Abstract
Rationale: Immunogenicity of new tuberculosis (TB) vaccines is commonly assessed by measuring the frequency and cytokine expression profile of T cells.
Objectives: We tested whether this outcome correlates with protection against childhood TB disease after newborn vaccination with bacillus Calmette-Guérin (BCG).
Methods: Whole blood from 10-week-old infants, routinely vaccinated with BCG at birth, was incubated with BCG for 12 hours, followed by cryopreservation for intracellular cytokine analysis. Infants were followed for 2 years to identify those who developed culture-positive TB—these infants were regarded as not protected against TB. Infants who did not develop TB disease despite exposure to TB in the household, and another group of randomly selected infants who were never evaluated for TB, were also identified—these groups were regarded as protected against TB. Cells from these groups were thawed, and CD4, CD8, and γδ T cell–specific expression of IFN-γ, TNF-α, IL-2, and IL-17 measured by flow cytometry.
Measurements and Main Results: A total of 5,662 infants were enrolled; 29 unprotected and two groups of 55 protected infants were identified. There was no difference in frequencies of BCG-specific CD4, CD8, and γδ T cells between the three groups of infants. Although BCG induced complex patterns of intracellular cytokine expression, there were no differences between protected and unprotected infants.
Conclusions: The frequency and cytokine profile of mycobacteria-specific T cells did not correlate with protection against TB. Critical components of immunity against Mycobacterium tuberculosis, such as CD4 T cell IFN-γ production, may not necessarily translate into immune correlates of protection against TB disease.
Keywords: mycobacteria immunity, pediatric settings
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Correlates of protection against tuberculosis (TB) remain unknown; hence, knowledge on the subject is incomplete.
What This Study Adds to the Field
The study emphasizes the need to explore beyond the classical immune markers thought to be important for protection against TB in humans.
Tuberculosis (TB) kills 1.7 million people worldwide each year (1). The current TB vaccine, Mycobacterium bovis bacillus Calmette-Guérin (BCG), affords approximately 80% protection against severe forms of childhood TB (2, 3). However, BCG's protection against pulmonary TB, particularly in adults, is highly variable and mostly poor (4). Adults with lung TB spread the disease; new, better TB vaccines that target pulmonary disease are therefore needed urgently.
Our knowledge of immune correlates of protection against TB remains incomplete. Consequently, assessment of immunogenicity of TB vaccines may, at best, be a measure of vaccine take. Current evaluation of vaccine-induced immunity focuses on immunity essential for protection against TB. For example, experimental and clinical evidence support a critical role for CD4 T cells (5, 6), particularly IFN-γ production by these cells (7, 8), in protection against TB. This outcome is, therefore, the most commonly measured when determining vaccine take. Because important roles for other type-1 cytokines, such as tumor necrosis factor (TNF)–α and IL-2 (9–11), and for CD8 T cells (12–14), in protection against TB have also been described, all these markers are commonly measured together, by multiparameter flow cytometry after short-term stimulation of whole blood or peripheral blood mononuclear cells (PBMCs) (15–21). Experimental animal studies assessing the efficacy of novel TB vaccines have reported an association between mycobacteria-specific polyfunctional T cells that coexpress IFN-γ, TNF-α, and IL-2 at the site of the infection and protection against TB (22, 23). These findings have stimulated much interest in evaluating this subset of T cells in clinical trials.
Our aim was to assess whether these markers correlate with protection against childhood TB after newborn vaccination with BCG. We complemented this assessment by also determining whether expression of IL-17 correlates with protection. Memory T helper (Th) 17 cells are present in peripheral blood of persons exposed to mycobacteria (24); experimental evidence supports a role for these cells in the induction of chemokine release in the lung, resulting in Th1 cell recruitment (25). Furthermore, the magnitude of IL-17 response has been shown to correlate with the clinical outcome of Mycobacterium tuberculosis (M.tb) infection (26). We also wished to determine whether γδ T cell activation correlates with protection. Our interest to evaluate γδ T cells was based on potent mycobacteria-specific activation of γδ T cells in 7-month-old infants who had received BCG vaccine at birth (27), and on experimental evidence that suggests an important role in protection against TB, possibly by activating antigen-presenting cells to prime T cell responses (28).
Some of the results of these studies have been previously reported in the form of a poster presentation (29).
METHODS
Participant Enrollment
We enrolled participants at the South African Tuberculosis Vaccine Initiative field site in the Worcester area, near Cape Town, South Africa. This area has one of the highest TB incidence rates in the world, documented to be in excess of 2,000/100,000/year in children under 2 years of age (30). The study was nested within a randomized controlled trial (30), which aimed to determine whether intradermal or percutaneous delivery of Japanese BCG at birth resulted in equivalent protection against TB. The following were exclusion criteria at 10 weeks of age: mother known to be infected with human immunodeficiency virus; BCG not received by infant within 24 hours of birth; significant perinatal complications in the infant; any acute or chronic disease in the infant at the time of enrollment; clinically apparent anemia in the infant; and household contact with any person with TB disease, or any person who was coughing. The study was conducted according to the U.S. Department of Health and Human Services and Good Clinical Practice guidelines, and included protocol approval by the University of Cape Town Research Ethics Committee and written informed consent from the parent or legal guardian.
