Abstract
Rationale: Enteral administration of probiotics may modify the gastrointestinal environment in a manner that preferentially favors the growth of minimally virulent species. It is unknown whether probiotic modification of the upper aerodigestive flora can reduce nosocomial infections.
Objectives: To determine whether oropharyngeal and gastric administration of Lactobacillus rhamnosus GG can reduce the incidence of ventilator-associated pneumonia (VAP).
Methods: We performed a prospective, randomized, double-blind, placebo-controlled trial of 146 mechanically ventilated patients at high risk of developing VAP. Patients were randomly assigned to receive enteral probiotics (n = 68) or an inert inulin-based placebo (n = 70) twice a day in addition to routine care.
Measurements and Main Results: Patients treated with Lactobacillus were significantly less likely to develop microbiologically confirmed VAP compared with patients treated with placebo (40.0 vs. 19.1%; P = 0.007). Although patients treated with probiotics had significantly less Clostridium difficile–associated diarrhea than patients treated with placebo (18.6 vs. 5.8%; P = 0.02), the duration of diarrhea per episode was not different between groups (13.2 ± 7.4 vs. 9.8 ± 4.9 d; P = 0.39). Patients treated with probiotics had fewer days of antibiotics prescribed for VAP (8.6 ± 10.3 vs. 5.6 ± 7.8 d; P = 0.05) and for C. difficile–associated diarrhea (2.1 ± 4.8 SD d vs. 0.5 ± 2.3 d; P = 0.02). No adverse events related to probiotic administration were identified.
Conclusions: These pilot data suggest that L. rhamnosus GG is safe and efficacious in preventing VAP in a select, high-risk ICU population.
Clinical trial registered with www.clinicaltrials.gov (NCT00613795).
Keywords: probiotics, ventilator-associated pneumonia, Lactobacillus , Clostridium difficile , bloodstream infection
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Although probiotics have been evaluated for prevention of ventilator-associated pneumonia, the existing data are limited in scope.
What This Study Adds to the Field
We provide evidence that Lactobacillus rhamnosus GG administration to critically ill patients is safe and is efficacious for the prevention of ventilator-associated pneumonia in a very high-risk population.
It is estimated that ventilator-associated pneumonia (VAP) complicates the care of up to 30% of patients receiving mechanical ventilation (1–4). Patients with VAP have increased morbidity, mortality, and hospital costs as well as prolonged intensive care unit (ICU) and hospital lengths of stay and increased costs (1, 5–7). The pathogenesis of VAP is complex but typically involves colonization of the aerodigestive tract with pathogenic bacteria, formation of biofilms, and microaspiration of contaminated secretions (5, 8). Current effective VAP prevention strategies target modifiable risk factors for colonization and aspiration, including elevation of the head of the bed, subglottic secretion draining or silver-coated endotracheal tubes, intensive oral care, and minimizing the duration of mechanical ventilation through regular use of sedation vacations and weaning protocols (3, 8–14).
In view of these events central to the pathogenesis of VAP, probiotic therapy is an intriguing option as a nonantibiotic strategy for maintenance of the host's aerodigestive microbial balance and VAP prevention. Probiotics are defined by the World Health Organization as living microbial agents of human origin that are able to tolerate the hostile gastrointestinal environment (acid and bile) such that they ultimately persist in the lower alimentary tract to confer health benefits to the host (15). Probiotics could potentially reduce the incidence of VAP through various local and systemic effects that minimize colonization by more virulent species or optimize host immune defenses. These effects include reduced overgrowth of potentially pathogenic microorganisms, improved gut mucosal barrier function, reduced bacterial translocation, and toll-like receptor–mediated up-regulation of immune function (16–21). Evidence supporting this theory is limited but promising (22).
Studies enrolling adult trauma, neurosurgical, liver transplant, and general surgery patients have demonstrated trends toward reduced rates of infections, including pneumonia, in patients treated with probiotics (23–27). However, almost all of these studies included coadministration of prebiotics (nondigestible ingredients that stimulate the growth and activity of bacteria in the gut), a practice known as synbiotic therapy. Because prebiotics have effects on the intestinal flora that are analogous to those seen with probiotic administration, the precise role of probiotics in reducing infectious complications remains unknown. Such an assessment is important because administration of living microbial species to critically ill individuals carries the inherent risk of iatrogenic infection. Therefore, we conducted a study with two main goals: (1) to determine the efficacy of isolated probiotic administration for the prophylaxis of VAP and (2) to examine the safety of probiotic therapy in a high-risk, critically ill population. Some of the results of these studies have been previously reported in the form of abstracts (28, 29).
METHODS
Creighton University's institutional review board approved the study protocol, including written, informed surrogate consent, before enrolling any patients.
All screening was performed daily by the lead investigator (LEM) or a dedicated study coordinator. Adults at least 19 years old (the age of majority in Nebraska) were eligible for enrollment if the lead investigator and the treating physician agreed that there was a 95% likelihood that the patient would require mechanical ventilation with an endotracheal tube for at least 72 hours. Exclusion criteria were selected to exclude patient subsets previously described as being at risk for iatrogenic probiotic infection: pregnancy; immunosuppression; prosthetic cardiac valve or vascular graft; cardiac trauma; history of rheumatic fever, endocarditis, or congenital cardiac abnormality; gastroesophageal or intestinal injury or foregut surgery during the current admission; oropharyngeal mucosal injury; and placement of a tracheostomy. The rationale underlying each of the many exclusion criteria is included in the online supplement. Patients were also excluded if the investigators were unable to obtain informed written consent and administer the first dose of the study drug within 24 hours of intubation. Patients were recruited from July 2004 to January 2009 at a 325-bed, university-based hospital that provides Level 1 trauma services.
