Abstract
Transgenic Brassica compestris L. spp. chinensis plants expressing a choline oxidase (codA) gene from Arthrobacter globiformis were obtained through Agrobacterium tumefaciens-mediated transformation. In the transgenic plants, codA gene expression and its product transportation to chloroplasts were detected by the enzyme-linked immunosorbent assay (ELISA) examination, immunogold localization, and 1H-nuclear magnetic resonance (1H-NMR). Stress tolerance was evaluated in the T3 plants under extreme temperature and salinity conditions. The plants of transgenic line 1 (L1) showed significantly higher net photosynthetic rate (P n) and P n recovery rate under high (45 °C, 4 h) and low temperature (1 °C, 48 h) treatments, and higher photosynthetic rate under high salinity conditions (100, 200, and 300 mmol/L NaCl, respectively) than the wild-type plants. The enhanced tolerance to high temperature and high salinity stresses in transgenic plants is associated with the accumulation of betaine, which is not found in the wild-type plants. Our results indicate that the introduction of codA gene from Arthrobacter globiformis into Brassica compestris L. spp. chinensis could be a potential strategy for improving the plant tolerance to multiple stresses.
Keywords: Brassica compestris L. spp. chinensis, codA, Stress, Glycine betaine, Net photosynthetic rate (Pn)
1. Introduction
Glycine betaine (betaine) is a quaternary ammonium compound that occurs naturally in a wide variety of plants, animals, and microorganisms. It can stabilize the structure of proteins, and maintain the integrity of cell membrane, thus enhancing the tolerance of organisms to high salt, high temperature, and cold injuries (Gorham, 1995). Many plant species, including rice, tomato, tobacco, and Arabidopsis, are not able to synthesize betaine, but may be engineered to accumulate betaine through a transgenic approach to improve their tolerance to various extreme conditions (Sakamoto and Murata, 2001; Prasad and Saradhi, 2004).
The first successful transformation of a plant for the synthesis of betaine in Arabidopsis thaliana was reported by Hayashi et al. (1997). Within chloroplasts, choline oxidase encoded by the codA gene produces betaine, which provides leaf protection from high temperature and other stresses in Arabidopsis thaliana (Alia et al., 1998a; Sakamoto and Murata, 2000). Higher betaine accumulation has been reported to endow higher salt tolerance in plants (Saneoka et al., 1995). For example, transgenic Arabidopsis expressing codA exhibits enhanced germination under high salinity conditions (Hayashi et al., 1998). Transgenic tobacco (Nicotiana tabacum) plants show improved tolerance to salt and drought stresses (Holmström et al., 2000; Huang et al., 2000; He et al., 2001). Transgenic rice is advantageous in maintaining stable osmotic pressure and promoting roots growth, thus enhancing the plant tolerance to water deficit (Sakamoto et al., 1998; Sawahel, 2003). In the transgenic codA plants, the enhanced performance in germination and tolerance to various stress conditions has been associated with the accumulation of betaine (Alia et al., 1998a; 1998b; 1999; Prasad and Saradhi, 2004). Moreover, it has been found that the transgenic codA plants show a significantly lower trend of electrolyte leakage, as well as hydrogen peroxide and malondialdehyde contents than wild-type plants (Parvanova et al., 2004).
Brassica compestris L. spp. chinensis is a vegetable crop widely cultivated in South China. It does not synthesize betaine in vivo, and is sensitive to salt, drought, and high temperature stresses. Via the Agrobacterium tumefaciens-mediated transformation procedure, we have successfully transferred the codA gene into the genome of Brassica compestris L. spp. chinensis var. Aikangqing. In this study, we evaluated the transgenic plants for their tolerance to high and low temperatures and high salinity stresses by examining their photosynthetic performance at the growth stage. We report here the enhanced tolerance observed in these transgenic plants.
2. Materials and methods
2.1. Construction of vector plasmids
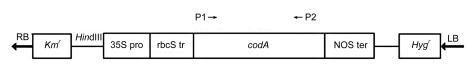
The binary vector pGAH/codA (a kind gift from Dr. Norio MURATA), harboring an expression cassette of the codA gene from Arthrobacter globiformis, was used for transformation. The construct contains the 35S promoter of cauliflower mosaic virus, the codA gene, and the terminator for nopaline synthase gene (Fig. 1). Its T-DNA region also contains genes encoding a neomycin phosphotransferase (NPTII), which confers kanamycin resistance, and a hygromycin phosphotransferase (HPT), which confers hygromycin resistance. In order to direct the expressed choline oxidase to chloroplasts, where betaine can be accumulated to a greater degree, the construct was modified by adding a complementary DNA (cDNA) sequence encoding the transit peptide of a tobacco Rubisco subunit to the codA coding region. The resultant binary vector plasmid was introduced into A. tumefaciens strain EHA101 containing the Ti plasmid, which was then used for plant transformation.
