Abstract
We previously reported that Fms-like tyrosine kinase 3 ligand (Flt3-L) reversed airway hyperresponsiveness (AHR) and airway inflammation, and increased the number of regulatory CD11chighCD8αhighCD11blow dendritic cells and CD4+CD25+ICOS+Foxp3+IL-10+ T-regulatory cells in the lung of allergen-sensitized and -challenged mice. In this study, we evaluated the effect of Flt3-L on Th17 cells and expression of suppressors of cytokine signaling (SOCS) proteins in the lungs of house dust mite (HDM)–sensitized and –challenged mice. BALB/c mice were sensitized and challenged with HDM, and AHR to methacholine was established. Mice were treated with Flt3-L (5 μg, intraperitoneal) daily for 10 days. Levels of IL-4, -5, -6, -8, and -13, and transforming growth factor (TGF)–β in the bronchoalveolar lavage fluid (BALF) were examined by ELISA. Flt3-L treatment reversed existing AHR to methacholine and substantially decreased eosinophils, neutrophils, IL-5, -6, -8, and IL-13, and TGF-β levels in the BALF. HDM-sensitized and -challenged mice showed a significant increase in lung CD4+IL-17+IL-23R+CD25− T cells with high expression of retinoic acid–related orphan receptor (ROR)–γt transcripts. However, administration of Flt3-L substantially decreased the number of lung CD4+IL-17+IL-23R+CD25− T cells, with significantly decreased expression of ROR-γt mRNA in these cells. HDM sensitization caused a significant increase in the expression of SOCS-1, -3, and -5 in the lung. Flt3-L treatment abolished the increase in SOCS-1 and SOCS-3 proteins, whereas SOCS-5 expression was significantly reduced. These data suggest that the therapeutic effect of Flt3-L in reversing the hallmarks of allergic asthma in a mouse model is mediated by decreasing IL-6 and TGF-β levels in the BALF, which, in turn, decrease CD4+IL-17+IL-23R+ROR-γt+CD25− T cells and the expression of SOCS-1 and SOCS-3 in the lung of HDM-sensitized and -challenged mice.
Keywords: airway hyperresponsiveness, house dust mite, retinoic acid–related orphan receptor–γt, suppressors of cytokine signaling, T helper cell type 17
CLINICAL RELEVANCE.
Fms-like tyrosine kinase 3 ligand (Flt3-L) is a potent immunomodulator in allergic airway inflammation and airway hyperresponsiveness in asthma. Here, we found that Flt3-L decreases lung T helper type 17 cells that are one of the major cells involved in allergic airway inflammation. These findings enhance our knowledge base of the disease process, and may be helpful in developing a better therapeutic approach.
Asthma is a chronic inflammatory lung disease of the airways, characterized by reversible airway obstruction (1), airway hyperresponsiveness (AHR) (2), and airway inflammation (3). CD4+ T cells have been found in the lungs of postmortem subjects who died of severe acute asthma attacks, suggesting that CD4+ T cells have a central role in the pathogenesis of asthma (4). An imbalanced ratio of T helper cell (Th) type 1 and Th2 cells leads to the development of a Th2 polarized response and pathophysiological changes seen in airways of subjects with asthma (5). The Th1 and Th2 model was first proposed over 2 decades ago (6, 7), and this paradigm has been the cornerstone of understanding cellular development of Th1 cells in response to viruses, intracellular bacteria, and cancer (8). On the other hand, the development of a Th2 polarized response is initiated by multicellular parasites and extracellular bacterial infections (9).
Th17 cells are the most recently identified member of CD4+ T cell subset, and it has been defined by its secreted product, IL-17 (10). There are several isoforms of IL-17 that include IL-17A to IL-17F, and they are homodimeric peptides between 35 and 52 kD (11). IL-17 is a proinflammatory cytokine (12) secreted by Th17 cells and consists of six isoforms—IL-17A through IL-17F (13)—but it can be produced by CD8+ T cells and NKT cells (14). Th17 cells develop in response to IL-6 and transforming growth factor (TGF)–β (15), and these differentiated cells express IL-23R, which is stabilized and/or maintained by IL-23 signaling (16). In general, Th17 cells mount a defense against extracellular bacteria (i.e., Klebsiella pneumoniae) (17) and fungi (i.e., Candida albicans) (18) by the secretion of IL-17 and the recruitment of neutrophils (19). Recent data have shown that Th17 cells may indirectly participate in virus-associated pathologies (20), such as human immunodeficiency virus (21). However, Th17 cells have been implicated in the development of autoimmune diseases, including multiple sclerosis (22), rheumatoid arthritis (23), and lupus (24), and accumulating data suggest a correlation with initiation and pathogenesis of allergic diseases, such as atopic dermatitis, allergic rhinitis, food allergy, and asthma (25).
There is a plethora of environmental antigens that trigger the development of proinflammatory T cells, and these lymphocytes play important roles in pathogenesis of asthma (26). House dust mite (HDM), a common indoor antigen consist of Dermatophagoides farinae (27) and Dermatophagoides pteronyssinus (28), is a potent inducer of atopic asthma (29), and mite allergy affects approximately 10–20% of the population (30). People who are allergic to dust mites respond to the proteins within the bodies and feces of the mites (31). Exposure to dust mite at high levels is an important factor in the development of asthma in children (32). The proteins of HDM are found mostly in pillows, mattresses, carpeting, and upholstered furniture (33); therefore, this household allergen is a high-risk factor for the initiation of allergic asthma.
Fms-like tyrosine kinase (Flt) 3 (or Flk2) is a member of the class III tyrosine kinase receptor family (34), and it was independently cloned by two groups of investigators (35). Flt3 was cloned from murine placenta based upon its similar sequence homology to c-fms (36). The c-fms (cellular) oncogene is a homolog of v-fms (viral) oncogenes, and it was originally encoded by the Susan McDonough strain of feline sarcoma virus (37). The mouse and human ligands (Flt3-L) for the Flt3/Flk2 receptor were cloned, and were found to share structural similarities with c-kit-L and M-CSF (38, 39). In vivo administration Flt3-L in mice leads to a significant increase of dendritic cells (DCs) in all primary and secondary lymphoid tissues (40), and in humans it induces both CD11c+ and CD11c− DC subsets (41).
Recently, our laboratory reported that treatment of Flt3-L in ovalbumin-sensitized and -challenged mice reversed AHR to methacholine and airway inflammation (42). In addition, we found that ovalbumin-sensitized mice treated with Flt3-L increased the number of regulatory CD11chighCD8αhighCD11blow DCs in the lung (42). We also found that administration of Flt3-L significantly increased the number of CD4+CD25+Foxp3+IL-10+ T cells in the lungs of cockroach-sensitized and -challenged mice (43). Furthermore, adoptive transfer of T-regulatory cells (Tregs) reverses AHR and airway inflammation (45). The secretion of cytokines has been shown to play a role in the regulation of T cell response. The regulation of Th1 and Th2 development in steady-state conditions has implied that suppressors of cytokine signaling (SOCS) proteins play a significant role in the proper immune response, and the expression of these protein molecules is also regulated by cytokines (44). Therefore, in this study, we examined the potential effect of Flt3-L on the expression of SOCS proteins and suppression of Th17 cells in the lungs of a clinically relevant model of HDM-induced allergic asthma.
