Abstract
Rationale: Single-site clinic-based studies suggest an increasing prevalence of pulmonary nontuberculous mycobacteria (NTM) disease, but systematic data are lacking.
Objectives: To describe prevalence and trends for NTM lung disease at four geographically diverse integrated heath care delivery systems in the United States.
Methods: We abstracted mycobacterial culture results from electronic laboratory databases and linked to other datasets containing clinical and demographic information. Possible cases were defined as a single positive NTM pulmonary isolate, and definite cases were defined as two positive sputum cultures, or one positive culture from a bronchoalveolar lavage or lung biopsy. Annual prevalence was calculated using United States census data; average annual prevalence is presented for 2004–2006. Poisson regression models were used to estimate the annual percent change in prevalence.
Measurements and Main Results: A total of 28,697 samples from 7,940 patients were included in the analysis. Of these, 3,988 (50%) were defined as possible cases, and 1,865 (47%) of these were defined as definite cases. Average annual (2004–2006) site-specific prevalence ranged from 1.4 to 6.6 per 100,000. Prevalence was 1.l- to 1.6-fold higher among women relative to men across sites. The prevalence of NTM lung disease was increasing significantly at the two sites where trends were studied, by 2.6% per year among women and 2.9% per year among men. Among persons aged greater than or equal to 60 years, annual prevalence increased from 19.6 per 100,000 during 1994–1996 to 26.7 per 100,000 during 2004–2006.
Conclusions: The epidemiology of nontuberculous mycobacterial lung disease is changing, with a predominance of women and increasing prevalence at the sites studied.
Keywords: epidemiology, prevalence, nontuberculous mycobacteria, atypical mycobacteria
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Current data on prevalence and trends for pulmonary nontuberculous mycobacteria disease in the United States are fragmentary. Although recent reports suggest rising numbers, the last population-based prevalence data were published more than 20 years ago.
What This Study Adds to the Field
The epidemiology of mycobacterial disease in the United States has changed over the last 20 years, with a decline in pulmonary tuberculosis and an increase identified in pulmonary nontuberculosis mycobacterial disease. These findings highlight the increased burden among women and older adults, and the need for regular evaluation and monitoring of patients with this condition.
The nontuberculous mycobacteria (NTM) represent a diverse group of mycobacterial species (e.g., Mycobacterium avium, Mycobacterium abscessus) widespread in the environment and readily isolated from soil and water; human-to-human transmission has not been documented. Available data suggest that NTM have increased in importance as a cause of mycobacterial lung disease over the last 25 or more years in the United States (1, 2) but current national data on disease burden and distribution are lacking. Genetic or acquired host defense defects are thought to be important factors in disease development. Lung disease caused by NTM is widely thought to be opportunistic and occurs commonly in patients with structural lung disease, such as chronic obstructive pulmonary disease (COPD), bronchiectasis, and cystic fibrosis (2–4).
The first national efforts to characterize NTM disease began in 1980; reports from state laboratories for that year indicated a ratio of NTM/Mycobacterium tuberculosis (MTB) of 1:2 (5). The first systematic national survey conducted, based on isolates sent to state laboratories during 1981–1983, estimated an NTM annual disease prevalence of 1.8 per 100,000 persons (5). Unlike patients with tuberculosis (TB), those with NTM disease were predominantly white, and among those aged more than 75 years, predominantly female. Several single-site studies have been conducted since then that provide evidence for an increasing predominance of NTM relative to MTB and an absolute increase in NTM and a decline in TB disease rates in the United States (1, 2, 6). The increasing use of rapid molecular probes for TB diagnostics has led to greater on-site identification of mycobacterial species by hospital and clinical laboratories, with a concurrent decrease in referral of specimens to state laboratories (7). Therefore, data from surveys of state laboratories no longer accurately represent trends and features of NTM disease. The health care systems involved in this study offered the following advantages: defined, enumerated source populations; capture of laboratory results for members; computerized laboratory results and medical records; and the capacity to link laboratory records to patient data. They thus provide a cost-effective and rapid method to study trends in mycobacterial disease and describe associated patient characteristics. We conducted a comprehensive study of NTM prevalence and trends to understand better the current epidemiology of this disease, using a large-linked database approach at four integrated health care delivery systems (IHDS): Kaiser Permanente Southern California (KPSC), Pasadena, CA; Group Health (GH), Seattle, WA; Kaiser Permanente Colorado (KPCO), Denver, CO; and Geisinger Health Systems (Geisinger), Danville, PA. The study period ranged from 7 (Geisinger) to 17 (Group Health) years at these sites (Table 1). Some of the results of these studies have been previously reported in the form of an abstract (8).
TABLE 1.
