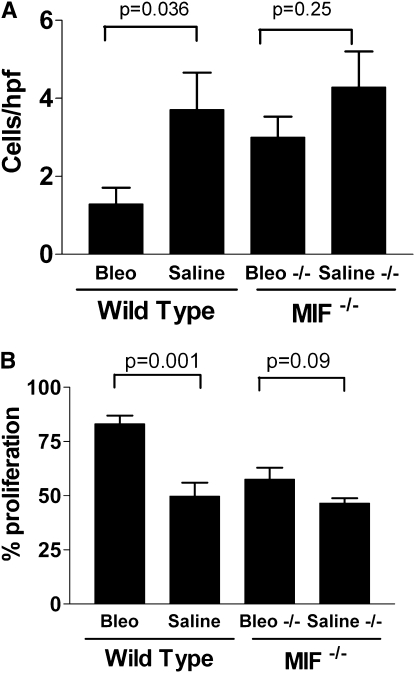
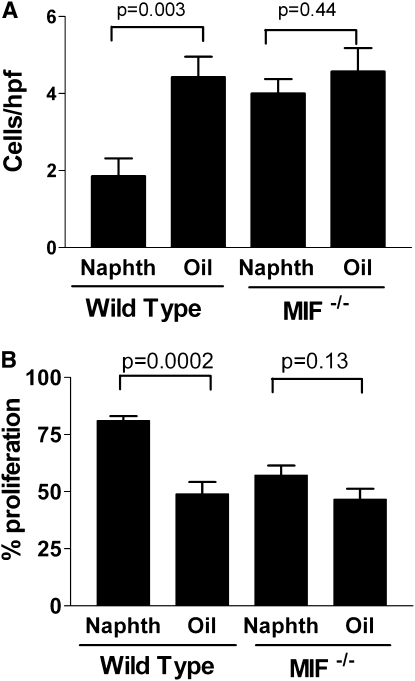
To determine whether MIF expression promoted increased tumor growth after tissue injury, we next examined the effect of lung injury and repair in MIF knockout (C57Bl6 background) and control mice. We initialed lung injury (or sham injury) with either bleomycin or naphthalene in MIF knockout or wild-type C57Bl6 mice, and then delivered LLC cells to the lungs intratracheally on Day 14. On Day 28 (14 d after LLC injection) there were significantly more tumors present in the lungs of lung-injured wild-type mice, compared with sham-injured wild-type mice. In MIF knockout mice, however, lung injury failed to promote tumor growth (Figures 6A and 6B). Additionally, neither protection from apoptosis nor increased proliferation was observed in tumors from MIF knockout mice after lung injury (Figures 7 and 8).
Figure 6.
Increased tumor growth during the response to (A) bleomycin or (B) naphthalene lung injury requires the presence of host-derived macrophage migration inhibitory factor (MIF). (MIF−/− are MIF knockout mice.) Each bar represents mean ± SEM of seven or eight mice per experimental group.
Figure 7.
Increased proliferation and protection from apoptosis during lung repair from bleomycin-induced injury requires the presence of macrophage migration inhibitory factor (MIF). (A) shows the number of apoptotic cells per high-power field (cleaved caspase 3) in tumors from wild-type or MIF knockout (−/−) mice. (B) shows the proliferative index (% of Ki6-positive cells) in tumors from wild-type or MIF knockout (−/−) mice. Indicated P values are from Student t test. Each bar represents the analysis of tumors from the lungs of at least five mice per group.
Figure 8.
Increased proliferation and protection from apoptosis during lung repair from naphthalene-induced injury requires the presence of macrophage migration inhibitory factor (MIF). (A) shows the number of apoptotic cells per high-power field (cleaved caspase 3) in tumors from wild-type or MIF knockout (−/−) mice. (B) shows the proliferative index (% of Ki6-positive cells) in tumors from wild-type or MIF knockout (−/−) mice. Indicated P values are from Student t test. Each bar represents the analysis of tumors from the lungs of at least five mice per group.
