Abstract
Rationale: An activated vasoconstrictive, proliferative, and fibrotic axis of the renin angiotensin system (angiotensin-converting enzyme [ACE]/angiotensin [Ang]II/AngII type 1 receptor) has been implicated in the pathophysiology of pulmonary fibrosis (PF) and pulmonary hypertension (PH). The recent discovery of a counterregulatory axis of the renin angiotensin system composed of ACE2/Ang-(1–7)/Mas has led us to examine the role of this vasoprotective axis on such disorders.
Objectives: We hypothesized that Ang-(1–7) treatment would exert protective effects against PF and PH.
Methods: Lentiviral packaged Ang-(1–7) fusion gene or ACE2 cDNA was intratracheally administered into the lungs of male Sprague Dawley rats. Two weeks after gene transfer, animals received bleomycin (2.5 mg/kg). In a subsequent study, animals were administered monocrotaline (MCT, 50 mg/kg).
Measurements and Main Results: In the PF study, bleomycin administration resulted in a significant increase in right ventricular systolic pressure, which was associated with the development of right ventricular hypertrophy. The lungs of these animals also exhibited excessive collagen deposition, decreased expression of ACE and ACE2, increased mRNA levels for transforming growth factor β and other proinflammatory cytokines, and increased protein levels of the AT1R. Overexpression of Ang-(1–7) significantly prevented all the above-mentioned pathophysiological conditions. Similar protective effects were also obtained with ACE2 overexpression. In the PH study, rats injected with MCT developed elevated right ventricular systolic pressure, right ventricular hypertrophy, right ventricular fibrosis, and pulmonary vascular remodeling, all of which were attenuated by Ang-(1–7) overexpression. Blockade of the Mas receptor abolished the beneficial effects of Ang-(1–7) against MCT-induced PH.
Conclusions: Our observations demonstrate a cardiopulmonary protective role for the ACE2/Ang-(1–7)/Mas axis in the treatment of lung disorders.
Keywords: renin angiotensin system, cardiopulmonary diseases, ACE2/Ang-(1-7)/Mas axis, gene therapy
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Current therapeutic strategies for treating lung fibrosis and pulmonary hypertension have not shown promising results either to abate the progression of the disease or to improve the quality of life.
What This Study Adds to the Field
This study suggests that the angiotensin-converting enzyme 2/angiotensin-(1–7)/Mas axis may serve as a novel therapeutic target for the treatment of lung fibrosis and pulmonary hypertension.
Pulmonary fibrosis (PF) is a fatal lung disease of unknown cause. The disease is characterized by progressive scarring of the lung tissue accompanied by fibroblast proliferation, excessive accumulation of matrix proteins, and abnormal alveolar structure, all leading to a loss of lung function and, ultimately, respiratory failure (1). Current treatments with antiinflammatory, immunosuppressive, and antifibrotic agents have not shown promising results to abate the progression of the disease or to improve the quality of life (2). Thus, it becomes essential to better understand the disease pathophysiology and identify novel therapeutic targets/agents for the treatment of PF. There is experimental and clinical evidence to indicate that an activated pulmonary renin angiotensin system (RAS) is involved in the pathophysiology of this disease (3, 4): (1) Polymorphism of the angiotensinogen (AGT) gene has been associated with the progression of idiopathic pulmonary fibrosis (5); (2) AGT has been found to be one of the most overexpressed genes in patients with PF (6); (3) angiotensin II (Ang II) has been shown to stimulate cell growth and proliferation of lung fibroblasts (7, 8); (4) Ang II is known to up-regulate the expression of transforming growth factor-β (TGF-β) (9), a central player in fibrogenesis; (5) Ang II–induced TGF-β generation is associated with the differentiation of fibroblast to myofibroblasts, which dramatically enhances extracellular matrix protein deposition (10); (6) Ang II is involved in lung inflammation through the generation of reactive oxygen species and the release of proinflammatory cytokines (11, 12); (7) Ang II has been shown to induce alveolar epithelial cell apoptosis (13), which is believed to initiate the fibrotic process; and (8) Ang II is also involved in promoting procoagulatory effects by activating plasminogen activator inhibitor-1 (14).
Another lung disease that is closely associated with an altered RAS is pulmonary hypertension (PH) (15). Polymorphism in the genes encoding for components of the RAS, such as AGT (16), angiotensin-converting enzyme (ACE) (16), and the Ang II type 1 receptor (AT1R) (17), have been linked to the development of PH. Local production of Ang II by ACE stimulates the growth of smooth muscle cells in the arterial wall to cause maladaptive changes and function of the pulmonary vasculature, which can result in the development of PH (18).
It is evident that modulation of components of the RAS appears to be a viable therapeutic strategy for the treatment of lung diseases such as PF and PH. However, direct targeting of the RAS components in these lung disorders using ACE inhibitors or AT1R blockers have produced mixed results in animal models and in humans (19, 20). Nevertheless, the recent discovery of a homolog of ACE, called ACE2, and its enzymatic product angiotensin-(1–7) ([Ang-[1–7]), offer an alternative pathway to regulate the intrapulmonary components of the RAS for producing beneficial effects. Ang-(1–7) is a heptapeptide that counteracts many of the detrimental effects of Ang II, including heart failure, kidney diseases, and diabetic complications (21, 22). However, the role of Ang-(1–7) in pulmonary disorders, especially in PF and PH, has not yet been investigated. Because Ang-(1–7) is a peptide that undergoes rapid proteolytic degradation in the circulation (23), we decided to adopt a novel strategy of overexpressing this peptide locally in the lung tissue through a lentiviral-mediated gene delivery approach. Thus, in this study we propose that lentiviral-mediated pulmonary overexpression of Ang-(1–7) exerts cardiopulmonary beneficial effects against two experimental models of lung disorders: PF and PH. Some of the results of these studies have been previously reported in the form of abstracts (24).
METHODS
Materials
Bleomycin sulfate was purchased from Calbiocem Labs (San Diego, CA). Mouse monoclonal anti–β-actin antibody, monocrotaline (MCT), and α smooth muscle actin (clone 1A4) were purchased from Sigma-Aldrich (St. Louis, MO). AT1R rabbit polyclonal antibody and ACE rabbit polyclonal antibody were procured from Santa Cruz Biotechnology (Santa Cruz, CA). Alzet osmotic pumps (models 2004 and 2ML4) were purchased from Durect Corporation (Cupertino, CA). The Mas-receptor antagonist A-779 was purchased from Bachem (Torrance, CA).