STUDY POPULATION, NUMBER OF POSSIBLE AND DEFINITE CASES, AND NUMBER OF SAMPLES BY CASE STATUS, FOUR INTEGRATED HEALTH CARE DELIVERY SYSTEMS, 1991–2007
| Kaiser Permanente Southern California | Group Health | Kaiser Permanente Southern Colorado | Geisinger | ||
|---|---|---|---|---|---|
| Study period |
1994–2006 |
1991–2006 |
2000–2007 |
2001–2007 |
Total |
| Beneficiary population, 2004–2006 (average) | 3.1 million | 350,000 | 400,000 | 250,000 | 4.1 million |
| Total samples | 23,183 | 3,774 | 519 | 1,221 | 28,697 |
| Possible cases | 3,349 | 452 | 93 | 94 | 3,988 |
| Definite cases | 1,561 | 212 | 39 | 53 | 1,865 |
| Average samples\possible case | 3.1 | 4.9 | 3.3 | 7.3 | 3.4 |
| Average samples\definite case | 4.5 | 6.2 | 3.9 | 7.3 | 4.8 |
METHODS
Study Population
Electronic laboratory records were queried to identify patients from whom one or more isolates of mycobacteria were recovered. Specimen and demographic information was linked through a unique patient identifier. At three sites (KPSC, GH, and KPCO) specimen information was further linked to International Classification of Diseases (ICD)-9 codes to assess comorbid conditions. Two approaches were used: a search of available datasets for a set list of known associated conditions, and identification of all ICD9 codes listed for the 3 months before and 6 months after a positive culture. Additional detail on this strategy is provided in the online supplement. At these same sites, pharmacy records were searched to identify treatment episodes of at least 3 months with any of the following drugs: ethambutol, rifampin, rifabutin, clarithromycin, azithromycin, moxifloxicin, linezolid, or amikacin (9). Institutional Review Board approval was obtained at all sites. Datasets were anonymized before sending to the National Institutes of Health; this study was exempt from Institutional Review Board review at the National Institutes of Health.
Laboratory Methods
Laboratory directors reported use of DNA probes for identification of Mycobacterium avium complex (MAC), M. avium, Mycobacterium intracellulare, Mycobacterium kansasii, and Mycobacterium gordonae; high-performance liquid chromatography and biochemicals were used to identify rapid growers. For trend analysis, no changes in methods for species identification during the study periods were reported.
Data Analysis
A “possible case” was defined as a person with at least one positive pulmonary mycobacterial isolate other than M. tuberculosis or M. gordonae. A “definite case” further met additional American Thoracic Society (ATS) microbiologic criteria (3): at least two sputum samples positive for an NTM species or a single positive culture from bronchoscopy or lung biopsy. To ascertain the radiographic criteria for disease (3), we reviewed radiology findings for all possible and definite cases identified from KPSC for 2006. Two independent reviewers scored the reports for the presence or absence of the following findings: (1) bronchiectasis, (2) nodules, or (3) cavities. Discordant findings were rereviewed.
Observed Prevalence
The annual observed prevalence of NTM was calculated as the number of observed (definite) NTM cases in a year divided by the total number of observed person-years for that year. Sex-specific prevalence was estimated across age groups by directly standardizing the age- and sex-specific prevalence using the 2000 U.S. Census Standard Population. Observed prevalence was calculated for 2004–2006, the most current comparable 3-year period, to best represent current prevalence. We also estimated the cumulative incidence by assuming that once a person was detected as a case, he or she remained a case for the remainder of the period of observation (until death, loss to follow-up, or end of the study period).
Trend Analysis
Poisson regression models with allowance for overdispersion were fit to the observed data to analyze prevalence as a function of site, sex, age group, and year. The annual percent change was calculated from the fitted models. Because of sparse longitudinal data, data from only two sites (KPSC and GH) were used in the time trend analysis. A model assuming a common time trend across the four age groups and two sites was used to produce an overall summary trend measure, the annual percent change, by sex.
RESULTS
Across the participating sites, the size of the beneficiary populations for 2004–2006 ranged from 250,000 to 3.1 million. Study sites varied in the period for which electronic mycobacteriology laboratory data were available, ranging from 17 years (1991–2006) to 7 years (2001–2007) (Table 1).
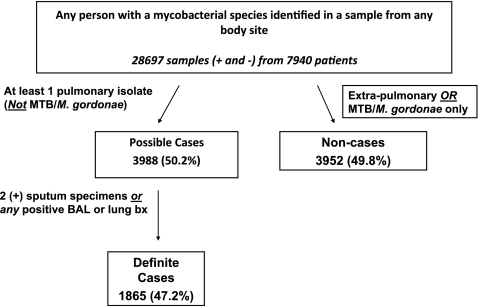
For all four sites combined, 28,697 samples from 7,940 patients with a mycobacterial sample from any body site and with known age and sex were included in the initial analysis, although our main analyses focused on patients with pulmonary isolates who were possible and definite cases (Figure 1). Patients may have had more than one species identified in their samples; all identified species were included in the analysis. Overall, 99.6% of definite cases were aged greater than or equal to 20 years, and 86.2% were aged greater than or equal to 60 years. A single site (KPSC) comprised 81% of all samples included. From all persons with a mycobacterial species identified from any body site, a total of 3,988 (50%) patients were defined as possible cases, and 1,865 (47% of possible cases) were further defined as definite cases (Figure 1). These proportions were similar for each of the sites. The average number of samples per definite case ranged from 4.5–7.3, and at most sites was slightly higher than the average number of samples per possible case, ranging from 3.1–7.3 (Table 1).
Figure 1.
Study population, four integrated health care delivery systems. BAL = bronchoalveolar lavage; MTB = Mycobacterium tuberculosis.