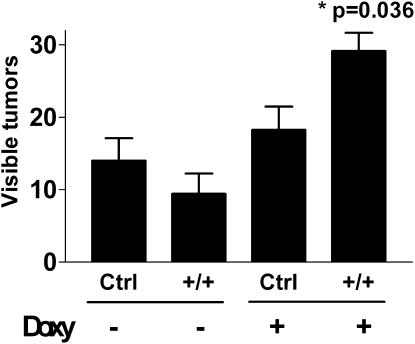
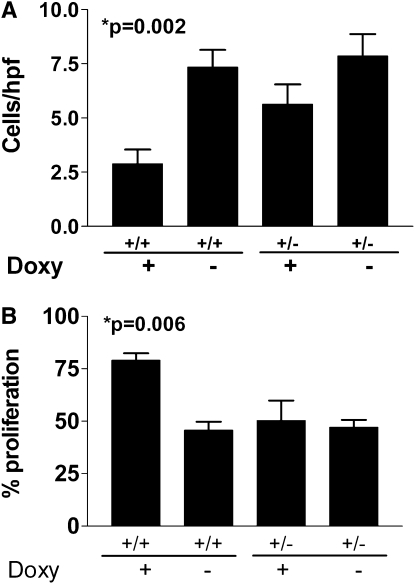
The above data suggest that host-derived MIF expression in response to lung injury was necessary to support the subsequent increased tumor growth. To determine if MIF expression was, by itself, sufficient to result in the acceleration of tumor growth of injected LLC cells, we used a double-transgenic mouse, in which MIF is expressed in a lung-specific, inducible manner. We administered doxycycline to double-transgenic mice bearing both the reverse tetracycline transactivator-VP16 transgene under the control of the CCSP promoter (CCSP-rtTA) and the MIF cDNA controlled by the tet-operator (tetO-MIF). Control groups included both double-transgenic mice without doxycycline and single-transgenic littermate mice treated with and without doxycycline. After 2 weeks the doxycycline was discontinued to mimic the pattern of expression seen after injury, and LLC cells were delivered to the lungs. Two weeks after injection of LLC cells, mice were killed and we enumerated lung tumors after removing the lungs. The overexpression of MIF was, by itself, sufficient to support increased growth of lung tumors even in uninjured lungs (Figure 9; P = 0.036 by analysis of variance) as well as to support increased proliferation and protection from apoptosis (Figure 10). MIF overexpression by itself, even for up to 2 months, did not result in any histologically detectable lung injury (Figures E1–E5).
Figure 9.
Doxycycline-induced overexpression of macrophage migration inhibitory factor (MIF) (on Days 1–14) in the lungs of Clara-cell secretory protein (CCSP)-rtTA/tet(O)-MIF double-transgenic mice results in increased growth of subsequently introduced orthotopic Lewis lung carcinoma tumors (Days 14–28). Control mice include double-transgenic mice receiving no doxycycline and single-transgenic mice with or without doxycycline (P = 0.036 by analysis of variance). +/+ designates mice bearing both the CCSP-rtTA and tet(O)MIF alleles respectively. +/− designates single-transgenic littermate mice. Each bar represents the analysis of tumors from the lungs of at least five mice per group.
Figure 10.
Doxycycline-induced overexpression of macrophage migration inhibitory factor (MIF) (on Days 1–14) in the lungs of Clara-cell secretory protein (CCSP)-rtTA/tet(O)-MIF double-transgenic mice results in increased proliferation and protection from apoptosis in subsequently introduced orthotopic Lewis lung carcinoma tumors (Days 14–28). (A) shows the number of apoptotic cells per high-power field (cleaved caspase 3) in tumors from double- (+/+) or single-transgenic (+/−) mice treated with or without doxycycline (P = 0.006 by analysis of variance [ANOVA]). (B) shows the proliferative index (% of Ki67-positive cells) in tumors from double- (+/+) or single-transgenic (+/−) mice treated with or without doxycycline (P = 0.002 by ANOVA). Control mice include double-transgenic mice receiving no doxycycline and single-transgenic mice with or without doxycycline. +/+ designates mice bearing both the CCSP-rtTA and tet(O)MIF alleles, respectively. +/− designates single-transgenic littermate mice. Each bar represents the analysis of tumors from the lungs of at least five mice per group.
