Abstract
Rationale: It is unclear if lung perfusion can predict response to lung volume reduction surgery (LVRS).
Objectives: To study the role of perfusion scintigraphy in patient selection for LVRS.
Methods: We performed an intention-to-treat analysis of 1,045 of 1,218 patients enrolled in the National Emphysema Treatment Trial who were non–high risk for LVRS and had complete perfusion scintigraphy results at baseline. The median follow-up was 6.0 years. Patients were classified as having upper or non–upper lobe–predominant emphysema on visual examination of the chest computed tomography and high or low exercise capacity on cardiopulmonary exercise testing at baseline. Low upper zone perfusion was defined as less than 20% of total lung perfusion distributed to the upper third of both lungs as measured on perfusion scintigraphy.
Measurements and Main Results: Among 284 of 1,045 patients with upper lobe–predominant emphysema and low exercise capacity at baseline, the 202 with low upper zone perfusion had lower mortality with LVRS versus medical management (risk ratio [RR], 0.56; P = 0.008) unlike the remaining 82 with high perfusion where mortality was unchanged (RR, 0.97; P = 0.62). Similarly, among 404 of 1,045 patients with upper lobe–predominant emphysema and high exercise capacity, the 278 with low upper zone perfusion had lower mortality with LVRS (RR, 0.70; P = 0.02) unlike the remaining 126 with high perfusion (RR, 1.05; P = 1.00). Among the 357 patients with non–upper lobe–predominant emphysema (75 with low and 282 with high exercise capacity) there was no improvement in survival with LVRS and measurement of upper zone perfusion did not contribute new prognostic information.
Conclusions: Compared with optimal medical management, LVRS reduces mortality in patients with upper lobe–predominant emphysema when there is low rather than high perfusion to the upper lung.
Keywords: perfusion, computed tomography, emphysema, mortality, lung volume reduction surgery
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
The role of perfusion scintigraphy in patient selection for lung volume reduction surgery (LVRS) has been investigated by multiple studies with conflicting results.
What This Study Adds to the Field
The current study reports that measurement of upper lung perfusion via perfusion scintigraphy adds new prognostic information, in addition to that available by visual examination of the chest computed tomogram, and helps identify patients likely to survive longer with LVRS rather than with medical management.
Lung volume reduction surgery (LVRS) can be an effective treatment for emphysema in carefully selected patients. It is well established that those with advanced upper lobe–predominant emphysema derive the greatest benefit from this surgical procedure (1–3). The current standard of care for defining upper lung–predominant emphysema is visual examination of the chest computed tomogram (CT) (1, 4). Perfusion scintigraphy is another commonly available test for assessing the distribution of emphysema. Unlike chest CT, which defines only the anatomic distribution of emphysema, perfusion imaging also reflects regional lung function (5). Although the role of perfusion scintigraphy in patient selection for LVRS has been investigated by multiple prior studies, the results have been inconclusive (6–11). These studies have examined perfusion scintigraphy as a replacement rather than a complementary test to the chest CT, and have been limited in sample size.
We used data from the National Emphysema Treatment Trial (NETT) to study the role of perfusion scintigraphy in patient selection for LVRS. We hypothesized that perfusion scintigraphy would complement visual examination of the chest CT in selecting patients for LVRS. Some of the results of this study have been previously reported in the form of an abstract (12).
METHODS
Study Design
A detailed design of NETT has been published previously and only the relevant aspects are reviewed here (13). Enrollment began in January 1998 and ended in July 2002. Major enrollment criteria are listed in the online supplement. All patients underwent a prerandomization (baseline) evaluation including a perfusion scintigram, chest CT, measurement of maximal exercise capacity on cardiopulmonary exercise testing, pulmonary function tests, and quality-of-life questionnaires (St. George's Respiratory Questionnaire [SGRQ] and the University of California at San Diego Shortness of Breath Questionnaire [SOBQ]). Patients were then randomized 1:1 to LVRS or optimal medical management and returned for postrandomization visits at 6 months, 12 months, and yearly thereafter through December 2002. During 2003, patients returned for examinations at 6 months and 2, 3, and 5 years after randomization. At each postrandomization visit assessment of exercise capacity, pulmonary function, and quality of life was repeated to assess the efficacy of the treatment provided.
Patient Cohort
One thousand two hundred and eighteen patients were enrolled into NETT. For the current analysis, we excluded the 140 patients known to be high risk for LVRS per previously published criteria because these patients are not considered candidates for LVRS or approved for LVRS by the Centers for Medicare and Medicaid Services or the Joint Commission on Accreditation (14, 15). An additional 33 patients were excluded because of incomplete perfusion scan results at baseline, resulting in a final sample size of 1,045.
Perfusion Scintigraphy
Participants were injected with 4 mCi of technetium-99 macroaggregated albumin over 5–10 respiratory cycles and imaged in the upright position. The anterior and posterior computerized scintigraphic lung images were enclosed by a rectangle abutting the superior, inferior, medial, and lateral aspects of the lung. This rectangle was divided into upper, middle, and lower zones of equal craniocaudal height, using two horizontal lines (11). The counts for a given zone on the anterior and posterior images were multiplied and then the square root was taken. This value was divided by the total of similar values for all six lung zones (three for each lung) and multiplied by 100 to obtain the percent perfusion to that zone.
Because LVRS is beneficial mostly for patients with upper lobe–predominant emphysema we decided a priori to focus on perfusion to the upper lung zones. The median combined perfusion to the two upper zones in the NETT cohort was 20% of total lung perfusion; therefore, we used 20% as the cutoff for classifying patients as having low or high upper zone perfusion. To check our assumption that 20% was a good cutoff value, we examined a number of different cutoffs spaced at 2.5% intervals as predictors of mortality after LVRS.
Chest CT
High-resolution chest CT images were obtained for each patient and interpreted by visual and densitometric methods (1). Upper versus non–upper lung predominance was assessed on the basis of the radiologist's visual examination of the CT scan (1). For densitometric measurements, the lung was divided into upper, middle, and lower zones of equal craniocaudal height analogous to the perfusion images, and the percent emphysema was assessed separately for each zone, using a threshold value of −950 Hounsfield units as described previously (4, 16).
Exercise Capacity
Patients were classified as having low exercise capacity if the baseline exercise capacity was not more than 40 W (men) or 25 W (women) on cardiopulmonary exercise testing, using a cycle ergometer, and vice versa as described previously (1, 15).
Outcomes after LVRS
To facilitate comparison with prior reports, the main outcomes of interest were mortality and improvement in exercise capacity by at least 10 W (1). Multiple other outcomes were explored: improvement in FEV1 by at least 100 ml, total SGRQ score by at least 8 points, and SOBQ score by at least 5 points from baseline. These outcomes were assessed 1, 2, and 3 years after randomization; they were not analyzed at 5 years or beyond because a significant proportion of the cohort (41%) had died. The cutoffs for defining improvement were chosen because they are thought to represent clinically important changes in the respective parameters after LVRS (17–20). To minimize potential for bias and to produce conservative estimates, patients who died or were missing at follow-up were assumed to have not improved.
Vital status, last updated in September 2008, was ascertained by reports from the clinical centers and review of the Social Security Administration's Death Master File.
Statistical Analysis
The analyses were performed post-hoc according to the intention-to-treat principle. The baseline characteristics of the 1,045 patients with low versus high upper zone perfusion were compared by univariate analysis.
Analysis of the role of upper zone perfusion in patient selection for LVRS was performed in four previously defined prognostic subgroups (1), that is, (1) those with upper lobe–predominant emphysema and low exercise capacity (n = 284 of 1,045), (2) upper lobe–predominant emphysema and high exercise capacity (n = 404 of 1,045), (3) non–upper lobe–predominant emphysema and low exercise capacity (n = 145 of 1,045), and (4) non–upper lobe–predominant emphysema and high exercise capacity (n = 212 of 1,045). In each of these four groups, patients were further classified as having low or high upper zone perfusion. Mortality and the other outcomes after randomization were compared in those with low versus high perfusion.
Mortality was examined on the basis of Kaplan-Meier survival curves. Log-rank tests were not used to compare survival curves because the hazard functions were expected to cross each other because of the early mortality from LVRS resulting in nonproportional hazards; instead, the proportion of patients who died by a given time point after randomization were compared by Fisher's exact tests (1). The risk ratio (RR) for mortality was estimated on the basis of the overall mortality in each subgroup after a median follow-up of 6.0 years.
Outcomes besides mortality were examined by comparing the proportions of patients (those with low vs. high upper zone perfusion) who demonstrated improvement in these outcomes 1, 2, and 3 years after randomization, using Fisher's exact tests. Bar charts with corresponding P values were used to summarize the results. The sample sizes for these outcomes were 10–15% smaller than those for the mortality analysis because patients who had not been in the study long enough to complete 1-, 2-, or 3-year assessments had to be excluded.
To determine whether there were differences in outcomes with LVRS versus medical management for patients with low versus high upper zone perfusion, logistic regression models were used. A separate model was created with mortality at 1, 3, 5, 7, and 9 years and improvement in exercise capacity and health-related quality of life 1 and 3 years after randomization as the outcome. Each model included a term for treatment group assignment (LVRS vs. medical management), upper zone perfusion (low vs. high), and an interaction term between the treatment group and upper zone perfusion. P values for the interaction terms were determined by exact score tests for logistic regression. Because statistical tests for interactions have low power these tests were performed separately in two groups (upper vs. non–upper lobe predominant) instead of the four groups described previously.