MAC was the most commonly isolated species from patients with a definite case of pulmonary NTM: 80% had at least a single isolate of MAC. The proportion of cases with MAC varied little across sites, ranging from 79–86% (Table 2). The next most common group were the rapidly growing mycobacteria (Mycobacterium chelonae/M. abscessus or Mycobacterium fortuitum), and their frequency was more variable across sites, ranging from 5.2–19.2%. Isolates of M. chelonae and M. abscessus could not be reliably differentiated from one another with the methods used by these sites (high-performance liquid chromatography or biochemicals), particularly over the long study period for most isolates identified in this study, and are therefore grouped. These organisms are among the most antibiotic-resistant species of the pathogenic rapidly growing mycobacteria, and M. abscessus is among the most virulent. Because chelonae is only rarely a cause of chronic lung disease, most of these isolates are likely M. abscessus; the clinical implications of infection with one or the other are greatly different: no effective treatment has been identified for M. abscessus and the prognosis is poor, whereas a greater range of antibiotics are effective against M. chelonae (3).
TABLE 2.
SPECIES DISTRIBUTION AMONG DEFINITE CASES OF NONTUBERCULOUS MYCOBACTERIA, FOUR INTEGRATED HEALTH CARE DELIVERY SYSTEMS, 1991–2007
| Species | Overall n (%) | Site Range (%) |
|---|---|---|
| M. avium complex | 1,495 (80.1) | 79–86 |
| M. chelonae/abscessus | 225 (12.1) | 2.6–13 |
| M. fortuitum | 106 (5.6) | 2.6–6.2 |
| M. kansasii | 102 (5.5) | 0–6.2 |
| M. simiae | 53 (2.8) | 0–5.1 |
| M. mucogenicum * | 22 (1.9) | 0–1.9 |
| M. xenopi | 33 (1.7) | 1.6–2.8 |
| Total cases | 1,865 |
Of the 22 patients with mucogenicum identified, 8 had this as the only species identified.
Other species identified were as follows: M. terrae (n = 11, for which nine patients had other species identified); M. flavenscens (n = 7, of which one was from a patient with no other species identified); M. scrofulaceum (n = 6); M. szulgai (n = 3); N. smegmatis (n = 2); M. lentiflavium (n = 2); M. peregrinum (n = 2); M. malmoense (n = 1); and M. marinum (n = 1).
Observed Prevalence Trends
Disease trends were analyzed for definite cases. The trends by age and sex groups are shown in Figure 2. Table 3 provides summary data and estimated annual percent change rates for the gender-specific models. Prevalence had increasing trends in most age- and sex-specific groups with the exception of the 70- to 79-year age group. The greatest annual percent change was observed among women aged greater than or equal to 80 years, who had a significant rate of 7.5% (3.4–11.8) (Figure 2A, Table 3). Within the less than 60-year age group the annual percent change showed a similar increasing trend among both men (4.3%) and women (3.7%), although this increase was significant only among men (Figure 2B). Trends were significantly different by age group among women (P = 0.021) but not among men (P = 0.48). For all age groups combined, the annual percent change was increasing overall, at a rate of 2.6% (95% confidence interval [CI], 0.76–4.4) per year among women and 2.9% (95% CI, 0.69–5.1) among men. These trends correspond to an annual increase among women from 4.5 per 100,000 during 1994–1996 to 7.5 per 100,000 during 2004–2006, and among men from 3.5 per 100,000 during 1994–1996 to 4.9 per 100,000 during 2004–2006. For all age groups and both sexes combined, the annual percent change was 2.7% (95% CI, 1.3–4.1).
Figure 2.
Observed annual prevalence with fitted trend from Poisson regression model among (A) women, (B) men, (C) women <60 years, and (D) men <60 years. Group Health and Kaiser Permanente Southern California combined, 1994–2006.
TABLE 3.
ANNUAL PERCENT CHANGE* IN NONTUBERCULOUS MYCOBACTERIA PREVALENCE, KAISER PERMANENTE SOUTHERN CALIFORNIA, GROUP HEALTH, 1994–2006
| Sex | Age Group | Annual Percent Change (95% confidence interval) |
|---|---|---|
| Women | 0–59 | 3.7 (−0.21 to 7.8) |
| 60–69 | 1.8 (−1.7 to 5.4) | |
| 70–79 | −0.29 (−3.1 to 2.6) | |
| 80+ | 7.5† (3.4 to 11.8) | |
| Standardized rate (US 2000) | 2.6* (0.76 to 4.4) | |
| Men | 0–59 | 4.3† (0.10 to 8.7) |
| 60–69 | 3.9 (−0.70 to 8.7) | |
| 70–79 | 0.20 (−3.7 to 4.3) | |
| 80+ | 3.9 (−1.3 to 9.4) | |
| Standardized rate (US 2000) | 2.9† (0.69 to 5.1) |
As estimated from the model.
Statistically significant at P < 0.05.
Observed Prevalence and Cumulative Incidence
Average annual age-adjusted prevalence estimates by sex for each site for 2004–2006 are shown in Table 4. Across all four sites, prevalence was 50% higher among women compared with men, with a range of 1.1- to 1.6-fold greater. Prevalence (cases per 100,000 person-years) ranged from 1.1 for men and 1.7 for women to 5.2 among men and 8.1 among women (Table 4).