Blood Collection, Stimulation, and Cryopreservation
At 10 weeks of age, heparinized blood was collected from all infants, and 1 ml was immediately incubated with BCG (SSI strain, 1.2 × 106 organisms/ml), as previously described (31). Medium alone served as negative control, whereas staphylococcal enterotoxin B (10 μg/ml; Sigma-Aldrich, St. Louis, MO) was used as positive control. The costimulatory antibodies, anti-CD28 and anti-CD49d (1 μg/ml each; BD Biosciences, San Jose, CA), were added to all conditions, as this results in enhancement of specific responses (31). Blood was incubated for 7 hours at 37°C. Brefeldin-A was then added, followed by incubation for an additional 5 hours. Cells were then harvested, fixed, and cryopreserved as previously described (31).
Participant Follow Up and Evaluation
Participants were followed for 2 years. Community-wide passive surveillance systems identified patients with TB disease and children with symptoms suggestive of TB disease, or from households where an adult had TB disease (30). Children who fell into the latter two categories were followed actively; among these, participants who never developed TB over the 2-year follow-up period were classified as household controls, whereas participants who developed TB disease over this period were classified as TB cases. Community controls were randomly selected from the passive surveillance arm, and were never evaluated for TB. The parent study (30) reports the detailed criteria employed to detect all cases of TB disease among participants up to the age of 2 years. All infants who had symptoms compatible with TB disease, or who had contact with an adult with TB disease, were admitted to a dedicated research ward for clinical examination, chest radiography, tuberculin skin testing, two early-morning gastric aspirations, and two sputum inductions for M.tb smear and culture (30). All infants admitted to the research ward were also tested for human immunodeficiency virus infection: a positive antibody test resulted in exclusion.
Intracellular Cytokine Staining and Multiparameter Flow Cytometry
Cryopreserved cells from the protected and unprotected groups only (see subsequent data analysis here) were thawed, washed, and permeabilized with Perm/wash solution (BD Biosciences). Cells were then incubated at 4°C for 1 hour with fluorescence-conjugated antibodies directed against surface antigens and intracellular cytokines. The following antibodies were used: anti-CD3 Pac Blue (clone UCHT1); anti-CD8 Cy5.5PerCP (SK-1); anti-γδ T cell receptor allophycocyanin (B1); anti–TNF-α Cy7PE (Mab11); anti–IFN-γ Alexa 700 (B27); anti–IL-2 fluorescein isothiocyanate (5,344.111) (all from BD Biosciences, San Jose, CA); anti-CD4 QDot605 (S3.5; Invitrogen, Eugene, OR); and anti–IL-17 phycoerythrin (eBio64CAP17; eBioscience, San Diego, CA). Cells were acquired on a LSR II flow cytometer (BD Biosciences) configured with 3 lasers and 10 detectors, with FACS Diva 6.1 software (San Jose, CA). Optimal photomultiplier tube settings were established for this study before sample analysis. Cytometer setting and tracking beads (BD Biosciences) were used to record the target median fluorescence intensity (MFI) values for the baseline settings, and these calibrations were performed each day before sample acquisition. Compensation settings were set with anti-mouse kappa-beads (BD Biosciences) labeled with the respective fluorochrome-conjugated antibodies. Flowjo version 8.8.4 (Treestar, Ashland, OR) was used to compensate and to analyze the flow cytometric data. Boolean gating was applied to generate combinations of cytokine-expressing CD4 and CD8 T cell subsets.
Data Analysis
Flow cytometric analysis was compared between three groups of infants: (1) those who ultimately developed culture-confirmed TB disease—regarded as not protected against TB and termed “TB cases”; (2) those who were evaluated for TB because of household exposure to an adult with TB disease, but found not to have TB—regarded as protected against TB and termed “household controls”; and (3) a randomly selected group, subjects who had no known exposure to an adult with TB disease, or symptoms compatible with TB disease, and were therefore never evaluated for TB—this was the second group regarded as protected against TB and termed “community controls.” A total of 20 TB cases were excluded from the analysis due to insufficient sample volumes. The microbiological, clinical, and radiological features of the excluded TB cases were comparable to those of the included TB cases (data not shown).
For flow cytometric analysis of cytokine-expressing cells, frequencies of cytokine expression from the negative control (i.e., blood incubated with costimulatory antibodies alone) were subtracted from the BCG-specific responses. Participants were excluded from the analysis for any of the following reasons: (1) a positive control (staphylococcal enterotoxin B) response less than the median plus 3 median average deviations of the negative control; (2) frequencies in the negative control and in the BCG sample in a similar range, suggesting possible contamination of the negative control; and (3) number of CD3 T cell events counted less than 75,000.