MATERIALS AND METHODS
Animals
Female BALB/c mice (4–5 wk old) were purchased from Harlan Laboratories (Indianapolis, IN), and were housed in separate cages according to treatment protocol. Food and water were provided ad libitum. In accord with National Institutes of Health guidelines, the research protocol of this study was approved by the Institutional Animal Care and Use Committee of Creighton University (Omaha, NE).
Sensitization and Treatment Protocol
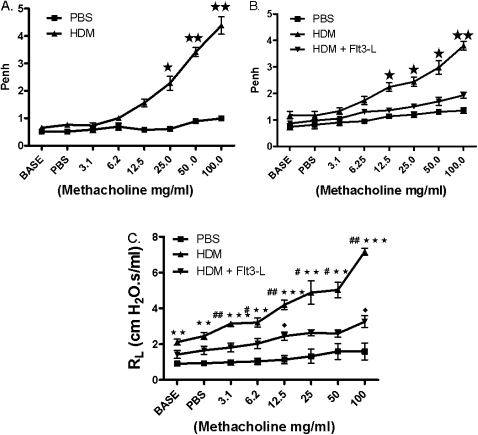
Allergic airway inflammation was induced by intranasal injections of 20 μg allergenic extract of whole bodies of mites, D. farinae and D. pteronyssines (Hollister-Stier Laboratories LLC, Spokane, WA), on Days 0 and 14, followed by aerosol sensitization with 1% HDM for 20 minutes on Days 28–30 with an Aeroneb Pro nebulizer (Aerogen, Somerset, PA). Mice were again challenged on Day 32 with 5% HDM for 20 minutes followed by the measurement of pulmonary functions 24 hours later to establish AHR to methacholine (Figure 1A), by the established methods in our laboratory (42–45). Starting Day 33, HDM-sensitized- and -challenged mice with established AHR to methacholine were randomized into two groups: the first experimental group received 5 μg Flt3-L (Peprotech, Inc Rocky Hill, NJ) intraperitoneal treatment for 10 days, and the second group received sterile PBS, the vehicle of Flt-3L. The nonsensitized control mice were sham treated with sterile PBS. On Day 45, mice in all groups were challenged with 5% aerosolized HDM, and nonsensitized control mice were administered only sterile PBS. On Day 46, AHR to methacholine was measured by whole-body plethysmography (Buxco Electronics, Troy, NY), and the data reported in enhanced pause (Penh) values. On Day 47, AHR to methacholine was confirmed by the invasive tracheotomy method of measuring specific airway resistance, followed by collection of bronchoalveolar lavage fluid (BALF), blood, lungs, and spleen.
Figure 1.
Airway hyperresponsiveness (AHR) to methacholine (Mch). (A) On Day 33, AHR to Mch was established, followed by 10-day treatment with Fms-like tyrosine kinase ligand (Flt3-L; 5 μg/d, intraperitoneal). (B) On Day 45, AHR to Mch was measured in enhanced pause (Penh) values after Flt3-L treatment. (C) AHR was confirmed by the measurement of specific airway resistance (RL). Data are shown as means (±SEM) from five mice in each experimental group. *P < 0.01, **P < 0.001, ***P < 0.0001 compared with PBS group; #P < 0.05, ##P < 0.01 compared with house dust mite (HDM); closed diamonds, P < 0.05 compared with PBS group.
Tissue Preparation and Isolation of Th17 Cells
To isolate Th17 cells, mice were killed and lungs were harvested. These tissues were cut into fragments and digested with collagenase-D (1 mg/ml; Roche Laboratories, Burlington, NC) and 5 ml of RPMI 1,640 (Cambrex, East Rutherford, NJ). The samples were incubated at 37°C in a CO2 incubator for 60 minutes. Tissues were disrupted with a 1-ml syringe, and red blood cells were removed from the suspension with Tris-buffered ammonium chloride solution and neutralized with PBS4 solution. The suspension was centrifuged at 350 × g for 15 minutes. Supernatant was discarded and the pellet washed in 10 ml Hanks' balanced buffered solution, centrifuged, and resuspended in AutoMACS running buffer. The cell suspension was labeled with the CD4+ microbead kit (Miltenyi Biotec, Auburn, CA) and sorted by AutoMACS (miltenyi Biotec, Auburn, CA). The sorted cells were labeled with conjugated peridinin chlorophyll protein (PerCP) CD4 antibody and allophycocyanin (APC) CD25 antibody, and further purified by FACSAria (BD Bioscience, San Diego, CA). The purified CD4+CD25− T cells were processed for Th17 cells by a three-step process: (1) the purified CD4+CD25− T cells were labeled with IL-17 catch reagent and incubated for 5 minutes at 37°C (warm 37°C serum-free medium was added to the CD4+CD25− T cells and incubated for an additional 45 minutes on a slow, continuous rocker); (2) CD4+CD25− T cells were washed, centrifuged, and resuspended in cold running buffer, and labeled with IL-17 detection antibody, incubated for 10 minutes on ice; and (3) cells were washed, centrifuged, and resuspended in buffer and label with anti-PE microbeads, and sorted for IL-17–secreting cells with AutoMACS. The CD4+CD25− T cells were analyzed for IL-17 expression by FACS (BD Bioscience). Overall purity and viability of the CD4+CD25− T cell population in the lung tissue ranged between 94 and 95% and 95 and 98%, respectively. The purity of the CD4+CD25− T cells used to analyze the expression of IL-23R was 60–70%, as the cells in these experiments were isolated with AutoMACS, and not the FACSAria cell sorter.
Flow Cytometry and Antibodies
A FACScan (Becton Dickenson, San Diego, CA) was used for analytical flow cytometry, and data were processed with CellQuest Pro (Becton Dickenson) by standard protocol for cell preparation. Cells were stained with PerCP CD4 (L3T4) and APC CD25 (BC96), purchased from eBioscience (San Diego, CA). In addition, cells were stained with mouse PE IL-17 Secretion and Detection assay kit (Miltenyi Biotec).
BALF and Cytokine Measurements
After death, lungs were gently lavaged with 1 ml of warm saline (37°C) via a tracheal cannula. Total cell counts were performed with a Coulter counter (Beckman and Coulter, Brea, CA). All samples were centrifuged at 400 rpm for 10 minutes, and the supernatant was stored in a −80°C freezer until assays were performed for IL-4, -5, -6, -13, and TGF-β levels with an ELISA Detection Ready-Set-Go kit (eBioscience), according to the manufacturer's protocol. The IL-8 levels were examined with the Mouse GRO/KC Assay kit—IBL 96 well (Immuno-Biological Labs Co., Ltd., Minneapolis, MN), according to the manufacturer's protocol.
Immunohistochemistry
Paraffin sections (5 μm) were cut from the tissue-embedded blocks, placed onto a warm (37°C) water bath, and placed onto superfrost plus slides (VWR International, Lutterworth, Leicestershire, UK). The sections were deparaffinized and hydrated in xylene and solutions of ethanol gradients (100, 95, 80, and 70%). For immunostaining, antigen was exposed with target retrieval solution by boiling the sections in a steam cooker for 30 minutes. Sections were cooled to room temperature for 15 minutes and washed three times for 5 minutes each. Endogenous peroxidase was blocked with 3% H2O2 in methanol for 30 minutes. All procedures for blocking of nonspecific binding sites, incubation with primary antibody (1–2 h), secondary antibodies, and avidin-biotin complex) protocol were performed as recommended by the manufacturer (Vectastain ABC elite kit, PK-7800; Vector Laboratories, Burlingame, CA). The substrate used was 2,2-diaminobenzene in which positive staining was indicated by the presence of brown precipitate. The sections were counterstained with Gills no. 2 hematoxylin for 7 seconds. Negative controls were run without the primary antibody. Results were examined by light microscopy.