Summary statistics are reported as proportions or medians with interquartile range. To compare continuous variables the t test or Mann-Whitney test was used after assessing the data for normality. Statistical significance was defined as two-tailed P < 0.05. All analyses were performed with SAS version 9.1.4 (SAS Institute, Cary, NC).
RESULTS
The comparison of baseline characteristics of the 1,045 participants included in our analysis with low versus high upper zone perfusion is included in Table 1. Clinically small but statistically significant differences were present for the diffusing capacity of carbon monoxide and PaO2. The percentage of female patients was slightly higher among those with high versus low upper zone perfusion. As expected, patients with low rather than high upper zone perfusion had more upper zone emphysema. Notably, spirometry results and exercise capacity at baseline were similar in the two groups.
TABLE 1.
COMPARISON OF BASELINE CHARACTERISTICS OF 1,045 PATIENTS INCLUDED IN CURRENT ANALYSIS WITH LOW VERSUS HIGH UPPER ZONE PERFUSION
| Upper Zone Perfusion <20% |
Upper Zone Perfusion ≥20% |
||
|---|---|---|---|
| Baseline Characteristic | (n = 555) | (n = 490) | P Value |
| Demographic factors | |||
| Age, median (IQR), yr | 68 (64, 71) | 68 (64, 71) | 0.61 |
| Female, % | 37 | 43 | 0.050 |
| Body mass index, median (IQR), kg/m2 | 25 (22, 27) | 25 (22, 28) | 0.47 |
| Smoking history | |||
| Pack-years of smoking, median (IQR) | 60 (42, 84) | 61 (42, 82) | 0.60 |
| Years since quitting, median (IQR) | 8 (4, 14) | 8 (4, 15) | 0.31 |
| Pulmonary function | |||
| FEV1, median (IQR), % predicted | 27 (22, 32) | 28 (23, 32) | 0.31 |
| FVC, median (IQR), % predicted | 68 (59, 78) | 69 (59, 79) | 0.50 |
| RV, median (IQR), % predicted | 212 (185, 245) | 208 (184, 238) | 0.20 |
| TLC, median (IQR), % predicted | 127 (116, 136) | 127 (117, 136) | 0.59 |
| DlCO, median (IQR), % predicted | 28 (22, 34) | 29 (24, 36) | 0.003 |
| Arterial blood gases | |||
| PaO2, median (IQR), mm Hg | 66 (59, 74) | 63 (56, 70) | <0.001 |
| PaCO2, median (IQR), mm Hg | 42 (38, 45) | 42 (39, 46) | 0.09 |
| Quality-of-life scores | |||
| SGRQ total score, median (IQR) | 53 (43, 62) | 53 (43, 61) | 0.69 |
| SOBQ score, median (IQR) | 63 (51, 74) | 61 (48, 37) | 0.99 |
| Maximal exercise capacity, median (IQR), W | 38 (25, 51) | 38 (25, 51) | 0.67 |
| LVEF <45%,* % | 4.0 | 3.5 | 0.74 |
| Percent emphysema in upper zone, median (IQR)† | 29 (18, 40) | 9 (3, 19) | <0.001 |
Definition of abbreviations: DlCO = diffusing capacity of carbon monoxide; IQR = interquartile range; LVEF = left ventricular ejection fraction; RV = residual volume; SGRQ = St. George Respiratory Questionnaire; SOBQ = University of California at San Diego Shortness-of-Breath Questionnaire; TLC = total lung capacity.
Measured by transthoracic echocardiography.
Measured by computed tomography densitometry and available for 606 patients.
In the 555 patients with low upper zone perfusion, blood flow to the right and left upper zones was symmetrically reduced: median perfusion to the right upper zone was 7% (interquartile range, 5–9%) and median perfusion to the left upper zone was 5% (interquartile range, 4–7%).
Upper Lobe–predominant Emphysema and Low Exercise Capacity
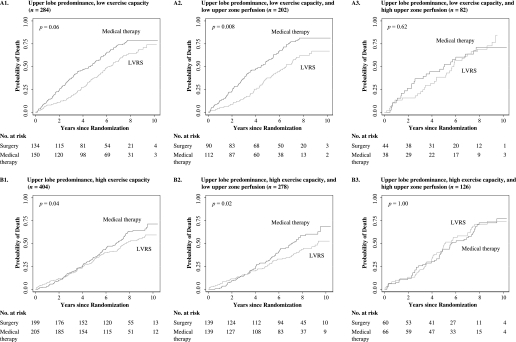
Among the 284 patients with upper lobe–predominant emphysema and low exercise capacity, mortality was lower with LVRS rather than optimal medical management (Figure 1, panel A1: RR, 0.66; P = 0.06). When these patients were further subclassified as having low or high upper zone perfusion this reduction in mortality was greater and was present only in the 202 patients with low perfusion (Figure 1, panel A2: RR, 0.56; P = 0.008). The survival curves for LVRS versus medical management separated early and the survival advantage was maintained until the end of follow-up in (Figure 1, panel A2). In contrast, for the remaining 82 patients who had high upper zone perfusion there was no reduction in mortality (Figure 1, panel A3: RR, 0.97; P = 0.62).
Figure 1.
Kaplan-Meier survival curves after randomization for patients with upper lobe–predominant emphysema (n = 688). Among those with low exercise capacity (panel A1), mortality was lower with lung volume reduction surgery (LVRS) than with optimal medical management (risk ratio [RR], 0.66; P = 0.06). On further classifying patients by upper zone perfusion, this reduction in mortality was greater and was restricted to patients with low upper zone perfusion (panel A2: RR, 0.56; P = 0.008) unlike those with high perfusion (panel A3: RR, 0.97; P = 0.62). Among those with high exercise capacity at baseline (panel B1), reduction in mortality was again greater with LVRS (RR, 0.80; P = 0.04). On further classification by upper zone perfusion this reduction in mortality was restricted to patients with low upper zone perfusion (panel B2: RR, 0.70; P = 0.02), unlike those with high perfusion (panel B3: RR, 1.05; P = 1.00).
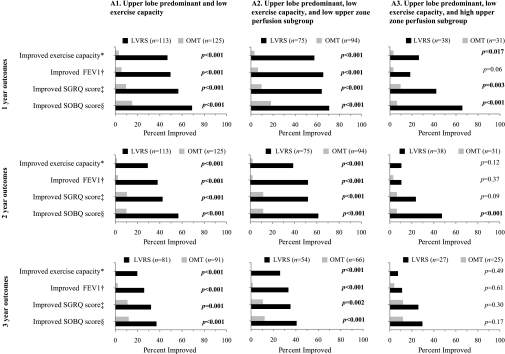
Outcomes besides survival were superior with LVRS in these patients (Figure 2, column A1). Among those with low upper zone perfusion (Figure 2, column A2), these improvements with LVRS were greater and were durable until Year 3 postrandomization, unlike those with high upper zone perfusion (Figure 2, column A3).
Figure 2.
Comparison of frequency of improvement in functional outcomes after randomization to lung volume reduction surgery (LVRS, solid bars) versus optimal medical treatment (OMT, gray bars) for patients with upper lobe–predominant emphysema and low exercise capacity at baseline. Outcomes were consistently better with LVRS through Year 3 (column A1). On further classification by upper zone perfusion, those with low (column A2) rather than high upper zone perfusion (column A3) derived greater benefit from LVRS. These improvements were durable to Year 3 in patients with low but not high upper zone perfusion. SGRQ = St. George's Respiratory Questionnaire; SORQ = University of California at San Diego Shortness of Breath Questionnaire. *Improvement in exercise capacity by at least 10 W; †improvement in FEV1 by at least 100 ml; ‡improvement in total SGRQ score by at least 8 points; §improvement in SOBQ score by at least 5 points from baseline.
Upper Lobe–predominant Emphysema and High Exercise Capacity
Among the 404 patients with upper lobe–predominant emphysema and high exercise capacity, mortality was lower with LVRS rather than optimal medical management (Figure 1, panel B1: RR, 0.80; P = 0.04). When these patients were further subclassified as having low or high upper zone perfusion this reduction in mortality was greater and was present only in the 278 patients with low upper zone perfusion (Figure 1, panel B2: RR, 0.70; P = 0.02). In these patients, there was an early increase in mortality with LVRS that was likely postsurgical, followed by a crossing of the curves and a reduced mortality beyond Year 4 with LVRS. In the remaining 126 patients with high perfusion there was no difference in mortality with LVRS versus medical management (Figure 1, panel B3: RR, 1.05; P = 1.00).
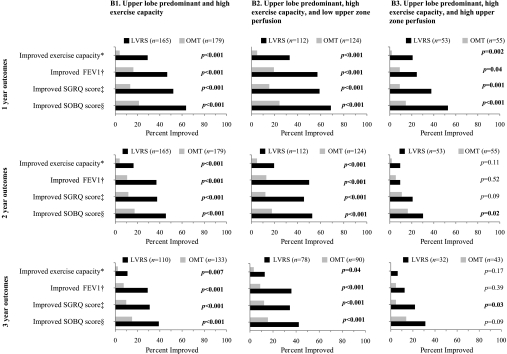
Outcomes besides survival were superior with LVRS in these patients (Figure 3, column B1). Among those with low upper zone perfusion (Figure 3, column B2) the improvements were greater and durable to the third year of follow-up, unlike those with high upper zone perfusion (Figure 3, column B3).
Figure 3.