For the analysis of MFI of the BCG-stimulated samples, further exclusions occurred if results from participants did not meet any of the following conditions: (1) ratio of BCG to unstimulated frequencies greater than 2; (2) frequencies of BCG-specific cells greater than 0.01%; and (3) number of positive events in the BCG-stimulated sample greater than 20. The Kruskal-Wallis test was used to assess differences between the three groups, and when differences had a P value less than 0.05, a Mann-Whitney U test was used to assess differences between individual groups. A P value less than 0.05 was considered significant.
RESULTS
Study Participants
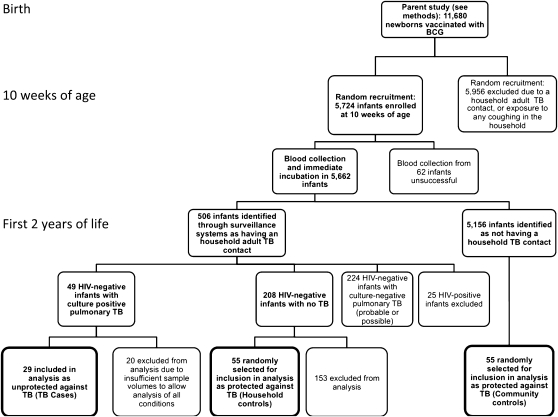
A total of 5,724 infants routinely vaccinated with BCG at birth were randomly enrolled from the parent cohort of 11,680 infants (30). Identification of the three infant groups, with clinical exclusions, is shown in Figure 1.
Figure 1.
Recruitment and enrollment of participants into the study.
Frequency and Cytokine Profile of BCG-Specific CD4 T Cells
We used an intracellular cytokine assay to evaluate the frequency and cytokine profiles of specific T cells: the flow cytometric gating strategy is shown in Figure 2. A median of 409,077 (154,687–802,636) CD3 T cells were evaluated. Results from seven participants were excluded from analysis, because the inclusion criteria for analysis were not met (see Table E1A in the online supplement). There were no differences in frequencies of CD4 T cells expressing any cytokine, or IFN-γ, TNF-α, IL-2, or IL-17 individually, between the three groups of infants (Figure 3A). When expression patterns of cytokines on an individual cell level were evaluated, no differences in polyfunctional CD4 T cells coexpressing IFN-γ, TNF-α, and IL-2 together, or CD4 T cells expressing any other combination of the four cytokines, could be shown (Figure 3B). We concluded that, with this assay system at 10 weeks of age, frequency of cytokine profiles of BCG-specific CD4 T cell responses did not correlate with protection against TB.
Figure 2.
Gating strategy Flow cytometric analysis of bacillus Calmette-Guérin (BCG)–induced T cell cytokine production. Whole blood was incubated with BCG for 12 hours. Representative dot plots from a single participant are shown. (A) Gating strategy used to identify CD4, CD8, and γδ T cells. From left to right, leukocytes from whole blood were acquired and cell doublets excluded with forward scatter area versus forward scatter height parameters. T cells were identified by assessing CD3 expression against IFN-γ expression, which enables inclusion of any T cells that may have down-regulated CD3 expression upon activation. Subsequently, CD3+ T cells were differentiated into conventional T cells, which did not express γδ T cell receptor, and γδ T cells, by assessing γδ expression against CD8 expression. Finally, the conventional T cell population was divided into CD4+ and CD8+ T cells. (B) Representative dot plots of cytokine coexpression patterns in CD4 T cells from unstimulated, BCG, and staphylococcal enterotoxin B (SEB)–stimulated conditions. APC = allophycocyanin; Cy5.5PerCP-A = cyanine peridinin chlorophyll protein; FSC = forward scatter.
Figure 3.
Frequency and cytokine expression profile of bacillus Calmette-Guérin (BCG)–specific CD4 T cells. (A) Frequencies of total, IL-2, IFN-γ, tumor necrosis factor (TNF)–α, and IL-17 cytokine-expressing CD4 T cells, as detected by an intracellular cytokine assay after incubation of whole blood with BCG. Total cytokine frequencies incorporate all cytokine-positive CD4 T cells. One participant had a very high total and IFN-γ CD4 T cell response (4.010 and 3.797%, respectively), and these values are shown individually on the graph. (B) Frequencies of distinct subsets of specific CD4 T cells, based on combinations of cytokine expression. After selecting CD4 T cells, boolean gating was used to generate 15 distinct cytokine-expressing subsets. The Kruskal-Wallis test was used to assess differences between the groups of infants. None of the parameters assessed was different between groups (all: P > 0.05). TB = tuberculosis.