DISCUSSION
Kim and colleagues demonstrated that naphthalene-induce lung injury favored the development of tumors in a model of oncogenic KRAS activation in mice (32). In their study they demonstrated expansion of a putative bronchoalveolar stem cell population after injury, and that this expansion was associated with increased tumor number after activation of oncogenic KRAS, suggesting that expansion of the stem cell niche also expanded the development of KRAS-transformed cells into tumors. In the current study, we demonstrate that the injured lung microenvironment further supports the growth of already-transformed lung cancer cells. This article demonstrates that lungs recovering from lung injury support increased growth of orthotopic lung tumors by promoting growth and inhibiting apoptosis in a MIF-dependent mechanism. We originally expected that the increased tumor growth would be attributed to enhanced angiogenesis as a result of the ongoing tissue repair. Our findings did not provide any evidence for increased vessel growth in the tumors, despite that levels of angiogenic CXC chemokines were increased in these tumors (Figure E6).
Dvorak originally proposed the paradigm that tumors were “wounds that do not heal” (1). This paradigm predicts common molecular mediators between tissue repair and tumor growth. The cytokine MIF plays a role in the response of a wide range of models of tissue injury. MIF activity is associated with tissue repair (10, 33–35), cell proliferation (36), and protection from apoptotic stimuli (21, 37–39), as well as angiogenesis (17, 30, 40, 41). We determined that MIF was expressed in both models of lung injury, and that host-derived MIF was necessary for the subsequent acceleration of tumor growth. We then used an inducible lung-targeted transgenic mouse to determine that MIF expression by itself was sufficient to promote the growth of tumors. In each case the protection from apoptosis and increased proliferation of tumor cells was dependent on the presence of host-derived MIF. We were unable to demonstrate an increase in vessel density to suggest that MIF-dependent promotion of angiogenesis was important in this model.
MIF is expressed in many models that recapitulate acute and chronic tissue injury, such as cutaneous wounds (33, 44), asthma (11), cystitis (45, 46), rheumatoid arthritis (47), sepsis (48), and atherosclerosis (49). Although traditional gene expression microarrays fail to identify up-regulation of MIF at the level of mRNA in response to tissue injury (50–53), proteomic methods have identified MIF as a key mediator of tissue repair (54), a finding confirmed by animal studies. This may reflect the fact that preformed MIF is often present in cells and may be released into the microenvironment in response to injury without the benefit of transcriptional up-regulation. One study has examined the role of MIF in bleomycin-induced lung injury. This study found that inhibition of MIF after bleomycin-induced lung injury improved survival and reduced the inflammatory response, but had no effect on deposition of collagen. This suggests that the processes of repair are dissociated from fibrosis (55). Our own findings show (in response to lung injury) increased immunolocalization of MIF to epithelium in the airway and airspaces, as well as in some endothelial cells in response to lung injury. In the context of tumor growth arising from the distal airways or airspaces, this increased local expression of MIF, combined with MIF-dependent effects on cell proliferation and protection from apoptosis, may have an important impact.
We have previously shown that MIF is expressed by lung cancer cells in vitro, and that it induces the expression of angiogenic CXC chemokines by cocultured monocytes, resulting in a significant increase in the angiogenic bioactivity of coculture supernatants (30). MIF expression is markedly increased in human NSCLC tumors, and its expression correlates with higher risk of disease recurrence and mortality (18, 56, 57). Furthermore, we recently demonstrated the presence of the putative receptor for MIF, CD74, in a specimen of human NSCLC (31).
Prior studies seeking a connection between inflammation and cancer have conclusively shown that activation of the nuclear factor (NF)-κB transcription factor pathway plays a role in the development of cancer. It is not clear whether there is a connection between the activity of MIF and the NF-κB pathway, though MIF may up-regulate NF-κB in endothelial cells (58). MIF is not known to activate the NF-κB pathway in epithelial or myeloid derived cells, but activation of NF-κB may induce the expression of MIF in tumor stromal cells (59). Our study did not examine the process of transformation at a cellular level. Rather, we sought to study the effect of a microenvironment undergoing repair on cells that were already transformed. Our data provide evidence for an additional potential target to attenuate the malignant phenotype and the tumor microenvironment by targeting the presence of MIF. Further studies will be focused on the role of MIF on the process of cellular transformation in the context of injury and repair.