RT-PCR
To analyze the expression of retinoic acid–related orphan receptor (ROR)–γt transcripts, the mRNA was prepared from isolated CD4+IL-23R+IL-17+ T cells with Trizol (Sigma-Aldrich, St. Louis, MO) reagent protocol. Gene Amp PCR System 2,400 (Perkin-Elmer, Waltham, MA) was used at 31 cycles for: (1) ROR-γt, forward 5′-TGA GGA AAC CAG GCA TCC TGA ACT-3′, reverse 5′-TGT GTG GTT GTT GGC ATT GTA GGC-3′ (melting temperature, Tm, 55°C); and (2) ROR-γt, forward 5′-GTT TGG CCG AAT GTC CAA GAA GCA-3′, reverse 5′-ATT GAT GAG AAC CAG GGG CCG TGT A (melting temperature, Tm, 55°C).
Statistical Analysis
Data were analyzed with GraphPad Prism statistical analysis and graphing software. Unpaired Student's t test was used to determine differences between two groups. Multiple group comparison was made with ANOVA. A P value of less than 0.05 was considered significant.
RESULTS
Effect of Flt3-L Treatment on AHR to Methacholine in HDM-Sensitized and -Challenged Mice
Before the treatment with Flt3-L or PBS, HDM-sensitized and -challenged mice showed elevated AHR to methacholine on Day 33, as measured by noninvasive whole-body plethysmography (Figure 1A). HDM-sensitized and -challenged mice treated with Flt3-L exhibited a significant reduction of AHR to methacholine compared with PBS control mice (Figure 1B). A more rigorous method with invasive tracheostomy in anesthetized mice to measure specific airway resistance confirmed the findings of AHR (Figure 1C).
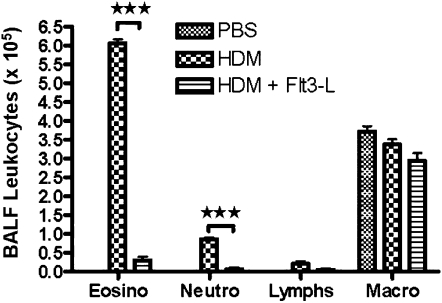
Effect of Flt3-L Treatment on Leukocytes in the BALF from HDM-Sensitized and -Challenged Mice
HDM sensitization and challenge significantly increased the number of eosinophils and neutrophils in the BALF compared with PBS control mice. The administration of Flt3-L substantially decreased the density of BALF eosinophils and neutrophils to the PBS control level (Figure 2).
Figure 2.
Bronchoalveolar lavage fluid (BALF) leukocytes after Flt3-L treatment. BALF was centrifuged, and pelleted cells were counted and differential analysis was performed with standard morphological criteria. A total of 300 cells was examined per cytospin slide, and absolute cell numbers were calculated per milliliter of the BALF based on the percentage of individual cell in a slide. Data are shown as means (±SEM) for five animals in each experimental group. ***P < 0.001.
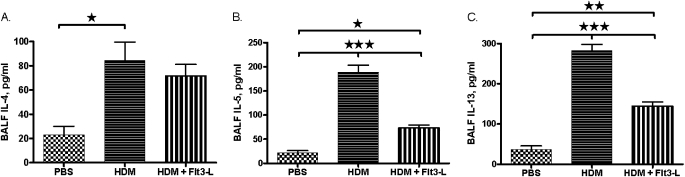
Effect of Flt3-L Treatment on Th2 cytokines in the BALF of HDM-Sensitized and -Challenged Mice
The levels of IL-5 and -13 were significantly increased in the BALF of HDM-sensitized and -challenged mice. However, treatment with Flt3-L in these mice substantially reduced the BALF levels of IL-5 and -13. There was no significant effect of Flt3-L on the level of BALF IL-4. (Figure 3).
Figure 3.
T helper (Th) 2 cytokines in the BALF of HDM-sensitized and -challenged mice. Th2 cytokine levels in the BALF of and HDM-sensitized and -challenged mice with and without Flt3-L treatment. (A) IL-4; (B) IL-5; and (C) IL-13. Data are shown as means (±SEM) for five animals in each experimental group. *P < 0.05, **P < 0.01, ***P < 0.001.
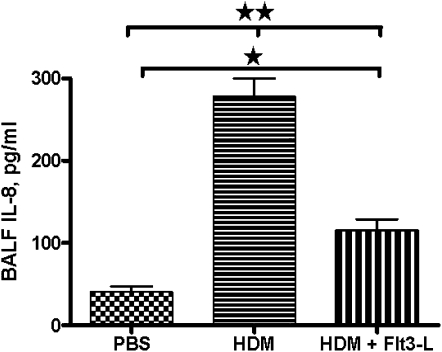
Effect of Flt3-L Treatment on IL-8 in the BALF of HDM-Sensitized and -Challenged Mice
Increased neutrophils in the BALF could be due to increases in IL-8 levels. Therefore, we evaluated the levels of IL-8 in the BALF of each experimental group. The IL-8 levels were substantially higher in the BALF of HDM-sensitized and -challenged mice than in PBS control mice. Treatment with Flt3-L in HDM-sensitized and -challenged mice significantly reduced the BALF IL-8 levels (Figure 4).
Figure 4.
IL-8, a potent chemokine for neutrophil recruitment. Collected BALF samples were analyzed for levels of IL-8 expression in HDM-sensitized and -challenged mice with and without Flt3-L treatment, and PBS control mice. Data are shown as means (±SEM) for five animals in each experimental group. *P < 0.05, **P < 0.01.
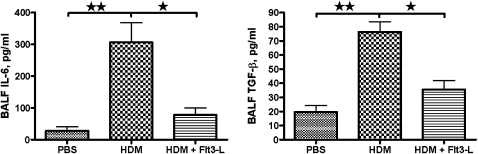
Effect of Flt3-L Treatment on IL-6 and TGF-β in the BALF of HDM-Sensitized and -Challenged Mice
HDM sensitization and challenge significantly increased the levels of BALF IL-6 and TGF-β compared with PBS control mice. In contrast, administration of Flt3-L in HDM-sensitized and -challenged mice significantly lowered the BALF IL-6 and TGF-β levels, almost to the levels in PBS control mice (Figures 5A and 5B).
Figure 5.
Transforming growth factor (TGF)–β and IL-6 levels in the BALF. BALF samples were immediately centrifuged and frozen. The cytokines, IL-6 (A) and TGF-β (B), in all samples were measured in the stored samples. Data are shown as means (±SEM) for five animals in each experimental group. *P < 0.05, **P < 0.01.