Animal Procedures and Treatment of Rats with Lenti-Ang-(1–7)
Five-week-old male Sprague Dawley rats were used in this study. All animals were housed in a temperature-controlled room (25 ± 1°C) and were maintained on a 12:12-hour light:dark cycle with free access to water and food. All procedures involving experimental animals were approved by the Institutional Animal Care and Use Committee at the University of Florida and complied with National Institutes of Health guidelines. For gene delivery into the lungs, rats were anesthetized with isoflurane and a midline incision was made to expose the trachea. Empty virus (control), lenti-ACE2, or lenti–Ang-(1–7) viral particles (3 × 108 TU in 100 μl of phosphate-buffered saline) were injected into the trachea followed by 300 μl of air so as to enhance the spread of virus in the rat lung. Two weeks after lentiviral treatment, animals were subjected to bleomycin or monocrotaline administration.
Bleomycin-induced Pulmonary Fibrosis
Animals under the influence of isoflurane anesthesia received a single intratracheal instillation of 2.5 mg/kg dose of bleomycin sulfate in 300 μl of sterile saline. The 300-μl solution was instilled at end expiration, and the liquid was immediately followed by 200 μl of air to increase delivery to the distal airways. Control animals received an equal volume of sterile saline.
MCT-induced Pulmonary Hypertension
PH was induced by single subcutaneous injection of MCT (50 mg/kg). Control rats received saline (500 μl, subcutaneously). At the same time of MCT administration, a subset of animals were implanted with Alzet mini-osmotic pumps (model 2ML4) containing A-779, a Mas antagonist, at an infusion rate of 60 μg/d (1 mg/ml stock solution) for 28 days.
Systemic and Right Ventricular Systolic Pressure Measurements
Weekly systemic blood pressure was measured in conscious rats using the noninvasive tail-cuff method (Narco Bio-Systems, Austin, TX) as described elsewhere (25). For measurement of right ventricular systolic pressure (RVSP), rats were anesthetized with a mixture of ketamine (30 mg/kg, subcutaneously) and xylazine (6 mg/kg, subcutaneously) and were placed in a supine position, breathing room air. The RVSP was measured using a silastic catheter inserted into the right descending jugular vein and forwarded to the RV. The data were recorded after stabilization of the tracing using a liquid pressure transducer, which was interfaced to a PowerLab (AD Instruments, Colorado Springs, CO) signal transduction unit. The waveform was used to confirm the positioning of the catheter in the RV. Data were analyzed by using the Chart program supplied with the PowerLab system. After RVSP was measured, the rats were killed, and the hearts and lungs were harvested.
Biochemical Studies for Measuring Lung Hydroxyproline Content
Lung collagen deposition was estimated by measuring hydroxyl-proline content (26). Briefly, frozen lung tissue was homogenized in 1.8 ml of glacial acetic acid (0.5 mol/L) and dried in a speed vacuum. The dried sample was weighed, dissolved in 2 ml of 6 N HCl, and hydrolyzed at 110°C overnight. The acid hydrolysates and standards were applied to an ELISA plate along with citric/acetate buffer and chloramine T solution. After 20 minutes, Ehrlich solution was added and incubated at 65°C for 15 minutes. The reaction product was read at 550 nm. Solutions of 0 to 1,000 μg/ml hydroxyproline (Sigma, St. Louis, MO) were used to construct the standard curve.
Statistical Analysis
Data are presented as means ± SEM. Statistical differences were evaluated by either one-way or two-way analysis of variance wherever applicable, followed by the Newman-Keuls test. P values less than 0.05 were considered statistically significant.
Production of ACE2 and Ang-(1–7) lentiviral particles, determination of transduction efficiency of the lung by lentivirus, cardiac hypertrophy, quantitative real-time reverse transcription–polymerase chain reaction, Western blot analysis, and electron spin resonance spectroscopy are described in the online data supplement.
RESULTS
Efficacy of the Lentivirus to Overexpress Ang-(1–7)
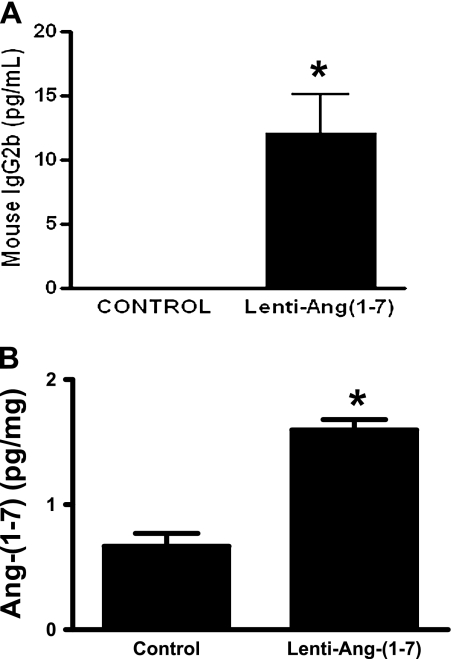
Infection of cardiac myoblasts with 10 multiplicity of infection of lenti–Ang-(1–7) fusion protein resulted in a robust expression of IgG2b isotype (Figure 1A). Furthermore, in vivo experiments also demonstrated increased levels of Ang-(1–7) peptide in the lenti–Ang-(1–7) infected rat lungs compared with control animals (Figure 1B). These data establish that lenti–Ang-(1–7) is active and effective in secreting Ang-(1–7), and this secretion of Ang-(1–7) was present for 6 weeks after administration of the viral vector–containing transgene.
Figure 1.
(A) Lenti–Ang-(1–7) caused a significant increase in the levels of mouse IgG2b after 72 hours of infection in the H9C2 myoblasts. (B) In vivo administration of lenti–angiotensin (Ang)-(1–7) significantly increased the levels of Ang-(1–7) in the rat lungs after 6 weeks of gene transfer. Data are represented as mean ± SEM. *P < 0.05 versus control group (n = 4–5 per group).
As for lenti-ACE2 efficacy, murine ACE2 mRNA was detected only in the lungs of rats treated with lenti-ACE2, which we have also previously reported to occur in mice with tracheally induced lenti-ACE2 (27). There was no murine ACE2 expression in the control animals (data not shown).