TABLE 4.
AVERAGE ANNUAL AGE-ADJUSTED* PERIOD PREVALENCE BY SITE AND SEX, FOUR INTEGRATED HEALTH CARE DELIVERY SYSTEMS, 2004–2006
| Men Cases per 100,000 Person Years | Women Cases per 100,000 Person Years | Overall Cases per 100,000 Person Years | |
|---|---|---|---|
| Kaiser Permanente Southern California | 5.2 | 8.1 | 6.7 |
| Group Health | 2.7 | 3.9 | 3.3 |
| Kaiser Permanente Colorado | 1.1 | 1.7 | 1.4 |
| Geisinger | 3.4 | 3.8 | 3.6 |
| Combined | 4.4 | 6.5 | 5.5 |
Standardized to the 2000 US Census Standard Population.
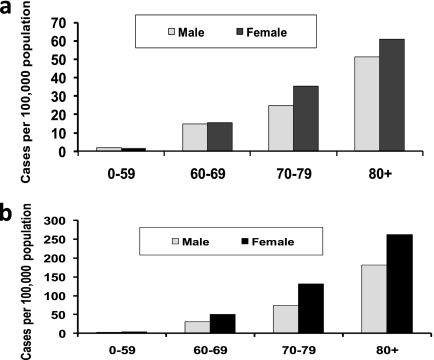
We calculated observed prevalence and cumulative incidence for the two sites with the longest period of observation, GH and KPSC. Cumulative incidence was estimated by assuming that once a person was diagnosed and “counted” as a case for our analysis, they would be counted for all subsequent years. Observed prevalence by age and sex for 2004–2006 is shown in Figure 3A. After age 60, prevalence increased more than sevenfold among both men and women, from 1.7 per 100,000 among persons aged less than 60 years to 15 per 100,000 among those aged 60–69 years, 30 per 100,000 among persons 70–79 years, and 57 per 100,000 among persons aged greater than or equal to 80 years. Within the greater than 60 age group, women had a 30% higher prevalence than men. Over age 60, 1 out of every 3,370 women and 1 out of every 4,347 men were affected by pulmonary NTM.
Figure 3.
(A) Average annual prevalence by age and sex. Group Health and Kaiser Permanente Southern California combined, 2004–2006. (B) Cumulative incidence by age and sex. Group Health and Kaiser Permanente Southern California combined, 2004–2006.
The cumulative incidence followed a similar age- and sex-specific pattern, with an increasing prevalence after age 60, and higher prevalence in women relative to men (Figure 3B). The cumulative incidence was twofold to fivefold higher than the observed prevalence for each age group, and the female/male difference was more marked compared with that seen for the observed prevalence. In the oldest age group, the cumulative incidence among women was 263 per 100,000, 1.4-fold higher than men in the same age group, and 4.3-fold higher than the observed prevalence among women aged greater than or equal to 80 years.
More than 80% of cases were represented in only a single year, likely explained by the lack of microbiologic sampling among patients. At KPSC, 87% of cases were cases in a single year only; this figure was 85% at GH. The proportion of cases who were never cultured in other years ranged from 40% at GH to 70% at KPSC. Patients who were cases in these sites represented a relatively stable population, with opportunity for microbiologic follow-up: at KPSC, 87% of cases were enrolled at least 5 years, and 58% were enrolled greater than or equal to 10 years; at GH these figures were 88% and 70%, respectively.
Overall, only 335 (18.5%) cases received antibiotic treatment of at least 3 months duration in association with their positive culture (site–specific range, 17.1–41%). Within the group of patients who received any treatment, 87 (26%) received greater than or equal to three drugs. Of these, 84 (97%) had MAC infection and all received at least clarithromycin or azithromycin, ethambutol, and rifabutin or rifampin.
The frequency of underlying conditions and symptoms identified from any ICD9 codes during the 3 months before or 6 months after collection of a positive culture was also similar for possible and definite cases (Table 5). Among possible cases, only 16% were coded as having pulmonary NTM (ICD9 031.0), and for definite cases this figure was 26.9%. Bronchiectasis (23.6%), COPD (28.2%), and any malignancy (25%) were among the most frequent conditions identified for definite cases around the time of specimen collection; 31.4% of possible and 33.3% of definite cases also had a diagnosis of pneumonia. Coding of symptoms was variable; cough, shortness of breath, and hemoptysis were coded for 10–28% of possible and definite cases (Table 5).
TABLE 5.