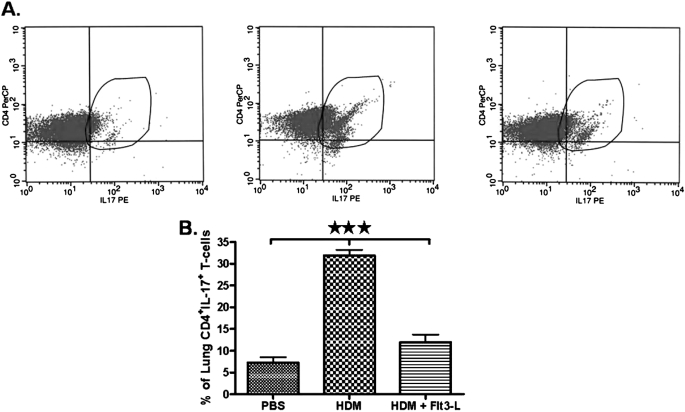
Effect of Flt3-L on CD4+CD25− T Cells Expressing IL-17 Isolated from the Lungs of HDM-Sensitized and -Challenged Mice, and from PBS Control Mice
Because IL-6 and TGF-β promote the development of Th17 cells, we isolated CD4+CD25− T cells from the lung and gated for expression of IL-17 to compare PBS, HDM, and HDM plus Flt3-L groups. The expression of IL-17 on lung CD4+CD25− T cells is shown in representative contour plots (Figure 6A). The percentage of lung CD4+CD25− T cells expressing IL-17 from PBS control mice was substantially lower (Figure 6B). However, HDM sensitization and challenge significantly increased the percentage of CD4+CD25− T cells expressing IL-17 in the lungs. CD4+CD25− T cells, isolated from the lungs of HDM-sensitized and -challenged mice treated with Flt3-L, exhibited a substantial decrease in IL-17 expression (Figure 6B).
Figure 6.
Expression of IL-17–producing lung CD4+CD25− T cells. (A) Dot plots showing a comparative analysis of IL-17–producing CD4+CD25− T cells isolated from lungs of PBS (left panel), HDM (middle panel), and HDM/Flt3-L (right panel). (B) Cumulative analysis of IL-17–producing CD4+CD25− cells in the lungs of mice in each experimental group. Data are shown as means (±SEM) from five mice in each experimental group. ***P < 0.001.
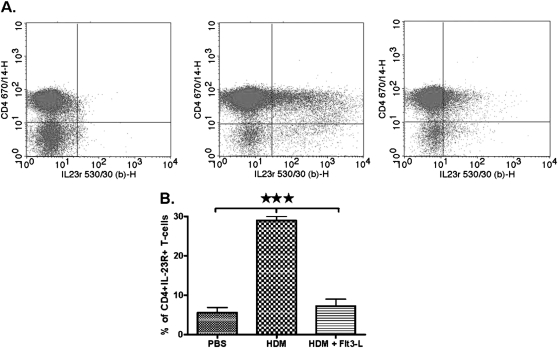
Effect of Flt3-L on the Expression of IL-23R on CD4+CD25− T Cells Isolated from the Lungs of HDM-Sensitized and -Challenged Mice
The expression of IL-23R on lung CD4+CD25− T cells is shown in representative dot plots (Figure 7A). CD4+CD25− T cells isolated from the lungs of PBS control mice had low to no expression of IL-23R (Figure 7B). HDM sensitization and challenge significantly increased the expression of IL-23R on lung CD4+CD25−. However, CD4+CD25− T cells isolated from the lungs of HDM-sensitized and -challenged mice treated with Flt3-L showed a substantial decrease in IL-23R expression (Figure 7B).
Figure 7.
Expression of IL-23R on lung CD4+CD25− T cells. (A) Dot plots showig a comparative analysis of IL-23R with CD4+CD25− T cells isolated from lungs of PBS (left panel), HDM (middle panel), and HDM/Flt3-L (right panel). (B) Cumulative analysis of IL-23R expression in each experimental group. Data are shown as means (±SEM) from five mice in each experimental group. ***P < 0.001.
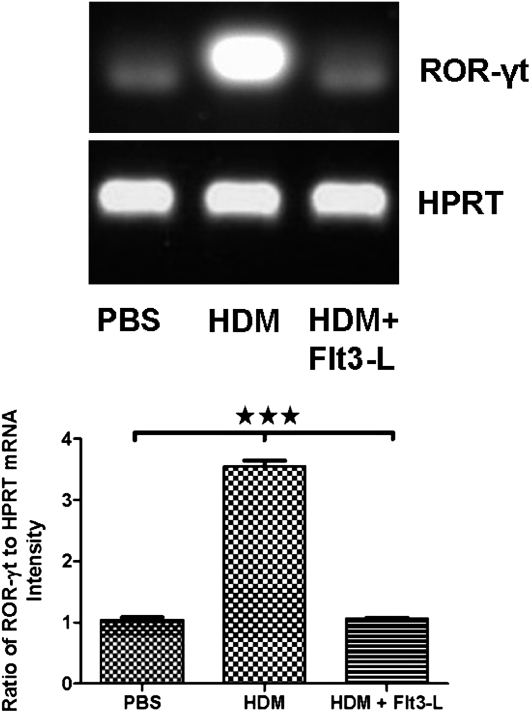
Therapeutic Effect of Flt3-L on the mRNA Expression of ROR-γt in Lung CD4+IL-17+CD25− T Cells
We analyzed the expression of ROR-γt transcripts in purified CD4+CD25−IL-17+ T cells from lung tissue of each experimental group. The expression of ROR-γt transcripts was substantially increased in the HDM-sensitized and -challenged mice without Flt3-L treatment. Treatment with Flt3-L significantly reduced the expression of ROR-γt mRNA to the PBS control level (Figure 8).
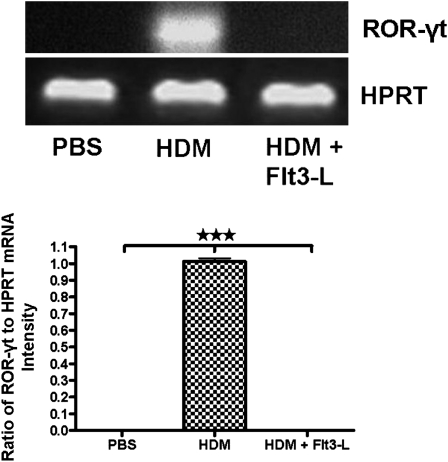
Figure 8.
Effect of Flt3-L on retinoic acid–related orphan receptor (ROR)–γt expression in CD4+CD25−IL-17+ T cells. Cells were isolated from the lungs HDM-sensitized and -challenged mice with and without Flt3-L for expression of ROR-γt mRNA (970 bp). The purity of these CD4+CD25−IL-17+ T cells was greater than 98%. Densitometric analyses confirmed the PCR data, as shown by the ratio of mRNA intensity of ROR-γt to intensity of the housekeeping gene hypoxanthine-guanine phosphoribosyltransferase (HPRT), Data are shown as means (±SEM) from five mice in each experimental group. ***P value < 0.001.
Effect of Flt3-L Treatment on mRNA Levels of ROR-γt in Lung CD4+IL23R+CD25− T Cells
Lung CD4+IL-23R+CD25− T cells isolated from PBS control mice exhibited no detectable levels of ROR-γt transcripts. However, sensitization and challenge with HDM antigen caused a significant increase in the levels of ROR-γt transcripts in the lung CD4+IL-23R+CD25− T cells. The administration of Flt3-L in HDM-sensitized and -challenged mice abolished the ROR-γt mRNA expression in the lung CD4+IL-23R+CD25− T cells (Figure 9).