Patients were randomly assigned in a 1:1 ratio to treatment groups using permutation blocks (n = 4 per block) within three severity of illness strata by Acute Physiology and Chronic Health Evaluation (APACHE) II scores (<18, 18–24, or >24). Investigators, bedside nurses, primary care clinicians, and microbiology laboratory personnel, were blinded to group assignments. Patients randomized to probiotic therapy received 2 × 109 colony-forming units (cfu) of Lactobacillus rhamnosus GG on a twice-daily basis. The contents of one capsule containing 109 cfu of Lactobacillus were suspended in sterile, water-based surgical lubricant and administered as a slurry to the oropharynx; the contents of a second capsule containing 109 cfu of Lactobacillus were suspended in sterile water and given through the nasogastric tube. The same methods were used to deliver the contents of identical appearing capsules containing the inert plant starch inulin to patients randomized to placebo.
Patients continued to receive active intervention or placebo until extubation, tracheostomy placement, or death. Patients received all routine care, including VAP-preventive measures as per hospital protocols and antibiotic therapy as deemed necessary, under the direction of their admitting physicians throughout the study. Institutional VAP-prevention measures remained unchanged throughout the study period and are described further in the online supplement.
The study protocol-mandated baseline data included demographic information, medical history, and the APACHE II score. Additional information collected on a daily basis included chest radiograph findings, clinical signs of VAP, adverse events, lengths of stay in the ICU and hospital, duration of mechanical ventilation, and mortality. If patients were diagnosed with VAP using the American College of Chest Physicians (ACCP) clinical criteria, quantitative cultures of distal airways samples were obtained by nonbronchoscopic bronchoalveolar lavage (BAL) using a protected catheter (Combicath; KOL Biomedical Instruments, Chantilly, VA). The ACCP clinical criteria require a new and persistent infiltrate on chest radiographs with two of three supporting findings: fever (>38.5°C or <35.0°C), leukocytosis (white blood cells > 10,000/mm3 or <3,000/mm3), and purulent sputum.
Nonbronchoscopic BAL was performed using previously described techniques (30). Samples obtained using this technique are collected blindly (i.e., they are not specifically collected from the site of radiographic abnormality). However, the diagnostic utility of nonbronchoscopic BAL is comparable to that of specimens obtained bronchoscopically (30).
The primary outcome was microbiologically confirmed VAP incidence based on quantitative BAL culture with at least 104 cfu/ml in patients intubated for 48 hours or longer. Secondary outcomes included mortality; time to occurrence of VAP; durations of mechanical ventilation, ICU stay, and hospital stay; Clostridium difficile–associated diarrhea; other ICU-associated diarrhea; antibiotic consumption (total, VAP-specific, and C. difficile-specific); and hospital charges. Antibiotic consumption was measured in antibiotic-days, a composite measure incorporating the number and duration of antibiotics prescribed. Antibiotic-days were calculated by summing the number of antibiotics administered across all of the days antibiotics were prescribed. This calculation is discussed further in the online supplement. All patients with diarrhea (three or more loose stools per 24-h period or placement of a fecal management system for continuous liquid stool) had a C. difficile cytotoxin assay sent. Each negative assay was repeated twice to minimize the rate of false-negative results. Patients with diarrhea but three negative C. difficile cytotoxin assays were classified as having “ICU-associated” diarrhea, presumably due to acute illness, dietary changes, and antibiotic administration.
To assess whether probiotic administration resulted in measurable changes in the oropharyngeal flora, patients had an oral swab, gastric aspirate, and nonbronchoscopic BAL collected before administration of the first dose of study medication, after 72 hours of study participation (immediately before dose 7 of study drug), and with the clinical diagnosis of VAP. Oral swabs and gastric aspirates were sent for semiquantitative cultures, and BAL fluid was sent for quantitative culture.
An independent data and safety monitoring board supervised the study investigation and reviewed interim data after the first 40 patients were enrolled and after the first 80 patients were enrolled. Board members had no financial relationship with the sponsor. The board had access to all data and made the final determination regarding whether the study would be continued, terminated, or modified based on study enrolment, trends toward futility or inferiority in the primary outcome (VAP), lack of measured effect on colonization, and safety. None of the interim analyses resulted in modifications or termination based on our application of the predefined early stopping rules of O'Brien and Fleming (31).
Statistical Analysis
Sample size calculations assumed a 38% incidence of VAP in the control arm based on historic trends from this ICU, a 50% reduction in VAP caused by the intervention based on existing published data, and a dropout rate of 5%. We calculated that approximately 146 patients should be enrolled to achieve statistical power of 80% with a two-sided significance level of 0.05. In modified intention-to-treat analyses, patients intubated for 48 hours or longer (those “at-risk” for VAP) were analyzed as the primary efficacy population. All patients enrolled were analyzed for safety.
Descriptive statistics using appropriate tests were used for all baseline characteristics. As determined by the data distribution, the t or Mann-Whitney test was used to compare between-group differences for continuous variables. A χ2 test was used for categorical variables. The primary outcome, VAP incidence, was analyzed by a univariate technique using PASW Statistics 17 (Chicago, IL). Only the initial episode of VAP for each patient was included in the analyses. Kaplan-Meier analyses were performed using product-limit survival estimates with the generalized Wilcoxon test for statistical comparisons. All P values were two sided, significance was set at P < 0.05, and the significance level was adjusted for the two interim analyses required by the data and safety monitoring plan.