Fig. 1.
Structure of T-DNA in binary vector pGAH/codA with the codA gene for choline oxidase
RB: right border; Kmr: kanamycin resistance gene; 35S pro: the 35S promoter of cauliflower mosaic virus; rbcS tr: the sequence that encodes transit peptide of subunit of Rubisco from tobacco; codA: the gene encoding choline oxidase (COD); NOS ter: NOS terminator; P1 and P2 are upstream and downstream primers, respectively; Hygr: hygromycin resistance gene; LB: left border
2.2. Transformation of Brassica compestris L. spp. chinensis
After germinating for 5–7 d, the hypocotyls or cotyledons of Brassica compestris L. spp. chinensis were pre-cultured for 1 d on the differentiation medium. A. tumefaciens strain EHA101 harboring the pGAH/codA plasmid was grown at 28 °C for 36 h in a 250-ml flask containing 50 ml of yeast extract broth (YEB) liquid medium supplemented with kanamycin 50 mg/L and hygromycin 50 mg/L until the late exponential phase. The explants pre-cultured for 1 d were co-cultured with the A. tumefaciens for 5 min, and transferred onto a differentiation Murashige and Skoog (MS) medium supplemented with 0.5 mg/L indole-3-acetic acid (IAA), 0.025 mg/L naphthaleneacetic acid (NAA), 5 mg/L 6-benzyladenine (6-BA), 300 mg/L carbenicillin, and 15 mg/L kanamycin. After co-incubation with A. tumefaciens for 2 d at 25 °C (14-h light period) and incubation without A. tumefaciens for 4–5 weeks, the shoots of the explants were excised and transplanted to the root induction medium containing kanamycin. The surviving explants were carefully transferred into plastic pots (10 cm×10 cm×10 cm) loaded with sterilized vermiculite, and grown in a phytotron with fixed temperature (25 °C), 16-h light period (350–400 μmol/(m2∙s)), and a relative humidity of 70%–80%. Transformants were obtained from the selection medium with 60 mg/L kanamycin.
2.3. Development of subsequent T1, T2, and T3 plants
T1 seeds harvested from the T0 transgenic plants were first washed in tap water for 3 h, soaked in 1.5% (w/v) Xiaojieling solution for 30 min and in 0.1% (w/v) HgCl2 solution for 2–3 min, and washed repeatedly in sterile water. The treated seeds were sowed in the MS basal (MS0) medium containing 60 mg/L kanamycin for testing and selecting T1 plants for antibiotic resistance. The same treatment described above was also applied to T2 seeds. Those plants without segregation for antibiotic resistance were considered as homozygous. The T3 seeds harvested from self-crossed T2 plants were used for subsequent stress experiments.
2.4. Polymerase chain reaction (PCR) examination of the transgenic plants
To detect the presence of the codA gene in the transgenic plants, a pair of primers was designed (P1: 5′-AACATCGAGAACCTGAGCGACAGG-3′; P2: 5′-AGCATCAACAGCTTCGGCGTATC-3′) using codA as template and used in the PCR. Each 25 μl PCR solution consisted of 10 pmol/L of each primer, 50 μmol/L of deoxyribonucleoside triphosphate, 2.5 μl 10× Taq buffer, 1 μl extracted DNA solution, 1 U Taq enzyme, 1% (v/v) dimethyl sulphoxide (DMSO), and 5% (v/v) glycerine. Assays were performed on the MJ Research Minicycler (Watertown, MA) with the following thermocycle profile: 94 °C for 5 min, 40 amplification cycles of 94 °C for 1 min, 59 °C for 1 min, 72 °C for 1 min, and finally 72 °C for 10 min.