Figure 9.
The effect of Flt3-L on lung CD4+ IL-23R+CD25− T cells expressing ROR-γt transcripts. CD4+IL-23+CD25− T cell isolated from the lungs of PBS control mice showed very low but detectable levels ROR-γt. However, HDM sensitization induced a significant increase in ROR-γt transcripts. Flt3-L significantly decreased ROR-γt expression compared with HDM and PBS control group. Densitometric analyses confirmed the PCR data, as shown by the ratio of mRNA intensity of ROR-γt to the intensity of the housekeeping gene, HPRT. Data are shown as means (±SEM) from five mice in each experimental group. ***P value < 0.001.
Expression of SOCS-1, -3, and -5 after Flt3-L Treatment in the Lungs of HDM-Sensitized and -Challenged mice
SOCS proteins negatively regulate cytokine signaling. IL-6 is a proinflammatory cytokine, and is known to be regulated by SOCS proteins, specifically SOCS-1 and -3 (45, 46). Because development of Th17 cells is regulated by the secretion and activity of IL-6 and TGF-β, we examined the expression of SOCS proteins in the lungs of HDM-sensitized and -challenged mice with and without Flt3-L treatment. PBS control mice expressed low to undetectable levels of SOCS-1, -3, and -5 in the lungs (Figures 10A–10C). However, the lungs of HDM-sensitized and -challenged mice exhibited substantially higher expression of SOCS-1, -3, and -5 compared with PBS control mice. Flt3-L treatment abolished the increase in SOCS-1 and -3 protein, whereas SOCS-5 expression was significantly reduced (Figures 10A–10C).
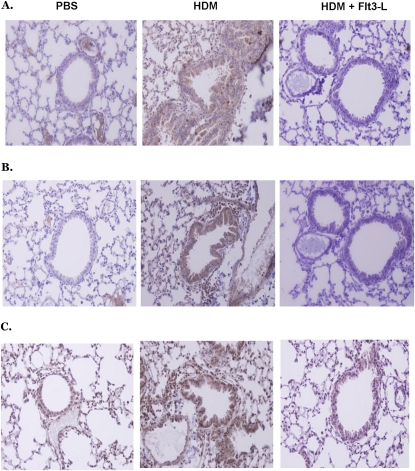
Figure 10.
Effect of Flt3-L on the expression of (A) suppressors of cytokine signaling (SOCS)–1, (B) SOCS-3, and (C) SOCS-5 in lungs of HDM-sensitized and -challenged and PBS control mice. Flt3-L caused a decrease in SOCS-1, -3, and -5 expression compared with that in HDM-sensitized and -challenged mice without Flt3-L treatment. However, SOCS-5 was not completely removed after Flt3-L treatment. The histological slides are representative of five mice in each experimental group. Left panels, PBS; middle panels, HDM; right panels, HDM plus Flt3-L.
DISCUSSION
In this study, the clinically relevant aeroallergen, HDM, induced the classical hallmarks of allergic airway inflammation and asthma, including elevated AHR, and high levels of BALF IL-6 and TGF-β. In addition, we found that sensitization with HDM antigen caused a substantial increase in CD4+IL23R+IL-17+CD25− T cells in the lungs of these mice, and these cells expressed significantly higher levels of ROR-γt transcripts, the master transcriptional factor for Th17 cells. In addition, HDM sensitization caused a significant increase in the expression of SOCS-1, -3, and -5 in the lungs of challenged mice. Flt3-L treatment significantly decreased CD4+IL-23R+IL-17+CD25− T cells in the lungs of HDM-sensitized and -challenged mice, and decreased expression of ROR-γt transcripts. In addition, Flt3-L treatment reversed AHR, airway inflammation, and BALF IL-6 and TGF-β, and completely abolished the expression of SOCS-1 and -3 in the lung of HDM-sensitized and -challenged mice. These data suggest that the therapeutic effect of Flt3-L in allergic asthma could involve a reduction in the cytokines that increases Th17 cells.
In the HDM model, we found significantly higher BAL eosinophilia and neutrophilia, and Flt3-L treatment inhibited the density of these inflammatory cells in the BALF. The increased level of neutrophils in the BALF of HDM-sensitized mice may correlate with the increased number of CD4+IL-17+CD25− T cells. IL-17 has been implicated in the recruitment of neutrophils in subjects with acute to severe asthma (47), and is expressed in BALF and sputum of patients with asthma (48, 49). Expression and activity of IL-23R on Th17 cells help in the maintenance of Th17 cells after their differentiation. In this study, HDM sensitization caused high expression of IL-23R on lung CD4+IL-17+CD25− T cells, and Fl3-L treatment significantly decreased the expression of IL-23R on lung CD4+IL-17+CD25− T cells to the PBS control mice.
The expression of ROR-γt mRNA was substantially increased in the HDM-sensitized and -challenged mice, and treatment with Flt3-L significantly reduced the expression of ROR-γt mRNA to control level. The low expression of ROR-γt transcripts in the PBS control group could be due to the quiescent and/or an intermediate state of CD4+CD25−IL-17+ T cells, and these cells are activated during immune response. Recently, studies have shown that CD4+ T cells can coexpress ROR-γt and Foxp3, and, under proper stimulation, these cells can differentiate into Tregs or Th17 cells (47). However, further investigation is warranted to elucidate these subsets and their functional role.