Study 1: Bleomycin-induced PF
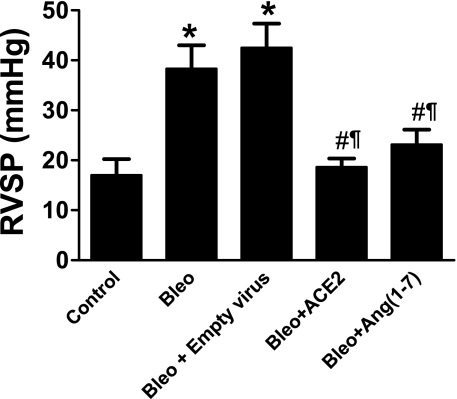
Attenuation of bleomycin-induced RVSP and right ventricular hypertrophy by ACE2 and Ang-(1–7).
Two weeks of bleomycin administration resulted in a significant increase in the RVSP (Figure 2), which was followed by the development of right ventricular hypertrophy (RVH) (0.27 ± 0.01 in control animals vs. 0.49 ± 0.02 in bleomycin). Gene therapy treatment with either ACE2 or Ang-(1–7) significantly attenuated the bleomycin-induced increase in RVSP (Figure 2) and RVH (0.37 ± 0.02 in bleomycin + ACE2 and 0.37 ± 0.03 in bleomycin + Ang-[1–7]).
Figure 2.
Effects of lentiviral-mediated overexpression of angiotensin-converting enzyme (ACE)2 and angiotensin (Ang)-(1–7) in bleomycin-induced pulmonary hypertension. Bleomycin administration caused a significant increase in the right ventricular systolic pressure (RVSP) after 2 weeks, which was completely attenuated by lenti-ACE2 and lenti–Ang-(1–7) treatment. Data are represented as mean ± SEM. *P < 0.05 versus control group, #P < 0.05 versus bleomycin, ¶P < 0.05 versus bleomycin + empty virus (n = 5–6 per group).
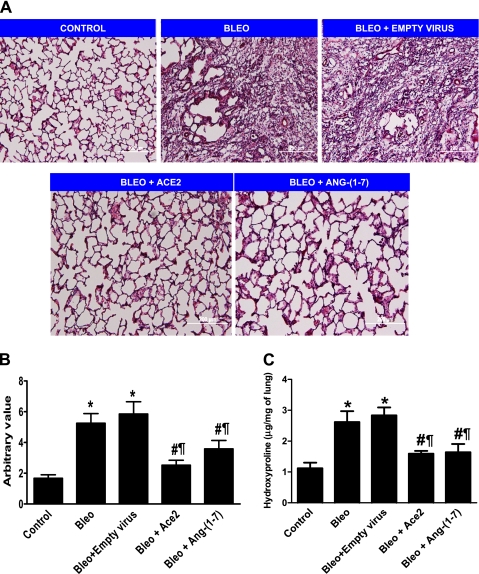
Pathological score and hydroxyproline content of lung tissue after bleomycin administration.
Lung sections from the different treatment groups were stained with hematoxylin-eosin, and representative sections are shown in Figure 3A. Ashcroft scoring was determined from five different nonoverlapping fields from each lung section of different treatment groups as described previously (28). The mean group results demonstrated a higher score for the bleomycin alone group compared with control animals, indicating a fibrotic response to bleomycin administration. However, animals treated with either lenti-ACE2 or Ang-(1–7) showed a significantly lower score, demonstrating a protective effect against the development of bleomycin-induced fibrosis. These results are summarized in Figure 3B. Along similar lines, an increase in lung collagen accumulation, as assessed by measurement of lung hydroxyproline, was observed in the bleomycin alone group, which was significantly reduced with either ACE2 or Ang-(1–7) overexpression (Figure 3C).
Figure 3.
Histological analysis and quantitative fibrosis scoring of lung sections. (A) Representative photographs of the lung tissue stained with hematoxylin and eosin. (B) Morphological changes in fibrotic lungs quantified using the Ashcroft score. (C) Lung hydroxyproline content, in the various treatment groups. Data are represented as mean + SEM. *P < 0.05 versus control group; #P < 0.05 versus bleomycin (bleo), ¶P < 0.05 versus bleomycin + empty virus (n = 4 per group). ACE = angiotensin-converting enzyme; ang = angiotensin.
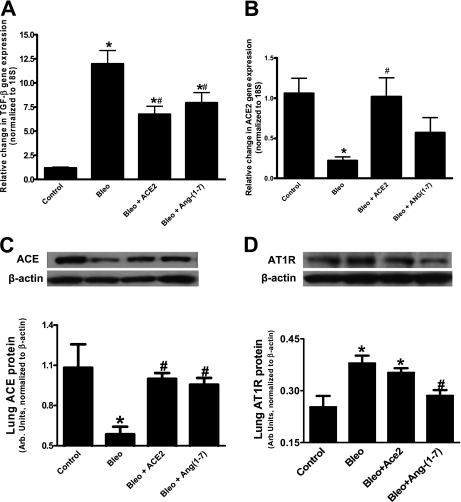
Effects of different treatments on the lung mRNA expressions of TGF-β and ACE2.
Figure 4A demonstrates that the expression of TGF-β was significantly elevated in the bleomycin group compared with those of normal lungs. TGF-β expression was significantly suppressed by either ACE2 or Ang-(1–7) overexpression. On the other hand, mRNA levels of endogenous ACE2, a membrane-bound enzyme, were significantly decreased in bleomycin-treated animals (Figure 4B). This decrease was completely prevented by overexpression of ACE2. Ang-(1–7) treatment resulted in an intermediate response in that these animals were not different from either control animals or the bleomycin-treated groups.
Figure 4.
Effect of various treatments on lung transforming growth factor (TGF)-β, angiotensin-converting enzyme (ACE)2, ACE, and angiotensin II type 1 receptor (AT1R) levels. (A) Lung TGF-β mRNA levels were significantly increased by bleomycin (bleo) administration, which was prevented by overexpression of murine ACE2 and angiotensin (Ang)-(1–7). (B) Bleomycin-induced decrease in lung ACE2 mRNA levels was completely prevented by overexpression of murine ACE2 but only partially by Ang-(1–7). (C) Bleomycin administration decreased lung ACE protein level, which was significantly restored by ACE2 and Ang-(1–7) overexpression. (D) Bleomycin administration increased lung AT1R protein level, which was significantly decreased by Ang-(1–7). However, ACE2 overexpression failed to decrease the elevated AT1R levels induced by bleomycin. *P < 0.05 versus control group; #P < 0.05 versus bleomycin (n = 3–5 per group).
Western analysis for comparative quantification of ACE and AT1R in the bleomycin study.