Comparison of frequency of improvement in functional outcomes after randomization to lung volume reduction surgery (LVRS, solid bars) versus optimal medical treatment (OMT, gray bars) for patients with upper lobe–predominant emphysema and high exercise capacity at baseline. Outcomes were better with LVRS through Year 3 after randomization (column B1). On further classification by upper zone perfusion, those with low upper zone perfusion (column B2) rather than high upper zone perfusion (column B3) derived greater benefit from LVRS. These improvements were durable to Year 3 in patients with low but not high upper zone perfusion. *Improvement in exercise capacity by at least 10 W; †improvement in FEV1 by at least 100 ml; ‡improvement in total SGRQ score by at least 8 points; §improvement in SOBQ score by at least 5 points from baseline.
Non–upper Lobe–predominant Emphysema and Low Exercise Capacity
Among the 145 patients with non–upper lobe–predominant emphysema and low exercise capacity, mortality was similar with LVRS or optimal medical management (see Figure E1, panel A1, in the online supplement: RR, 0.78; P = 0.12). The same remained true for patients with either low (Figure E1, panel A2: RR, 0.88; P = 0.38; n = 36) or high upper zone perfusion (Figure E1, panel A3: RR, 0.76; P = 0.21; n = 109).
Outcomes besides mortality were superior with LVRS in these patients (Figure E2, column A) although the improvements did not persist until Year 3 of follow-up. The same pattern was present in those with either low or high upper zone perfusion (Figure E2, columns B and C, respectively). P values were nonsignificant for those with low perfusion 1 year postrandomization, likely because of the smaller sample size compared with the high-perfusion group (n = 39 vs. 100).
Non–upper Lobe–predominant Emphysema and High Exercise Capacity
Among the 212 patients with non–upper lobe–predominant emphysema and high exercise capacity, mortality was nonsignificantly higher with LVRS rather than optimal medical management (Figure E1, panel B1: RR, 1.2; P = 0.20). The same remained true for patients with either low (Figure E1, panel B2: RR, 1.8; P = 0.33) or high upper zone perfusion (Figure E1, panel B3: RR, 1.1; P = 0.35).
Outcomes besides mortality were superior with LVRS in these patients (Figure E3, column A) although the improvements did not persist until Year 3 of follow-up. The same pattern was present in those with either low or high upper zone perfusion (Figure E3, columns B and C, respectively). P values were nonsignificant for those with low perfusion 1 year postrandomization, likely because of the smaller sample size compared with the high perfusion group (n = 34 vs. 96).
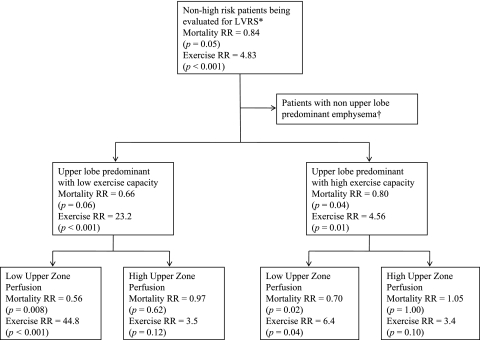
The key findings from the analysis so far have been summarized in Figure 4.
Figure 4.
Flow diagram summarizing the main results of our analysis. Risk ratios (RRs) for mortality were estimated on the basis of the overall mortality in each subgroup after a median follow-up of 6.0 years. The RR for improvement in exercise capacity was assessed by maximal workload achieved on a cycle ergometer 2 years after randomization (less than 10 W vs. at least 10 W).*High risk were patients with FEV1 not exceeding 20% predicted and either diffusing capacity of carbon monoxide not exceeding 20% predicted or nonheterogeneous distribution of emphysema on computed tomography. †Patients with non–upper lobe–predominant emphysema and low exercise capacity can experience more frequent improvement in functional outcomes with lung volume reduction surgery (LVRS) rather than optimal medical management though these are not durable beyond the first 2 years after LVRS, unlike in patients with upper lobe–predominant emphysema. There is no improvement in survival with surgery. In the current analysis upper zone perfusion did not help further define prognosis in this group of patients.
Differential Reduction in Mortality and Other Outcomes with LVRS in Patients with Low versus High Upper Zone Perfusion
In the 688 patients with upper lobe predominance, P values for interaction terms testing a differential survival advantage with LVRS in those with low rather than high upper zone perfusion (n = 284 and 404) were significant after 5 years of follow-up (P = 0.01 at Year 7 and P = 0.03 at Year 9) unlike those before 5 years (P = 0.56 at Year 1, P = 0.83 at Year 3, and P = 0.23 at Year 5).
In contrast, P values for the same interaction terms for the 357 patients with non–upper lobe–predominant emphysema were nonsignificant throughout follow-up (P = 0.70 at Year 1, P = 0.36 at Year 3, P = 0.30 at Year 5, P = 0.59 at Year 7, and P = 0.77 at Year 9).
Selection of a Cutoff Value for Defining Low versus High Upper Zone Perfusion
Examination of the effect of using different cutoff values on odds ratios predicting mortality 1 year post-LVRS confirmed that 20% was a good cutoff because the odds ratios started to approach 1 and the confidence intervals started to widen if the cutoff exceeded or was less than 20% (Figure E4).
DISCUSSION
We examined the role of perfusion scintigraphy in patient selection for LVRS, using data from a large randomized control trial. Among those with upper lobe–predominant emphysema and either low or high exercise capacity mortality was reduced after LVRS if there was low and not high upper zone perfusion (Figure 4). In contrast, among patients with non–upper lobe–predominant emphysema there was no reduction in mortality with LVRS, and performing perfusion scintigraphy did not contribute new prognostic information.
Cooper and colleagues revived interest in LVRS in the 1990s; they examined both the perfusion scintigram and chest CT in selecting surgical candidates with heterogeneous distribution of diseased areas amenable to surgical resection (21). Subsequent research compared perfusion scintigraphy with visual examination of the chest CT to determine which one technique was superior in identifying upper lobe–predominant emphysema. However, these studies were limited in sample size and produced conflicting results (2, 6–9, 22).
Similar to our findings from this updated NETT data set, results from the analysis of the initial data from NETT found that the (upper)/(middle + lower) zone perfusion ratio did not identify differential improvements in survival, exercise capacity, and health-related quality of life up to 2 years after randomization. In our analysis, the statistical tests for interaction become significant only after Year 5 of follow-up. This is not surprising because these statistical tests have optimal power when about 50% of the cohort has had events (death in our study), that is, about Year 6, which was the median survival in our cohort. Also, these tests were significant only for those with upper lobe predominance, whereas the tests for interaction in the early NETT data were performed in the entire cohort (upper and non–upper lobe predominant) because the importance of upper lobe predominance as a prognostic variable was not established at that time.
At our medical center in Boston and at the University of Pennsylvania, even prior to our results being known, perfusion scintigrams have been obtained and visually examined for disease distribution in all patients being evaluated for LVRS. Our results now provide the evidence and an objective framework for the interpretation of these scintigrams. We recommend that patients with upper lobe–predominant emphysema on visual examination of the chest CT undergo perfusion scintigraphy in addition to measurement of maximal exercise capacity. This will allow the clinician to classify them into one of the four subgroups described at the bottom of Figure 4. For patients with non–upper lobe–predominant emphysema perfusion scintigraphy should not be performed because no additional prognostic information is obtained.
The likely reason why lung perfusion provides additional prognostic information in patients known to have upper lobe predominance on chest CT is because unlike chest CT, which is a purely anatomical measure of emphysema distribution, lung perfusion also reflects regional lung function (5, 23). A combination of structural (chest CT) and functional (perfusion scintigraphy) upper lobe predominance may give a better summary of emphysema distribution for the purpose of patient selection for LVRS rather than structural disease alone. Therefore, surgical resection of emphysematous and poorly perfused lung produces better results than resection of emphysematous but better perfused parenchyma.
Our data suggest that patients with low versus high upper zone perfusion are part of the same clinical phenotype of advanced emphysema because a number of important clinical variables were similar in the two groups (Table 1).
Our study has a number of limitations. The division of each lung into three zones of equal craniocaudal height was arbitrary; however, this method is easy to remember and apply in clinical practice. Even though the same method was used to define lung zones in scintigraphic and CT images, the lung zones may not have corresponded exactly when assessed by these different imaging techniques. Finally, the long duration of follow-up and data on a number of outcomes resulted in a large number of comparisons and P values. We attempted to minimize the number of tests by being selective, for example, tests for interaction were performed at alternative years instead of annually during follow-up. Also, the P values obtained indicated consistent patterns in the data with no one “standout” value that would suggest a chance finding.
Conclusion
Perfusion scintigraphy should be considered in patients with upper lobe–predominant emphysema being evaluated for LVRS, because those with upper lung perfusion less than 20% rather than 20% or more of total lung perfusion are likely to live longer and have more frequent improvement in functional outcomes with LVRS rather than continued medical management.
Supplementary Material
Acknowledgments
Members of the National Emphysema Treatment Trial Research Group : Office of the Chair of the Steering Committee, University of Pennsylvania, Philadelphia, PA: Alfred P. Fishman, M.D. (Chair); Betsy Ann Bozzarello; Ameena Al-Amin.