Frequency and Cytokine Profile of BCG-Specific CD8 T Cells
The frequency of specific CD8 T cells and pattern of activation of these cells did not differ between cases and controls (Figures 4A and 4B). In contrast to CD4 T cell responses, where a complex pattern of BCG-specific subsets coexpressing cytokines was shown, most CD8 T cells expressed IFN-γ, whereas expression of TNF-α or IL-2 or -17 was very low or undetectable (Figure 4A).
Figure 4.
Frequency and cytokine expression profile of bacillus Calmette-Guérin (BCG)–specific CD8 T cells. (A) Frequencies of total, IL-2, IFN-γ, tumor necrosis factor (TNF)–α, and IL-17 cytokine-expressing CD8 T cells, as detected by an intracellular cytokine assay after incubation of whole blood with BCG. Total cytokine frequencies incorporate all cytokine-positive CD8 T cells One participant had a very high total and IFN-γ CD4 T cell response (6.198 and 6.157%, respectively), and these values are shown individually on the graph. (B) Frequencies of distinct subsets of specific CD8 T cells, based on combinations of cytokine expression. After selecting CD8 T cells, boolean gating was used to generate 15 distinct cytokine-expressing subsets. One participant had a very high IL-2 CD4 T cell response (0.199%), and this value is shown individually on the graph. The Kruskal-Wallis test was used to assess differences between the groups of infants. None of the parameters assessed was different between groups (all: P > 0.05).
BCG-Induced γδ T Cell Responses
The γδ T cells induced by BCG stimulation almost exclusively produced IFN-γ; no differences were seen between the three groups (Figure 5). A very small number of γδ T cells expressed TNF-α; again, there were no differences between the groups (Figure 5).
Figure 5.
Bacillus Calmette-Guérin (BCG)–induced γδ T cell responses. Frequencies of γδ T cells expressing cytokines after incubation of whole blood with BCG. The Kruskal-Wallis test was used to assess differences between the groups of infants. None of the parameters assessed was different between groups (all: P > 0.05). TNF = tumor necrosis factor.
Median Fluorescent Intensity of Cytokine Expression in BCG-Specific T Cells
MFI of cytokine expression has been suggested as a useful readout of quality of the T cell response, because the intensity of cytokine expression appears to be highest in polyfunctional T cells (32). We therefore compared the MFI of cytokine expression of BCG-induced CD4, CD8, and γδ T cells between the three groups. Participants were excluded from analysis if the previously described inclusion criteria were not fulfilled for the flow cytometric data (Tables E1B–E1G). We found no differences in any of the MFI values evaluated between the TB cases and the control groups (CD4 T cell MFI is shown in Figure E1; other data not shown).
DISCUSSION
We report that, after newborn BCG vaccination, the magnitude and profile of cytokine expression of BCG-specific CD4 and CD8 T cells did not correlate with protection against childhood TB. Importantly, there were no differences in polyfunctional BCG-specific CD4 T cells, which coexpress IFN-γ, TNF-α, and IL-2. We also confirm production of IFN-γ by γδ T cells when whole blood is stimulated with BCG; however, expression of this cytokine by this subset was not associated with protection against childhood TB.
Multiple experimental studies have shown that the Th1 cytokines, IFN-γ and TNF-α, are required for immunity against M.tb infection and disease (10, 11, 33). This is supported by findings from experimental TB vaccine studies that evaluated biomarkers of protection (34–36). For example, in a heterologous prime-boost strategy with BCG followed by adenovirus-expressing Ad85A, Forbes and colleagues (23) reported a correlation between magnitude of polyfunctional Ad85A-specific Th1 responses in the lungs after M.tb challenge and protection against disease. Transfer of early secretory antigenic target (ESAT)-6–specific Th1 memory cells to recipient mice before M.tb challenge enhanced protection, suggesting the importance of the quantity of antigen-specific T cells at the disease site (37). In contrast, multiple other experimental studies have shown that IFN-γ production at the disease site does not correlate with protection against TB; rather, expression of the cytokine may be a marker of the magnitude of the inflammatory response (38–40). For example, Mittrucker and colleagues (38) reported no correlation of BCG-induced T cell responses and protection in a mouse TB challenge model. Furthermore, in a clinical study, Sutherland and colleagues (41) reported that patients with TB disease had a higher polyfunctional CD4+ T cell response after overnight stimulation of whole blood with ESAT-6 and purified protein derivative compared with healthy individuals with a positive tuberculin skin test.
