Abstract
Objective
Preoperatively, it is difficult to differentiate between parathyroid cancer (PtCa) and severe primary hyperparathyroidism (PHPT) due to a benign tumor. Human chorionic gonadotropin (hCG) is a tumor marker in trophoblastic and nontrophoblastic cancers and hyperglycosylated hCG is increased in hCG-secreting malignancies. We investigated whether hCG can distinguish PtCa cancer from benign disease and add prognostic information.
Design
Observational study.
Methods
Measurement of urinary hCG (total and malignant isoforms) and serum malignant hCG in 8 subjects with PtCa and in 18 subjects with PHPT (measurement of urine in ten and serum in eight).
Results
Total urinary hCG was normal in the benign PHPT control subjects (range: 0–17 fmol/mg Cr; nl < 50). In the PtCa subjects, three had normal total urinary hCG levels and survived complication free for at least 2 years; three had persistently elevated total urinary hCG levels (range: 217–1986 fmol/mg Cr) and sustained hip fracture (n = 3) and died (n = 2) within 3 and 6 months respectively; two had a rise in total urinary hCG and had hip fracture (n = 1) and died (n = 2) within 4 and 10 months respectively. Elevated urinary hCG was of the malignant hyperglycosylated isoform. Serum malignant hyperglycosylated hCG values in all of the cancer patients exceeded the maximal serum malignant hCG level of the PHPT subjects with benign disease (3.77 pmol/l).
Conclusion
hCG, especially itshyperglycosylated isoform, might add diagnostic and prognostic information in PtCa. Further studies would help to elucidate the role of hCG as a potential tumor marker in this disease.
Introduction
Parathyroid carcinoma is a rare presentation of primary hyperparathyroidism (PHPT) (1). In most patients with PHPT today, the disease is benign and asymptomatic. Nevertheless, PHPT is still occasionally seen in the more classical form in which severe biochemical and clinical manifestations predominate. In these patients, a high index of suspicion for parathyroid cancer (PtCa) is important, because the best chance for cure is associated with complete resection at the time of initial surgery (2–5). However, on clinical grounds alone, it is very difficult to distinguish between very active benign parathyroid disease and its malignant counterpart. A mutation in a tumor suppressor gene, HRPT2, has been demonstrated in many cases of parathyroid carcinoma (6). While further studies are ongoing to determine the clinical utility of HRPT2 and its encoded parafibromin measurements, a readily available biochemical marker that reliably distinguishes between malignant and benign parathyroid disease would be useful.
Human chorionic gonadotropin (hCG), a glycoprotein hormone, is normally produced by placental trophoblasts. However, sera from many patients with trophoblastic and certain nontrophoblastic tumors also contain hCG immunoreactivity, usually of the free β-subunit (7). In hCG-secreting malignancies, an isoform of hCG that is hyperglycosylated is preferentially secreted (8, 9). In these diseases, elevated expression of standard or hyperglycosylated hCG is an adverse prognostic indicator and helps to predict recurrence (7).
In the course of a therapeutic trial with the calcimimetic, cinacalcet, we discovered among baseline indices elevated levels of hCG in the serum of non-pregnant women and men with PtCa. This unexpected observation raised the possibility that hCG is a marker for PtCa and might correlate with disease activity. The purpose of this paper is to report findings that support this hypothesis.
Methods
Subjects
Eight consecutive patients with PtCa and unknown hCG levels were recruited and enrolled. Five of the cancer patients had histological features at initial parathyroid surgery, which were consistent with carcinoma; of those five, four had either concomitant or subsequent metastatic disease (mediastinal and lung) while the other subject had persistent hypercalcemia (> 3.5 mmol/l) and high parathyroid hormone (PTH) levels (> 100 pmol/l). The other three cancer patients had initial histological features that were consistent with parathyroid adenomas, but had recurrences of hypercalcemia with subsequent surgeries and histological findings consistent with PtCa, as well as neck metastases in two of the patients. Parathyroid carcinoma was thus confirmed on histology in all of the patients, either on initial or repeat parathyroid surgery, with classic pathological characteristics, such as capsular invasion, being seen in all eight patients. One subject (patient #1) had a suggestive history for hyperparathyroidism–jaw tumor syndrome, with a jaw tumor and a brother with benign PHPT. He was subsequently tested for HRPT2 mutation and was found to be positive. Since commercial testing was not available at the time of study initiation, other patients were not routinely tested for HRPT2.