The specific mechanism(s) that mediates the mobilization and recruitment of neutrophils to the lung airways is ill defined. However, the secretion of IL-17 has been implicated in granulopoiesis and specific recruitment of neutrophils in various tissue types (50). Caldwell and colleagues (51) showed that the depletion of CD4+ T cells with antibody significantly reduced recruitment of neutrophils and injury after hepatic ischemia–reperfusion. However, little is known about the molecular mechanism(s) by which CD4+ T cells orchestrate neutrophil recruitment in the airways in response to stimuli other than allergens (52). To date, data suggest that Th17 cells are specific for recruiting neutrophils to the airways in the presence of exogenous antigen (52), and the Th17/neutrophil signaling axis could be mediated by airway epithelial cells (53). In this study, we found a significant increase in CD4+IL-23R+IL-17+CD25− T cells in the lungs of HDM-sensitized and -challenged mice, and increased levels of neutrophils in the BALF of the same mice. However, the mechanism underlying the recruitment of neutrophils by Th17 cells remains to be elucidated. Recent evidence reported by Fielding and colleagues (54) suggests that IL-6 may regulate the mobilization and recruitment of neutrophils to the site of inflammation during acute-phase response. The BALF of HDM-sensitized and -challenged mice showed significantly higher levels of IL-6 and TGF-β, and this could synergistically drive the development of Th17 cells (55, 56), and may explain the increased density of neutrophils in the BALF of the HDM-sensitized and -challenged mice. Alternatively, neutrophil recruitment to the airways could be mediated directly by HDM allergen. HDMs produce enzymes called proteases that cleave peptide bonds of tight junction in the epithelial cell layer (57–59), and this action occurs by the binding of proteases to protease activator receptors that are found on airway epithelial cells (60). The binding of proteases causes the production and secretion of IL-6 and -8 (61). In addition, protease activator receptor–2 receptors are found on fibroblasts, and activated fibroblasts secrete TGF-β (62). In addition, it is well established that IL-6 and TGF-β are responsible for development of Th17 cells, and the maintenance of Th17 cells is regulated by IL-23R on Th17 cells. Therefore, it is tempting to speculate that the increase in Th17 in the lungs of HDM-sensitized and -challenged mice is due to IL-6 and TGF-β, and the secretion of IL-17 from Th17 cells and IL-8 from epithelial cells lead to the recruitment of neutrophils. In this study, we found IL-8 levels to be substantially increased in the BALF of HDM-sensitized and -challenged mice. IL-8 is a potent chemokine to recruit neutrophils. However, IL-17 could be a potential stimulus for the release of IL-8 and -6 from the structural cells in the lungs, such as epithelial cells and macrophages (65). Indeed, IL-17 substantially increases IL-8 secretion from epithelial cells and induces migration of neutrophils. In the presence of IL-17 antibody, IL-8 secretion was substantially reduced together with the inhibition of neutrophil recruitment the lungs (66). Ye and colleagues (17) demonstrated in IL-17 knockout mice that IL-17 was essential for the up-regulation of granulocyte colony-stimulating factor (G-CSF) to induce granulopoiesis, macrophage-inflammatory protein-2 (MIP-2) MIP-2, and TNF-α, which promoted the recruitment of neutrophils (17). In a model of rheumatoid arthritis, IL-17 mediates IL-8 and -6 secretion via activation of phosphatidylinositol 3-kinase/Akt and NF-κB (67). Thus, most of the data support the view that IL-17 causes neutrophil recruitment via IL-8 secretion in the lung. Nonetheless, the therapeutic effect of Flt3-L in the HDM-sensitized mice substantially decreased the levels of IL-6, -8, and TGF-β in the BALF; however, TGF-β and IL-8 were not lowered to the PBS control level. The remaining levels of TGF-β in the BALF may be a result of a skewed development of CD4+CD25+ Tregs, which also use TGF-β for development and function, or Flt3-L treatment may require a longer duration of therapy to completely remove TGF-β. Interestingly, we found an increase in Th2 cytokines, IL-4, -5, and -13, in HDM-sensitized and -challenged mice without Flt3-L treatment. Although not examined in the study, a recent new IL-17 family constituent, IL-25, has been shown to decrease significantly eosinophilia in the lung by increased production of IL-5 and -13, but not IL-4 (63). In addition, we found a significant decrease in eosinophilia in the lungs of HDM-sensitized and -challenged mice after treatment with Flt3-L.
IL-6 has also been implicated in the expression of SOCS proteins in several disease pathologies. Neuwirt and colleagues (65) demonstrated in prostate cancer cells that IL-6 induced a significant up-regulation of SOCS-1, which promoted the differentiation and survival of these cells. In addition, insulin resistance in adipocytes showed a substantial increase in SOCS-1 expression after treatment with IL-6 (64). SOCS-1 is highly expressed in committed Th17 cells, and it has been suggested that SOCS-1 could be a mechanism for IL-4 and IFN-γ resistance by committed Th17 cells (66). To this end, IL-6 and SOCS-1 expression appears to be pivotal in the development of Th17 cells, and Flt3-L was able to reverse or block their development by inhibiting IL-6, SOCS-1, and/or TGF-β.
SOCS-3 has been reported to be a negative regulator of Th17 cells due to its ability to suppress IL-6 and -23 in a STAT3-dependent pathway (46). However, SOCS-3 has been shown to preferentially promote the development of Th2 cells (67). Kinjyo and colleagues (68) demonstrated that SOCS-3–deficient CD4+ T cells secreted high levels of TGF-β and moderate levels of IL-10, suggesting the development of Th3 Treg phenotype. In contrast, the presence of IL-6 with TGF-β blocks the development of Tregs and promotes development of Th17 cells (69). To this end, we have found that Flt3-L completely inhibited the expression of SOCS-3 induced by HDM sensitization, significantly decreased IL-6 and TGF-β, as well as CD4+CD25−IL-17+ T cells, and the effect Flt3-L may arise from the increased differentiation and development of Tregs. In addition, we found high expression of SOCS-5 in the lungs of HDM-sensitized mice; however, Flt3-L treatment did not abolish the expression of SOCS-5. The role of SOCS-5 in allergic asthma remains to be elucidated, but data suggest that SOCS-5 may have overlapping roles with other SOCS proteins. Alternatively, SOCS-5 might not be essential in the differentiation of either Th1 or Th2 cells (70).
In summary, we have delineated that HDM sensitization and challenge causes a substantial increase in CD4+IL-23R+IL-17+ROR-γt+ T cells in the lungs. However, Flt3-L therapy significantly decreased CD4+IL-23R+IL-17+ROR-γt+ T cells in the lung after HDM sensitization and challenge. The therapeutic effect of Flt3-L could be due to significant decreases in IL-6 and TGF-β levels in the lung, and by decreased expression of IL-23R on Th17 cells. This mechanism could be modulated by the expression of SOCS proteins, and possibly by the development of regulatory DCs and/or Tregs in the lungs. Nonetheless, the interaction of Th17 cells and Tregs warrants further investigation.
This work was supported by National Institutes of Health grant R01HL070885.