In our study, we did not measure IFN-γ at a disease site—this is not possible in healthy 10-week-old infants—but in peripheral blood. We found no association between the frequency of BCG-specific Th1 cells and protection against TB. This finding is of particular importance, because this peripheral blood outcome is used to assess vaccine-induced immunity in most clinical trials of new TB vaccines. The latter studies often focus on the quality of the CD4 T cell response, with the hypothesis that polyfunctionality (i.e., combined expression of IFN-γ, TNF-α, and IL-2 by individual cells) is a marker of protective immunity. The interest to evaluate mycobacteria-specific polyfunctional T cells is based on observations from experimental mouse models of protection against other intracellular organisms, such as Leishmania major (32), and, to a limited extent, from animal studies of novel TB vaccination (23). We showed no correlation between polyfunctional BCG-specific CD4 T cell responses in peripheral blood and protection against TB. In an experimental mouse model with L. major infection, Darrah and colleagues (32) reported that MFI of cytokine expression could be used as an additional measure of quality of the T cell response, as polyfunctional cells had the highest MFI of cytokine expression. In this study, we assessed the MFI of BCG-specific T cell cytokine expression, and showed no association with protection against TB.
We evaluated IL-17 expression in CD4 T cells based on evidence that this cytokine plays a protective role against TB (25, 26). This is the first study to demonstrate induction of BCG-specific IL-17 cells in infants after BCG vaccination at birth; however, frequencies of BCG-specific Th17 cells did not correlate with protection against TB.
We investigated CD8 T cell responses as possible correlates of protection, based on the important role of this subset suggested by recent experimental and clinical studies (13, 14). For example, Chen and colleagues (13) reported that depletion of CD8 T cells in BCG-vaccinated rhesus macaques led to a decrease in induced immunity upon subsequent challenge of the animals with M.tb. Furthermore, Bruns and colleagues (14) showed that patients undergoing anti-TNF therapy had decreased antimicrobial activity against M.tb due to diminished numbers of antigen-specific effector memory CD8 T cells, with an associated increased incidence of TB disease. We observed no differences when comparing specific CD8 T cell responses between protected and unprotected infants.
We assessed γδ T cell responses based on a report that only the γδ T cells expanded from PBMCs of purified protein derivative–positive donors incubated with BCG, and not γδ T cells expanded by phosphoantigen, were able to inhibit growth of M.tb in autologous macrophages (42). However, we found no association between the frequency of BCG-induced γδ T cells and protection against TB in our study.
Our results strongly suggest that aspects of BCG-specific CD4 and CD8 T cell immunity, or γδ T cell immunity, measured in this whole blood assay at 10 weeks of age, may not correlate with protection against TB. We cannot exclude the possibility that these outcomes, measured at another time point after newborn BCG vaccination, or with different antigens or assay systems, might correlate with protection. An infant biomarker study of this size (n = 5,662) has not been reported to date; the magnitude of the project required that we limit our blood collection to one practical time point, before M.tb infection, and at an age before significant exposure to environmental mycobacteria. This was also the reason for selecting a single viable bacterial antigen that can be processed for recognition by a wide range of lymphocytes. The results generated by using BCG as an antigen are likely to be specific, as we have recently demonstrated that responses with BCG in a whole blood assay are detectable at 10 weeks of age only in infants who have been vaccinated at birth, and not in unvaccinated infants (43). Regardless, individual mycobacterial antigens might yield different results. Furthermore, the T cell response to mycobacteria is complex (44–46), and involves cytotoxic activity (46–48), for example, in addition to cytokine production. These additional aspects of T cell immunity might correlate with outcome, whereas routine vaccine take measurements focus on cytokine production, using a short-term whole blood assay. Similarly, innate host responses may also be important. We propose that biomarkers of protection against TB may only be unraveled when multiple host factors are examined together in a system biology approach. Ongoing, complementary studies will address whether biomarkers of protection against TB may be identified through other approaches. These include measuring soluble levels of cytokines, chemokines, and growth factors after 7-hour incubation of whole blood with BCG, the cytotoxic, proliferative, and cytokine-expressing capacity of T cells after incubation of PBMCs with BCG for 3–6 days, and gene expression profiles in PBMCs incubated with BCG for 12 hours.
Overall, our results strongly suggest caution when interpreting T cell immune markers commonly evaluated in new TB vaccine clinical trials. More importantly, our results indicate that protective immunity against M.tb may be very complex, and suggest a need to look beyond the classical Th1 immunity when assessing the efficacy of novel TB vaccines in clinical trials.