ASSOCIATED CONDITIONS AND SYMPTOMS CODED DURING THE 3 MONTHS BEFORE OR 6 MONTHS AFTER DETECTION OF A POSITIVE SPECIMEN, POSSIBLE AND DEFINITE CASES, THREE INTEGRATED HEALTH CARE DELIVERY SYSTEMS
| Diagnosis or Symptom (ICD9 code) | Possible Cases | Definite Cases |
|---|---|---|
| Pulmonary nontuberculous mycobacteria (031.0) | 638 (16) | 488 (26.9) |
| Bronchiectasis (494, 494.0, 494.1) | 628 (16) | 427 (23.6) |
| Chronic obstructive pulmonary disease (496, 491) | 1,256 (32.3) | 511 (28.2) |
| Pneumonia, unspec (486) | 1,224 (31.4) | 603 (33.3) |
| Asthma (493.2, 493.9) | 446 (11.5) | 223 (12.3) |
| Other lung disease not elsewhere classified (518.89) | 419 (10.8) | 249 (13.7) |
| Malignancies (140-239, excluding 173) | 838 (21.5) | 454 (25) |
| Congestive heart failure (428.0) | 422 (10.8) | 188 (10.4) |
| Gastroesophageal reflux (530.81) | 388 (10.8) | 207 (11.4) |
| Cough (786.2) | 916 (23.5) | 508 (28) |
| Shortness of breath (786.05) | 401 (10.3) | 215 (11.9) |
| Hemoptysis (786.3) | 443 (11.4) | 264 (14.6) |
| Total | 3,894 | 1,812 |
The frequency of ever–diagnosed chronic predisposing conditions identified through a search of the entire time span of available data with a set list of ICD9 codes was quite similar for both possible and definite cases. Overall, 3.9% of possible cases and 3.4% of definite cases were identified as having HIV-AIDS, and 9.4% of possible cases and 11.5% of definite cases were diagnosed with a malignant neoplasm of the trachea, bronchus, or lung; 1.2% of both groups were identified as having an immune disorder, and 0.6% of both groups were diagnosed with cystic fibrosis.
The variation by sex in coding of pulmonary NTM, COPD, and bronchiectasis was analyzed for cases from KPSC and GHC. Bronchiectasis was more commonly coded among women (31.6%) compared with men (15.8%). The frequency of a COPD code was similar for men (38.9%) and women (35.5%). Women were more likely to be coded as having pulmonary NTM (31.4%) compared with men (20%).
Radiology
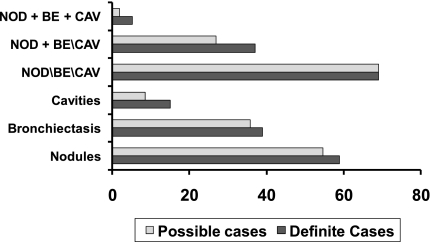
Among 388 possible and definite cases identified from one site with available radiographic data for 2006, 131 (33.7%) had a chest radiograph only and 257 (66.2%) had at least one chest CT scan. Of 151 definite cases, 89 (58.9%) had nodules; 60 (39.7%) had bronchiectasis; 9 (8.5%) had cavities; and 105 (69.5%) had at least one of these three findings. The frequency of these radiographic findings was very similar for possible and definite cases, with the exception of cavities, which was nearly twice as frequent among definite cases (15.2% vs. 8.5%) (Figure 4).
Figure 4.
Radiographic findings among possible and definite cases with at least one chest CT (n = 257). Kaiser Permanente Southern California, 2006. BE = bronchiectasis; CAV = cavities; NOD = nodules.
Tuberculosis
For the most recent 3-year period (2004–2006) the prevalence of TB at these sites was calculated and compared with the prevalence of NTM in the same period. All rates were adjusted to the United States population using 2000 census data. A case of pulmonary TB was defined as any person who had M. tuberculosis isolated from a pulmonary site. The prevalence of pulmonary NTM disease was 2- to 2.9-fold greater than pulmonary TB across sites.
DISCUSSION
This first population-based estimate of trends in NTM pulmonary disease in the United States using current ATS microbiologic criteria demonstrates an overall increasing prevalence of pulmonary NTM. The burden estimated here is similar to other recently published estimates, and has increased since the last estimates were published 20 years ago (5). This analysis also identified infrequent microbiologic sampling for this condition, likely leading to an underestimate of the true burden. In addition, treatment for this condition was infrequent. Comorbid conditions were very similar for persons with a single pulmonary isolate compared with those who met ATS microbiologic criteria; the frequency of underlying conditions was similar to that described previously (1, 3, 5), with the additional identification of rare conditions associated with this disease.
The only prior population-based study of NTM trends in the United States, conducted in Massachusetts during 1972–1983, identified an increase in disease prevalence (10). Our trends in this analysis are consistent with those for Ontario, Canada, 1997–2003 (11), which reflected an annual percent increase in isolation prevalence of 8.4% per year, from 9.4 per 100,000 to 14.1 per 100,000. Only approximately 33% of persons with isolates met ATS microbiologic criteria, corresponding to an estimated increase in disease prevalence in Ontario from 3.1 to 4.6 per 100,000 during that time. At our two West coast sites combined, we found an average annual increase from 1994–1996 to 2004–2006 of 4.5 to 7.5 per 100,000 among women and 3.5 to 4.9 among men. Our population-based data from these large United States health care systems confirm prior clinic-based reports identifying an increase in the numbers of patients with pulmonary NTM disease (1, 2).
The reasons for the increasing prevalence likely reflect a combination of increased awareness and detection and true increases in disease. More rapid and specific diagnostics developed over the last 20 years (12) have increased the ease and capability for identification of mycobacterial species, leading to greater specificity in disease diagnosis. In addition, the recognition of pulmonary NTM among women aged greater than or equal to 60 years with no known underlying risk factors (2) has led to increasing awareness among physicians, patients, and advocacy groups. The increased use of CT scans also may have facilitated increased diagnosis. Data from serial assessment of skin test sensitization to M. intracellulare suggest an increasing prevalence of exposure (13). However, the link between sensitization and overt disease is still unclear. The increasing chlorination of the water supply has been hypothesized to select for NTM over other bacteria, potentially increasing their concentration and the potential for exposure (14).