Clinical centers: Baylor College of Medicine, Houston, TX: Marcia Katz, M.D. (Principal Investigator); Carolyn Wheeler, R.N., B.S.N. (Principal Clinic Coordinator); Elaine Baker, R.R.T., R.P.F.T.; Peter Barnard, Ph.D., R.P.F.T.; Phil Cagle, M.D.; James Carter, M.D.; Sophia Chatziioannou, M.D.; Karla Conejo-Gonzales; Kimberly Dubose, R.R.T.; John Haddad, M.D.; David Hicks, R.R.T., R.P.F.T.; Neal Kleiman, M.D.; Mary Milburn-Barnes, C.R.T.T.; Chinh Nguyen, R.P.F.T.; Michael Reardon, M.D.; Joseph Reeves-Viets, M.D.; Steven Sax, M.D.; Amir Sharafkhaneh, M.D.; Owen Wilson, Ph.D.; Christine Young P.T.; Rafael Espada, M.D. (Principal Investigator, 1996–2002); Rose Butanda (1999–2001); Minnie Ellisor (2002); Pamela Fox, M.D. (1999–2001); Katherine Hale, M.D. (1998–2000); Everett Hood, R.P.F.T. (1998–2000); Amy Jahn (1998–2000); Satish Jhingran, M.D. (1998–2001); Karen King, R.P.F.T. (1998–1999); Charles Miller III, Ph.D. (1996–1999); Imran Nizami, M.D. (Co-Principal Investigator, 2000–2001); Todd Officer (1998–2000); Jeannie Ricketts (1998–2000); Joe Rodarte, M.D. (Co-Principal Investigator, 1996–2000); Robert Teague, M.D. (Co-Principal Investigator, 1999–2000); Kedren Williams (1998–1999).
Brigham and Women's Hospital, Boston, MA: John Reilly, M.D. (Principal Investigator); David Sugarbaker, M.D. (Co-Principal Investigator); Carol Fanning, R.R.T. (Principal Clinic Coordinator); Simon Body, M.D.; Sabine Duffy, M.D.; Vladmir Formanek, M.D.; Anne Fuhlbrigge, M.D.; Philip Hartigan, M.D.; Sarah Hooper, E.P.; Andetta Hunsaker, M.D.; Francine Jacobson, M.D.; Marilyn Moy, M.D.; Susan Peterson, R.R.T.; Roger Russell, M.D.; Diane Saunders; Scott Swanson, M.D. (Co-Principal Investigator, 1996–2001).
Cedars-Sinai Medical Center, Los Angeles, CA: Rob McKenna, M.D. (Principal Investigator); Zab Mohsenifar, M.D. (Co-Principal Investigator); Carol Geaga, R.N. (Principal Clinic Coordinator); Manmohan Biring, M.D.; Susan Clark, R.N., M.N.; Jennifer Cutler, M.D.; Robert Frantz, M.D.; Peter Julien, M.D.; Michael Lewis, M.D.; Jennifer Minkoff-Rau, M.S.W.; Valentina Yegyan, B.S., C.P.F.T.; Milton Joyner, B.A. (1996–2002).
Cleveland Clinic Foundation, Cleveland, OH: Malcolm DeCamp, M.D. (Principal Investigator); James Stoller, M.D. (Co-Principal Investigator); Yvonne Meli, R.N.C. (Principal Clinic Coordinator); John Apostolakis, M.D.; Darryl Atwell, M.D.; Jeffrey Chapman, M.D.; Pierre DeVilliers, M.D.; Raed Dweik, M.D.; Erik Kraenzler, M.D.; Rosemary Lann, L.I.S.W.; Nancy Kurokawa, R.R.T., C.P.F.T.; Scott Marlow, R.R.T.; Kevin McCarthy, R.C.P.T.; Priscilla McCreight, R.R.T., C.P.F.T.; Atul Mehta, M.D.; Moulay Meziane, M.D.; Omar Minai, M.D.; Mindi Steiger, R.R.T.; Kenneth White, R.P.F.T.; Janet Maurer, M.D. (Principal Investigator, 1996–2001); Terri Durr, R.N. (2000–2001); Charles Hearn, D.O. (1998–2001); Susan Lubell, P.A.-C. (1999–2000); Peter O'Donovan, M.D. (1998–2003); Robert Schilz, D.O. (1998–2002).
Columbia University, New York, NY in consortium with Long Island Jewish Medical Center, New Hyde Park, NY: Mark Ginsburg, M.D. (Principal Investigator); Byron Thomashow, M.D. (Co-Principal Investigator); Patricia Jellen, M.S.N., R.N. (Principal Clinic Coordinator); John Austin, M.D.; Matthew Bartels, M.D.; Yahya Berkmen, M.D.; Patricia Berkoski, M.S., R.R.T. (Site Coordinator, LIJ); Frances Brogan, M.S.N., R.N.; Amy Chong, B.S., C.R.T.; Glenda DeMercado, B.S.N.; Angela DiMango, M.D.; Sandy Do, M.S., P.T.; Bessie Kachulis, M.D.; Arfa Khan, M.D.; Berend Mets, M.D.; Mitchell O'Shea, B.S., R.T., C.P.F.T.; Gregory Pearson, M.D.; Leonard Rossoff, M.D.; Steven Scharf, M.D., Ph.D. (Co-Principal Investigator, 1998–2002); Maria Shiau, M.D.; Paul Simonelli, M.D.; Kim Stavrolakes, M.S., P.T.; Donna Tsang, B.S.; Denise Vilotijevic, M.S., P.T.; Chun Yip, M.D.; Mike Mantinaos, M.D. (1998–2001); Kerri McKeon, B.S., R.R.T., R.N. (1998–1999); Jacqueline Pfeffer, M.P.H., P.T. (1997–2002).
Duke University Medical Center, Durham, NC: Neil MacIntyre, M.D. (Principal Investigator); R. Duane Davis, M.D. (Co-Principal Investigator); John Howe, R.N. (Principal Clinic Coordinator); R. Edward Coleman, M.D.; Rebecca Crouch, R.P.T.; Dora Greene; Katherine Grichnik, M.D.; David Harpole, Jr., M.D.; Abby Krichman, R.R.T.; Brian Lawlor, R.R.T.; Holman McAdams, M.D.; John Plankeel, M.D.; Susan Rinaldo-Gallo, M.E.D.; Sheila Shearer, R.R.T.; Jeanne Smith, A.C.S.W.; Mark Stafford-Smith, M.D.; Victor Tapson, M.D.; Mark Steele, M.D. (1998–1999); Jennifer Norten, M.D. (1998–1999).
Mayo Foundation, Rochester, MN: James Utz, M.D. (Principal Investigator); Claude Deschamps, M.D. (Co-Principal Investigator); Kathy Mieras, C.C.R.P. (Principal Clinic Coordinator); Martin Abel, M.D.; Mark Allen, M.D.; Deb Andrist, R.N.; Gregory Aughenbaugh, M.D.; Sharon Bendel, R.N.; Eric Edell, M.D.; Marlene Edgar; Bonnie Edwards; Beth Elliot, M.D.; James Garrett, R.R.T.; Delmar Gillespie, M.D.; Judd Gurney, M.D.; Boleyn Hammel; Karen Hanson, R.R.T.; Lori Hanson, R.R.T.; Gordon Harms, M.D.; June Hart; Thomas Hartman, M.D.; Robert Hyatt, M.D.; Eric Jensen, M.D.; Nicole Jenson, R.R.T.; Sanjay Kalra, M.D.; Philip Karsell, M.D.; Jennifer Lamb; David Midthun, M.D.; Carl Mottram, R.R.T.; Stephen Swensen, M.D.; Anne-Marie Sykes, M.D.; Karen Taylor; Norman Torres, M.D.; Rolf Hubmayr, M.D. (1998–2000); Daniel Miller, M.D. (1999–2002); Sara Bartling, R.N. (1998–2000); Kris Bradt (1998–2002).
National Jewish Medical and Research Center, Denver, CO: Barry Make, M.D. (Principal Investigator); Marvin Pomerantz, M.D. (Co-Principal Investigator); Mary Gilmartin, R.N., R.R.T. (Principal Clinic Coordinator); Joyce Canterbury; Martin Carlos; Phyllis Dibbern, P.T.; Enrique Fernandez, M.D.; Lisa Geyman, M.S.P.T.; Connie Hudson; David Lynch, M.D.; John Newell, M.D.; Robert Quaife, M.D.; Jennifer Propst, R.N.; Cynthia Raymond, M.S.; Jane Whalen-Price, P.T.; Kathy Winner, O.T.R.; Martin Zamora, M.D.; Reuben Cherniack, M.D. (Principal Investigator, 1997–2000).
Ohio State University, Columbus, OH: Philip Diaz, M.D. (Principal Investigator); Patrick Ross, M.D. (Co-Principal Investigator); Tina Bees (Principal Clinic Coordinator); Jan Drake; Charles Emery, Ph.D.; Mark Gerhardt, M.D., Ph.D.; Mark King, M.D.; David Rittinger; Mahasti Rittinger.
Saint Louis University, Saint Louis, MO: Keith Naunheim, M.D. (Principal Investigator); Robert Gerber, M.D. (Co-Principal Investigator); Joan Osterloh, R.N., M.S.N. (Principal Clinic Coordinator); Susan Borosh; Willard Chamberlain, D.O.; Sally Frese; Alan Hibbit; Mary Ellen Kleinhenz, M.D.; Gregg Ruppel; Cary Stolar, M.D.; Janice Willey; Francisco Alvarez, M.D. (Co-Principal Investigator, 1999–2002); Cesar Keller, M.D. (Co-Principal Investigator, 1996–2000).
Temple University, Philadelphia, PA: Gerard Criner, M.D. (Principal Investigator); Satoshi Furukawa, M.D. (Co-Principal Investigator); Anne Marie Kuzma, R.N., M.S.N. (Principal Clinic Coordinator); Roger Barnette, M.D.; Neil Brister, M.D.; Kevin Carney, R.N., C.C.T.C.; Wissam Chatila, M.D.; Francis Cordova, M.D.; Gilbert D'Alonzo, D.O.; Michael Keresztury, M.D.; Karen Kirsch; Chul Kwak, M.D.; Kathy Lautensack, R.N., B.S.N.; Madelina Lorenzon, C.P.F.T.; Ubaldo Martin, M.D.; Peter Rising, M.S.; Scott Schartel, M.D.; John Travaline, M.D.; Gwendolyn Vance, R.N., C.C.T.C.; Phillip Boiselle, M.D. (1997–2000); Gerald O'Brien, M.D. (1997–2000).