Eighteen subjects with benign PHPT were also enrolled. In the benign PHPT urinary control group, ten patients had measurement of urinary hCG and of the urinary ratio of the malignant hCG isoform. In the benign PHPT serum control group, eight other patients had measurement of the malignant hCG isoform in the serum. In both control groups, benign PHPT was characterized by asymptomatic mild hypercalcemia and elevated PTH levels. The benign PHPT patients were seen in our Bone Unit at the same time that the study data were being collected on the PtCa subjects. There was no known bias in terms of the way the PHPT subjects were selected. None had clinical features of PtCa, such as a palpable neck mass or significant renal or skeletal disease (1). The Institutional Review Board of Columbia University Medical Center approved the protocol. All patients gave written informed consent.
Protocol
Serial measurements of urinary and serum hCG were performed in the cancer patients at study entry and then at varying intervals after exposure to cinacalcet (administered on an escalating dosage regimen 30 mg twice daily to 90 mg four times daily) over 16 weeks. The 18 patients with benign parathyroid disease had a single measurement of hCG (ten of urine and eight of serum). The PHPT patients did not receive cinacalcet. All urine samples were measured in a first morning void and all blood samples were obtained fasting in the morning. In the cancer patients, samples were collected prior to the morning cinacalcet dose. Serum and urine were immediately frozen and kept at −80 degrees. Serum calcium and PTH measurements were made in all patients.
Biochemical determinations
Serum calcium was measured at Covance Laboratory (Indianapolis, IN, USA). Intact PTH was measured using an IRMA (coefficient of variation, CV = 4.2–6.4%; Allegro PTH, Nichols Institute Diagnostics; San Juan Capistrano, CA, USA).
The hCG measurement in urine was performed with two-site IRMAs measuring hyperglycosylated hCG (B152 capture), hCG, and β subunit (CTP104 capture) using a detection antibody directed to hCG and hCG β but not to the CTP region (B207). Monoclonal antibodies B152 and B207 have been described along with procedures for their use in two site immunometric assays (10, 11). The hCG employed as standard for these assays was the new WHO 1st Reference Reagent for Immunoassay 99/688 (12). B207 was used as detection (radiolabeled antibody) and B152 as capture antibody for measuring hyperglycosylated (core 2) hCG (12), while CTP104 was used as capture antibody for measuring total hCG (core 1 and core 2 forms) (12, 13). Capture antibodies B152 and CTP104 recognize epitopes on the CTP and are sensitive to the glycosylation status of this portion of hCG (13, 14). The presence of hyperglycosylated hCG isoforms was detected by a ratio method that involved two sandwich immunoassay measurements on the same specimen using the hCG RP as standard. The amount determined in an assay using B152 for capture and 125I-B207 for detection was divided by that measured in an assay using CTP104 for capture and 125I-B207 for detection. The ratios found ranged between 0.5 and 4 with the highest ratio indicative of the highest content of B152 isoforms.
In serum, a direct analysis for hyperglycosylated hCG used an automatic assay system standardized with a hyperglycosylated hCG form isolated from a cancer patient (the immunogen for antibody B152) (15).
Statistical analysis
Statistical analyses were performed using SPSS (version 11). Continuous data are presented as mean value ± S.D.; categorical data are reported as absolute number. A Cox proportional hazards model was used to estimate the survivor function as predicted by urinary hCG levels with four observed and four right-censored observations. The levels of urinary hCG for hypothetical patients with PtCa were entered into the resultant model to show the failure curves that would be predicted.
Results
Urinary hCG
The ten benign PHPT urinary control subjects (two males, eight females; age: 63 ± 15 years) had mildly elevated serum calcium (2.7 ± 0.1 mmol/l; nl: 2.1–2.6 mmol/l) and PTH (9.7 ± 0.2 pmol/l; nl: 1.0–6.8 pmol/l) levels, while the PtCa patients (five males, three females; age: 56 ± 13 years) had markedly elevated serum calcium (3.9 ± 0.5 mmol/l; P < 0.0001 versus benign PHPT urinary controls) and PTH levels (81.1 ± 64 pmol/l; P = 0.003 versus benign PHPT urinary controls) at presentation (Table 1).