2.5. Enzyme-linked immunosorbent assay (ELISA) examination of the transgenic plants
Fresh leaves of Brassica compestris L. spp. chinensis were ground in liquid nitrogen. The powder was suspended with a ratio of 3 ml/g fresh weight (FW) in a protein extraction buffer containing 0.1 mol/L Tris-HCl (pH 8.0), 0.01 mol/L MgCl2, 18% (w/v) sucrose, and 40 mmol/L 2-mercaptoethanol, and cell debris were removed by centrifugation at 10 000×g for 15 min at 4 °C. The antigen supernatant containing the crude protein extraction of choline oxidase was subjected to ELISA examination, as previously described by Kramer et al. (1995) with some modification. After 50 μl of antigen solution was moved from the incubated wells and washed three times, nonspecific protein used in blocking buffer was incubated for 2 h at room temperature. After discarding the blocking buffer and triple washing, the 50 μl primary antibody (rabbit immune body of choline oxidase) with a 1/200 (v/v) dilution in blocking buffer was added into each well and incubated for 2 h at room temperature. Following triple washing with sterilized water, 50 μl of goat anti-rabbit immunoglobulin G (IgG)-alkaline phosphatase (AP) conjugate (Sino-American Biotechnology Co., China) with a 1/1000 (v/v) dilution in blocking buffer was added into each well and incubated for 2 h at room temperature. After triple washing, dye conversion was initiated by charging the wells with 50 μl of the para-nitrophenylphosphate (pNPP) at 37 °C for 1 h, to determine if choline oxidase existed in the transgenic plants. The reaction was terminated by adding 25 μl 0.5 mol/L NaOH.
2.6. Immunological examination of the expressed choline oxidase
The localization of the expressed choline oxidase was examined immunocytochemically as previously described by Mustardy et al. (1990) with some modifications. For preparation of a soluble fraction, small pieces (2 mm×2 mm) of five-leaf-stage leaves of the transgenic and non-transgenic plants were cut and fixed in 2% (v/v) glutaraldehyde overnight at 4 °C and in 1% (w/v) osmium tetroxide for 2 h at 4 °C. Following triple washing with 5% (w/v) phosphate buffer solution (PBS), 20 min each time, samples were dehydrated in serial concentrations of ethanol (50%→70%→80%→90%→95%→100%) and 100% acetone, infiltrated overnight at room temperature in an acetone-Epon 812 mixture (1:1, v/v), embedded in fresh Epon 812, and subjected to heat-polymerization for 12 h at 37 °C, 12 h at 45 °C, and 24 h at 60 °C. Ultrathin sections (~60 nm) were cut with ultramicrotomy (speed 2–3 mm/s).
Antibodies against choline oxidase raised in a rabbit were purchased commercially from Sigma (St. Louis, MO, USA). Initially, the ultrathin sections were mounted on uncoated nickel grids, rinsed with 5% H2O2 and PBS buffer for three times (5 min per time), subsequently treated for 1 h at 25 °C with a blocking solution consisting of 0.25% (w/v) bovine serum albumin (BSA) (fraction V, Sigma), 0.05% (v/v) Tween 20 (Bio-Rad), 0.05% (w/v) NaN3, 1 mmol/L ethylenediaminetetraacetic acid (EDTA), 0.17 mol/L H3BO4, and 0.12 mol/L NaCl (pH 8.5), and then exposed to the primary antiserum diluted in 1:200 (v/v) in PBS buffer and incubated for 30 min. This was followed by 30 min incubation with the 10 nm-gold-conjugated secondary antiserum (goat anti-rabbit) in a 1:20 (v/v) dilution in PBS containing 1% (w/v) BSA. Samples were required to be vigorously rinsed six times with 1% PBS between the incubations, 5 min per time for optimum labeling, and finally were rinsed in sterilization ddH2O. After dried in the air, ultrathin sections were stained with 4% (v/v) uranyl acetate for 15 min and 2% (w/v) lead citrate for 5 min, and then examined in a transmission electron microscope (H-600-4, Hitachi Ltd., Japan).
2.7. Betaine measurement
Approximately 5 g fresh leaf materials were ground to a fine powder in liquid nitrogen. The powder was suspended in 25 ml of 1.0 mol/L H2SO4 and incubated at 25 °C for 2 h. Cell debris were removed by centrifugation at 1000×g for 10 min. The supernatant was incubated in 10 ml of KI-I2 solution (15.7 g I2 and 20 g KI dissolved in 100 ml of 1 mol/L HCl) at 0 °C for 2 h, and centrifuged at 1000×g for 30 min and the periodide adducts of betaine were collected. The resulting periodide adducts of betaine were then dissolved in 0.5 ml of D2O, which contained 0.5 mmol 2-methyl-2-propanol (tBA) as an internal standard, and were used for the determination of 1H-nuclear magnetic resonance (1H-NMR). Detailed procedure can be found in Hayashi et al. (1997).