University of California, San Diego, San Diego, CA: Andrew Ries, M.D., M.P.H. (Principal Investigator); Robert Kaplan, Ph.D. (Co-Principal Investigator); Catherine Ramirez, B.S., R.C.P. (Principal Clinic Coordinator); David Frankville, M.D.; Paul Friedman, M.D.; James Harrell, M.D.; Jeffery Johnson; David Kapelanski, M.D.; David Kupferberg, M.D., M.P.H.; Catherine Larsen, M.P.H.; Trina Limberg, R.R.T.; Michael Magliocca, R.N., C.N.P.; Frank J. Papatheofanis, M.D., Ph.D.; Dawn Sassi-Dambron, R.N.; Melissa Weeks.
University of Maryland at Baltimore, Baltimore, MD in consortium with Johns Hopkins Hospital, Baltimore, MD: Mark Krasna, M.D. (Principal Investigator); Henry Fessler, M.D. (Co-Principal Investigator); Iris Moskowitz (Principal Clinic Coordinator); Timothy Gilbert, M.D.; Jonathan Orens, M.D.; Steven Scharf, M.D., Ph.D.; David Shade; Stanley Siegelman, M.D.; Kenneth Silver, M.D.; Clarence Weir; Charles White, M.D.
University of Michigan, Ann Arbor, MI: Fernando Martinez, M.D. (Principal Investigator); Mark Iannettoni, M.D. (Co-Principal Investigator); Catherine Meldrum, B.S.N., R.N., C.C.R.N. (Principal Clinic Coordinator); William Bria, M.D.; Kelly Campbell; Paul Christensen, M.D.; Kevin Flaherty, M.D.; Steven Gay, M.D.; Paramjit Gill, R.N.; Paul Kazanjian, M.D.; Ella Kazerooni, M.D.; Vivian Knieper; Tammy Ojo, M.D.; Lewis Poole; Leslie Quint, M.D.; Paul Rysso; Thomas Sisson, M.D.; Mercedes True; Brian Woodcock, M.D.; Lori Zaremba, R.N.
University of Pennsylvania, Philadelphia, PA: Larry Kaiser, M.D. (Principal Investigator); John Hansen-Flaschen, M.D. (Co-Principal Investigator); Mary Louise Dempsey, B.S.N., R.N. (Principal Clinic Coordinator); Abass Alavi, M.D.; Theresa Alcorn, Selim Arcasoy, M.D.; Judith Aronchick, M.D.; Stanley Aukberg, M.D.; Bryan Benedict, R.R.T.; Susan Craemer, BS, R.R.T., C.P.F.T.; Ron Daniele, M.D.; Jeffrey Edelman, M.D.; Warren Gefter, M.D.; Laura Kotler-Klein, M.S.S.; Robert Kotloff, M.D.; David Lipson, M.D.; Wallace Miller, Jr., M.D.; Richard O'Connell, R.P.F.T.; Staci Opelman, M.S.W.; Harold Palevsky, M.D.; William Russell, R.P.F.T.; Heather Sheaffer, MSW; Rodney Simcox, B.S.R.T., R.R.T.; Susanne Snedeker, R.R.T., C.P.F.T.; Jennifer Stone-Wynne, M.S.W.; Gregory Tino, M.D.; Peter Wahl; James Walter, R.P.F.T.; Patricia Ward; David Zisman, M.D.; James Mendez, M.S.N., C.R.N.P. (1997–2001); Angela Wurster, M.S.N., CR.N.P (1997–1999).
University of Pittsburgh, Pittsburgh, PA: Frank Sciurba, M.D. (Principal Investigator); James Luketich, M.D. (Co-Principal Investigator); Colleen Witt, M.S. (Principal Clinic Coordinator); Gerald Ayres; Michael Donahoe, M.D.; Carl Fuhrman, M.D.; Robert Hoffman, M.D.; Joan Lacomis, M.D.; Joan Sexton; William Slivka; Diane Strollo, M.D.; Erin Sullivan, M.D.; Tomeka Simon; Catherine Wrona, R.N., B.S.N.; Gerene Bauldoff, R.N., M.S.N. (1997–2000); Manuel Brown, M.D. (1997–2002); Elisabeth George, R.N., M.S.N. (Principal Clinic Coordinator, 1997–2001); Robert Keenan, M.D. (Co-Principal Investigator, 1997–2000); Theodore Kopp, M.S. (1997–1999); Laurie Silfies (1997–2001).
University of Washington, Seattle, WA: Joshua Benditt, M.D. (Principal Investigator), Douglas Wood, M.D. (Co-Principal Investigator); Margaret Snyder, M.N. (Principal Clinic Coordinator); Kymberley Anable; Nancy Battaglia; Louie Boitano; Andrew Bowdle, M.D.; Leighton Chan, M.D.; Cindy Chwalik; Bruce Culver, M.D.; Thurman Gillespy, M.D.; David Godwin, M.D.; Jeanne Hoffman; Andra Ibrahim, M.D.; Diane Lockhart; Stephen Marglin, M.D.; Kenneth Martay, M.D.; Patricia McDowell; Donald Oxorn, M.D.; Liz Roessler; Michelle Toshima; Susan Golden (1998–2000).
Other participants: Agency for Healthcare Research and Quality, Rockville, MD: Lynn Bosco, M.D., M.P.H.; Yen-Pin Chiang, Ph.D.; Carolyn Clancy, M.D.; Harry Handelsman, D.O.
Centers for Medicare and Medicaid Services, Baltimore, MD: Steven M. Berkowitz, Ph.D.; Tanisha Carino, Ph.D.; Joe Chin, M.D.; JoAnna Baldwin; Karen McVearry; Anthony Norris; Sarah Shirey; Claudette Sikora Steven Sheingold, Ph.D. (1997–2004).
Coordinating Center, Johns Hopkins University, Baltimore, MD: Steven Piantadosi, M.D., Ph.D. (Principal Investigator); James Tonascia, Ph.D. (Co-Principal Investigator); Patricia Belt; Amanda Blackford, Sc.M.; Karen Collins; Betty Collison; Ryan Colvin, M.P.H.; John Dodge; Michele Donithan, M.H.S.; Vera Edmonds; Gregory L. Foster, M.A.; Julie Fuller; Judith Harle; Rosetta Jackson; Shing Lee, Sc.M.; Charlene Levine; Hope Livingston; Jill Meinert; Jennifer Meyers; Deborah Nowakowski; Kapreena Owens; Shangqian Qi, M.D.; Michael Smith; Brett Simon, M.D.; Paul Smith; Alice Sternberg, Sc.M.; Mark Van Natta, M.H.S.; Laura Wilson, Sc.M.; Robert Wise, M.D.
Cost Effectiveness Subcommittee: Robert M. Kaplan, Ph.D. (Chair); J. Sanford Schwartz, M.D. (Co-Chair); Yen-Pin Chiang, Ph.D.; Marianne C. Fahs, Ph.D.; A. Mark Fendrick, M.D.; Alan J. Moskowitz, M.D.; Dev Pathak, Ph.D.; Scott Ramsey, M.D., Ph.D.; Steven Sheingold, Ph.D.; A. Laurie Shroyer, Ph.D.; Judith Wagner, Ph.D.; Roger Yusen, M.D.
Cost Effectiveness Data Center, Fred Hutchinson Cancer Research Center, Seattle, WA: Scott Ramsey, M.D., Ph.D. (Principal Investigator); Ruth Etzioni, Ph.D.; Sean Sullivan, Ph.D.; Douglas Wood, M.D.; Thomas Schroeder, M.A.; Karma Kreizenbeck; Kristin Berry, M.S.; Nadia Howlader, M.S.
CT Scan Image Storage and Analysis Center, University of Iowa, Iowa City, IA: Eric Hoffman, Ph.D. (Principal Investigator); Janice Cook-Granroth, B.S.; Angela Delsing, R.T.; Junfeng Guo, Ph.D.; Geoffrey McLennan, M.D.; Brian Mullan, M.D.; Chris Piker, B.S.; Joseph Reinhardt, Ph.D.; Blake Wood; Jered Sieren, R.T.R.; William Stanford, M.D.
Data and Safety Monitoring Board: John A. Waldhausen, M.D. (Chair); Gordon Bernard, M.D.; David DeMets, Ph.D.; Mark Ferguson, M.D.; Eddie Hoover, M.D.; Robert Levine, M.D.; Donald Mahler, M.D.; A. John McSweeny, Ph.D.; Jeanine Wiener-Kronish, M.D.; O. Dale Williams, Ph.D.; Magdy Younes, M.D.
Marketing Center, Temple University, Philadelphia, PA: Gerard Criner, M.D. (Principal Investigator); Charles Soltoff, M.B.A.
Project Office, National Heart, Lung, and Blood Institute, Bethesda, MD: Gail Weinmann, M.D. (Project Officer); Joanne Deshler (Contracting Officer); Dean Follmann, Ph.D.; James Kiley, Ph.D.; Margaret Wu, Ph.D. (1996–2001).
Other acknowledgments: Arthur Gelb, M.D., Lakewood Regional Medical Center, Lakewood, CA.