Table 1.
Individual urinary human chorionic gonadotropin (hCG) levels (total levels and ratio of malignant hCG:total hCG), calcium, parathyroid hormone (PTH), and clinical outcomes for ten primary hyperparathyroidism (PHPT) subjects and eight parathyroid carcinoma subjects.
| Subjects | Urinary hCG fmol/mg Cr nl:< 50 |
Urinary malignant isoform nl: <0.5 |
Calcium nl: 2.1–2.6 mmol/ |
PTH nl: 1.0–6.8 pmol/l |
Hip fracture/ death |
|
|---|---|---|---|---|---|---|
| PHPT subjects (n = 10) | 6.1 ± 6 | 0 | 2.7 ± 0.1 | 9.7 ± 0.2 | No/No | |
| Parathyroid carcinoma subjects (n = 8) | ||||||
| Persistently low hCG (n = 3) | 1 | 0 | 0 | 2.6 | 118 | No/No |
| 2 | 0 | 0 | 3.1 | 18.2 | No/No | |
| 3 | 3 | 0 | 3.3 | 35 | No/No | |
| Persistently high hCG (n = 3) | 4 | 800 | 0.9 | 2.9 | 128 | Yes/Yes |
| 5 | 1986 | 0.8 | 3.2 | 87 | Yes/Yes | |
| 6 | 217 | 0.6 | 3.9 | 54 | Yes/No | |
| Rise in hCG (n = 2) | 7 | 0 | 0 | 3.2 | 36 | |
| 2662 | 1.7 | 3.3 | 130 | No/Yes | ||
| 8 | 0 | 0 | 3.8 | 222 | ||
| 956 | 1.5 | 2.9 | 227 | Yes/Yes | ||
Carcinoma subjects are grouped according to whether their hCG levels were persistently low, high, or increased over time. The values in the cancer subjects were those obtained at initial hCG measurement, which were measured at varying stages of cinacalcet treatment. Repeat hCG levels are shown for subjects 7 and 8; the levels remained unchanged in the first six subjects. Values are mean ± S.D.
Total urinary hCG was normal in the benign PHPT urinary controls subjects (range: 0–17 fmol/mg Cr; nl < 50). In the subjects with PtCa, the initial hCG levels were measured at a mean of 9 ± (range: 4–26) years after parathyroid carcinoma surgery (Figure 1). In these subjects, three of the eight patients (one male) had persistently normal total urinary hCG levels (< 50 fmol/mg Cr). These three subjects all survived without major complications in 2 years of follow-up.
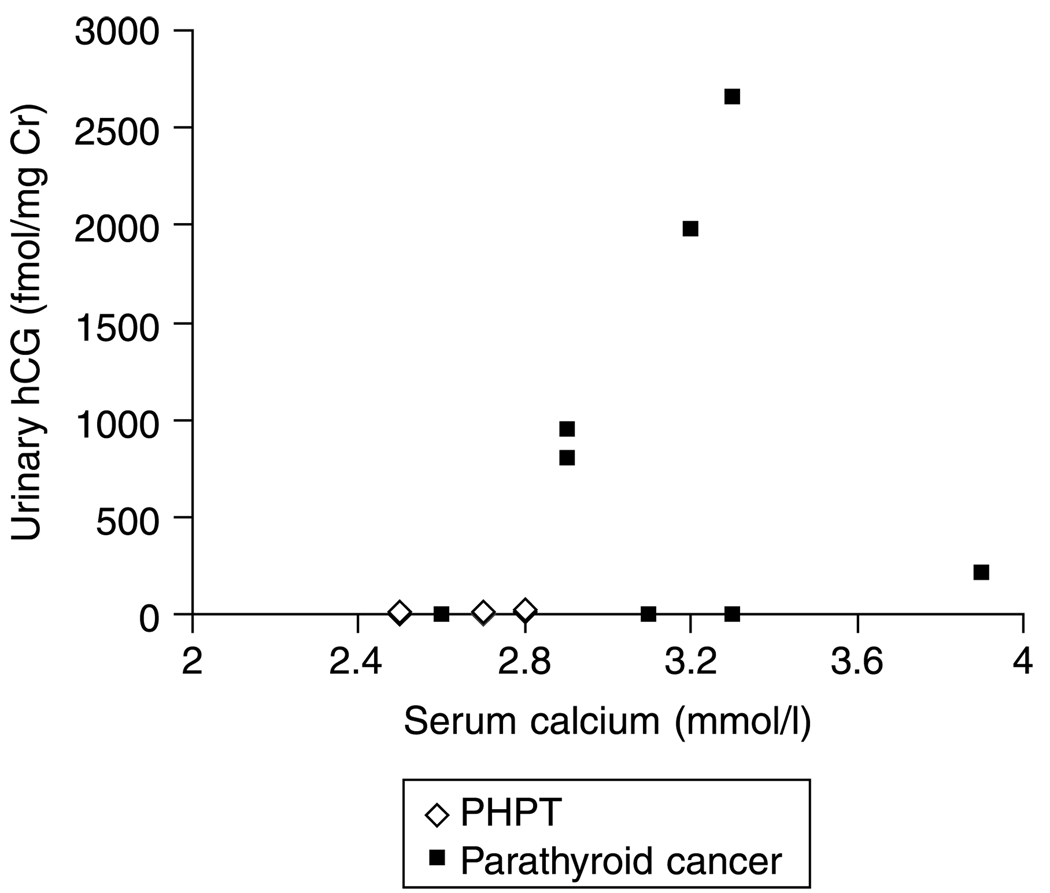
Figure 1.
The distribution of urinary hCG values in PHPT and parathyroid carcinoma subjects. A normal urinary hCG does not rule out the possibility of parathyroid cancer (sensitivity is low), but an elevated urinary hCG confirms it (specificity is high).