Supplementary Material
Originally Published in Press as DOI: 10.1164/rccm.201003-0334OC on June 17, 2010
Supported by National Institutes of Health grant RO1-AI065653, European and Developing Countries Clinical Trial Partnership, Aeras Global Tuberculosis Vaccine Foundation, and the Bill and Melinda Gates Foundation through Grand Challenges in Global Health grant 37772 (“Biomarkers of Protective Immunity against TB in the context of HIV/AIDS in Africa”).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author Disclosure: B.M.N.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; B.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; T.J.S. received more than $100,001 from the Wellcome Trust Fellowship in sponsored grants as a Research Training Fellowship; E.J.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; A.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; A.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; H.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; M.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; M.H. received $1,001–$5,000 from GlaxoSmithKline in consultancy fees for an expert meeting in March 2010, and up to $1,000 from the National Institutes of Health (NIH) in consultancy fees as a reviewer; S.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; H.M. received more than $100,001 from the Aeras Global Tuberculosis Foundation in sponsored grants; A.H. is an employee of the Aeras Global Tuberculosis (TB) Vaccine Foundation; G.H. received $5,001–$10,000 from GlaxoSmithKline in advisory board fees (Independent Data Monitoring Committee), $50,001–$100,001 from Sanofi, and $50,001–$100,000 from GlaxoSmithKline in industry-sponsored grants for a vaccinology course, more than $100,001 from Aeras Global TB Vaccine in sponsored grants for TB vaccine research, more than $100,001 from NIH Fogarty in sponsored grants as a training grant, and more than $100,001 from European and Developing Countries Clinical Trials Partnership in sponsored grants for vaccine research; G.K. received $10,001–$50,000 from the Celgene Corporation as a board member, and holds more than $100,001 in options accumulated over a number of years from the Celgene Corporation; W.A.H. received up to $1,000 from GlaxoSmithKline Bio in consultancy fees, up to $1,000 from National Foundation for Infectious Diseases in lecture fees, and more than $100,001 from the NIH, more than $100,001 from the Aeras Global TB Foundation, and more than $100,001 from the Gates Foundation in sponsored grants.
References
- 1.World Health Organization [Internet]. Who report 2009. —global tuberculosis control 2009 [accessed 2009 December 22]. Available from: http://www.who.int/tb/publications/global_report/2009/en/
- 2.Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet 2006;367:1173–1180. [DOI] [PubMed] [Google Scholar]
- 3.Bonifachich E, Chort M, Astigarraga A, Diaz N, Brunet B, Pezzotto SM, Bottasso O. Protective effect of bacillus Calmette-Guerin (BCG) vaccination in children with extra-pulmonary tuberculosis, but not the pulmonary disease a case–control study in Rosario, Argentina. Vaccine 2006;24:2894–2899. [DOI] [PubMed] [Google Scholar]
- 4.Fine P. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 1995;346:1339–1345. [DOI] [PubMed] [Google Scholar]
- 5.Andersen P, Smedegaard B. CD4+ T-cell subsets that mediate immunological memory to Mycobacterium tuberculosis infection in mice. Infect Immun 2000;68:621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hengel RL, Allende MC, Dewar RL, Metcalf JA, Mican JM, Lane HC. Increasing CD4+ T cells specific for tuberculosis correlate with improved clinical immunity after highly active antiretroviral therapy. AIDS Res Hum Retroviruses 2002;18:969–-975. [DOI] [PubMed] [Google Scholar]
- 7.Lammas DA, Casanova J-L, Kumararatne DS. Clinical consequences of defects in the IL-12–dependent interferon-gamma (IFN-γ) pathway. Clin Exp Immunol 2000;121:417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vallinoto AC, Graca ES, Araujo MS, Azevedo VN, Cayres-Vallinoto I, Machado LF, Ishak MO, Ishak R. IFN-γ +874T/A polymorphism and cytokine plasma levels are associated with susceptibility to Mycobacterium tuberculosis infection and clinical manifestation of tuberculosis. Hum Immunol 2010;71:692–696. [DOI] [PubMed] [Google Scholar]
- 9.Ottenhoff THM, Kumararatne D, Casanova JL. Novel human immunodeficiencies reveal the essential role of type-1 cytokines in immunity to intracellular bacteria. Immunology Today 1998;19:491–494. [DOI] [PubMed] [Google Scholar]
- 10.Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, Schrelber R, Mak TW, Bloom BR. Tumor necrosis factor-α is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 1995;2:561–572. [DOI] [PubMed] [Google Scholar]
- 11.Bean AGD, Roach DR, Briscoe H, France MP, Korner H, Sedgwick JD, Britton WJ. Structural deficiencies in granuloma formation in TNF-a gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin1. J Immunol 1999;162:3504–3511. [PubMed] [Google Scholar]