Clinical centers: Baylor College of Medicine, Houston, TX: Marcia Katz, M.D. (Principal Investigator); Carolyn Wheeler, R.N., B.S.N. (Principal Clinic Coordinator); Elaine Baker, R.R.T., R.P.F.T.; Peter Barnard, Ph.D., R.P.F.T.; Phil Cagle, M.D.; James Carter, M.D.; Sophia Chatziioannou, M.D.; Karla Conejo-Gonzales; Kimberly Dubose, R.R.T.; John Haddad, M.D.; David Hicks, R.R.T., R.P.F.T.; Neal Kleiman, M.D.; Mary Milburn-Barnes, C.R.T.T.; Chinh Nguyen, R.P.F.T.; Michael Reardon, M.D.; Joseph Reeves-Viets, M.D.; Steven Sax, M.D.; Amir Sharafkhaneh, M.D.; Owen Wilson, Ph.D.; Christine Young P.T.; Rafael Espada, M.D. (Principal Investigator, 1996–2002); Rose Butanda (1999–2001); Minnie Ellisor (2002); Pamela Fox, M.D. (1999–2001); Katherine Hale, M.D. (1998–2000); Everett Hood, R.P.F.T. (1998–2000); Amy Jahn (1998–2000); Satish Jhingran, M.D. (1998–2001); Karen King, R.P.F.T. (1998–1999); Charles Miller III, Ph.D. (1996–1999); Imran Nizami, M.D. (Co-Principal Investigator, 2000–2001); Todd Officer (1998–2000); Jeannie Ricketts (1998–2000); Joe Rodarte, M.D. (Co-Principal Investigator, 1996–2000); Robert Teague, M.D. (Co-Principal Investigator, 1999–2000); Kedren Williams (1998–1999).
Brigham and Women's Hospital, Boston, MA: John Reilly, M.D. (Principal Investigator); David Sugarbaker, M.D. (Co-Principal Investigator); Carol Fanning, R.R.T. (Principal Clinic Coordinator); Simon Body, M.D.; Sabine Duffy, M.D.; Vladmir Formanek, M.D.; Anne Fuhlbrigge, M.D.; Philip Hartigan, M.D.; Sarah Hooper, E.P.; Andetta Hunsaker, M.D.; Francine Jacobson, M.D.; Marilyn Moy, M.D.; Susan Peterson, R.R.T.; Roger Russell, M.D.; Diane Saunders; Scott Swanson, M.D. (Co-Principal Investigator, 1996–2001).
Cedars-Sinai Medical Center, Los Angeles, CA: Rob McKenna, M.D. (Principal Investigator); Zab Mohsenifar, M.D. (Co-Principal Investigator); Carol Geaga, R.N. (Principal Clinic Coordinator); Manmohan Biring, M.D.; Susan Clark, R.N., M.N.; Jennifer Cutler, M.D.; Robert Frantz, M.D.; Peter Julien, M.D.; Michael Lewis, M.D.; Jennifer Minkoff-Rau, M.S.W.; Valentina Yegyan, B.S., C.P.F.T.; Milton Joyner, B.A. (1996–2002).
Cleveland Clinic Foundation, Cleveland, OH: Malcolm DeCamp, M.D. (Principal Investigator); James Stoller, M.D. (Co-Principal Investigator); Yvonne Meli, R.N.C. (Principal Clinic Coordinator); John Apostolakis, M.D.; Darryl Atwell, M.D.; Jeffrey Chapman, M.D.; Pierre DeVilliers, M.D.; Raed Dweik, M.D.; Erik Kraenzler, M.D.; Rosemary Lann, L.I.S.W.; Nancy Kurokawa, R.R.T., C.P.F.T.; Scott Marlow, R.R.T.; Kevin McCarthy, R.C.P.T.; Priscilla McCreight, R.R.T., C.P.F.T.; Atul Mehta, M.D.; Moulay Meziane, M.D.; Omar Minai, M.D.; Mindi Steiger, R.R.T.; Kenneth White, R.P.F.T.; Janet Maurer, M.D. (Principal Investigator, 1996–2001); Terri Durr, R.N. (2000–2001); Charles Hearn, D.O. (1998–2001); Susan Lubell, P.A.-C. (1999–2000); Peter O'Donovan, M.D. (1998–2003); Robert Schilz, D.O. (1998–2002).
Columbia University, New York, NY in consortium with Long Island Jewish Medical Center, New Hyde Park, NY: Mark Ginsburg, M.D. (Principal Investigator); Byron Thomashow, M.D. (Co-Principal Investigator); Patricia Jellen, M.S.N., R.N. (Principal Clinic Coordinator); John Austin, M.D.; Matthew Bartels, M.D.; Yahya Berkmen, M.D.; Patricia Berkoski, M.S., R.R.T. (Site Coordinator, LIJ); Frances Brogan, M.S.N., R.N.; Amy Chong, B.S., C.R.T.; Glenda DeMercado, B.S.N.; Angela DiMango, M.D.; Sandy Do, M.S., P.T.; Bessie Kachulis, M.D.; Arfa Khan, M.D.; Berend Mets, M.D.; Mitchell O'Shea, B.S., R.T., C.P.F.T.; Gregory Pearson, M.D.; Leonard Rossoff, M.D.; Steven Scharf, M.D., Ph.D. (Co-Principal Investigator, 1998–2002); Maria Shiau, M.D.; Paul Simonelli, M.D.; Kim Stavrolakes, M.S., P.T.; Donna Tsang, B.S.; Denise Vilotijevic, M.S., P.T.; Chun Yip, M.D.; Mike Mantinaos, M.D. (1998–2001); Kerri McKeon, B.S., R.R.T., R.N. (1998–1999); Jacqueline Pfeffer, M.P.H., P.T. (1997–2002).
Duke University Medical Center, Durham, NC: Neil MacIntyre, M.D. (Principal Investigator); R. Duane Davis, M.D. (Co-Principal Investigator); John Howe, R.N. (Principal Clinic Coordinator); R. Edward Coleman, M.D.; Rebecca Crouch, R.P.T.; Dora Greene; Katherine Grichnik, M.D.; David Harpole, Jr., M.D.; Abby Krichman, R.R.T.; Brian Lawlor, R.R.T.; Holman McAdams, M.D.; John Plankeel, M.D.; Susan Rinaldo-Gallo, M.E.D.; Sheila Shearer, R.R.T.; Jeanne Smith, A.C.S.W.; Mark Stafford-Smith, M.D.; Victor Tapson, M.D.; Mark Steele, M.D. (1998–1999); Jennifer Norten, M.D. (1998–1999).
Mayo Foundation, Rochester, MN: James Utz, M.D. (Principal Investigator); Claude Deschamps, M.D. (Co-Principal Investigator); Kathy Mieras, C.C.R.P. (Principal Clinic Coordinator); Martin Abel, M.D.; Mark Allen, M.D.; Deb Andrist, R.N.; Gregory Aughenbaugh, M.D.; Sharon Bendel, R.N.; Eric Edell, M.D.; Marlene Edgar; Bonnie Edwards; Beth Elliot, M.D.; James Garrett, R.R.T.; Delmar Gillespie, M.D.; Judd Gurney, M.D.; Boleyn Hammel; Karen Hanson, R.R.T.; Lori Hanson, R.R.T.; Gordon Harms, M.D.; June Hart; Thomas Hartman, M.D.; Robert Hyatt, M.D.; Eric Jensen, M.D.; Nicole Jenson, R.R.T.; Sanjay Kalra, M.D.; Philip Karsell, M.D.; Jennifer Lamb; David Midthun, M.D.; Carl Mottram, R.R.T.; Stephen Swensen, M.D.; Anne-Marie Sykes, M.D.; Karen Taylor; Norman Torres, M.D.; Rolf Hubmayr, M.D. (1998–2000); Daniel Miller, M.D. (1999–2002); Sara Bartling, R.N. (1998–2000); Kris Bradt (1998–2002).
National Jewish Medical and Research Center, Denver, CO: Barry Make, M.D. (Principal Investigator); Marvin Pomerantz, M.D. (Co-Principal Investigator); Mary Gilmartin, R.N., R.R.T. (Principal Clinic Coordinator); Joyce Canterbury; Martin Carlos; Phyllis Dibbern, P.T.; Enrique Fernandez, M.D.; Lisa Geyman, M.S.P.T.; Connie Hudson; David Lynch, M.D.; John Newell, M.D.; Robert Quaife, M.D.; Jennifer Propst, R.N.; Cynthia Raymond, M.S.; Jane Whalen-Price, P.T.; Kathy Winner, O.T.R.; Martin Zamora, M.D.; Reuben Cherniack, M.D. (Principal Investigator, 1997–2000).
Ohio State University, Columbus, OH: Philip Diaz, M.D. (Principal Investigator); Patrick Ross, M.D. (Co-Principal Investigator); Tina Bees (Principal Clinic Coordinator); Jan Drake; Charles Emery, Ph.D.; Mark Gerhardt, M.D., Ph.D.; Mark King, M.D.; David Rittinger; Mahasti Rittinger.
Saint Louis University, Saint Louis, MO: Keith Naunheim, M.D. (Principal Investigator); Robert Gerber, M.D. (Co-Principal Investigator); Joan Osterloh, R.N., M.S.N. (Principal Clinic Coordinator); Susan Borosh; Willard Chamberlain, D.O.; Sally Frese; Alan Hibbit; Mary Ellen Kleinhenz, M.D.; Gregg Ruppel; Cary Stolar, M.D.; Janice Willey; Francisco Alvarez, M.D. (Co-Principal Investigator, 1999–2002); Cesar Keller, M.D. (Co-Principal Investigator, 1996–2000).