Three of the other subjects with PtCa (one male) had persistently abnormal elevations in total urinary hCG (range: 217–1986 fmol/mg Cr). The three patients showed a trend towards higher serum calcium levels when compared with the three PtCa patients with normal hCG (3.3 ± 0.5 vs 3.0 ± 0.3 mmol/l). The three patients with elevated hCG all had a poor prognosis with hip fracture (n = 3) and death (n = 2) occurring within 3 and 6 months respectively of their highest hCG measurements.
In the two remaining cancer patients (both males), total urinary hCG became abnormally elevated over time. Again the presence of high hCG levels heralded a poor outcome. In one patient (patient #7), PTH increased, while in the other (patient #8), marked decreases in BMD (7.2% at the spine, 5.4% at the total hip, and 3.5% at the distal 1/3 radius) were observed over the interval in which hCG levels rose. This patient sustained a hip fracture within 4 months and both patients died within 10 months of the rise in hCG levels (Figure 2).
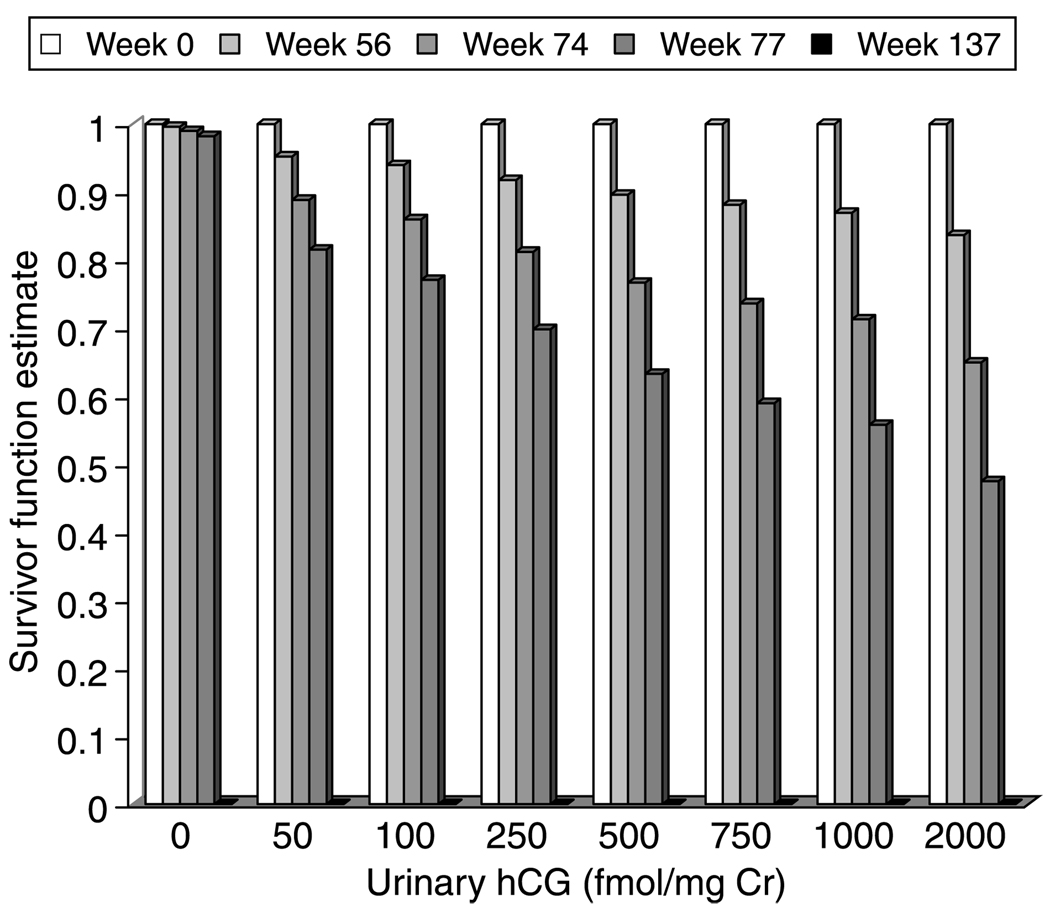
Figure 2.
The survivor function predicted from the Cox proportional hazards model estimated from the study observations. The survivor function estimate was applied to urinary hCG measurements of 0, 50, 100, 250, 500, 750, 1000, or 2000 fmol/mg Cr at weeks 0, 56, 74, 77, and 137. Increasing urinary hCG measurements are associated with a decreasing survivor function.
All of the five cancer patients with an elevated total urinary hCG had a high level of the urinary malignant hCG hyperglycosylated isoform. In contrast, this isoform was undetectable in the other three cancer patients with the better clinical outcomes and in the benign PHPT subjects.
Serum hCG
The benign PHPT serum control subjects (three males, five females; age: 62 ± 13) had mildly elevated serum calcium (2.7 ± 0.2 mmol/l; P < 0.0001 versus cancer subjects) and PTH (12.3 ± 6.2 pmol/l; P = 0.009 versus cancer subjects) levels. In the serum, only the malignant isoform, and not total serum hCG, was measured. The normal range for serum malignant hCG is not defined. In the benign PHPT subjects, the range of serum malignant