- 12.Flynn JL, Goldstein MM, Triebold KJ, Koller B, Bloom BR. Major histocompatibility complex class I- restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA 1992;89:12013–12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen CY, Huang D, Wang RC, Shen L, Zeng G, Yao S, Shen Y, Halliday L, Fortman J, McAllister M, et al. A critical role for CD8 T cells in a nonhuman primate model of tuberculosis. PLoS Pathog 2009;5:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruns H, Meinken C, Schauenberg P, Härter G, Kern P, Modlin RL, Antoni C, Stenger S. T cell-mediated antimicrobial activity against Mycobacterium tuberculosis in humans. J Clin Invest 2009;119:1167–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tena-Coki NG, Scriba TJ, Peteni N, Eley B, Wilkinson RJ, Andersen P, Hanekom WA, Kampmann B. CD4 and CD8 T cell responses to mycobacterial antigens in African children. Am J Respir Crit Care Med 2010;182:120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoft DF, Blazevic A, Abate G, Hanekom WA, Kaplan G, Soler JH, Weichold F, Geiter L, Sadoff JC, Horwitz MA. A new recombinant Bacille Calmette-Guérin vaccine safely induces significantly enhanced tuberculosis-specific immunity in human volunteers. J Infect Dis 2008;198:1491–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beveridge NER, Price DA, Casazza JP, Pathan AA, Sander CR, Asher TE, Ambrozak DR, Precopio ML, Scheinberg P, Alder NC, et al. Immunization with BCG and recombinant MVA85A induces long-lasting, polyfunctional Mycobacterium tuberculosis-specific CD4+ memory T lymphocyte populations. Eur J Immunol 2007;37:3089–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawkridge T, Scriba TJ, Gelderbloem S, Smit E, Tameris M, Moyo S, Lang T, Veldsman A, Hatherill M, Merwe L, et al. Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in healthy adults in South Africa. J Infect Dis 2008;198:544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sander CR, Pathan AA, Beveridge NER, Poulton I, Minassian A, Alder N, Wijgerden JV, Hill AVS, Gleeson FV, Davies RJO, et al. Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in Mycobacterium tuberculosis infected individuals. Am J Respir Crit Care Med 2009;179:724–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scriba TJ, Tameris M, Mansoor N, Smit E, Merwe L, Isaacs F, Keyser A, Moyo S, Brittain N, Lawrie A, et al. Modified vaccinia ankara-expressing Ag85A, a novel tuberculosis vaccine, is safe in adolescents and children, and induces polyfunctional CD4(+) T cells. Eur J Immunol 2010;40:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abel B, Tameris M, Mansoor N, Gelderbloem S, Hughes J, Abrahams D, Makhethe L, Erasmus M, de Kock M, van der Merwe L, et al. The novel TB vaccine, Aeras-402, induces robust and polyfunctional CD4 and CD8 T cells in adults. Am J Respir Crit Care Med 2010;181:1407–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aagaard C, Hoang TTKT, Izzo A, Billeskov R, Troudt J, Arnett K, Keyser A, Elvang T, Andersen P, Dietrich J. Protection and polyfunctional T cells induced by Ag85-Tb10.4/IC31 against Mycobacterium tuberculosis is highly dependent on the antigen dose. PloS One 2009;4:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forbes EK, Sander C, Ronan EO, McShane H, Hill AVS, Beverley PCL, Tchilian EZ. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J Immunol 2008;181:4955–4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scriba TJ, Kalsdorf B, Abrahams D-A, Isaacs F, Hofmeister J, Black G, Hassan HY, Wilkinson RJ, Walzl G, Gelderbloem SJ, et al. Distinct, specific IL-17– and IL-22–producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J Immunol 2008;180:1962–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol 2007;8:369–377. [DOI] [PubMed] [Google Scholar]
- 26.Chen X, Zhang M, Liao M, Graner MW, Wu C, Yang Q, Liu H, Zhou B. Reduced Th17 response in patients with tuberculosis correlates with IL-6R expression on CD4+ T cells. Am J Respir Crit Care Med 2010;181:734–742. [DOI] [PubMed] [Google Scholar]
- 27.Mazzola TN, Silva MTND, Moreno YMF, Lima SCBS, Carniel EF, Morcillo AM, Antonio MARGM, Zanolli ML, Netto AA, Blotta MH, et al. Robust γδ+ T cell expansion in infants immunized at birth with BCG vaccine. Vaccine 2007;25:6313–6320. [DOI] [PubMed] [Google Scholar]
- 28.Caccamo N, Sireci G, Meraviglia S, Dieli F, Ivanyi J, Salerno A. γδ T cells condition dendritic cells in vivo for priming pulmonary CD8 T cell responses against Mycobacterium tuberculosis. Eur J Immunol 2006;36:2681–2690. [DOI] [PubMed] [Google Scholar]
- 29.Kagina BMN. BCG-specific T cell responses do not correlate with protection against tuberculosis following BCG vaccination of newborns. Poster No. 205, presented at the keystone meeting of Overcoming the Crisis of TB and AIDS. Arusha, Tanzania; October 20–25, 2009.