Temple University, Philadelphia, PA: Gerard Criner, M.D. (Principal Investigator); Satoshi Furukawa, M.D. (Co-Principal Investigator); Anne Marie Kuzma, R.N., M.S.N. (Principal Clinic Coordinator); Roger Barnette, M.D.; Neil Brister, M.D.; Kevin Carney, R.N., C.C.T.C.; Wissam Chatila, M.D.; Francis Cordova, M.D.; Gilbert D'Alonzo, D.O.; Michael Keresztury, M.D.; Karen Kirsch; Chul Kwak, M.D.; Kathy Lautensack, R.N., B.S.N.; Madelina Lorenzon, C.P.F.T.; Ubaldo Martin, M.D.; Peter Rising, M.S.; Scott Schartel, M.D.; John Travaline, M.D.; Gwendolyn Vance, R.N., C.C.T.C.; Phillip Boiselle, M.D. (1997–2000); Gerald O'Brien, M.D. (1997–2000).
University of California, San Diego, San Diego, CA: Andrew Ries, M.D., M.P.H. (Principal Investigator); Robert Kaplan, Ph.D. (Co-Principal Investigator); Catherine Ramirez, B.S., R.C.P. (Principal Clinic Coordinator); David Frankville, M.D.; Paul Friedman, M.D.; James Harrell, M.D.; Jeffery Johnson; David Kapelanski, M.D.; David Kupferberg, M.D., M.P.H.; Catherine Larsen, M.P.H.; Trina Limberg, R.R.T.; Michael Magliocca, R.N., C.N.P.; Frank J. Papatheofanis, M.D., Ph.D.; Dawn Sassi-Dambron, R.N.; Melissa Weeks.
University of Maryland at Baltimore, Baltimore, MD in consortium with Johns Hopkins Hospital, Baltimore, MD: Mark Krasna, M.D. (Principal Investigator); Henry Fessler, M.D. (Co-Principal Investigator); Iris Moskowitz (Principal Clinic Coordinator); Timothy Gilbert, M.D.; Jonathan Orens, M.D.; Steven Scharf, M.D., Ph.D.; David Shade; Stanley Siegelman, M.D.; Kenneth Silver, M.D.; Clarence Weir; Charles White, M.D.
University of Michigan, Ann Arbor, MI: Fernando Martinez, M.D. (Principal Investigator); Mark Iannettoni, M.D. (Co-Principal Investigator); Catherine Meldrum, B.S.N., R.N., C.C.R.N. (Principal Clinic Coordinator); William Bria, M.D.; Kelly Campbell; Paul Christensen, M.D.; Kevin Flaherty, M.D.; Steven Gay, M.D.; Paramjit Gill, R.N.; Paul Kazanjian, M.D.; Ella Kazerooni, M.D.; Vivian Knieper; Tammy Ojo, M.D.; Lewis Poole; Leslie Quint, M.D.; Paul Rysso; Thomas Sisson, M.D.; Mercedes True; Brian Woodcock, M.D.; Lori Zaremba, R.N.
University of Pennsylvania, Philadelphia, PA: Larry Kaiser, M.D. (Principal Investigator); John Hansen-Flaschen, M.D. (Co-Principal Investigator); Mary Louise Dempsey, B.S.N., R.N. (Principal Clinic Coordinator); Abass Alavi, M.D.; Theresa Alcorn, Selim Arcasoy, M.D.; Judith Aronchick, M.D.; Stanley Aukberg, M.D.; Bryan Benedict, R.R.T.; Susan Craemer, BS, R.R.T., C.P.F.T.; Ron Daniele, M.D.; Jeffrey Edelman, M.D.; Warren Gefter, M.D.; Laura Kotler-Klein, M.S.S.; Robert Kotloff, M.D.; David Lipson, M.D.; Wallace Miller, Jr., M.D.; Richard O'Connell, R.P.F.T.; Staci Opelman, M.S.W.; Harold Palevsky, M.D.; William Russell, R.P.F.T.; Heather Sheaffer, MSW; Rodney Simcox, B.S.R.T., R.R.T.; Susanne Snedeker, R.R.T., C.P.F.T.; Jennifer Stone-Wynne, M.S.W.; Gregory Tino, M.D.; Peter Wahl; James Walter, R.P.F.T.; Patricia Ward; David Zisman, M.D.; James Mendez, M.S.N., C.R.N.P. (1997–2001); Angela Wurster, M.S.N., CR.N.P (1997–1999).
University of Pittsburgh, Pittsburgh, PA: Frank Sciurba, M.D. (Principal Investigator); James Luketich, M.D. (Co-Principal Investigator); Colleen Witt, M.S. (Principal Clinic Coordinator); Gerald Ayres; Michael Donahoe, M.D.; Carl Fuhrman, M.D.; Robert Hoffman, M.D.; Joan Lacomis, M.D.; Joan Sexton; William Slivka; Diane Strollo, M.D.; Erin Sullivan, M.D.; Tomeka Simon; Catherine Wrona, R.N., B.S.N.; Gerene Bauldoff, R.N., M.S.N. (1997–2000); Manuel Brown, M.D. (1997–2002); Elisabeth George, R.N., M.S.N. (Principal Clinic Coordinator, 1997–2001); Robert Keenan, M.D. (Co-Principal Investigator, 1997–2000); Theodore Kopp, M.S. (1997–1999); Laurie Silfies (1997–2001).
University of Washington, Seattle, WA: Joshua Benditt, M.D. (Principal Investigator), Douglas Wood, M.D. (Co-Principal Investigator); Margaret Snyder, M.N. (Principal Clinic Coordinator); Kymberley Anable; Nancy Battaglia; Louie Boitano; Andrew Bowdle, M.D.; Leighton Chan, M.D.; Cindy Chwalik; Bruce Culver, M.D.; Thurman Gillespy, M.D.; David Godwin, M.D.; Jeanne Hoffman; Andra Ibrahim, M.D.; Diane Lockhart; Stephen Marglin, M.D.; Kenneth Martay, M.D.; Patricia McDowell; Donald Oxorn, M.D.; Liz Roessler; Michelle Toshima; Susan Golden (1998–2000).
Other participants: Agency for Healthcare Research and Quality, Rockville, MD: Lynn Bosco, M.D., M.P.H.; Yen-Pin Chiang, Ph.D.; Carolyn Clancy, M.D.; Harry Handelsman, D.O.
Centers for Medicare and Medicaid Services, Baltimore, MD: Steven M. Berkowitz, Ph.D.; Tanisha Carino, Ph.D.; Joe Chin, M.D.; JoAnna Baldwin; Karen McVearry; Anthony Norris; Sarah Shirey; Claudette Sikora Steven Sheingold, Ph.D. (1997–2004).
Coordinating Center, Johns Hopkins University, Baltimore, MD: Steven Piantadosi, M.D., Ph.D. (Principal Investigator); James Tonascia, Ph.D. (Co-Principal Investigator); Patricia Belt; Amanda Blackford, Sc.M.; Karen Collins; Betty Collison; Ryan Colvin, M.P.H.; John Dodge; Michele Donithan, M.H.S.; Vera Edmonds; Gregory L. Foster, M.A.; Julie Fuller; Judith Harle; Rosetta Jackson; Shing Lee, Sc.M.; Charlene Levine; Hope Livingston; Jill Meinert; Jennifer Meyers; Deborah Nowakowski; Kapreena Owens; Shangqian Qi, M.D.; Michael Smith; Brett Simon, M.D.; Paul Smith; Alice Sternberg, Sc.M.; Mark Van Natta, M.H.S.; Laura Wilson, Sc.M.; Robert Wise, M.D.
Cost Effectiveness Subcommittee: Robert M. Kaplan, Ph.D. (Chair); J. Sanford Schwartz, M.D. (Co-Chair); Yen-Pin Chiang, Ph.D.; Marianne C. Fahs, Ph.D.; A. Mark Fendrick, M.D.; Alan J. Moskowitz, M.D.; Dev Pathak, Ph.D.; Scott Ramsey, M.D., Ph.D.; Steven Sheingold, Ph.D.; A. Laurie Shroyer, Ph.D.; Judith Wagner, Ph.D.; Roger Yusen, M.D.
2Cost Effectiveness Data Center, Fred Hutchinson Cancer Research Center, Seattle, WA: Scott Ramsey, M.D., Ph.D. (Principal Investigator); Ruth Etzioni, Ph.D.; Sean Sullivan, Ph.D.; Douglas Wood, M.D.; Thomas Schroeder, M.A.; Karma Kreizenbeck; Kristin Berry, M.S.; Nadia Howlader, M.S.
CT Scan Image Storage and Analysis Center, University of Iowa, Iowa City, IA: Eric Hoffman, Ph.D. (Principal Investigator); Janice Cook-Granroth, B.S.; Angela Delsing, R.T.; Junfeng Guo, Ph.D.; Geoffrey McLennan, M.D.; Brian Mullan, M.D.; Chris Piker, B.S.; Joseph Reinhardt, Ph.D.; Blake Wood; Jered Sieren, R.T.R.; William Stanford, M.D.
Data and Safety Monitoring Board: John A. Waldhausen, M.D. (Chair); Gordon Bernard, M.D.; David DeMets, Ph.D.; Mark Ferguson, M.D.; Eddie Hoover, M.D.; Robert Levine, M.D.; Donald Mahler, M.D.; A. John McSweeny, Ph.D.; Jeanine Wiener-Kronish, M.D.; O. Dale Williams, Ph.D.; Magdy Younes, M.D.