hCG was 1.76–3.77 pmol/l. The cancer patients tended to have higher serum malignant hCG values than the PHPT patients, ranging from 2.01–10.81 pmol/l. Although values below 3.77 pmol/l (the maximum value in a PHPT subject) were observed in the cancer patients, all of the cancer patients had serum malignant hCG values that exceeded that maximal PHPT value at least at one of the serial time points.
Effects of cinacalcet
The hCG levels were obtained at varying time points of cinacalcet administration (range of time points: 0–120 weeks). Nevertheless, there did not appear to be an effect of cinacalcet on the hCG levels.
Discussion
We found that the hCG levels were elevated at certain time points in most patients with PtCa. The malignant isoform of hCG, the predominant form in these patients, was not seen at all in subjects with PHPT. In patients with malignant disease, increased urinary hCG or a rise in urinary hCG levels appeared to signal a more aggressive stage of PtCa. High urinary hCG levels were associated with a greater likelihood of hip fracture and death. Hip fractures and/or death occurred in the five cancer subjects with persistently high or rising hCG levels, while neither occurred in the three cancer subjects with persistently low hCG levels.
These data suggest that the urinary hCG levels might have the potential to discriminate between parathyroid adenomas and carcinomas. In our pilot sample, the sensitivity was ~40% (three of eight patients with carcinomas had raised urinary hCG), but the specificity was 100% (none of the ten urinary measurements done in PHPT were above the diagnostic threshold). Future studies with larger numbers of subjects would help to better define the properties of urinary hCG as a diagnostic test in parathyroid carcinoma. Even if the sensitivity in a larger population were found to be no higher than 50%, the negative predictive value (the probability that a cancer-free subject will test negative) would still be high because of the very low prevalence of parathyroid carcinoma. In other words, a normal urinary hCG at diagnosis does not rule out the possibility of PtCa, but an elevated urinary hCG confirms it.