Originally Published in Press as DOI:10.1165/rcmb.2009-0241OC on November 20, 2009
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Boulet LP. Irreversible airway obstruction in asthma. Curr Allergy Asthma Rep 2009;9:168–173. [DOI] [PubMed] [Google Scholar]
- 2.Meurs H, Gosens R, Zaagsma J. Airway hyperresponsiveness in asthma: lessons from in vitro model systems and animal models. Eur Respir J 2008;32:487–502. [DOI] [PubMed] [Google Scholar]
- 3.Fischer R, Tome D, McGhee JR, Boyaka PN. Th1 and Th2 cells are required for both eosinophil- and neutrophil-associated airway inflammatory responses in mice. Biochem Biophys Res Commun 2007;357:44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamid QA, Cameron LA. Recruitment of T cells to the lung in response to antigen challenge. J Allergy Clin Immunol 2000;106:S227–S234. [DOI] [PubMed] [Google Scholar]
- 5.Hartenstein B, Teurich S, Hess J, Schenkel J, Schorpp-Kistner M, Angel P. Th2 cell–specific cytokine expression and allergen-induced airway inflammation depend on JunB. EMBO J 2002;21:6321–6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood 2008;112:1557–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Awasthi A, Murugaiyan G, Kuchroo VK. Interplay between effector Th17 and regulatory T cells. J Clin Immunol 2008;28:660–670. [DOI] [PubMed] [Google Scholar]
- 8.Lehar SM, Bevan MJ. Immunology: polarizing a T-cell response. Nature 2004;430:150–151. [DOI] [PubMed] [Google Scholar]
- 9.Taylor JJ, Krawczyk CM, Mohrs M, Pearce EJ. Th2 cell hyporesponsiveness during chronic murine schistosomiasis is cell intrinsic and linked to GRAIL expression. J Clin Invest 2009;119:1019–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weaver CT. Th17: the ascent of a new effector T-cell subset. Preface. Eur J Immunol 2009;39:634–636. [DOI] [PubMed] [Google Scholar]
- 11.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 2008;28:454–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McAllister F, Henry A, Kreindler JL, Dubin PJ, Ulrich L, Steele C, Finder JD, Pilewski JM, Carreno BM, Goldman SJ, et al. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-alpha and granulocyte colony-stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J Immunol 2005;175:404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Ramli W, Prefontaine D, Chouiali F, Martin JG, Olivenstein R, Lemiere C, Hamid QT. (H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol 2009;123:1185–1187. [DOI] [PubMed] [Google Scholar]
- 14.Nistala K, Wedderburn LR. Th17 and regulatory T cells: rebalancing pro- and anti-inflammatory forces in autoimmune arthritis. Rheumatology (Oxford) 2009;48:602–606. [DOI] [PubMed] [Google Scholar]
- 15.Cooke A. Th17 cells in inflammatory conditions. Rev Diabet Stud 2006;3:72–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gamelli RL, He LK, Liu H. Recombinant human granulocyte colony-stimulating factor treatment improves macrophage suppression of granulocyte and macrophage growth after burn and burn wound infection. J Trauma 1995;39:1141–1146, discussion 1146–1147. [DOI] [PubMed] [Google Scholar]
- 17.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med 2001;194:519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti–Candida albicans host defense in mice. J Infect Dis 2004;190:624–631. [DOI] [PubMed] [Google Scholar]
- 19.Oukka M. Th17 cells in immunity and autoimmunity. Ann Rheum Dis 2008;67:iii26–iii29. [DOI] [PubMed] [Google Scholar]
- 20.Louten J, Boniface K, de Waal Malefyt R. Development and function of TH17 cells in health and disease. J Allergy Clin Immunol 2009;123:1004–1011. [DOI] [PubMed] [Google Scholar]
- 21.Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, Asher TE, Scheinberg P, Price DA, Hage CA, Kholi LM, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood 2008;112:2826–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klemann C, Raveney BJ, Klemann AK, Ozawa T, von Horsten S, Shudo K, Oki S, Yamamura T. Synthetic retinoid AM80 inhibits Th17 cells and ameliorates experimental autoimmune encephalomyelitis. Am J Pathol 2009;174:2234–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shahrara S, Huang Q, Mandelin AM II, Pope RM. TH-17 cells in rheumatoid arthritis. Arthritis Res Ther 2008;10:R93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emamaullee JA, Davis J, Merani S, Toso C, Elliott JF, Thiesen A, Shapiro AM. Inhibition of Th17 cells regulates autoimmune diabetes in NOD mice. Diabetes 2009;58:1302–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Cesare A, Di Meglio P, Nestle FO. A role for Th17 cells in the immunopathogenesis of atopic dermatitis? J Invest Dermatol 2008;128:2569–2571. [DOI] [PubMed] [Google Scholar]
- 26.Falcone M, Bloom BRA. T helper cell 2 (Th2) immune response against non-self antigens modifies the cytokine profile of autoimmune T cells and protects against experimental allergic encephalomyelitis. J Exp Med 1997;185:901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen CL, Lee CT, Liu YC, Wang JY, Lei HY, Yu CK. House dust mite Dermatophagoides farinae augments proinflammatory mediator productions and accessory function of alveolar macrophages: implications for allergic sensitization and inflammation. J Immunol 2003;170:528–536. [DOI] [PubMed] [Google Scholar]
- 28.Chang JW, Lin CY, Chen WL, Chen CT. Higher incidence of Dermatophagoides pteronyssinus allergy in children of Taipei city than in children of rural areas. J Microbiol Immunol Infect 2006;39:316–320. [PubMed] [Google Scholar]
- 29.Huss K, Adkinson NF Jr, Eggleston PA, Dawson C, Van Natta ML, Hamilton RG. House dust mite and cockroach exposure are strong risk factors for positive allergy skin test responses in the Childhood Asthma Management Program. J Allergy Clin Immunol 2001;107:48–54. [DOI] [PubMed] [Google Scholar]
- 30.Platts-Mills TA, Rakes G, Heymann PW. The relevance of allergen exposure to the development of asthma in childhood. J Allergy Clin Immunol 2000;105:S503–S508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nuhoglu Y, Ozumut SS, Ozdemir C, Ozdemir M, Nuhoglu C, Erguven M. Sublingual immunotherapy to house dust mite in pediatric patients with allergic rhinitis and asthma: a retrospective analysis of clinical course over a 3-year follow-up period. J Investig Allergol Clin Immunol 2007;17:375–378. [PubMed] [Google Scholar]
- 32.Arshad SH. Indoor allergen exposure in the development of allergy and asthma. Curr Allergy Asthma Rep 2003;3:115–120. [DOI] [PubMed] [Google Scholar]
- 33.Schei MA, Hessen JO, Lund E. House-dust mites and mattresses. Allergy 2002;57:538–542. [DOI] [PubMed] [Google Scholar]
- 34.Asadi A, Hedman E, Widen C, Zilliacus J, Gustafsson JA, Wikstrom AC. FMS-like tyrosine kinase 3 interacts with the glucocorticoid receptor complex and affects glucocorticoid dependent signaling. Biochem Biophys Res Commun 2008;368:569–574. [DOI] [PubMed] [Google Scholar]
- 35.Namikawa R, Muench MO, Roncarolo MG. Regulatory roles of the ligand for Flk2/Flt3 tyrosine kinase receptor on human hematopoiesis. Stem Cells 1996;14:388–395. [DOI] [PubMed] [Google Scholar]
- 36.Rosnet O, Marchetto S, deLapeyriere O, Birnbaum D. Murine Flt3, a gene encoding a novel tyrosine kinase receptor of the PDGFR/CSF1R family. Oncogene 1991;6:1641–1650. [PubMed] [Google Scholar]
- 37.Scheijen B, Griffin JD. Tyrosine kinase oncogenes in normal hematopoiesis and hematological disease. Oncogene 2002;21:3314–3333. [DOI] [PubMed] [Google Scholar]