Bleomycin administration significantly decreased ACE protein levels, and this decrease was prevented by overexpression with either ACE2 or Ang-(1–7) (Figure 4C). In contrast, bleomycin treatment resulted in a significant increase in lung AT1R protein as compared with control animals, and this increase was significantly attenuated by Ang-(1–7) overexpression, but not by ACE2 overexpression (Figure 4D).
Study 2: Monocrotaline-induced PH
Prevention of MCT-induced PH and associated cardiopulmonary remodeling by Ang-(1–7).
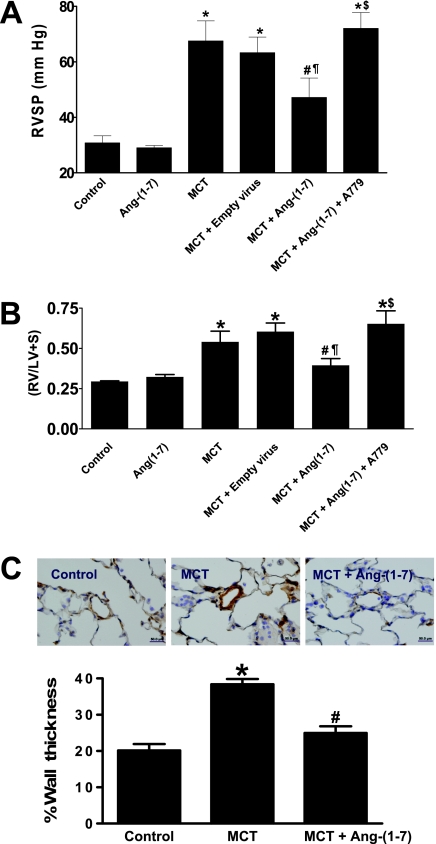
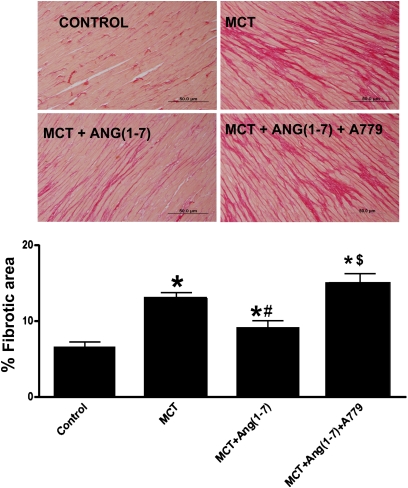
A single subcutaneous administration of MCT in rats resulted in robust increase in RVSP in 4 weeks (30.8 ± 2.6 mm Hg in control vs. 67.5 ± 7.3 mm Hg in MCT, P < 0.05, n = 4–7; Figure 5A). Overexpression of Ang-(1–7) significantly attenuated the MCT-induced elevation in RVSP (47 ± 7 mm Hg, P < 0.05, n = 9). MCT-challenged animals also developed RVH as measured by the right ventricle/left ventricle + septum (RV/LV+S) ratio (0.29 ± 0.004 in control vs. 0.55 ± 0.05 in MCT, P < 0.05, n = 4–7) (Figure 5B), which likewise was prevented by overexpression of Ang-(1–7) (0.41 ± 0.04, P < 0.05, n = 9). Empty virus was without any effect on any parameters assessed. Furthermore, MCT treatment resulted in increase in the medial wall thickness, which also was significantly attenuated by Ang-(1–7) overexpression (Figure 5C). Rats treated with MCT exhibited increased interstitial right ventricular fibrosis as evaluated by percent fibrotic area (5.3 ± 0.6% in control vs. 13.0 ± 0.7% in MCT, P < 0.05). This effect was partially blunted by overexpression of Ang-(1–7) (10.1 ± 0.4%, P < 0.05). The biological effects of Ang-(1–7) are believed to be mediated through stimulation of the putative Mas receptor. Coadministration of A-779, an Mas antagonist, in MCT-challenged rats completely abolished the improvements in RVSP, ventricular hypertrophy, and fibrosis induced by the overexpression of Ang-(1–7) (Figures 5A, 5B, and 6, respectively). In contrast to RVSP, the systemic blood pressure was unchanged with either MCT treatment or overexpression of Ang-(1–7) (mean arterial pressure [MAP]: control, 122 ± 5 mm Hg, n = 4; MCT, 118 ± 2 mm Hg, n = 8; MCT + Ang-(1–7), 118 + 2 mm Hg, n = 9).
Figure 5.
Effects of lenti–angiotensin (Ang)-(1–7) on monocrotaline (MCT)-induced pulmonary hypertension. (A) MCT administration caused a significant increase in the right ventricular systolic pressure (RVSP), which was significantly attenuated by lenti–Ang-(1–7) overexpression. This beneficial effect was, however, lost on blockade of the Mas receptor with A-779. (B) MCT administration resulted in the development of right ventricular hypertrophy as indicated by a significant increase in the RV/LV+S ratio. This increase in ratio was prevented by Ang-(1–7) overexpression. Blockade of the Mas receptor with A-779 abolished the beneficial effect of Ang-(1–7). (C) The vascular hypertrophic effect of MCT, indicated by the brown staining of the pulmonary vasculature, was significantly blunted by lenti–Ang-(1–7) overexpression. Data are represented as mean ± SEM. *P < 0.05 versus control group, #P < 0.05 versus MCT, ¶P < 0.05 versus MCT + empty virus, $P < 0.05 versus MCT + Ang-(1–7), (n = 3–9 per group).
Figure 6.
Effect of lenti–angiotensin (Ang)-(1–7) on right ventricular fibrosis. Pico Sirius red staining revealed increased collagen deposition (red) in the right ventricle of monocrotaline (MCT)-challenged rats, which was partially blocked by lenti–Ang-(1–7) administration. The beneficial effect of Ang-(1–7) was totally lost on coadministration of A-779, a Mas antagonist. Data are represented as mean ± SEM. *P < 0.05 versus control group, #P < 0.05 versus MCT, $P < 0.05 versus MCT + Ang-(1–7), (n = 3–5 per group).
Effect of Ang-(1–7) overexpression on MCT-induced lung RAS and inflammatory cytokines.