hCG is well-known to be expressed in gestational trophoblastic disease and in germ cell tumors (7). However, it can be expressed by nontrophoblastic cancers as well. These include transitional cell carcinoma of the bladder and urinary tract (16), renal cancer (17), prostate cancer (18), gastrointestinal cancer (19), carcinoid (20), lung cancer (21), breast cancer (22), gynecologic cancer (23), oral cancer (24), and lymphoma (25). In general, hCG ismore likely to be expressed when these tumors are poorly differentiated. βhCG appears to enhance the growth of tumor cells in culture by preventing apoptosis (26), possibly explaining why the expression of βhCG is associated with aggressive cancer. Hyperglycosylated hCG, the malignancy-associated isoform, is the predominant isoform of hCG in a healthy pregnant woman until the 6th week of gestation (11, 14). It is also produced in choriocarcinomas and can be detected by an antibody that was raised using a choriocarcinoma-derived form of hCG (10). The concentration of hyperglycosylated hCG has been shown to correlate with malignancy (8).
In a previous study, Stock and colleagues measured the concentration of hCG subunits in the serum of 70 patients with PHPT, 3 of whom had PtCa (27). Two of the cancer patients had elevations in α- and β-hCG, which fell after surgical cure. In the same study, selective venous catheterization in 42 patients with benign PHPT yielded tenfold gradients of PTH, as expected, but also a mild elevation of hCG α-subunit in one patient (27). In another report, immunostaining of parathyroid tissue was positive for hCG in 6 out of 31 patients with parathyroid adenomas and in 1 out of 3 cancer patients (28). These immunohistochemical data suggest that parathyroid cells can acquire the capacity to synthesize and express hCG.
Cinacalcet did not appear to affect hCG levels, although a limitation of this study is that the hCG levels were obtained at varying time points of cinacalcet administration. Cinacalcet has been shown to lower serum calcium levels in PtCa (29), but its effect on the progression of the underlying disease is unknown. An additional limitation is the small number of PHPT subjects; it is conceivable that evaluation of additional PHPT subjects might have revealed elevated hCG levels in some patients with benign disease. A larger study with more PHPT subjects would be able to extend our preliminary observations.
In conclusion, this brief report calls attention to the need for a more detailed investigation of hCG in malignant and benign parathyroid disease. A future area of investigation would be immunolocalization of hCG in the histological samples of the cancers themselves, a finding that would confirm that PtCa is indeed the source of the hCG measured in serum and urine. These early results suggest that hCG might have the potential to become a marker of disease progression in malignant parathyroid disease. Investigation of patients who have clinically and biochemically severe, but pathologically benign, parathyroid disease and those with malignant pathology, despite mild clinical features, will help to elucidate further the utility of hCG as a marker for parathyroid carcinoma.
Acknowledgments
Funding
This study was supported by NIH NIDDK32333, DK074457, and NCI R21 CA 98350.
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
References
- 1.Shane E. Clinical review 122: parathyroid carcinoma. Journal of Clinical Endocrinology and Metabolism. 2001;86:485–493. doi: 10.1210/jcem.86.2.7207. [DOI] [PubMed] [Google Scholar]
- 2.Obara T, Fujimoto Y. Diagnosis and treatment of patients with parathyroid carcinoma: an update and review. World Journal of Surgery. 1991;15:738–744. doi: 10.1007/BF01665308. [DOI] [PubMed] [Google Scholar]
- 3.Lumachi F, Basso SM, Basso U. Parathyroid cancer: etiology, clinical presentation and treatment. Anticancer Research. 2006;26:4803–4807. [PubMed] [Google Scholar]