2.8. Net photosynthetic rate (P n) measurement
The plants of 6–8-leaf stage were treated with low temperature (1 °C for 48 h) or high temperature (45 °C for 4 h), then moved into the phytotron to recover for 1 h, and measured for P n with a portable CO2 gas analyzer (Model CI-301, CID Inc., Vancouver, WA, USA). The antepenultimate leaves were selected for the P n measurement. According to the method of Yang (1999), 10 leaf disks from the leaves, which were measured for photosynthesis, were used to analyze the contents of chlorophyll a and b (mg/g FW). All measurements were done with three replicates.
2.9. Salt tolerance
At 8–9-leaf stage, 20 plants of T3 generation from the transgenic lines 1 and 2 (L1 and L2) and wild-type were subjected to salt treatments by adding NaCl to the growth medium with 50 mmol/L increments every 24 h until a final concentration of 0, 100, 200, and 300 mmol/L, respectively, and maintained for 7 d (Xu et al., 2001). The survival rate in each treatment was analyzed to compare the salt tolerance between transgenic and wild-type plants, and the antepenultimate leaves were measured for P n. The survival rate (Su) was evaluated by Su (%)=Ns/Na×100, where N s is the number of survival plants per treatment, and N a is the number of all plants per treatment.
3. Results
3.1. Transformation with the codA gene
We used 769 Brassica compestris L. spp. chinensis explants (339 cotyledons and 430 hypocotyls) to conduct the transformation experiments with the codA gene carried by the vector plasmid shown in Fig. 1, and eventually obtained 129 transgenic plants (T0), which were derived from 45 cotyledons and 84 hypocotyls. The differentiation rates for the cotyledon and hypocotyl in our experiments were extremely high (13.27% and 19.53%, respectively). We have developed an efficient codA transformation system for Brassica compestris L. spp. chinensis through screening of the compositions of differentiation and selection media (data not shown).
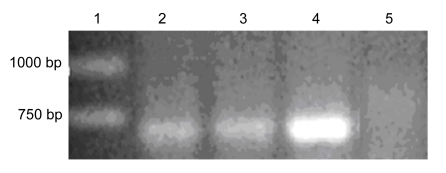
From the 129 transgenic plants (T0), we obtained 10 transgenic plants (T1 plants) showing resistance to both kanamycin and hygromycin. Subsequent T2 and T3 plants were obtained by the procedure as described in Section 2.3. In each generation from T0 to T3, the presence of the transgene (codA) was confirmed by PCR (Fig. 2). The band with same molecular weight (M W) size amplified in L1 and L2 plants was also amplified from the plasmid pBinMoBc, which was used as the positive control, but no bands appeared in the wild-type plants, suggesting that the codA has been successfully inserted into the Brassica compestris L. spp. chinensis genome by A. tumefaciens-mediated transformation.
Fig. 2.
PCR test of genomic DNA from the transgenic and wild-type plants
Lane 1: DNA marker which has been purchased commercially (TaKaRa); Lane 2: transgenic line 1 (L1); Lane 3: transgenic line 2 (L2); Lane 4: PCR fragment of plasmid pGAH/codA; Lane 5: wild-type plant
We further evaluated the existence of the choline oxidase, the product of the codA gene through ELISA examination of the leaves of L1 and L2 plants. The results showed that only L1 and L2 plants had the positive reaction (Fig. 3), indicating that the coda gene had been efficiently expressed in each line of the transgenic plants, and that the expressed precursor had been effectively processed to the mature protein.
Fig. 3.