RESULTS
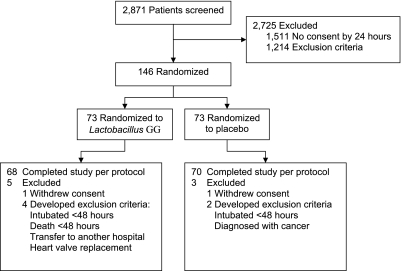
A total of 2,871 patients were screened (Figure 1); 2,725 were not enrolled because informed surrogate consent could not be obtained during the first 24 hours of mechanical ventilation, patients had exclusion criteria, or patients were unlikely to require intubation for at least 72 hours. Patients were evenly distributed between groups based on demographic and other baseline characteristics (Table 1). Although most VAP risk factors were balanced, the prevalence of chest trauma was higher in the Lactobacillus arm of the study (2.9 vs. 19.1%; P = 0.002).
Figure 1.
Study participants.
TABLE 1.
DEMOGRAPHICS AND BASELINE CHARACTERISTICS FOR THE INTENTION-TO-TREAT POPULATION
| Placebo (n = 73) | Lactobacillus GG (n = 73) | P Value | |
|---|---|---|---|
| Female sex | 30 (41.1%) | 30 (41.1%) | 1.00 |
| Age, mean ± SD (range), yr | 54.6 ± 16.3 (21–(91) | 52.5 ± 19.3 (19–(88) | 0.47 |
| APACHE II score, mean ± SD (range) | 23.7 ± 8.0 (8–41) | 22.7 ± 7.5 (8–38) | 0.45 |
| Race | 0.97 | ||
| Caucasian | 58 (79.5%) | 57 (78.1%) | |
| African American | 9 (12.3%) | 10 (13.7%) | |
| Hispanic | 6 (8.2%) | 6 (8.2%) | |
| VAP risk factors | |||
| Smoker | 17 (23.3%) | 20 (27.4%) | 0.52 |
| COPD | 12 (16.4%) | 11 (15.1%) | 0.82 |
| Chest trauma | 2 (2.7%) | 13 (17.8%) | 0.003 |
| Nursing home resident | 4 (5.5%) | 10 (13.7%) | 0.09 |
| Alcohol abuse | 12 (16.4%) | 17 (23.3%) | 0.30 |
| Reason for ICU admission | 0.30 | ||
| Trauma | 23 (31.5%) | 31 (42.4%) | |
| Respiratory failure | 20 (27.4%) | 16 (21.9%) | |
| Infection | 5 (6.8%) | 2 (2.7%) | |
| Cardiology | 8 (11.0%) | 6 (8.2%) | |
| Neurology/neurosurgery | 12 (16.5%) | 13 (17.8%) | |
| Gastrointestinal | 4 (5.5%) | 3 (4.1%) | |
| Renal | 1 (1.4%) | 0 (0.0%) | |
| Endocrine | 0 (0.0%) | 2 (2.7%) |
Definition of abbreviations: APACHE = Acute Physiology and Chronic Health Evaluation; COPD = chronic obstructive pulmonary disease VAP = ventilator-associated pneumonia.
Primary Outcome
Among the 138 patients in the modified intention-to-treat (mITT) analysis, 50 were diagnosed with VAP using the clinical criteria and underwent nonbronchoscopic BAL (33/70 placebo patients [47.1% incidence; 95% confidence interval (CI), 35.1–59.1] vs. 17/68 patients treated with Lactobacillus [25.0% incidence; 95% CI, 14.4–35.6]; P < 0.001) (Table 2). Of these, 28 patients receiving placebo had microbiologically confirmed VAP (40.0% incidence; 95% CI, 28.2–51.8), compared with 13 patients receiving Lactobacillus (19.1% incidence; 95% CI, 9.4–28.6; P = 0.007).
TABLE 2.
INCIDENCE AND MICROBIOLOGY OF VENTILATOR-ASSOCIATED PNEUMONIA
| Placebo | Lactobacillus GG | P Value | |
|---|---|---|---|
| Subjects with at least one episode of clinically diagnosed VAP | |||
| Intention-to-treat analysis | 33/73 (45.2%) | 17/73 (23.3%) | 0.005 |
| Modified intention-to-treat analysis | 33/70 (47.1%) | 17/68 (25%) | <0.001 |
| Subjects with at least one episode of microbiologically confirmed VAP | |||
| Intention-to-treat analysis | 28/73 (38.4%) | 13/73 (17.8%) | 0.006 |
| Modified intention-to-treat analysis | 28/70 (40.0%) | 13/68 (19.1%) | 0.007 |
| Subjects with Gram-positive pneumonia | 9/70 (12.8%) | 4/68 (5.8%) | 0.16 |
| Subjects with Gram-negative pneumonia | 16/70 (22.8%) | 6/68 (8.8%) | 0.02 |
| Subjects with mixed (Gram-positive, Gram-negative, and/or other) pneumonia | 3/70 (4.2%) | 3/68 (4.4%) | 0.97 |
| Microbiology | |||
| Gram-positive pathogens isolated | 15 | 10 | |
| MSSA | 8 | 4 | |
| MRSA | 6 | 4 | |
| Streptococcus species | 1 | 2 | |
| Gram-negative pathogens isolated | 31 | 9 | |
| Pseudomonas | 6 | 0 | |
| Enterobacteriaceae | 3 | 2 | |
| Haemophilus influenza | 3 | 1 | |
| Acinetobacter | 2 | 3 | |
| Klebsiella | 3 | 1 | |
| Proteus | 2 | 0 | |
| Escherichia coli | 3 | 0 | |
| Serratia | 4 | 0 | |
| Citrobacter | 1 | 0 | |
| Stenotrophomonas | 4 | 0 | |
| Burkholderia | 0 | 1 | |
| Alcaligenese | 0 | 1 | |
| Other pathogens isolated | 1 | 0 | |
| Yeast | 1 | 0 |
Definition of abbreviations: MRSA = methicillin-resistant Staphylococcus aureus; MSSA = methicillin-susceptible Staphylococcus aureus; VAP = ventilator-associated pneumonia.