- 4.Lee PK, Jarosek SL, Virnig BA, Evasovich M, Tuttle TM. Trends in the incidence and treatment of parathyroid cancer in the United States. Cancer. 2007;109:1736–1741. doi: 10.1002/cncr.22599. [DOI] [PubMed] [Google Scholar]
- 5.Wang CA, Gaz RD. Natural history of parathyroid carcinoma. Diagnosis, treatment, and results. American Journal of Surgery. 1985;149:522–527. doi: 10.1016/s0002-9610(85)80050-7. [DOI] [PubMed] [Google Scholar]
- 6.Shattuck TM, Valimaki S, Obara T, Gaz RD, Clark OH, Shoback D, Wierman ME, Tojo K, Robbins CM, Carpten JD, Farnebo LO, Larsson C, Arnold A. Somatic and germ-line mutations of the HRPT2 gene in sporadic parathyroid carcinoma. New England Journal of Medicine. 2003;349:1722–1729. doi: 10.1056/NEJMoa031237. [DOI] [PubMed] [Google Scholar]
- 7.Stenman UH, Alfthan H, Hotakainen K. Human chorionic gonadotropin in cancer. Clinical Biochemistry. 2004;37:549–561. doi: 10.1016/j.clinbiochem.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Khanlian SA, Smith HO, Cole LA. Persistent low levels of human chorionic gonadotropin: a premalignant gestational trophoblastic disease. American Journal of Obstetrics and Gynecology. 2003;188:1254–1259. doi: 10.1067/mob.2003.271. [DOI] [PubMed] [Google Scholar]
- 9.Birken S. Specific measurement of o-linked core 2 sugar-containing isoforms of hyperglycosylated human chorionic gonadotropin by antibody b152. Tumour Biology. 2005;26:131–141. doi: 10.1159/000086484. [DOI] [PubMed] [Google Scholar]
- 10.Birken S, Krichevsky A, O’Connor J, Schlatterer J, Cole L, Kardana A, Canfield R. Development and characterization of antibodies to a nicked and hyperglycosylated form of hCG from a choriocarcinoma patient: generation of antibodies that differentiate between pregnancy hCG and choriocarcinoma hCG. Endocrine. 1999;10:137–144. doi: 10.1385/ENDO:10:2:137. [DOI] [PubMed] [Google Scholar]
- 11.Kovalevskaya G, Birken S, Kakuma T, O’Connor JF. Early pregnancy human chorionic gonadotropin (hCG) isoforms measured by an immunometric assay for choriocarcinoma-like hCG. Journal of Endocrinology. 1999;161:99–106. doi: 10.1677/joe.0.1610099. [DOI] [PubMed] [Google Scholar]
- 12.Birken S, Berger P, Bidart JM, Weber M, Bristow A, Norman R, Sturgeon C, Stenman UH. Preparation and characterization of new WHO reference reagents for human chorionic gonadotropin and metabolites. Clinical Chemistry. 2003;49:144–154. doi: 10.1373/49.1.144. [DOI] [PubMed] [Google Scholar]
- 13.Krichevsky A, Birken S, O’Connor J, Acevedo HF, Bikel K, Lustbader J, Hartree A, Canfield RE. Development, characterization, and application of monoclonal antibodies to the native and synthetic beta COOH-terminal portion of human chorionic gonadotropin (hCG) that distinguish between the native and desialylated forms of hCG. Endocrinology. 1994;134:1139–1145. doi: 10.1210/endo.134.3.7509735. [DOI] [PubMed] [Google Scholar]
- 14.Kovalevskaya G, Birken S, Kakuma T, Ozaki N, Sauer M, Lindheim S, Cohen M, Kelly A, Schlatterer J, O’Connor JF. Differential expression of human chorionic gonadotropin (hCG) glycosylation isoforms in failing and continuing pregnancies: preliminary characterization of the hyperglycosylated hCG epitope. Journal of Endocrinology. 2002;172:497–506. doi: 10.1677/joe.0.1720497. [DOI] [PubMed] [Google Scholar]
- 15.Pandian R, Lu J, Ossolinska-Plewnia J. Fully automated chemiluminometric assay for hyperglycosylated human chorionic gonadotropin (invasive trophoblast antigen) Clinical Chemistry. 2003;49:808–810. doi: 10.1373/49.5.808. [DOI] [PubMed] [Google Scholar]
- 16.Wurzel RS, Yamase HT, Nieh PT. Ectopic production of human chorionic gonadotropin by poorly differentiated transitional cell tumors of the urinary tract. Journal of Urology. 1987;137:502–504. doi: 10.1016/s0022-5347(17)44088-2. [DOI] [PubMed] [Google Scholar]