Because cytokines have been shown to play a key role in the pathophysiology of PH, we evaluated the effects of Ang-(1–7) overexpression on inflammatory cytokines and oxidative stress. Table 1 summarizes the quantitative measurement of mRNA for inflammatory cytokines in MCT and MCT + Ang-(1–7)–treated rats. MCT treatment resulted in significant increases in the mRNA levels of proinflammatory cytokines (TNF-α, IL-1β, IL-6). gp91phox, a subunit of the NADPH oxidase, tended to be increased by MCT treatment, but this increase was not statistically significant (Table 1). Ang-(1–7) overexpression in the MCT-challenged rats resulted in a reversal of this pattern with decreased levels of TNF-α, IL-1β, IL-6, and gp91phox. In contrast, there was a significant increase in the antiinflammatory cytokine (IL-10) in MCT-treated rats overexpressing Ang-(1–7). Electron spin resonance spectroscopy revealed increased oxidative stress in the pulmonary artery and lungs of MCT-challenged animals, which was significantly attenuated by Ang-(1–7) overexpression (Figure E3).
TABLE 1.
RELATIVE FOLD-CHANGE IN LUNG RENIN ANGIOTENSIN SYSTEM AND CYTOKINE MRNA LEVELS
| Gene | Control | MCT | MCT+ Ang-(1–7) |
|---|---|---|---|
| ACE | 0.85 ± 0.37 (4) | 2.89 ± 0.45* (7) | 1.22 ± 0.1† (4) |
| ACE2 | 0.82 ± 0.28 (4) | 3.18 ± 0.69 (6) | 4.89 ± 1.93* (4) |
| IL-10 | 1.22 ± 0.38 (4) | 2.68 ± 0.44 (6) | 4.13 ± 1.33* (5) |
| IL-1β | 1.6 ± 0.57 (4) | 4.62 ± 1.00* (7) | 1.02 ± 0.41† (4) |
| IL-6 | 1.63 ± 0.74 (4) | 16.37 ± 4.59* (7) | 2.1 ± 0.61† (4) |
| Phox 91 | 1.96 ± 0.97 (4) | 3.60 ± 1.22 (7) | 1.78 ± 0.84 (4) |
| TNF-α | 1.08 ± 0.53 (4) | 6.7 ± 1.53* (7) | 2.34 ± 0.65† (6) |
Definition of abbreviations: ACE = angiotensin-converting enzyme; MCT = monocrotaline; TNF = tumor necrosis factor.
Numbers within parentheses refer to the sample size.
P < 0.05 versus control group.
P < 0.05 versus MCT.
As for the lung RAS, the MCT treatment resulted in an increase in ACE mRNA levels that was significantly decreased by overexpression of Ang-(1–7). The levels of ACE2 mRNA tended to increase with MCT treatment, possibly in a compensatory manner; however, this increase was significantly higher than control animals in the MCT + Ang-(1–7) group.
DISCUSSION
The most significant finding of this study is that overexpression of ACE2, the enzyme that generates Ang-(1–7), or Ang-(1–7) itself, by targeted gene transfer protects the lungs from lung fibrosis and pulmonary hypertension. To our knowledge, this is the first study to report beneficial effects of Ang-(1–7) against lung diseases using a novel lentiviral-mediated gene delivery approach. Ang-(1–7) is not a gene product but an enzymatic breakdown metabolite of Ang II. Here, we devised a synthetic gene for Ang-(1–7) and packaged it into a lentivirus.
In the current study, endotracheal instillation of bleomycin evoked a severe fibrotic response, characterized by accumulation of interstitial lung collagen. Collagen deposition was significantly decreased by overexpression of ACE2 or Ang-(1–7). It has been reported that an increase in lung AT1R levels may considerably contribute to the fibrotic process as collagen-synthesis activity of the fibroblasts is enhanced in response to AT1R stimulation (8). Lung AT1R was significantly increased after bleomycin administration, which was consistent with previous reports (29). This effect of bleomycin was attenuated by pulmonary overexpression of Ang-(1–7). Down-regulation of AT1R by Ang-(1–7) has been reported in other tissues too (30). However, no reduction in the lung AT1R protein level was observed in the ACE2 treatment group. We speculate that ACE2 overexpression may either inhibit components of the AT1R signaling pathway or stimulate the degradation of collagen, to bring about the beneficial antifibrotic effects. Lung protein analysis revealed decreased ACE levels in bleomycin-treated rats. Decreased pulmonary ACE activity associated with bleomycin injury has been reported in both animal and human studies (31–33), which confirm our current findings.
The mRNA levels of ACE2 were also significantly lower with bleomycin treatment, which is also in agreement with other published data (34). Accordingly, the observed decreases in ACE and ACE2, which are predominantly expressed on the endothelial cells, may be attributed to endothelial injury. In fact, the endothelium has been identified as the primary site of injury after bleomycin toxicity (35).
TGF-β is an important cytokine that plays a key role in fibrogenesis (36). Both in vitro and in vivo studies have demonstrated increased TGF-β levels with bleomycin treatment (37, 38). In line with these studies, we also observed increased lung mRNA levels of this profibrotic cytokine after bleomycin instillation, which was significantly reduced by overexpression of both ACE2 and Ang-(1–7). We and others have demonstrated that Ang-(1–7) and ACE2 can reduce TGF-β levels in fibroblast cell culture experiments (39, 40). Clinically, the presence of PH secondary to fibrotic lung diseases, called cor pulmonale, indicates poor prognosis with a compromised cardiac function. In our study, we did detect pulmonary hypertension and RVH after bleomycin administration. However, treatment with Ang-(1–7) prevented the development of both PH and RVH. Not only did we find protective effects with Ang-(1–7), but overexpression of ACE2 also conferred similar beneficial effects, possibly mediated via generation of Ang-(1–7). It is conceivable that the protective effects of ACE2 and Ang-(1–7) on the heart may be secondary to the reduction in the lung fibrosis. However, it is also possible that ACE2 and Ang-(1–7) may have direct effects on the heart. We have previously reported direct protective effects of ACE2 overexpression or Ang-(1–7) treatment against heart remodeling (26, 27). Also, we have demonstrated that lentiviral administration of ACE2 in MCT-treated mice prevented the development of pulmonary hypertension and cardiac remodeling (41). Likewise, similar findings were observed with a synthetic activator of the ACE2 enzyme in the MCT model of PH (42). These collective results and the results of the current PF study demonstrating a beneficial effect of ACE2 and Ang-(1–7) on pulmonary pressure encouraged us to perform a subsequent experiment to determine whether Ang-(1–7) overexpression would prevent the development of PH in the monocrotaline model.