None of the patients who were randomized but excluded from the mITT analysis (n = 8) met the clinical criteria for VAP during hospitalization. As such, when the intention-to-treat population was analyzed, significant between-group differences remained for clinically diagnosed VAP (33 placebo cases [45.2% incidence; 95% CI, 33.4–57.0] vs.17 patients with Lactobacillus [23.3% incidence; 95% CI, 13.3–33.3]; P = 0.005) and for microbiologically diagnosed VAP (28 placebo cases [38.4% incidence; 95% CI, 27.0–49.8] vs.13 patients with Lactobacillus [17.8% incidence; 95% CI, 8.8–26.8]; P = 0.006).
Secondary Outcomes
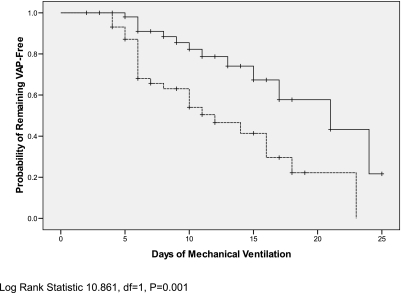
In this study cohort, Lactobacillus administration resulted in a significant delay in the time to onset of microbiologically confirmed VAP (P < 0.001 by generalized Wilcoxon Test) (Figure 2). Probiotic use led to significant reductions in rates of C. difficile cytotoxin assay–positive diarrhea (13 patients [18.6%] vs. four patients [5.8%]; P = 0.02). Patients treated with probiotics who tested positive for C. difficile did not have fewer days of diarrhea compared with patients treated with placebo who tested positive for C. difficile (9.8 ± 4.9 d vs. 13.2 ± 7.4 d; P = 0.39). Diarrhea not caused by C. difficile (so-called “ICU-associated diarrhea”) was common in both groups (42 [61.8%] patients treated with Lactobacillus vs. 44 [62.9%] patients treated with placebo; P = 0.81). The number of days of ICU-associated diarrhea was significantly reduced in patients receiving Lactobacillus therapy (4.1 ± 3.7 vs. 5.9 ± 3.8 d; P = 0.03).
Figure 2.
Kaplan-Meier analysis of time to microbiologically confirmed ventilator-associated pneumonia (VAP). Solid line represents patients receiving Lactobacillus GG; dashed line represents patients receiving placebo.
Among patients with confirmed VAP, those treated with probiotics had 16.1 ± 7.9 antibiotic-days for their VAP, whereas patients treated with placebo had 15.3 ± 10.7 antibiotic-days (P = 0.81). Among patients with confirmed C. difficile infection, probiotic patients had 6.3 ± 5.4 antibiotic-days for their C. difficile, whereas placebo patients had 9.5 ± 5.9 antibiotic-days (P = 0.35). However, the differences in nosocomial infection rates translated into trends toward reductions in total antibiotic consumption (16.3 ± 14.4 vs. 13.3 ± 10.4 antibiotic-days; P = 0.16) and antibiotics prescribed for VAP (8.6 ± 10.3 vs. 5.6 ± 7.8 antibiotic-days; P = 0.05) in patients randomized to probiotic treatment. There was a significant reduction in antibiotic consumption for C. difficile (2.1 ± 4.8 vs. 0.5 ± 2.3 antibiotic-days; P = 0.02) in the group of patients receiving probiotics (Table 3).
TABLE 3.
SECONDARY OUTCOMES
| Placebo (n = 70) | Lactobacillus GG (N = 68) | P Value | |
|---|---|---|---|
| Death | 15 (21.4%) | 12 (17.6%) | 0.47 |
| Clostridium difficile diarrhea | 13 (18.6%) | 4 (5.8%) | 0.02 |
| Days of Clostridium difficile diarrhea, mean ± SD* | 13.2 ± 7.4 | 9.8 ± 4.9 | 0.39 |
| ICU-associated diarrhea | 44 (62.9%) | 42 (61.8%) | 0.81 |
| Days of ICU-associated diarrhea, mean ± SD† | 5.9 ± 3.8 | 4.1 ± 3.7 | 0.03 |
| Total antibiotic-days, mean ± SD | 16.3 ± 14.4 | 13.3 ± 10.4 | 0.16 |
| Prescribed for VAP | 8.6 ± 10.3 | 5.6 ± 7.8 | 0.05 |
| Prescribed for Clostridium difficile | 2.1 ± 4.8 | 0.5 ± 2.3 | 0.02 |
| Hospital length of stay in days, mean ± SD | 21.7 ± 17.4 | 21.4 ± 14.9 | 0.90 |
| ICU length of stay in days, mean ± SD | 14.6 ± 11.6 | 14.8 ± 11.8 | 0.87 |
| Duration of mechanical ventilation in days, mean ± SD | 9.6 ± 7.2 | 9.5 ± 6.3 | 0.91 |
| Hospital charges | $416,446 ± 359,701 | $350,847 ± 258,087 | 0.22 |
Definition of abbreviation: VAP = ventilator-associated pneumonia.
Among patients with a positive Clostridium difficile cytotoxin assay.
Among patients with at least 1 d of ICU-associated diarrhea.