Our overall age-adjusted average annual prevalence of 5.4 per 100,000 for 2004–2006 is higher than the last published estimates of 1.8 per 100,000 in 1984 (5) and is consistent with a recently published report from a single state, Oregon (15). The overall prevalence of 4.4 per 100,000 among men and 6.5 per 100,000 among women is nearly identical with that of 4.7 per 100,000 among men and 6.4 per 100,000 among women found in Oregon. The only other current estimate is from one area of Northern Manhattan, which estimated an annual prevalence of pulmonary NTM of 2 per 100,000 for the period 2000–2003 (16). Because the IHDS studied did not include populations from the Southeastern United States, which have been found to have higher prevalence of NTM isolation and of disease (17, 18) and also have a high population density, the overall prevalence found here could represent an underestimate of the prevalence for the United States as a whole. However, given the unavailability of comparable disease prevalence estimate from other regions of the United States, we cannot evaluate the degree of this bias with any certainty. With respect to individual level factors, the IHDS studied have a relatively similar age and sex distribution relative to the underlying population in the coverage area, with a slight overrepresentation of persons aged greater than 60 years at KPCO (20% vs. 12% in coverage area). In the one IHDS where this has been systematically assessed (KPSC), the poorest, nonworking population was least likely to be enrolled, which would explain the lower rates of TB in the IHDS population, with an underrepresentation of the lowest (<$35,000/yr) and highest (>$75,000/yr) income groups (D. Strickland, personal communication).
The cases identified in our study more likely represent incident than prevalent cases: greater than or equal to 80% of cases were cases in only a single year, even over a 13- to 16-year period, most likely because they were not routinely cultured for NTM. Persons may not be routinely cultured because their disease is mild and slowly progressing, a lack of awareness by physicians or patients about this condition, or because of a decision not to treat the infection (3). In a recent large study at a tertiary referral center, patients went an average of 5 years between onset of symptoms and diagnosis (19). Rates of mycobacterial clearance are low and relapse or reinfection rates are high (20, 21), contributing to the chronic nature of this condition. For these reasons, our observed prevalence estimates likely underestimate true prevalence. Therefore, to obtain a more complete estimate of the true burden of disease, we estimated cumulative incidence by assuming that once a person was diagnosed and “counted” as a case for our analysis, they would be counted for all subsequent years. Using this approach, the estimated overall age-adjusted cumulative incidence was 2.5-fold greater than the average annual prevalence among men and 3.5-fold greater than that for women. However, this approach does not consider cure rates; some proportion of patients may clear their infection. Therefore, true prevalence may be intermediate between the observed prevalence and that estimated by the cumulative incidence approach.
The geographic differences in estimated prevalence in our study support prior findings showing geographic variability (17, 18). In 1999, the Centers for Disease Control and Prevention published estimates of isolate prevalence based on patient isolates referred to the state laboratories, demonstrating marked geographic variability; the highest rates were in the Southeastern United States (17). A study of NTM-associated hospitalizations also found geographic differences in the prevalence of NTM-associated hospitalizations and prevalence trends, with the highest rates in the Southeastern United States (18). We found the lowest estimates of 1–2 per 100,000 from the site in the Rocky Mountain Region and the highest of 5.2 (men) to 8.1 (women) in Southern California. On the West coast, where the most beneficiaries were observed for the longest time, the prevalence was very similar at the two participating sites, and quite similar to the rates reported from Oregon (15).
Treatment for pulmonary NTM disease is lengthy and complex. For the most common mycobacteria, MAC, the ATS has recommended treatment with a multidrug regimen to achieve persistently negative sputum cultures for 12 months; sputum conversion should be observed within 3–6 months (3). Some patients may meet diagnostic criteria but do not have progressive or severe disease and therefore may be closely monitored with regular sputum collection (3). We found a low proportion of antibiotic treatment of at least 3 months duration, a minimum estimate of treatment. Physicians may have chosen a “wait and see” approach before prescribing antibiotics. This approach is partially justified by the fact that the reported efficacy of treatment is low. In a recent review of 12 clinical trials with regimens containing macrolides, the median reported efficacy was 56% with a range from 26–71%; the median sample size in these studies was 43 (range, 10–103) (20). In a recently published large clinical trial, the proportion of patients with M. avium infection treated with a currently recommended regimen who were alive and reported “cured” at 5 years was 31% (21). Drug intolerance and dose-dependent toxicity are common, particularly in the elderly: from 10–50% of patients experience side effects of varying severity to the commonly prescribed drugs for MAC disease (3, 10, 21, 22). Therefore, for many patients and their physicians, even when the disease is recognized, the perceived benefit of treatment is low.