Four weeks of MCT treatment resulted in significant increase in RVSP, accompanied by cardiac remodeling in terms of development of RVH and fibrosis. Overexpression of Ang-(1–7), before induction of PH resulted in prevention of the elevated pressure and attenuation of the remodeling effects on the heart. By preventing RVH and fibrosis, Ang-(1–7) offers a cardioprotective role against pathobiology of PH. Structural changes in the pulmonary vasculature, characterized by medial wall thickening of the pulmonary arterioles, is a prominent feature of human PH. Thickening of the pulmonary arterioles increase pulmonary arterial resistance contributing to the elevated pressure (43). MCT-challenged animals exhibited marked increase in the wall thickness of pulmonary resistant vessels and overexpression of Ang-(1–7) resulted in near-normal vessel morphology. It is likely that inhibition of vascular remodeling is a direct effect of Ang-(1–7), because previous findings have demonstrated antiproliferative properties of this peptide in vascular smooth muscle cells (30, 44). Ang-(1–7) is believed to mediate its biological effects by interacting with the G protein-coupled receptor Mas (45). Expression of Mas has been observed in the lung tissues (46), and this receptor can be blocked using a specific and potent antagonist, A-779. Coadministration of A-779 abolished the beneficial effects of Ang-(1–7), strongly suggesting a protective role for the Ang-(1–7)/Mas axis in PH.
Increase in the expression of proinflammatory cytokines is a characteristic hallmark of PH (47). In line with these findings, elevated lung mRNA levels of proinflammatory cytokines were observed in the MCT alone group. Overexpression of Ang-(1–7) appeared to prevent the increase in the mRNA levels of proinflammatory cytokines (IL-1β, IL-6, and TNF-α) and produced an increase in the antiinflammatory cytokine (IL-10). We have recently demonstrated that XNT, an ACE2 activator, also increased lung IL-10 expression in the MCT model of PH (42). This alteration of the inflammatory system by a peptide of the renin angiotensin system is of significance, as antiinflammatory therapy is currently being investigated for the treatment of PH. A recent report by Ito and colleagues (48) has shown that overexpression of IL-10 exerts beneficial effects against PH. One reason for the observed favorable changes in cytokine levels with Ang-(1–7) overexpression may be due to modulation of intrapulmonary RAS, which is known to be a potent regulator of cytokines and inflammation (49). However, a direct effect of Ang-(1–7) on the immunomodulatory system may not be ruled out. Ang-(1–7) treatment prevented the MCT-induced increase in ACE mRNA levels, and significantly up-regulated ACE2 levels. It was unexpected to observe that ACE2 expression was increased by the overexpression of Ang-(1–7), although a similar increase in ACE2 expression was also observed with XNT treatment (42). Also, cardiac-specific overexpression of Ang-(1–7) resulted in increased ACE2 levels in the heart (unpublished data). It is purely speculative that a positive feedback mechanism may be responsible for this observed phenomenon.
Nevertheless, this explanation clearly warrants further evaluation. Lung overexpression of Ang-(1–7) did not adversely affect the basal systemic blood pressure. Clinically, this lack of effect on systemic blood pressure becomes more relevant because systemic hypotension could worsen prognosis in these patients with compromised cardiopulmonary functions (50).
Studies of Kuba and colleagues (51) demonstrated the beneficial role of ACE2 against acute lung injury, importantly against Severe Acute Respiratory Syndrome–corona virus infections. Similarly, studies by Li and colleagues (34) showed that ACE2 is protective but is down-regulated in experimental and human lung fibrosis. Also, studies from our laboratory have demonstrated that overexpression of ACE2 (41) or its activation (42) prevented the development of PH and related cardiac pathophysiologies. The present study extends the findings of our earlier experiments and suggests that the beneficial effects of ACE2 overexpression/activation are most likely mediated via generation of the Ang-(1–7) peptide, which in turn stimulates the Mas receptor to bring about protection. Taken together, previous and the current studies indicate that targeting of the pulmonary ACE2/Ang-(1–7)/Mas axis could provide novel therapeutic strategy in the treatment of lung diseases, particularly involving pulmonary fibrosis and pulmonary hypertension. Because some of the findings suggest that ACE2 and Ang-(1–7) mediate their protective effects by different pathways, it appears that most effective therapeutic effect may be accomplished with a dual treatment regimen.
Perspectives
We have demonstrated that Angiotensin-(1–7) has a cardiopulmonary protective role against recognized animal models of lung fibrosis and pulmonary hypertension. Clinical trials of Ang-(1–7) are currently underway for treating cancer patients (52), so the use of Ang-(1–7) as a new therapy for pulmonary diseases such as lung fibrosis/hypertension might be plausible. The medical use of bleomycin as a tumor suppressant is limited due to induction of PF. Thus, it is possible to have a combination therapy with Ang-(1–7) to enhance the therapeutic efficacy of bleomycin by preventing the pulmonary toxicity.
Supplementary Material
Acknowledgments
The authors thank Bert Herrera for tissue culture assistance.
Funded by NIH grant HL56921 (M.J.K. and M.K.R.).
Originally Published in Press as DOI: 10.1164/rccm.200912-1840OC on June 25, 2010
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org.
Author Disclosure: V.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.J.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. Y.Q. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.A.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.Y.J. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. N.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.S.V. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. T.R. has received lecture fees from Novartis ($1,001–$5,000); he has received industry-sponsored grants from Pfizer ($50,001–$100,000); he has received sponsored grants from the Canadian Institute of Health Research (more than $100,000). R.A.S. has received industry-sponsored grants from Daiichi-Sankyo ($10,001–$50,000). J.M.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.K.R. has received sponsored grants from NIH (more than $100,000). M.J.K. has received sponsored grants from the American Heart Association and NIH (more than $100,000).