Durations of mechanical ventilation, ICU stay, hospital stay, and total charges were not different between groups (Table 3). Although mortality was not significantly different between the two study arms (21.4% in the placebo arm vs. 17.6% in the probiotic arm; P = 0.42), patients with VAP showed a strong trend toward increased mortality when compared with patients without VAP (23.7 vs. 9.8%; P = 0.06). We did not observe any adverse events attributable to probiotic administration. Specifically, no cases of Lactobacillus bacteremia or pneumonia were seen in the intervention arm of the study. We obtained permission for autopsy in three patients treated with Lactobacillus who died while participating in the study. There was no evidence of Lactobacillus infection in any of these patients.
Surveillance Culture Data
Rates of oral colonization with pathogenic species were not significantly different between study arms at baseline (41.4% for placebo vs. 42.6% for Lactobacillus; P = 0.88) (Table 4). Rates of gastric colonization were also similar at baseline (31.4% for placebo vs. 32.3% for Lactobacillus; P = 0.49). After 72 hours of study participation, patients given placebo had significantly higher oral colonization rates compared with patients given Lactobacillus (70.0% for placebo vs. 38.2% for Lactobacillus; P < 0.001). Rates of gastric colonization were also higher at 72 hours in patients treated with placebo (45.7% for placebo vs. 32.3% for Lactobacillus; P = 0.03). Changes in oral colonization were significantly correlated with the development of microbiologically confirmed VAP (Pearson correlation coefficient, 0.22; P = 0.009).
TABLE 4.
SURVEILLANCE CULTURE DATA
| None* | Rare | Few | Moderate | Many | P Value | |
|---|---|---|---|---|---|---|
| Oral swab pathogen† density at baseline | ||||||
| Placebo | 41 | 2 | 7 | 14 | 6 | 0.88 |
| Lactobacillus | 39 | 8 | 4 | 7 | 10 | |
| Gastric aspirate pathogen† density at baseline | ||||||
| Placebo | 48 | 4 | 7 | 6 | 5 | 0.49 |
| Lactobacillus | 46 | 3 | 3 | 7 | 9 | |
| Oral swab pathogen density at 72 h | ||||||
| Placebo | 21 | 4 | 12 | 16 | 17 | <0.001 |
| Lactobacillus | 42 | 7 | 2 | 5 | 12 | |
| Gastric aspirate pathogen density at 72 h | ||||||
| Placebo | 38 | 5 | 6 | 9 | 12 | 0.03 |
| Lactobacillus | 46 | 7 | 6 | 4 | 5 |
None if no growth is seen; rare if growth is restricted to only the first quadrant; few if growth extends into the second quadrant; moderate if growth extends into the third quadrant; many if growth extends into the fourth quadrant.
Pathogens from oral and gastric aspirates included Staphylococcus aureus (including methicillin-resistant strains), Enterobacteriaceae, and nonfermenting Gram-negative bacteria.
DISCUSSION
In this very select, high-risk cohort, probiotic administration was associated with a statistically significant reduction in the incidence of VAP based on rigorous diagnostic criteria requiring microbiological confirmation on invasive lower respiratory tract samples. The estimated number of patients needed to treat with Lactobacillus to prevent one case of VAP is approximately 5 (95% CI, 3–250), based on the high-risk patients we studied. This novel finding builds on the observations of others who suggest that probiotic therapy is safe for administration in a properly selected, critically ill population. In addition to the reduction of VAP in this cohort, Lactobacillus therapy led to statistically significant reductions in C. difficile–associated diarrhea. Probiotic therapy also showed less use of antibiotics for the treatment of C. difficile diarrhea. Collectively, these data suggest that Lactobacillus may represent a novel, inexpensive (retail price $2.13/d for a total of four tablets as administered per protocol), and nonantibiotic approach to prevent nosocomial infections in properly selected ICU patients.
There have been five randomized controlled trials of probiotic therapy as a strategy to prevent VAP (24, 32–35). These studies had a mean sample size of 159 patients (range, 50–300) and an average APACHE II score of 17. Three of the studies used double blinding (24, 32, 35), and four analyzed single-center data (24, 32–34). Although four of the five studies showed trends toward reduced VAP rates in patients treated with probiotics, the difference was statistically significant in only two of the studies (34, 35). These studies have significant heterogeneity in their inclusion criteria, populations studied, probiotic agent(s) used, probiotic dosing, route of probiotic administration, and, most importantly, the diagnostic criteria used in establishing VAP. Four of the five studies required only qualitative cultures of tracheal aspirates (24, 33–35). The lone study that used quantitative cultures allowed testing of samples from tracheal aspirate, protected specimen brush, or BAL (33). These studies, when combined using metaanalysis methods, suggest that administration of probiotics results in a 39% reduction in VAP (36). Significant differences were also seen in length of ICU stay and colonization of the respiratory tract with Pseudomonas aeruginosa.
Because the presumed mechanisms of probiotic therapy are inherently based on an ability to alter the host flora, the results of these studies must be interpreted within this context. In the three negative VAP prevention trials to date, two were unable to demonstrate significant effects on oropharyngeal colonization (24, 33), and the remaining study favorably altered colonization patterns but was underpowered to detect differences in VAP (32). Neither of the positive studies reported data regarding changes in the pathogen colonization rates (34, 35). In the present study, probiotic administration significantly reduced oropharyngeal and gastric colonization. This is an important observation because changes in colonization were significantly correlated with the development of microbiologically confirmed VAP in the present study.
Probiotic administration appeared to have preferential effects on reducing rates of microbiologically confirmed VAP caused by Gram-negative pathogens. Although VAP caused by gram-positive organisms did not differ between groups (12.8 vs. 5.8%; P=0.16), cases of VAP caused by gram–negative organisms were dramatically different (22.8 vs. 8.8%; P < 0.02). Our present lack of understanding regarding the mechanisms of probiotics precludes speculation regarding why this observation exists. However, the data on pathogen colonization from the present study imply that changes in the host flora are relevant. This observation is consistent with two other studies that have shown decreased colonization in patients administered probiotic therapy (32, 33). It remains unknown which anatomic sites are the most important targets for modifying the host flora with probiotic therapy.