Supplementary Material
Acknowledgments
The authors thank Galina Gavrilia for preparation of transgenic mice and the Transgenic Animal Model Core of the University of Michigan's Biomedical research Core Facilities. Core support was provided by The University of Michigan Cancer Center NIH Grant CA46592.
Supported by NIH grant R01-CA94121 (D.A.); a Sidney Kimmel foundation grant (D.A.); the Flight Attendants Medical Research Institute; Michigan Tri-technology corridor MTTC grant GR-687 for the Proteomics Alliance for Cancer Research; The University of Michigan Cancer Center NIH Grant CA46592; the Veteran's Administration Research Service (A.E.K.); NIH R01-AR48267 (A.E.K.); the Frederick G.L. Huetwell and William D. Robinson, M.D. Professorship (A.E.K.); and NIH R03 AR052482 (M.A.A.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI:10.1164/rccm.201001-0120OC on June 25, 2010
Author Disclosure: D.A. has received sponsored grants from NCI (more than $100,000). T.R.L. has received sponsored grants from NIH (more than $100,000). S.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. L.Z. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.A.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.E.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Dvorak HF. Tumors: wounds that do not heal. N Engl J Med 1986;315:1650–1659. [DOI] [PubMed] [Google Scholar]
- 2.Barnes PJ. Mechanisms in COPD: differences from asthma. Chest 2000;117:10S−14S. [DOI] [PubMed]
- 3.Bhowmik A, Seemungal TAR, Sapsford RJ, Wedzicha JA. Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbations. BMJ 2000;55:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pesci A, Balbi B, Majori M, Cacciani G, Bertacco S, Alciato P, Donner CF. Inflammatory cells and mediators in bronchial lavage of patients with chronic obstructive pulmonary disease. Eur Respir J 1998;12:380–386. [DOI] [PubMed] [Google Scholar]
- 5.Wasswa-Kintu S, Gan WQ, Man SFP, Pare PD, Sin DD. Relationship between reduced forced expiratory volume in one second and the risk of lung cancer: a systematic review and meta-analysis. Thorax 2005;60:570–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spitz MR, Hong WK, Amos CI, Wu X, Schabath MB, Dong Q, Shete S, Etzel CJ. A risk model for prediction of lung cancer. J Natl Cancer Inst 2007;99:715–726. [DOI] [PubMed] [Google Scholar]
- 7.Zielinski J, MacNee W, Wedzicha J, Ambrosino N, Braghiroli A, Dolensky J, Howard P, Gorzelak K, Lahdensuo A, Strom K, et al. Causes of death in patients with COPD and chronic respiratory failure. Monaldi Arch Chest Dis 1997;52:43–47. [PubMed] [Google Scholar]
- 8.Hubbard R, Venn A, Lewis S, Britton J. Lung cancer and cryptogenic fibrosing alveolitis. A population-based cohort study. Am J Respir Crit Care Med 2000;161:5–8. [DOI] [PubMed] [Google Scholar]
- 9.Raghu G, Nyberg F, Morgan G. The epidemiology of interstitial lung disease and its association with lung cancer. Br J Cancer 2004;91:S3–S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardman MJ, Waite A, Zeef L, Burow M, Nakayama T, Ashcroft GS. Macrophage migration inhibitory factor: A central regulator of wound healing. Am J Pathol 2005;167:1561–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishihira J. Novel pathophysiological aspects of macrophage migration inhibitory factor. Int J Mol Med 1998;2:17–28. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Y, Shimizu T, Nishihira J, Koyama Y, Kushibiki T, Honda A, Watanabe H, Abe R, Tabata Y, Shimizu H. Tissue regeneration using macrophage migration inhibitory factor-impregnated gelatin microbeads in cutaneous wounds. Am J Pathol 2005;167:1519–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamp R, Sun X, Garcia JGN. Making genomics functional: deciphering the genetics of acute lung injury. Proc Am Thorac Soc 2008;5:348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wachi S, Yoneda K, Wu R. Interactome-transcriptome analysis reveals the high centrality of genes differentially expressed in lung cancer tissues. Bioinformatics 2005;21:4205–4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bando H, Matsumoto G, Bando M, Muta M, Ogawa T, Funata N, Nishihira J, Koike M, Toi M. Expression of macrophage migration inhibitory factor in human breast cancer: association with nodal spread. Jpn J Cancer Res 2002;93:389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer-Siegler K. Increased stability of macrophage migration inhibitory factor (MIF) in du-145 prostate cancer cells. J Interferon Cytokine Res 2000;20:769–778. [DOI] [PubMed] [Google Scholar]
- 17.Nishihira J, Ishibashi T, Fukushima T, Sun B, Sato Y, Todo S. Macrophage migration inhibitory factor (MIF): its potential role in tumor growth and tumor-associated angiogenesis. Ann N Y Acad Sci 2003;995:171–182. [DOI] [PubMed] [Google Scholar]
- 18.White ES, Flaherty KR, Carskadon S, Brant A, Iannettoni MD, Yee J, Orringer MB, Arenberg DA. Macrophage migration inhibitory factor and CXC chemokine expression in non-small cell lung cancer: role in angiogenesis and prognosis. Clin Cancer Res 2003;9:853–860. [PubMed] [Google Scholar]
- 19.Yao K, Shida S, Selvakumaran M, Zimmerman R, Simon E, Schick J, Haas NB, Balke M, Ross H, Johnson SW, et al. Macrophage migration inhibitory factor is a determinant of hypoxia-induced apoptosis in colon cancer cell lines. Clin Cancer Res 2005;11:7264–7272. [DOI] [PubMed] [Google Scholar]
- 20.Fingerle-Rowson G, Petrenko O, Metz CN, Forsthuber TG, Mitchell R, Huss R, Moll U, Muller W, Bucala R. The p53-dependent effects of macrophage migration inhibitory factor revealed by gene targeting. Proc Natl Acad Sci USA 2003;100:9354–9359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hudson JD, Shoaibi MA, Maestro R, Carnero A, Hannon GJ, Beach DH. A proinflammatory cytokine inhibits p53 tumor suppressor activity. J Exp Med 1999;190:1375–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore BB, Paine R, Christensen PJ, Moore TA, Sitterding S, Ngan R, Wilke CA, Kuziel WA, Toews GB. Protection from pulmonary fibrosis in the absence of CCR2 signaling. J Immunol 2001;167:4368–4377. [DOI] [PubMed] [Google Scholar]
- 23.Stripp BR, Maxson K, Mera R, Singh G. Plasticity of airway cell proliferation and gene expression after acute naphthalene injury. Am J Physiol 1995;269:L791–L799. [DOI] [PubMed] [Google Scholar]
- 24.McClelland MR, Carskadon SL, Zhao L, White ES, Beer DG, Orringer MB, Pickens A, Chang AC, Arenberg DA. Diversity of the angiogenic phenotype in non-small cell lung cancer. Am J Respir Cell Mol Biol 2007;36:343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hogan B, Beddington R, Constantini F, Lacy E. Manipulating the mouse embryo: a laboratory manual. New York: Cold Spring Harbor Press; 1994.