References
- 1.Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med 2001;345:517–525. [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment: international consensus statement. Am J Respir Crit Care Med 2000;161:646–664. [DOI] [PubMed] [Google Scholar]
- 3.Antoniu SA. Targeting the angiotensin pathway in idiopathic pulmonary fibrosis. Expert Opin Ther Targets 2000;12:1587–1590. [DOI] [PubMed] [Google Scholar]
- 4.Aras O, Dilsizian V. Targeting tissue angiotensin-converting enzyme for imaging cardiopulmonary fibrosis. Curr Cardiol Rep 2008;10:128–134. [DOI] [PubMed] [Google Scholar]
- 5.Molina-Molina M, Xaubet A, Li X, Abdul-Hafez A, Friderici K, Jernigan K, Fu W, Ding Q, Pereda J, Serrano-Mollar A, et al. Angiotensinogen gene G-6A polymorphism influences idiopathic pulmonary fibrosis disease progression. Eur Respir J 2008;32:1004–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selman M, Pardo A, Barrera L, Estrada A, Watson SR, Wilson K, Aziz N, Kaminski N, Zlotnik A. Gene expression profiles distinguish idiopathic pulmonary fibrosis from hypersensitivity pneumonitis. Am J Respir Crit Care Med 2006;173:188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marshall RP, McAnulty RJ, Laurent GJ. Angiotensin II is mitogenic for human lung fibroblasts via activation of the type 1 receptor. Am J Respir Crit Care Med 2000;161:1999–2004. [DOI] [PubMed] [Google Scholar]
- 8.Marshall RP, Gohlke P, Chambers RC, Howell DC, Bottoms SE, Unger T, McAnulty RJ, Laurent GJ. Angiotensin II and the fibroproliferative response to acute lung injury. Am J Physiol Lung Cell Mol Physiol 2004;286:L156–L164. [DOI] [PubMed] [Google Scholar]
- 9.Uhal BD, Kim JK, Li X, Molina-Molina M. Angiotensin-TGF-beta 1 crosstalk in human idiopathic pulmonary fibrosis: autocrine mechanisms in myofibroblasts and macrophages. Curr Pharm Des 2007;13:1247–1256. [DOI] [PubMed] [Google Scholar]
- 10.Guo W, Shan B, Klingsberg RC, Qin X, Lasky JA. Abrogation of TGF-beta1-induced fibroblast-myofibroblast differentiation by histone deacetylase inhibition. Am J Physiol Lung Cell Mol Physiol 2009;297:L864–L870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mastruzzo C, Crimi N, Vancheri C. Role of oxidative stress in pulmonary fibrosis. Monaldi Arch Chest Dis 2002;57:173–176. [PubMed] [Google Scholar]
- 12.Okada M, Suzuki K, Matsumoto M, Takada K, Nakanishi T, Horikoshi H, Higuchi T, Hosono Y, Nakayama M, Ohsuzu F. Effects of angiotensin on the expression of fibrosis-associated cytokines, growth factors, and matrix proteins in human lung fibroblasts. J Clin Pharm Ther 2009;34:288–299. [DOI] [PubMed] [Google Scholar]
- 13.Wang R, Zagariya A, Ibarra-Sunga O, Gidea C, Ang E, Deshmukh S, Chaudhary G, Baraboutis J, Filippatos G, Uhal BD. Angiotensin II induces apoptosis in human and rat alveolar epithelial cells. Am J Physiol 1999;276:L885–L889. [DOI] [PubMed] [Google Scholar]
- 14.Liu RM. Oxidative stress, plasminogen activator inhibitor 1, and lung fibrosis. Antioxid Redox Signal 2008;10:303–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cargill RI, Lipworth BJ. The role of the renin-angiotensin and natriuretic peptide systems in the pulmonary vasculature. Br J Clin Pharmacol 1995;40:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solari V, Puri P. Genetic polymorphisms of angiotensin system genes in congenital diaphragmatic hernia associated with persistent pulmonary hypertension. J Pediatr Surg 2004;39:302–306. [DOI] [PubMed] [Google Scholar]
- 17.Chung WK, Deng L, Carroll JS, Mallory N, Diamond B, Rosenzweig EB, Barst RJ, Morse JH. Polymorphism in the angiotensin II type 1 receptor (AGTR1) is associated with age at diagnosis in pulmonary arterial hypertension. J Heart Lung Transplant 2009;28:373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orte C, Polak JM, Haworth SG, Yacoub MH, Morrell NW. Expression of pulmonary vascular angiotensin-converting enzyme in primary and secondary plexiform pulmonary hypertension. J Pathol 2000;192:379–384. [DOI] [PubMed] [Google Scholar]
- 19.Cassis LA, Rippetoe PE, Soltis EE, Painter DJ, Fitz R, Gillespie MN. Angiotensin II and monocrotaline-induced pulmonary hypertension: effect of losartan (DuP 753), a nonpeptide angiotensin type 1 receptor antagonist. J Pharmacol Exp Ther 1992;262:1168–1172. [PubMed] [Google Scholar]
- 20.Kreutz R, Fernandez-Alfonso MS, Ganten D, Paul M. Effect of losartan on right ventricular hypertrophy and cardiac angiotensin I-converting enzyme activity in pulmonary hypertensive rats. Clin Exp Hypertens 1996;18:101–111. [DOI] [PubMed] [Google Scholar]
- 21.Katovich MJ, Grobe JL, Raizada MK. Angiotensin-(1–7) as an antihypertensive, antifibrotic target. Curr Hypertens Rep 2008;10:227–232. [DOI] [PubMed] [Google Scholar]
- 22.Iusuf D, Henning RH, van Gilst WH, Roks AJ. Angiotensin-(1–7): pharmacological properties and pharmacotherapeutic erspectives. Eur J Pharmacol 2008;585:303–312. [DOI] [PubMed] [Google Scholar]
- 23.Yamada K, Iyer SN, Chappell MC, Ganten D, Ferrario CM. Converting enzyme determines plasma clearance of angiotensin-(1–7). Hypertension 1998;32:496–502. [DOI] [PubMed] [Google Scholar]
- 24.Shenoy V, Ferreira AJ, Qi Y, Fraga-Silva RA, Dooies A, Francis J, Reudelhuber T, Raizada MK, Katovich MJ. Prevention of monocrotaline-induced pulmonary hypertension by lenti-viral mediated gene delivery of Angiotensin-(1–7). Circ Res 2008;103:1493–1501. [Google Scholar]
- 25.Grobe JL, Mecca AP, Lingis M, Shenoy V, Bolton TA, Machado JM, Speth RC, Raizada MK, Katovich MJ. Prevention of angiotensin II-induced cardiac remodeling by angiotensin-(1–7). Am J Physiol Heart Circ Physiol 2007;292:H736–H742. [DOI] [PubMed] [Google Scholar]