The frequency of underlying conditions was similar to that described previously, and was similar between possible and definite cases. Approximately a third of cases had COPD, commonly associated with NTM (1, 3, 5). The frequency of bronchiectasis was also high. The higher frequency of bronchiectasis among women than men is consistent with the greater proportion of women without other known underlying risk factors (1). Less than half of all cases were coded as having pulmonary NTM; because NTM is a relatively rare and often unrecognized condition, the codes may not be listed on standard discharge or billing sheets and physicians or coders may not be as likely to assign this code. The higher proportion of definite cases coded as NTM among women (31%) compared with men (20%) suggests that NTM in men may be considered as incidental to their underlying condition (and therefore not coded), whereas among women without other underlying risk factors NTM is considered primary.
Underlying and associated conditions were quite similar among possible cases (persons with only a single positive sputum sample) compared with definite cases (persons with two positive sputum samples, or a positive culture from bronchoalveolar lavage or lung biopsy). The radiographic findings in these two groups were also quite similar, which could be explained in part by the similar frequency of underlying conditions, such as malignancies and COPD, or by a similar frequency of NTM disease in both groups. Both groups had a similar number of specimens collected: overall, on average, 3.4 samples per possible case, and 4.8 samples per definite case. These data suggest that the current ATS criteria, which recommend the collection of three sputum samples for diagnosis, and the use of two positive sputum samples rather than a single positive sputum culture to establish the diagnosis of disease, may differentiate poorly between those who do not have disease and those who have milder indolent disease that may progress to a more severe disease state. Although the ATS recommends follow-up with regular collection of sputum samples for patients with a single sputum sample, in this study the low average number of samples over a long time period indicates that most patients were not receiving standard recommended follow-up cultures. Approximately one fifth of definite cases had been possible cases in another year. In the absence of routine sputum cultures, a single positive sputum sample may be sufficient evidence of disease if radiographic findings and clinical features are consistent with disease.
Evaluation of our case definition is limited. One recent study in Denmark evaluated a small subset of patients who met ATS microbiologic criteria: 90% of those who met microbiologic criteria and were classified as “possible cases” in our study met additional radiographic and clinical criteria. Thus, by using microbiologic criteria alone, we may slightly overestimate disease prevalence. In addition, the use of this definition may underestimate the proportion of disease from organisms with a relatively high pathogenicity, such as M. kansasii (23).
The increasing prevalence of pulmonary NTM disease and declining rates of TB represent the new epidemiology of mycobacterial disease in the United States. The incidence of TB in the United States declined to 3.8 cases per 100,000 population in 2009, for a decrease of 11.4% from 2008, continuing a progressive decline since the 1990s (24). NTM seems to have surpassed TB as the leading cause of mycobacterial lung disease in the United States.
Supplementary Material
Acknowledgments
The authors thank Ellenie Tuazon (KPSC) and Patti Benson (GH) for their assistance with study coordination; and Tony Yiu (KPSC), Lora Bounds (GH), Heather M. Tavel (KPCO), Marian Bailey (KPCO), and Judy Reardon (Geisinger) for their efforts in data extraction and management.
Supported in part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, and by a grant from NTM Info and Research, a 501C3 nonprofit organization devoted to pulmonary nontuberculous mycobacteria disease.
The views expressed in this article are those of the authors and do not necessarily reflect those of the U.S. Department of Health and Human Services.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201002-0310OC on June 10, 2010
Author Disclosure: D.R.P. is an employee of the National Institutes of Health (NIH). P.A.S. is an employee of the NIH. D.S. is a full-time employee of the Southern California Permanente Medical Group, and received more than $100,001 from Sanofi-Aventis in industry-sponsored grants for the study of diabetes medications and cancer risk and $5,000 in contract funds from the NIH. L.A.J. received $10,001–$50,000 from Wyeth, $5,001–$10,000 from Novartis, and $1,001–$5,000 from GlaxoSmithKline in consultancy fees; more than $100,001 from Wyeth, more than $100,001 from Sanofi Pasteur, more than $100,001 from Novartis, and more than $100,001 from GlaxoSmithKline in industry-sponsored grants; and more than $100,001 from the NIH and more than $100,001 from the Centers for Disease Control and Prevention in sponsored grants. M.A.R.'s primary employer is the Kaiser Foundation Health Plan of Colorado and received $50,001–$100,000 from ICON in industry-sponsored grants for a research project on Clostridium difficile prevalence. M.A.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.M.D.O. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. Y.R.S. is employed by the NIH as a Microbiologist at NIH/DLM/Micro service. A.E.S. is employed by the NIH as a contractor through Kelly Services. S.M.H. is an employee of the NIH. K.N.O. is an employee of the NIH.