- 26.Arenberg DA, Kunkel SL, Polverini PJ, Morris SB, Burdick MD, Glass MC, Taub DT, Iannettoni MD, Whyte RI, Strieter RM. Interferon-gamma-inducible protein 10 (ip-10) is an angiostatic factor that inhibits human non-small cell lung cancer (NSCLC) tumorigenesis and spontaneous metastases. J Exp Med 1996;184:981–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phan SH, Kunkel SL. Lung cytokine production in bleomycin-induced pulmonary fibrosis. Exp Lung Res 1992;18:29–43. [DOI] [PubMed] [Google Scholar]
- 28.Plopper C, Suverkropp C, Morin D, Nishio S, Buckpitt A. Relationship of cytochrome p-450 activity to clara cell cytotoxicity. I. Histopathologic comparison of the respiratory tract of mice, rats and hamsters after parenteral administration of naphthalene. J Pharmacol Exp Ther 1992;261:353–363. [PubMed] [Google Scholar]
- 29.Arenberg DA, Keane MP, DiGiovine B, Kunkel SL, Morris SB, Xue YY, Burdick MD, Glass MC, Iannettoni MD, Strieter RM. Epithelial-neutrophil activating peptide (ena-78) is an important angiogenic factor in non-small cell lung cancer. J Clin Invest 1998;102:465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White ES, Strom SRB, Wys NL, Arenberg DA. Non-small cell lung cancer cells induce monocytes to increase expression of angiogenic activity. J Immunol 2001;166:7549–7555. [DOI] [PubMed] [Google Scholar]
- 31.McClelland M, Zhao L, Carskadon S, Arenberg D. Expression of CD74, the receptor for macrophage migration inhibitory factor, in non-small cell lung cancer. Am J Pathol 2009;174:638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim CFB, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 2005;121:823–835. [DOI] [PubMed] [Google Scholar]
- 33.Abe R, Shimizu T, Ohkawara A, Nishihira J. Enhancement of macrophage migration inhibitory factor (MIF) expression in injured epidermis and cultured fibroblasts. Biochim Biophys Acta 2000;1500:1–9. [DOI] [PubMed] [Google Scholar]
- 34.Ashcroft GS, Mills SJ, Lei K, Gibbons L, Jeong MJ, Taniguchi M, Burow M, Horan MA, Wahl SM, Nakayama T. Estrogen modulates cutaneous wound healing by downregulating macrophage migration inhibitory factor. J Clin Invest 2003;111:1309–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuda A, Tagawa Y, Matsuda H, Nishihira J. Expression of macrophage migration inhibitory factor in corneal wound healing in rats. Invest Ophthalmol Vis Sci 1997;38:1555–1562. [PubMed] [Google Scholar]
- 36.Liao H, Bucala R, Mitchell RA. Adhesion-dependent signaling by macrophage migration inhibitory factor (MIF). 2003;278:7–81. [DOI] [PubMed]
- 37.Lue H, Thiele M, Franz J, Dahl E, Speckgens S, Leng L, Fingerle-Rowson G, Bucala R, Luscher B, Bernhagen J. Macrophage migration inhibitory factor (MIF) promotes cell survival by activation of the akt pathway and role for csn5/jab1 in the control of autocrine MIF activity. Oncogene 2007;26:5046–5059. [DOI] [PubMed] [Google Scholar]
- 38.Oda S, Oda T, Nishi K, Takabuchi S, Wakamatsu T, Tanaka T, Adachi T, Fukuda K, Semenza GL, Hirota K. Macrophage migration inhibitory factor activates hypoxia-inducible factor in a p53-dependent manner. PLoS ONE 2008;3:e2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petrenko O, Moll UM. Macrophage migration inhibitory factor MIF interferes with the rb-e2f pathway. Mol Cell 2005;17:225–236. [DOI] [PubMed] [Google Scholar]
- 40.Hagemann T, Robinson SC, Thompson RG, Charles K, Kulbe H, Balkwill FR. Ovarian cancer cell-derived migration inhibitory factor enhances tumor growth, progression, and angiogenesis. Mol Cancer Ther 2007;6:1993–2002. [DOI] [PubMed] [Google Scholar]
- 41.Xu X, Wang B, Ye C, Yao C, Lin Y, Huang X, Zhang Y, Wang S. Overexpression of macrophage migration inhibitory factor induces angiogenesis in human breast cancer. Cancer Lett 2008;261:147–157. [DOI] [PubMed] [Google Scholar]