- 17.Hotakainen K, Ljungberg B, Paju A, Rasmuson T, Alfthan H, Stenman UH. The free beta-subunit of human chorionic gonadotropin as a prognostic factor in renal cell carcinoma. British Journal of Cancer. 2002;86:185–189. doi: 10.1038/sj.bjc.6600050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah VM, Newman J, Crocker J, Antonakopoulos GN, Chapple CR, Collard MJ. Production of beta-human chorionic gonadotropin by prostatic adenocarcinoma and transitional cell carcinoma of the upper urinary tract. British Journal of Experimental Pathology. 1987;68:871–878. [PMC free article] [PubMed] [Google Scholar]
- 19.Alfthan H, Haglund C, Roberts P, Stenman UH. Elevation of free beta subunit of human choriogonadotropin and core beta fragment of human choriogonadotropin in the serum and urine of patients with malignant pancreatic and biliary disease. Cancer Research. 1992;52:4628–4633. [PubMed] [Google Scholar]
- 20.Grossmann M, Trautmann ME, Poertl S, Hoermann R, Berger P, Arnold R, Mann K. Alpha-subunit and human chorionic gonadotropin-beta immunoreactivity in patients with malignant endocrine gastroenteropancreatic tumours. European Journal of Clinical Investigation. 1994;24:131–136. doi: 10.1111/j.1365-2362.1994.tb00978.x. [DOI] [PubMed] [Google Scholar]
- 21.Bepler G, Jaques G, Oie HK, Gazdar AF. Human chorionic gonadotropin and related glycoprotein hormones in lung cancer cell lines. Cancer Letters. 1991;58:145–150. doi: 10.1016/0304-3835(91)90037-i. [DOI] [PubMed] [Google Scholar]
- 22.Agnantis NJ, Patra F, Khaldi L, Filis S. Immunohistochemical expression of subunit beta HCG in breast cancer. European Journal of Gynaecological Oncology. 1992;13:461–466. [PubMed] [Google Scholar]
- 23.Braunstein GD, Vaitukaitis JL, Carbone PP, Ross GT. Ectopic production of human chorionic gonadotrophin by neoplasms. Annals of Internal Medicine. 1973;78:39–45. doi: 10.7326/0003-4819-78-1-39. [DOI] [PubMed] [Google Scholar]
- 24.Bhalang K, Kafrawy AH, Miles DA. Immunohistochemical study of the expression of human chorionic gonadotropin-beta in oral squamous cell carcinoma. Cancer. 1999;85:757–762. [PubMed] [Google Scholar]
- 25.Acevedo HF, Krichevsky A, Campbell-Acevedo EA, Galyon JC, Buffo MJ, Hartsock RJ. Expression of membrane-associated human chorionic gonadotropin, its subunits, and fragments by cultured human cancer cells. Cancer. 1992;69:1829–1842. doi: 10.1002/1097-0142(19920401)69:7<1829::aid-cncr2820690727>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 26.Butler SA, Ikram MS, Mathieu S, Iles RK. The increase in bladder carcinoma cell population induced by the free beta subunit of human chorionic gonadotrophin is a result of an anti-apoptosis effect and not cell proliferation. British Journal of Cancer. 2000;82:1553–1556. doi: 10.1054/bjoc.2000.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stock JL, Weintraub BD, Rosen SW, Aurbach GD, Spiegel AM, Marx SJ. Human chorionic gonadotropin subunit measurement in primary hyperparathyroidism. Journal of Clinical Endocrinology and Metabolism. 1982;54:57–63. doi: 10.1210/jcem-54-1-57. [DOI] [PubMed] [Google Scholar]
- 28.Carlinfante G, Lampugnani R, Azzoni C, Aprile MR, Brandi ML, Bordi C. Expression of the alpha- and beta-subunits of human chorionic gonadotropin by subsets of parathyroid cells in states of hyperparathyroidism. Journal of Pathology. 1998;185:389–393. doi: 10.1002/(SICI)1096-9896(199808)185:4<389::AID-PATH116>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 29.Silverberg SJ, Rubin MR, Faiman C, Peacock M, Shoback DM, Smallridge RC, Schwanauer LE, Olson KA, Klassen P, Bilezikian JP. Cinacalcet hydrochloride reduces the serum calcium concentration in inoperable parathyroid carcinoma. Journal of Clinical Endocrinology and Metabolism. 2007;92:3803–3808. doi: 10.1210/jc.2007-0585. [DOI] [PubMed] [Google Scholar]