The present study is unique in that we used lower respiratory tract sampling with quantitative cultures to establish the microbiologic diagnosis of VAP. This study also differs from previous studies in that we intentionally selected a very high-risk population, as evidenced by the cohort's high APACHE II scores and prolonged duration of mechanical ventilation. The choice of the specific probiotic agent used in this study (Lactobacillus rhamnosus GG) also differed from the agent(s) used in existing trials. This agent was chosen because it had the most robust safety data and had cursory data suggesting that it may have preferential activity in the upper airways (37–39). Given the paucity of comparative data in this area, it remains unknown whether other agents would have similar or superior results.
These data should be viewed as preliminary and cannot be generalized to the general ICU population given the prolonged period of enrolment, the rigorous inclusion criteria, the large number of exclusion criteria, and the small number of patients included. Furthermore, the current study has multiple limitations. First, these data come from a single center and carry inherent biases related to local practice habits and the population served. Creighton University Medical Center serves an urban community with limited resources and has a patient population with many risk factors for colonization with healthcare-associated pathogens (frequent readmissions, homelessness, high antibiotic consumption, and use of hospital-based clinics and the emergency room for primary care). This is reflected in the high rate of colonization seen in the baseline cultures. Second, in enrolling patients who were very likely to require more than 72 hours of mechanical ventilation, we selected patients who were very sick (mean APACHE ∼23) and had prolonged mechanical ventilation (mean duration ∼10 d), placing these patients at high risk for VAP. The assumptions used in our power calculations and our limited resources required us to exclude patients who would receive short courses of mechanical ventilation. This was necessary because individuals intubated for less than 48 hours cannot, by definition, develop VAP and would have an “immortality bias,” thereby skewing the results toward the null hypothesis. The anticipated consequence of such selective enrolment was that study completion was prolonged (54 mo), and a very large proportion of patients requiring mechanical ventilation were not included (95%). Because of these limitations and the extensive exclusion criteria, the results of this study cannot be generalized to the broader ICU population. Third, the sample size was not large enough to allow for adequate power when assessing most of our secondary endpoints. Accordingly, the described trends should be interpreted only as observations that merit further investigation. Fourth, like most other existing VAP-prevention strategies, probiotic therapy requires compliance and is inherently susceptible to human error. We rigorously monitored study adherence in real time, resulting in 97% of doses being administered within strict, protocol-specified time limits. Such compliance may not be obtained in routine practice. Finally, given the concurrent administration of probiotics to two anatomically distinct sites (i.e., the oropharynx and the stomach), the critical site of delivery is unknown. Similarly, the lack of samples for mechanistic studies limits further inference regarding mechanisms of action. Furthermore, the data regarding differences in antibiotic use are limited by the methods used to calculate our composite measure of antibiotic prescription (antibiotic-days).
The potential harms of probiotic therapy also require comment. Historically, the consensus has been that probiotic therapy was of questionable value but was safe. However, this latter assumption was shown to not be true in the PROPATRIA trial, which was a randomized, double-blind, placebo-controlled clinical trial evaluating the efficacy of a novel probiotic combination in preventing infections in predicted severe acute pancreatitis (40). Although the investigators in this multicenter Dutch trial have been criticized regarding the trial's design, execution, and analysis, even detractors agree that probiotic administration led to increased mortality when compared with placebo (15.7 vs. 6.3%; P = 0.01) (41). Contrary to the prevailing safety concern that probiotic administration could lead to iatrogenic infection, the increased mortality seen in the probiotic arm of PROPATRIA was attributed to a significantly higher rate of intestinal ischemia (6.3 vs. 0%; P = 0.004). Although this observation may be unique to the novel probiotic preparation used, its specific route of administration, the disease state studied, or other unknown factors, this trial should remind us of the risks (potentially unanticipated) of probiotic therapy research and highlights our need for meticulous monitoring of subjects. Although no safety issues have been identified in the investigations using probiotics for VAP prevention, the findings of the PROPATRIA investigators reinforces the need to view the current study as preliminary observations that merit further investigation and structured safety monitoring.
Probiotic prophylaxis of VAP using Lactobacillus rhamnosus GG appears safe and efficacious in a select population of patients who are at very high risk for contracting VAP. This therapy may also offer an opportunity to prevent related ICU complications, such as C. difficile and ICU-associated diarrhea. Ultimately, probiotics may fulfill a role in antimicrobial stewardship programs given the reductions in antibiotic consumption. Larger, multicenter clinical trials with more liberal inclusion criteria are needed to further establish the efficacy of probiotics and to allow for extrapolation to a larger at-risk population. Such studies should include collaboration with basic scientists to more rigorously study the potential mechanisms of probiotics' effects.
Supported by NIH grant K23 HL81491-04 and the Creighton University Health Futures Foundation (L.E.M.) and by the Barnes-Jewish Hospital Foundation (M.H.K.).