- 30.Hawkridge A, Hatherill M, Little F, Goetz MA, Barker L, Mahomed H, Sadoff J, Hanekom W, Geiter L, Hussey G, and the South African BCG trial team. Efficacy of percutaneous versus intradermal BCG in the prevention of tuberculosis in South African infants: randomised trial. BMJ 2008;337:1–8. [Google Scholar]
- 31.Hanekom WA, Hughes J, Mavinkurve M, Mendillo M, Watkins M, Gamieldien H, Gelderbloem SJ, Sidibana M, Mansoor N, Davids V, et al. Novel application of a whole blood intracellular cytokine detection assay to quantitate specific T-cell frequency in field studies. J Immunol Methods 2004;291:185–195. [DOI] [PubMed] [Google Scholar]
- 32.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, et al. Multifunctional Th1 cells defne a correlate of vaccine-mediated protection against Leishmania major. Nat Med 2007;13:843–850. [DOI] [PubMed] [Google Scholar]
- 33.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role of interferon g in resistance to Mycobacterium tuberculosis infection. J Exp Med 1993;178:2249–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reed SG, Coler RN, Dalemans W, Tan EV, Cruz ECD, Basaraba RJ, Orme IM, Skeiky YAW, Alderson MR, Cowgill KD, et al. Defined tuberculosis vaccine, Mtb72f/As02a, evidence of protection in cynomolgus monkeys. Proc Natl Acad Sci USA 2009;106:2301–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goonetilleke NP, McShane H, Hannan CM, Anderson RJ, Brookes RH, Hill AVS. Enhanced immunogenicity and protective efficacy against Mycobacterium tuberculosis of bacille Calmette-Guérin vaccine using mucosal administration and boosting with a recombinant modified vaccinia virus ankara. J Immunol 2003;171:1602–1609. [DOI] [PubMed] [Google Scholar]
- 36.Hervas-Stubbs S, Majlessi L, Simsova M, Morova J, Rojas M-J, Nouzé CM, Brodin P, Sebo P, Leclerc C. High frequency of CD4+ T cells specific for the Tb10.4 protein correlates with protection against Mycobacterium tuberculosis infection. Infect Immun 2006;74:3396–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gallegos AM, Pamer EG, Glickman MS. Delayed protection by ESAT-6–specific effector CD4+ T cells after airborne M. tuberculosis infection. J Exp Med 2008;205:2359–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mittrucker H-W, Steinhoff U, Kohler A, Krause M, Lazar D, Mex P, Miekley D, Kaufmann SHE. Poor correlation between BCG vaccination-induced T cell responses and protection against tuberculosis. Proc Natl Acad Sci USA 2007;104:12434–12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vordermeier HM, Chambers MA, Cockle PJ, Whelan AO, Simmons J, Hewinson RG. Correlation of ESAT-6–specific gamma interferon production with pathology in cattle following Mycobacterium bovis BCG vaccination against experimental bovine tuberculosis. Infect Immun 2002;70:3026–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fuller CL, Flynn JL, Reinhart TA. In situ study of abundant expression of proinflammatory chemokines and cytokines in pulmonary granulomas that develop in cynomolgus macaques experimentally infected with Mycobacterium tuberculosis. Infect Immun 2003;71:7023–7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sutherland JS, Adetifa IM, Hill PC, Adegbola RA, Ota MOC. Pattern and diversity of cytokine production differentiates between Mycobacterium tuberculosis infection and disease. Eur J Immunol 2009;39:723–729. [DOI] [PubMed] [Google Scholar]
- 42.Spencer CT, Abate G, Blazevic A, Hoft DF. Only a subset of phosphoantigen-responsive γ9δ2 T cells mediate protective tuberculosis immunity. J Immunol 2008;181:4471–4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kagina BMN, Abel B, Bowmaker M, Scriba TJ, Gelderbloem S, Smit E, Erasmus M, Nene N, Walzl G, Black G, et al. Delaying BCG vaccination from birth to 10 weeks of age may result in an enhanced memory CD4 T cell response. Vaccine 2009;27:5488–5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soares AP, Scriba TJ, Joseph S, Harbacheuski R, Murray RA, Gelderbloem SJ, Hawkridge A, Hussey GD, Maecker H, Kaplan G, et al. Bacillus Calmette-Guérin vaccination of human newborns induces T cells with complex cytokine and phenotypic profiles. J Immunol 2008;180:3569–3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fletcher HA, Keyser A, Bowmaker M, Sayles PC, Kaplan G, Hussey G, Hill AV, Hanekom WA. Transcriptional profiling of mycobacterial antigen-induced responses in infants vaccinated with BCG at birth. BMC Med Genomics 2009;2:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joosten SA, van Meijgaarden KE, van Weeren PC, Kazi F, Geluk A, Savage ND, Drijfhout JW, Flower DR, Hanekom WA, Klein MR, et al. Mycobacterium tuberculosis peptides presented by HLA-E molecules are targets for human CD8 T-cells with cytotoxic as well as regulatory activity. PLoS Pathog 2010;6:e1000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murray RA, Mansoor N, Harbacheuski R, Soler J, Davids V, Soares A, Hawkridge A, Hussey GD, Maecker H, Kaplan G, et al. Bacillus Calmette Guerin vaccination of human newborns induces a specific, functional CD8+ T cell response. J Immunol 2006;177:5647–5651. [DOI] [PubMed] [Google Scholar]
- 48.Hussey GD, Watkins MLV, Goddard EA, Gottschalk S, Hughes EJ, Iloni K, Kibel MA, Ress SR. Neonatal mycobacterial specific cytotoxic T-lymphocyte and cytokine profiles in response to distinct BCG vaccination strategies. Immunology 2002;105:314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.