- 26.Edwards CA, O'Brien WD Jr. Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Clin Chim Acta 1980;104:161–167. [DOI] [PubMed] [Google Scholar]
- 27.Yamazato Y, Ferreira AJ, Hong KH, Sriramula S, Francis J, Yamazato M, Yuan L, Bradford CN, Shenoy V, Oh SP, et al. Prevention of pulmonary hypertension by angiotensin-converting enzyme 2 gene transfer. Hypertension 2009;54:365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol 1988;41:467–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Otsuka M, Takahashi H, Shiratori M, Chiba H, Abe S. Reduction of bleomycin induced lung fibrosis by candesartan cilexetil, an angiotensin II type 1 receptor antagonist. Thorax 2004;59:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clark MA, Diz DI, Tallant EA. Angiotensin-(1–7) downregulates the angiotensin II type 1 receptor in vascular smooth muscle cells. Hypertension 2001;37:1141–1146. [DOI] [PubMed] [Google Scholar]
- 31.Chen FP, Gong LK, Zhang L, Wang H, Qi XM, Wu XF, Xiao Y, Cai Y, Liu LL, Li XH, et al. Early lung injury contributes to lung fibrosis via AT1 receptor in rats. Acta Pharmacol Sin 2007;28:227–237. [DOI] [PubMed] [Google Scholar]
- 32.Newman RA, Kimberly PJ, Stewart JA, Kelley J. Assessment of bleomycin lung toxicity using angiotensin-converting enzyme in pulmonary lavage. Cancer Res 1980;40:3621–3626. [PubMed] [Google Scholar]
- 33.Orfanos SE, Psevdi E, Stratigis N, Langleben D, Catravas JD, Kyriakidis M, Moutsopoulos HM, Roussos C, Vlachoyiannopoulos PG. Pulmonary capillary endothelial dysfunction in early systemic sclerosis. Arthritis Rheum 2001;44:902–911. [DOI] [PubMed] [Google Scholar]
- 34.Li X, Molina-Molina M, Abdul-Hafez A, Uhal V, Xaubet A, Uhal BD. Angiotensin converting enzyme-2 is protective but downregulated in human and experimental lung fibrosis. Am J Physiol Lung Cell Mol Physiol 2008;295:L178–L185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lazo JS. Endothelial injury caused by antineoplastic agents. Biochem Pharmacol 1986;35:1919–1923. [DOI] [PubMed] [Google Scholar]
- 36.Goodwin A, Jenkins G. Role of integrin-mediated TGFbeta activation in the pathogenesis of pulmonary fibrosis. Biochem Soc Trans 2009;37:849–854. [DOI] [PubMed] [Google Scholar]
- 37.Breen E, Shull S, Burne S, Absher M, Kelley J, Phan S, Cutroneo KR. Bleomycin regulation of transforming growth factor-beta mRNA in rat lung fibroblasts. Am J Respir Cell Mol Biol 1992;6:146–152. [DOI] [PubMed] [Google Scholar]
- 38.Azuma A, Li YJ, Abe S, Usuki J, Matsuda K, Henmi S, Miyauchi Y, Ueda K, Izawa A, Sone S, et al. Interferon-{beta} inhibits bleomycin-induced lung fibrosis by decreasing transforming growth factor-{beta} and thrombospondin. Am J Respir Cell Mol Biol 2005;32:93–98. [DOI] [PubMed] [Google Scholar]
- 39.Grobe JL, Der Sarkissian S, Stewart JM, Meszaros JG, Raizada MK, Katovich MJ. ACE2 overexpression inhibits hypoxia-induced collagen production by cardiac fibroblasts. Clin Sci 2007;113:357–364. [DOI] [PubMed] [Google Scholar]
- 40.Iwata M, Cowling RT, Gurantz D, Moore C, Zhang S, Yuan JX, Greenberg BH. Angiotensin-(1–7) binds to specific receptors on cardiac fibroblasts to initiate antifibrotic and antitrophic effects. Am J Physiol Heart Circ Physiol 2005;289:H2356–H2363. [DOI] [PubMed] [Google Scholar]
- 41.Díez-Freire C, Vázquez J, Correa de Adjounian MF, Ferrari MF, Yuan L, Silver X, Torres R, Raizada MK. ACE2 gene transfer attenuates hypertension-linked pathophysiological changes in the SHR. Physiol Genomics 2006;27:12–19. [DOI] [PubMed] [Google Scholar]
- 42.Ferreira AJ, Shenoy V, Yamazato Y, Sriramula S, Francis J, Yuan L, Castellano RK, Ostrov DA, Oh SP, Katovich MJ, et al. Evidence for angiotensin-converting enzyme 2 as a therapeutic target for the prevention of pulmonary hypertension. Am J Respir Crit Care Med 2009;179:1048–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR, McMurtry IF, Stenmark KR, Thistlethwaite PA, Weissmann N, et al. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol 2009;30:S20–S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freemann EJ, Chisolm GM, Ferrario CM, Tallant EA. Angiotensin-(1–7) inhibits vascular smooth muscle cell growth. Hypertension 1996;28:104–108. [DOI] [PubMed] [Google Scholar]
- 45.Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M, et al. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. T. Proc Natl Acad Sci USA 2003;100:8258–8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gallagher PE, Tallant EA. Inhibition of human lung cancer cell growth by angiotensin-(1–7). Carcinogenesis 2004;25:2045–2052. [DOI] [PubMed] [Google Scholar]
- 47.Dorfmüller P, Perros F, Balabanian K, Humbert M. Inflammation in pulmonary arterial hypertension. Eur Respir J 2003;22:358–363. [DOI] [PubMed] [Google Scholar]
- 48.Ito T, Okada T, Miyashita H, Nomoto T, Nonaka-Sarukawa M, Uchibori R, Maeda Y, Urabe M, Mizukami H, Kume A, et al. Interleukin-10 expression mediated by an adeno-associated virus vector prevents monocrotaline-induced pulmonary arterial hypertension in rats. Circ Res 2007;101:734–741. [DOI] [PubMed] [Google Scholar]
- 49.Marchesi C, Paradis P, Schiffrin EL. Role of the renin-angiotensin system in vascular inflammation. Trends Pharmacol Sci 2008;29:367–374. [DOI] [PubMed] [Google Scholar]
- 50.Rubin LJ, Badesch DB. Evaluation and management of the patient with pulmonary arterial hypertension. Ann Intern Med 2005;143:282–292. [DOI] [PubMed] [Google Scholar]
- 51.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 2005;11:875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petty WJ, Miller AA, McCoy TP, Gallagher PE, Tallant EA, Torti FM. Phase I and pharmacokinetic study of angiotensin-(1–7), an endogenous antiangiogenic hormone. Clin Cancer Res 2009;15:7398–7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.