References
- 1.Prince DS, Peterson DD, Steiner RM, Gottlieb JE, Scott R, Israel HL, Figueroa WG, Fish JE. Infection with Mycobacterium avium complex in patients without predisposing conditions. N Engl J Med 1989;321:863–868. [DOI] [PubMed] [Google Scholar]
- 2.Cox JN, Brenner ER, Bryan CS. Changing patterns of mycobacterial disease at a teaching community hospital. Infect Control Hosp Epidemiol 1994;15:513–515. [DOI] [PubMed] [Google Scholar]
- 3.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland S, Horsburgh R, Huitt G, Iademarco MF, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007;175:367–416. [DOI] [PubMed] [Google Scholar]
- 4.Olivier KN, Weber DJ, Wallace RJ, Faiz AR, Lee JH, Zhang Y, Brown-Elliot BA, Handler A, Wilson RW, Schecter MS, et al. Nontuberculous mycobacteria: I. Multicenter study in cystic fibrosis. Am J Respir Crit Care Med 2003;167:828–834. [DOI] [PubMed] [Google Scholar]
- 5.O'Brien RJ, Geiter LJ, Snider DE Jr. The epidemiology of nontuberculous mycobacterial diseases in the United States: results from a national survey. Am Rev Respir Dis 1987;135:1007–1014. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy TP, Weber DJ. Nontuberculous mycobacteria: an underappreciated cause of geriatric lung disease. Am J Respir Crit Care Med 1994;149:1654–1658. [DOI] [PubMed] [Google Scholar]
- 7.Tokars JI, Rudnick JR, Kroc K, Manangan L, Pugliesse G, Huebner RE, Chan J, Jarvis WR. US hospital mycobacteriology laboratories: status and comparison with state public health department laboratories. J Clin Microbiol 1996;34:680–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prevots DR, Strickland D, Jackson L, Shaw P, Shea YR, Montes de Oca R, Olivier K. Prevalence of nontuberculous mycobacterial disease, Kaiser Permanente Southern California, and Group Health Cooperative, Seattle, Washington, 1991–2006. Presented at the annual meeting of the American Thoracic Society, May 15–20, 2009, San Diego, CA. Abstract, p. A5266.
- 9.Ballarino GJ, Olivier KN, Claypool RJ, Holland SM, Prevots DR. Pulmonary nontuberculous mycobacterial infections: antibiotic treatment and associated costs. Respir Med 2009;103:1448–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.du Moulin GC, Sherman IH, Hoaglin DC, Stottmeier KD. Mycobacterium avium complex: an emerging pathogen in Massachusetts. J Clin Microbiol 1985;22:9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marras TK, Chedore P, Ying AM, Jamieson F. Isolation prevalence of pulmonary non-tuberculous mycobacteria in Ontario, 1997–2003. Thorax 2007;62:661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Musial CE, Tice LS, Stockman L, Roberts GD. Identification of mycobacteria from culture by using the Gen-Probe Rapid Diagnostic System for Mycobacterium avium complex and Mycobacterium tuberculosis complex. J Clin Microbiol 1988;26:2120–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan K, Wang J, Marras TK. Nontuberculous mycobacterial sensitization in the United States: national trends over three decades. Am J Respir Crit Care Med 2007;176:306–313. [DOI] [PubMed] [Google Scholar]
- 14.Falkinham JO III. Nontuberculous mycobacteria in the environment. Clin Chest Med 2002;23:529–551. [DOI] [PubMed] [Google Scholar]
- 15.Cassidy PM, Hedberg K, Saulson A, McNelly E, Winthrop KL. Nontuberculous mycobacterial disease prevalence and risk factors: a changing epidemiology. Clin Infect Dis 2009;49:e124–e129. [DOI] [PubMed] [Google Scholar]
- 16.Bodle EE, Cunningham JA, Della-Latta P, Schluger NW, Saiman L. Epidemiology of nontuberculous mycobacteria in patients without HIV infection, New York City. Emerg Infect Dis 2008;14:390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Nontuberculous mycobacteria reported to the public health lab information system by state public health labs, United States, 1993–1996. 1999 Jul [cited 2009 Jun 2]. Available from: http://www.cdc.gov/tb/Laboratory_Services/NontuberculousMycobacteria.pdf.
- 18.Billinger ME, Olivier KN, Viboud C, de Oca RM, Steiner C, Holland SM, Prevots DR. Nontuberculous mycobacteria-associated lung disease in hospitalized persons, United States, 1998–2005. Emerg Infect Dis 2009;15:1562–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim RD, Greenberg DE, Ehrmantraut ME, Guide SV, Ding L, Shea Y, Brown MR, Chernick M, Ssteagall WK, Glasgow CG, et al. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med 2008;178:1066–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Field SK, Fisher D, Cowie RL. Mycobacterium avium complex pulmonary disease in patients without HIV infection. Chest 2004;126:566–581. [DOI] [PubMed] [Google Scholar]
- 21.Jenkins PA, Campbell IA, Banks J, Gelder CM, Prescott RJ, Smith AP. Clarithromycin vs ciprofloxacin as adjuncts to rifampicin and ethambutol in treating opportunist mycobacterial lung diseases and an assessment of Mycobacterium vaccae immunotherapy. Thorax 2008;63:627–634. [DOI] [PubMed] [Google Scholar]
- 22.Griffith DE, Brown BA, Cegielski P, Murphy DT, Wallace RJ Jr. Early results (at 6 months) with intermittent clarithromycin-including regimens for lung disease due to Mycobacterium avium complex. Clin Infect Dis 2000;30:288–292. [DOI] [PubMed] [Google Scholar]
- 23.Andrejak C, Thomsen VO, Johansen IS, Rils A, Benfield TL, Duhaut P, Sorensen HT, Lescure FX, Thomasen RW. Nontuberculous pulmonary mycobacteriosis in Denmark: incidence and prognostic factors. Am J Respir Crit Care Med 2010;181:514–521. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. Trends in tuberculosis—United States, 2008. MMWR 2010;59:289–294.20300055 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.