Marketing Center, Temple University, Philadelphia, PA: Gerard Criner, M.D. (Principal Investigator); Charles Soltoff, M.B.A.
Project Office, National Heart, Lung, and Blood Institute, Bethesda, MD: Gail Weinmann, M.D. (Project Officer); Joanne Deshler (Contracting Officer); Dean Follmann, Ph.D.; James Kiley, Ph.D.; Margaret Wu, Ph.D. (1996–2001).
Other acknowledgments: Arthur Gelb, M.D., Lakewood Regional Medical Center, Lakewood, CA.
The National Emphysema Treatment Trial (NETT) is supported by contracts with the National Heart, Lung, and Blood Institute (N01HR76101 through N01HR76119), the Centers for Medicare and Medicaid Services (CMS); and the Agency for Healthcare Research and Quality (AHRQ). Also supported by NIH grant 1K23HL089353-01A1 and a grant from the Parker B. Francis Foundation.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201001-0043OC on June 10, 2010
Author Disclosure: D.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.A.L. is a full-time employee of GlaxoSmithKline (GSK) and he owns stocks or options of GSK ($10,001–$50,000). E.A.H. was a consultant for Q12 ($10,001–$50,000), AstraZeneca, Sanofi-Aventis, and Grifols ($1,001–$5,000). He received travel expenses from Siemens Medical Systems (up to $1,000) and received lecture fees from Q12 ($10,001–$50,000), AstraZeneca, Sanofi-Aventis, and Grifols ($1,001–$5,000). He owns patents through the University of Iowa/VIDA Diagnostics for texture analysis of medical images and the use of color to detect pathology of the airway, and through Marval Therapeutics on nanometer-sized Stealth Liposomes as an imaging contrast agent. He is the founder and is a shareholder of VIDA Diagnostics and Marval Diagnostics (more than $100,001). He received grant support from the NIH (more than $100,001). E.A.H. declares that his primary, potential conflict of interest is as a founder and shareholder of VIDA Diagnostics, which is commercializing image analysis software, developed in his university laboratory, which has been used in this study. J.H.-F. receives royalties from Up-to-Date (up to $1,000) and received grant support from the Will Rogers Institute. F.C.S. was a consultant for Pfizer and served on the Board or Advisory Board for GlaxoSmithKline, AstraZeneca, Merck, and PneumRx ($1,001–$5,000). He received grant support from GSK, Pfizer, and Boehringer Ingelheim (more than $100,001). M.M.D.'s spouse/life partner is a full-time employee of Thoratec Corporation. He was a consultant for Portaero Inc. and was on the Board or Advisory Board for PneumRx ($5,001–$10,000). He receives royalties from Up-to-Date (up to $1,000) and his spouse/life partner owns stocks or options of Thoratec ($10,001–$50,000). J.J.R. has a patent on a software algorithm for CT scan analysis submitted; no financial benefit through Brigham and Women's Hospital. He receives royalties from Up-to-Date (up to $1,000). He received grant support from the NIH (more than $100,001) and is the Chairman of the Clinical Trials Study Section of the NHLBI; paid $200/day for service. G.R.W. was a consultant for MedImmune ($1,001–$5,000) and received grant support from the NHLBI (more than $100,001).
References
- 1.Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, Weinmann G, Wood DE. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059–2073. [DOI] [PubMed] [Google Scholar]
- 2.Thurnheer R, Engel H, Weder W, Stammberger U, Laube I, Russi EW, Bloch KE. Role of lung perfusion scintigraphy in relation to chest computed tomography and pulmonary function in the evaluation of candidates for lung volume reduction surgery. Am J Respir Crit Care Med 1999;159:301–310. [DOI] [PubMed] [Google Scholar]
- 3.Berger RL, Wood KA, Cabral HJ, Goodnight-White S, Ingenito EP, Gray A, Miller J, Springmeyer SC. Lung volume reduction surgery: a meta-analysis of randomized clinical trials. Treat Respir Med 2005;4:201–209. [DOI] [PubMed] [Google Scholar]
- 4.Washko GR, Hoffman E, Reilly JJ. Radiographic evaluation of the potential lung volume reduction surgery candidate. Proc Am Thorac Soc 2008;5:421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chenuel B, Haouzi P, Olivier P, Marie PY, Chalon B, Borrelly J. Effect of exercise on lung-perfusion scanning in patients with bronchogenic carcinoma. Eur Respir J 2002;20:710–716. [DOI] [PubMed] [Google Scholar]
- 6.Hunsaker AR, Ingenito EP, Reilly JJ, Costello P. Lung volume reduction surgery for emphysema: Correlation of CT and V/Q imaging with physiologic mechanisms of improvement in lung function. Radiology 2002;222:491–498. [DOI] [PubMed] [Google Scholar]
- 7.Jamadar DA, Kazerooni EA, Martinez FJ, Wahl RL. Semi-quantitative ventilation/perfusion scintigraphy and single-photon emission tomography for evaluation of lung volume reduction surgery candidates: description and prediction of clinical outcome. Eur J Nucl Med 1999;26:734–742. [DOI] [PubMed] [Google Scholar]
- 8.Cederlund K, Hogberg S, Jorfeldt L, Larsen F, Norman M, Rasmussen E, Tylen U. Lung perfusion scintigraphy prior to lung volume reduction surgery. Acta Radiol 2003;44:246–251. [DOI] [PubMed] [Google Scholar]
- 9.Cleverley JR, Desai SR, Wells AU, Koyama H, Eastick S, Schmidt MA, Charrier CL, Gatehouse PD, Goldstraw P, Pepper JR, et al. Evaluation of patients undergoing lung volume reduction surgery: ancillary information available from computed tomography. Clin Radiol 2000;55:45–50. [DOI] [PubMed] [Google Scholar]
- 10.Wang SC, Fischer KC, Slone RM, Gierada DS, Yusen RD, Lefrak SS, Pilgram TK, Cooper JD. Perfusion scintigraphy in the evaluation for lung volume reduction surgery: correlation with clinical outcome. Radiology 1997;205:243–248. [DOI] [PubMed] [Google Scholar]
- 11.Kotloff RM, Hansen-Flaschen J, Lipson DA, Tino G, Arcasoy SM, Alavi A, Kaiser LR. Apical perfusion fraction as a predictor of short-term functional outcome following bilateral lung volume reduction surgery. Chest 2001;120:1609–1615. [DOI] [PubMed] [Google Scholar]
- 12.Chandra D, Lipson D, Hansen-Flaschen J, DeCamp M, Reilly J, Washko G. Perfusion scintigraphy as a prognostic tool for lung volume reduction surgery outcomes [abstract]. Am J Respir Crit Care Med 2009;179:A6172. [Google Scholar]
- 13.National Emphysema Treatment Trial Research Group. Rationale and design of the National Emphysema Treatment Trial: a prospective randomized trial of lung volume reduction surgery. Chest 1999;116:1750–1761. [DOI] [PubMed] [Google Scholar]
- 14.Criner GJ, Sternberg AL. A clinician's guide to the use of lung volume reduction surgery. Proc Am Thorac Soc 2008;5:461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Emphyema Treatment Trial Research Group. Patients at high risk of death after lung-volume-reduction surgery. N Engl J Med 2001;345:1075–1083. [DOI] [PubMed] [Google Scholar]
- 16.Washko GR, Criner GJ, Mohsenifar Z, Sciurba FC, Sharafkhaneh A, Make BJ, Hoffman EA, Reilly JJ. Computed tomographic-based quantification of emphysema and correlation to pulmonary function and mechanics. COPD 2008;5:177–186. [DOI] [PubMed] [Google Scholar]
- 17.Donohue JF. Minimal clinically important differences in COPD lung function. COPD 2005;2:111–124. [DOI] [PubMed] [Google Scholar]
- 18.MacIntyre N. New data on lung volume reduction surgery and outcomes from the National Emphysema Treatment Trial. Available at http://www.Medscape.Com/viewarticle/507434 (accessed July 2010).
- 19.Kupferberg DH, Kaplan RM, Slymen DJ, Ries AL. Minimal clinically important difference for the UCSD Shortness of Breath Questionnaire. J Cardiopulm Rehabil 2005;25:370–377. [DOI] [PubMed] [Google Scholar]
- 20.Reese PR, Stynes G, Friel KR. Minimal clinically important difference (MCID) for the St. George's Respiratory Questionnaire (SGRQ) in chronic obstructive pulmonary disease (COPD). Available at http://www.Ers-education.Org/lr/abstract.Aspx?Idmedia=79572&nw=off (accessed July 2010).
- 21.Cooper JD, Trulock EP, Triantafillou AN, Patterson GA, Pohl MS, Deloney PA, Sundaresan RS, Roper CL. Bilateral pneumectomy (volume reduction) for chronic obstructive pulmonary disease. J Thorac Cardiovasc Surg 1995;109:106–116; discussion 116–119. [DOI] [PubMed] [Google Scholar]
- 22.Ingenito EP, Loring SH, Moy ML, Mentzer SJ, Swanson SJ, Hunsaker A, McKee CC, Reilly JJ. Comparison of physiological and radiological screening for lung volume reduction surgery. Am J Respir Crit Care Med 2001;163:1068–1073. [DOI] [PubMed] [Google Scholar]
- 23.Wu MT, Pan HB, Chiang AA, Hsu HK, Chang HC, Peng NJ, Lai PH, Liang HL, Yang CF. Prediction of postoperative lung function in patients with lung cancer: comparison of quantitative CT with perfusion scintigraphy. AJR Am J Roentgenol 2002;178:667–672. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.