- 38.Lyman SD, James L, Johnson L, Brasel K, de Vries P, Escobar SS, Downey H, Splett RR, Beckmann MP, McKenna HJ. Cloning of the human homologue of the murine flt3 ligand: a growth factor for early hematopoietic progenitor cells. Blood 1994;83:2795–2801. [PubMed] [Google Scholar]
- 39.Hannum C, Culpepper J, Campbell D, McClanahan T, Zurawski S, Bazan JF, Kastelein R, Hudak S, Wagner J, Mattson J, et al. Ligand for FLT3/FLK2 receptor tyrosine kinase regulates growth of haematopoietic stem cells and is encoded by variant RNAs. Nature 1994;368:643–648. [DOI] [PubMed] [Google Scholar]
- 40.Maraskovsky E, Brasel K, Teepe M, Roux ER, Lyman SD, Shortman K, McKenna HJ. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med 1996;184:1953–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maraskovsky E, Daro E, Roux E, Teepe M, Maliszewski CR, Hoek J, Caron D, Lebsack ME, McKenna HJ. In vivo generation of human dendritic cell subsets by Flt3 ligand. Blood 2000;96:878–884. [PubMed] [Google Scholar]
- 42.Edwan JH, Perry G, Talmadge JE, Agrawal DK. Flt-3 ligand reverses late allergic response and airway hyper-responsiveness in a mouse model of allergic inflammation. J Immunol 2004;172:5016–5023. [DOI] [PubMed] [Google Scholar]
- 43.McGee HS, Edwan JH, Agrawal DK. Flt3-L increases CD4+CD25+Foxp3+ICOS+ cells in the lung of cockroach-sensitized and challenged mice. Am J Respir Cell Mol Biol 2009;42:331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alexander WS, Hilton DJ. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu Rev Immunol 2004;22:503–529. [DOI] [PubMed] [Google Scholar]
- 45.Senn JJ, Klover PJ, Nowak IA, Zimmers TA, Koniaris LG, Furlanetto RW, Mooney RA. Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleukin-6–dependent insulin resistance in hepatocytes. J Biol Chem 2003;278:13740–13746. [DOI] [PubMed] [Google Scholar]
- 46.Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, Yoshimura A, Hennighausen L, O'Shea JJ. Selective regulatory function of SOCS3 in the formation of IL-17–secreting T cells. Proc Natl Acad Sci USA 2006;103:8137–8142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity 2004;21:467–476. [DOI] [PubMed] [Google Scholar]
- 48.Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Page N, Olivenstein R, Elias J, Chakir J. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol 2001;108:430–438. [DOI] [PubMed] [Google Scholar]
- 49.Sun YC, Zhou QT, Yao WZ. Sputum interleukin-17 is increased and associated with airway neutrophilia in patients with severe asthma. Chin Med J (Engl) 2005;118:953–956. [PubMed] [Google Scholar]
- 50.Ferretti S, Bonneau O, Dubois GR, Jones CE, Trifilieff A. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J Immunol 2003;170:2106–2112. [DOI] [PubMed] [Google Scholar]
- 51.Caldwell CC, Okaya T, Martignoni A, Husted T, Schuster R, Lentsch AB. Divergent functions of CD4+ T lymphocytes in acute liver inflammation and injury after ischemia–reperfusion. Am J Physiol Gastrointest Liver Physiol 2005;289:G969–G976. [DOI] [PubMed] [Google Scholar]
- 52.Miyamoto M, Prause O, Sjostrand M, Laan M, Lotvall J, Linden A. Endogenous IL-17 as a mediator of neutrophil recruitment caused by endotoxin exposure in mouse airways. J Immunol 2003;170:4665–4672. [DOI] [PubMed] [Google Scholar]
- 53.Woolard MD, Hensley LL, Kawula TH, Frelinger JA. Respiratory Francisella tularensis live vaccine strain infection induces Th17 cells and prostaglandin E2, which inhibits generation of gamma interferon–positive T cells. Infect Immun 2008;76:2651–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fielding CA, McLoughlin RM, McLeod L, Colmont CS, Najdovska M, Grail D, Ernst M, Jones SA, Topley N, Jenkins BJ. IL-6 regulates neutrophil trafficking during acute inflammation via STAT3. J Immunol 2008;181:2189–2195. [DOI] [PubMed] [Google Scholar]
- 55.Yoshimura T, Sonoda KH, Ohguro N, Ohsugi Y, Ishibashi T, Cua DJ, Kobayashi T, Yoshida H, Yoshimura A. Involvement of Th17 cells and the effect of anti-IL-6 therapy in autoimmune uveitis. Rheumatology (Oxford) 2009;48:347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006;441:235–238. [DOI] [PubMed] [Google Scholar]
- 57.Wan H, Winton HL, Soeller C, Gruenert DC, Thompson PJ, Cannell MB, Stewart GA, Garrod DR, Robinson C. Quantitative structural and biochemical analyses of tight junction dynamics following exposure of epithelial cells to house dust mite allergen Der p 1. Clin Exp Allergy 2000;30:685–698. [DOI] [PubMed] [Google Scholar]
- 58.Wan H, Winton HL, Soeller C, Tovey ER, Gruenert DC, Thompson PJ, Stewart GA, Taylor GW, Garrod DR, Cannell MB, et al. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J Clin Invest 1999;104:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Donnelly S, Dalton JP, Loukas A. Proteases in helminth- and allergen-induced inflammatory responses. Chem Immunol Allergy 2006;90:45–64. [DOI] [PubMed] [Google Scholar]
- 60.D'Andrea MR, Derian CK, Leturcq D, Baker SM, Brunmark A, Ling P, Darrow AL, Santulli RJ, Brass LF, Andrade-Gordon P. Characterization of protease-activated receptor–2 immunoreactivity in normal human tissues. J Histochem Cytochem 1998;46:157–164. [DOI] [PubMed] [Google Scholar]
- 61.Kauffman HF, Tamm M, Timmerman JA, Borger P. House dust mite major allergens Der p 1 and Der p 5 activate human airway-derived epithelial cells by protease-dependent and protease-independent mechanisms. Clin Mol Allergy 2006;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gordon JR. TGFbeta1 and TNFalpha secreted by mast cells stimulated via the FcepsilonRI activate fibroblasts for high-level production of monocyte chemoattractant protein-1 (MCP-1). Cell Immunol 2000;201:42–49. [DOI] [PubMed] [Google Scholar]
- 63.Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, Menon S, Seymour B, Jackson C, Kung TT, et al. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol 2002;169:443–453. [DOI] [PubMed] [Google Scholar]
- 64.Fasshauer M, Kralisch S, Klier M, Lossner U, Bluher M, Klein J, Paschke R. Insulin resistance-inducing cytokines differentially regulate SOCS mRNA expression via growth factor– and Jak/Stat-signaling pathways in 3T3-L1 adipocytes. J Endocrinol 2004;181:129–138. [DOI] [PubMed] [Google Scholar]
- 65.Neuwirt H, Puhr M, Cavarretta I, Mitterberger M, Hobisch A, Culig Z. Suppressor of cytokine signalling is up-regulated by androgen in prostate cancer cell lines and inhibits androgen mediated proliferation and secretion. Endocrine-Related Cancer 2007;14:1007–1019. [DOI] [PubMed] [Google Scholar]
- 66.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol 2007;7:454–465. [DOI] [PubMed] [Google Scholar]
- 67.Kubo M, Inoue H. Suppressor of cytokine signaling 3 (SOCS3) in Th2 cells evokes Th2 cytokines, IgE, and eosinophilia. Curr Allergy Asthma Rep 2006;6:32–39. [DOI] [PubMed] [Google Scholar]
- 68.Kinjyo I, Inoue H, Ohishi M, Hamano S, Fukuyama S, Yoshimura T, Koga K, Takakis H, Himeno K, Takaesu G, et al. Loss of SOCS3 in T helper cells resulted in reduced immune responses and hyperproduction of interleukin 10 and transforming growth factor-β 1. J Exp Med 2006;203:1021–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sonderegger I, Iezzi G, Maier R, Schmitz N, Kurrer M, Kopf M. GM-CSF mediates autoimmunity by enhancing IL-6–dependent Th17 cell development and survival. J Exp Med 2008;205:2281–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brender C, Columbus R, Metcalf D, Handman E, Starr R, Huntington N, Tarlinton D, Odum N, Nicholson SE, Nicola NA, et al. SOCS5 is expressed in primary B and T lymphoid cells but is dispensable for lymphocyte production and function. Mol Cell Biol 2004;24:6094–6103. [DOI] [PMC free article] [PubMed] [Google Scholar]