Originally Published in Press as DOI: 10.1164/rccm.200912-1853OC on June 3, 2010
Author Disclosure: L.E.M. has received consultancy fees from C.R. Bard, Inc. ($5001–$10,000), advisory board fees from Schering-Plough and Cadence (both up to $1000), lecture fees from Pfizer ($1001–$5000) and Medicon ($5000–$10,000), industry-sponsored grants from Pfizer ($50,001–$100,000), and sponsored grants from NIH (over $100,000). M.H.K. has received consultancy fees from Merck, Pfizer, and Astellas (all $5001–$10,000); advisory board fees from Pfizer and Merck (both $1001–$5000); lecture fees from Merck ($10,001–$50,000), Pfizer ($50,001–$100,000), C.R. Bard, Inc. ($5001–$10,000), and AstraZeneca ($10,001–$50,000); and industry-sponsored grants from Johnson and Johnson ($10,001–$50,000). T.B.C. has received advisory board fees from MedImmune ($5001–$10,000) and Roche ($1001–$5000); industry-sponsored institutional grants from Pfizer (over $100,000), Boehringer Ingelheim ($50,001–$100,000), Novartis ($50,001–$100,000), Amgen ($10,001–$50,000), and MedImmune ($10,001–$50,000); an institutional grant, category F, from Merck ($50,001–$100,000); and advisory fees from WAO (up to $1000). T.B.C. is employed by the American Academy of Allergy, Asthma and Immunology.
References
- 1.Rello J, Ollendorf DA, Oster G, Vera-Llonch M, Bellm L, Redman R, Kollef MH; VAP Outcomes Scientific Advisory Group. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest 2002;122:2115–2121. [DOI] [PubMed] [Google Scholar]
- 2.Cook DJ, Walter SD, Cook RJ, Griffith LE, Guyatt GH, Leasa D, Jaeschke RZ, Brun-Buisson C. Incidence of and risk factors for ventilator-associated pneumonia in critically ill patients. Ann Intern Med 1998;129:433–440. [DOI] [PubMed] [Google Scholar]
- 3.American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388–416. [DOI] [PubMed] [Google Scholar]
- 4.Ibrahim EH, Tracy L, Hill C, Fraser VJ, Kollef MH. The occurrence of ventilator-associated pneumonia in a community hospital: risk factors and clinical outcomes. Chest 2001;120:555–561. [DOI] [PubMed] [Google Scholar]
- 5.Kollef MH. What is ventilator-associated pneumonia and why is it important? Respir Care 2005;506:714–721. [PubMed] [Google Scholar]
- 6.Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. Epidemiology and outcomes of healthcare-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest 2005;128:3854–3862. [DOI] [PubMed] [Google Scholar]
- 7.Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in medical intensive care units in the United States: National Nosocomial Infections Surveillance System. Crit Care Med 1999;27:887–892. [DOI] [PubMed] [Google Scholar]
- 8.Kollef MH. Prevention of hospital-associated pneumonia and ventilator-associated pneumonia. Crit Care Med 2004;32:1396–1405. [DOI] [PubMed] [Google Scholar]
- 9.Craven DE. Preventing ventilator-associated pneumonia in adults: sowing seeds of change. Chest 2006;130:251–260. [DOI] [PubMed] [Google Scholar]
- 10.Cook D. Ventilator associated pneumonia: perspectives on the burden of illness. Intensive Care Med 2000;26:S31–S37. [DOI] [PubMed] [Google Scholar]
- 11.Dodek P, Keenan S, Cook D, Heyland D, Jacka M, Hand L, Muscedere J, Foster D, Mehta N, Hall R, et al.; Canadian Critical Care Trials Group. Canadian Critical Care Society. Evidence-based clinical practice guideline for the prevention of ventilator-associated pneumonia. Ann Intern Med 2004;141:305–313. [DOI] [PubMed] [Google Scholar]
- 12.Tablan OC, Anderson LJ, Besser R, Bridges C, Hajjeh R. Guidelines for preventing health-care-associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep 2004;53:1–36. [PubMed] [Google Scholar]
- 13.Babcock HM, Zack JE, Garrison T, Trovillion E, Jones M, Fraser VJ, Kollef MH. An educational intervention to reduce ventilator-associated pneumonia in an integrated health system: a comparison of effects. Chest 2004;125:2224–2231. [DOI] [PubMed] [Google Scholar]
- 14.Kollef MH, Afessa B, Anzueto A, Veremakis C, Kerr KM, Margolis BD, Craven DE, Roberts PR, Arroliga AC, Hubmayr RD, et al.; NASCENT Investigation Group. Silver-coated endotracheal tubes and incidence of ventilator-associated pneumonia: the NASCENT randomized trial. JAMA 2008;300:805–813. [DOI] [PubMed] [Google Scholar]
- 15.Guidelines for the evaluation of probiotics in food: report of a joint FAO/WHO working group on drafting guidelines for the evaluation of probiotics in food (accessed October 3, 2009) Available from: http://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf
- 16.Ghosh S, van Heel D, Playford RJ. Probiotics in inflammatory bowel disease: it is all gut flora modulation? Gut 2004;53:620–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNaught CE, Woodcock NP, MacFie J, Mitchell CJ. A prospective randomized study of the probiotic Lactobacillus plantarum 299V on indices of guy barrier function in elective surgical patients. Gut 2002;51:827–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Kleij D, Latz E, Brouwers JF, Kruize YC, Schmitz M, Kurt-Jones EA, Espevik T, de Jong EC, Kapsenberg ML, Golenbock DT, et al. A novel host-parasite lipid cross-talk: schistosomal lysto-phosphatidylserine activates toll-like receptor 2 and affects immune polarization. J Biol Chem 2002;277:48122–48129. [DOI] [PubMed] [Google Scholar]