- 42.Mitchell RA, Liao H, Chesney J, Fingerle-Rowson G, Baugh J, David J, Bucala R. Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: regulatory role in the innate immune response. Proc Natl Acad Sci USA 2002;99:345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, Dewor M, Georgiev I, Schober A, Leng L, et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med 2007;3:587–596. [DOI] [PubMed]
- 44.Shimizu T, Ohkawara A, Mizue Y, Nishihira J. Alpha-thrombin stimulates expression of macrophage migration inhibitory factor in skin fibroblasts. Semin Thromb Hemost 1999;25:569–573. [DOI] [PubMed] [Google Scholar]
- 45.Meyer-Siegler KL, Leifheit EC, Vera PL. Inhibition of macrophage migration inhibitory factor decreases proliferation and cytokine expression in bladder cancer cells. BMC Cancer 2004;4:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meyer-Siegler KL, Ordorica RC, Vera PL. Macrophage migration inhibitory factor is upregulated in an endotoxin-induced model of bladder inflammation in rats. J Interferon Cytokine Res 2004;24:55–63. [DOI] [PubMed] [Google Scholar]
- 47.Morand EF, Leech M. Macrophage migration inhibitory factor in rheumatoid arthritis. Front Biosci 2005;10:12–22. [DOI] [PubMed] [Google Scholar]
- 48.Calandra T, Echtenacher B, Roy DL, Pugin J, Metz CN, Hultner L, Heumann D, Mannel D, Bucala R, Glauser MP. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat Med 2000;6:164–170. [DOI] [PubMed] [Google Scholar]
- 49.Morand EF, Leech M, Bernhagen J. MIF: a new cytokine link between rheumatoid arthritis and atherosclerosis. Nat Rev Drug Discov 2006;5:399–410. [DOI] [PubMed]
- 50.Lewis CC, Yang JYH, Huang X, Banerjee SK, Blackburn MR, Baluk P, McDonald DM, Blackwell TS, Nagabhushanam V, Peters W, et al. Disease-specific gene expression profiling in multiple models of lung disease. Am J Respir Crit Care Med 2008;177:376–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han FM, Zhang XM, Xia QS, Wang JJ, Chen Y. Gene expression profiling of mice liver tissue after intragastric administration of Chinese nutgall extract. Fen Zi Xi Bao Sheng Wu Xue Bao 2009;42:101–108. [PubMed] [Google Scholar]
- 52.Thompson CA, Burcham PC. Genome-wide transcriptional responses to acrolein. Chem Res Toxicol 2008;21:2245–2256. [DOI] [PubMed] [Google Scholar]
- 53.Tseng LH, Chen I, Lin YH, Liang CC, Lloyd LK. Genome-based expression profiling study following spinal cord injury in the rat: An array of 48-gene model. Neurourol Urodyn [published ahead of print 17 Jul 2009 as DOI: 10.1002/nau.20769]. [DOI] [PubMed]
- 54.King MW, Neff AW, Mescher AL. Proteomics analysis of regenerating amphibian limbs: changes during the onset of regeneration. Int J Dev Biol 2009;53:955. [DOI] [PubMed] [Google Scholar]
- 55.Tanino Y, Makita H, Miyamoto K, Betsuyaku T, Ohtsuka Y, Nishihira J, Nishimura M. Role of macrophage migration inhibitory factor in bleomycin-induced lung injury and fibrosis in mice. Am J Physiol Lung Cell Mol Physiol 2002;283:L156–L162. [DOI] [PubMed] [Google Scholar]
- 56.Kamimura A, Kamachi M, Nishihira J, Ogura S, Isobe H, Dosaka-Akita H, Ogata A, Shindoh M, Ohbuchi T, Kawakami Y. Intracellular distribution of macrophage migration inhibitory factor predicts the prognosis of patients with adenocarcinoma of the lung. Cancer 2000;89:334–341. [PubMed] [Google Scholar]
- 57.Tomiyasu M, Yoshino I, Suemitsu R, Okamoto T, Sugimachi K. Quantification of macrophage migration inhibitory factor mRNA expression in non-small cell lung cancer tissues and its clinical significance. Clin Cancer Res 2002;8:3755–3760. [PubMed] [Google Scholar]
- 58.Amin MA, Haas CS, Zhu K, Mansfield PJ, Kim MJ, Lackowski NP, Koch AE. Migration inhibitory factor up-regulates vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 via SRC, pi3 kinase, and NFkappaB. Blood 2006;107:2252–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cao WG, Morin M, Metz C, Maheux R, Akoum A. Stimulation of macrophage migration inhibitory factor expression in endometrial stromal cells by interleukin 1, beta involving the nuclear transcription factor nfkappab. Biol Reprod 2005;73:565–570. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.