- 19.Chapat L, Chemin K, Dubois B, Bourdet-Sicard R, Kaiserlian D. Lactobacillus casei reduces CD8+ T cell-mediated skin inflammation. Eur J Immunol 2004;34:2520–2528. [DOI] [PubMed] [Google Scholar]
- 20.Isolauri E, Sutas Y, Kanaanpaa P, Arvilommi J, Salminen S. Probiotics: effects on immunity. Am J Clin Nutr 2001;73:444S–450S. [DOI] [PubMed] [Google Scholar]
- 21.Walker WA. Mechanisms of action of probiotics. Clin Infect Dis 2008;46:S87–S91. [DOI] [PubMed] [Google Scholar]
- 22.Wolvers D, Antoine JM, Myllyluoma E, Schrezenmeir J, Szajewska H, Rijkers JT. Guidance for substantiating the evidence for beneficial effects of probiotics: prevention and management of infections by probiotics. J Nutr 2010;140:698S–712S. [DOI] [PubMed] [Google Scholar]
- 23.Pitsouni E, Alexious V, Saridakis V, Peppas G, Falagas M. Does the use of probiotics/synbiotics prevent postoperative infections in patients undergoing abdominal surgery? A meta-analysis of randomized controlled trials. Eur J Pharmacol 2009;65:561–570. [DOI] [PubMed] [Google Scholar]
- 24.Knight DJ, Gardiner D, Banks A, Snape SE, Weston VC, Bengmark S, Girling KJ. Effect of synbiotic therapy on the incidence of ventilator associated pneumonia in critically ill patients: a randomised, double-blind, placebo-controlled trial. Intensive Care Med 2009;35:854–861. [DOI] [PubMed] [Google Scholar]
- 25.van Santvoort HC, Besselink MG, Timmerman HM, van Minnen LP, Akkermans LM, Gooszen HG. Probiotics in surgery. Surgery 2008;143:1–7. [DOI] [PubMed] [Google Scholar]
- 26.Rayes N, Seehofer D, Theruvath T, Schiller RA, Langrehr JM, Jonas S, Bengmark S, Neuhaus P. Supply of pre- and probiotics reduces bacterial infection rates after liver transplantation: a randomized, double-blind trial. Am J Transplant 2005;5:125–130. [DOI] [PubMed] [Google Scholar]
- 27.Falcao de Arruda IS, de Aguilar-Nascimenta JE. Benefits of early enteral nutrition with glutamine and probiotics in brain injury patients. Clin Sci 2004;106:287–292. [DOI] [PubMed] [Google Scholar]
- 28.Morrow LE. Probiotic manipulation of the endogenous flora as prophylaxis for ventilator-associated pneumonia. J Investig Med 2008;56:658–659. [Google Scholar]
- 29.Morrow LE, Kollef MH, Bowers JB, Casale TB. Probiotic manipulation of the native flora in critically ill patients: an opportunity for ventilator-associated pneumonia prophylaxis? Chest 2005;128:144S. [Google Scholar]
- 30.Kollef MH, Bock KR, Richards RD, Hearns ML. The safety and diagnostic accuracy of minibronchoalveolar lavage in patients with suspected ventilator-associated pneumonia. Ann Intern Med 1995;122:743–748. [DOI] [PubMed] [Google Scholar]
- 31.O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics 1979;35:549–556. [PubMed] [Google Scholar]
- 32.Forestier C, Guelon D, Cluytens V, Gillart T, Sirot J, De Champs C. Oral probiotic and prevention of Pseudomonas aeruginosa infections: a randomized, double-blind, placebo controlled pilot study in intensive care unit patients. Crit Care 2008;12:R69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klarin B, Molin G, Jeppson B, Larsson A. Use of the probiotic Lactobacillus plantarum 299 to reduce pathogenic bacteria in the oropharynx of intubated patients: a randomized controlled open pilot study. Crit Care 2008;12:R136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spindler-Vesel A, Benkgmark S, Vovk I, Cerovic O, Kompan L. Synbiotics, prebiotics, glutamine, or peptide in early enteral nutrition: a randomized study in trauma patients. J Parenter Enteral Nutr 2007;31:119–126. [DOI] [PubMed] [Google Scholar]
- 35.Kotzampassi K, Giamarellos-Bourboulis EJ, Voudouris A, Kazamanias P, Eleftheriadis E. Benefits of a synbiotic formula (Synbiotic 2000Forte) in critically ill trauma patients: early results of a randomized controlled trial. World J Surg 2006;30:1848–1855. [DOI] [PubMed] [Google Scholar]
- 36.Siempos II, Ntaidou TK, Falagas ME. Impact of the administration of probiotics on the incidence of ventilator-associated pneumonia: a meta-analysis of randomized, controlled trials. Crit Care Med 2010;38:954–962. [DOI] [PubMed] [Google Scholar]
- 37.Gluck U, Gebbers JO. Ingested probiotics reduce nasal colonization with pathogenic bacteria (Staphylococcus aureus, Streptococcus pneumoniae, and β-hemolytic streptococci). Am J Clin Nutr 2003;77:517–520. [DOI] [PubMed] [Google Scholar]
- 38.Hatakka K, Savilahti E, Ponka A, Meurman JH, Poussa T, Nase L, Saxelin M, Korpela R. Effect of long-term consumption of probiotic milk on infections in children attending day care centres: double blind, randomised trial. BMJ 2001;322:1327–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brandtzaeg P. Humoral immune response patterns of human mucosae: induction and relation to bacterial respiratory tract infections. J Infect Dis 1992;165:S167–S176. [DOI] [PubMed] [Google Scholar]
- 40.Besselink MGH, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet 2008;371:651–659. [DOI] [PubMed] [Google Scholar]
- 41.The Editors of the Lancet. Expression of concern: probiotic prophylaxis in predicted severe acute pancreatitis: a randomized, double-blind, placebo-controlled trial. Lancet 2010;375:875–876. [DOI] [PubMed] [Google Scholar]