Immunocytochemical detection of choline oxidase in leaves of the transgenic and wild-type plants
Lane 1: negative control (substrate+buffer+water); Lane 2: non-transgenic wild-type plant; Lane 3: transgenic line 1 (L1); Lane 4: transgenic line 2 (L2)
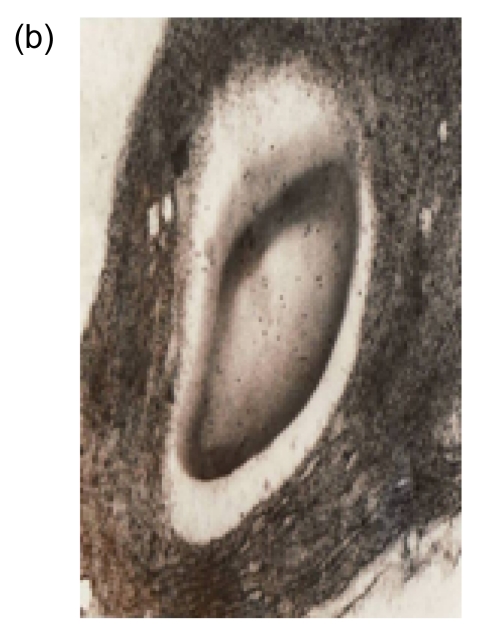
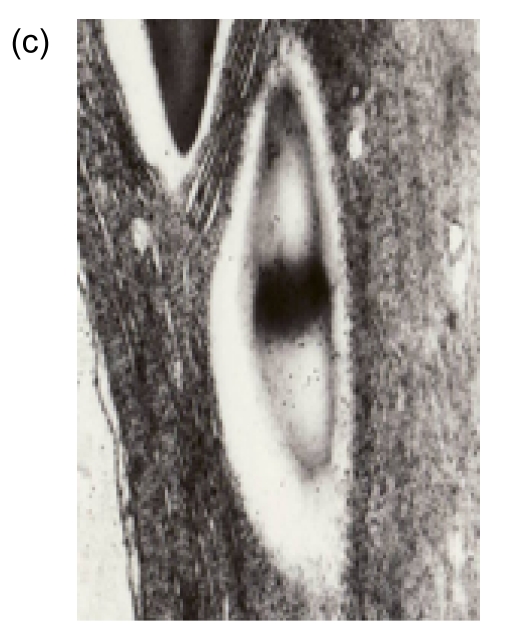
To determine the localization of the expressed choline oxidase, the immunogold labeling technique was applied in the transgenic and wild-type plants. Many colloid gold particles were observed in the chloroplasts of the L1 and L2 plants with the immunoelectron microscope (Figs. 4b and 4c). In contrast, the density of gold particles in the chloroplasts of the wild-type plants was at the background level (Fig. 4a). The results confirm that choline oxidase was located truly in the chloroplasts of the transgenic plants.
Fig. 4.
Immunoelectron microscope of choline oxidase in the chloroplasts of the wild-type and transgenic plants
(a) Wild-type plant; (b) Transgenic line 1 (L1) plant; (c) Transgenic line 2 (L2) plant
3.2. Betaine accumulation
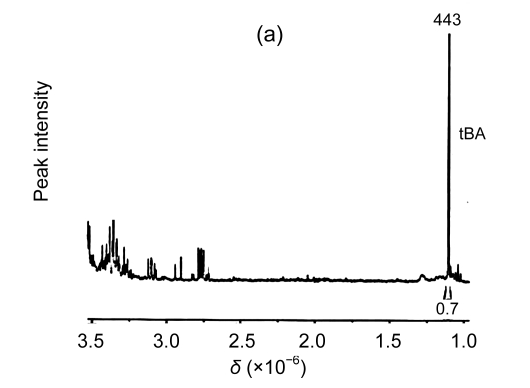
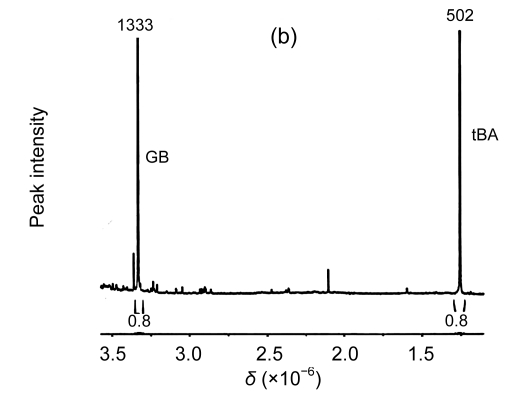
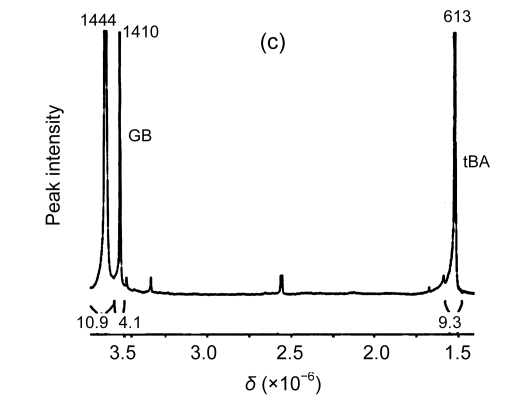
The results from the 1H-NMR spectrometry experiment showed that there were characteristic peaks of betaine with accumulation levels of 0.224 μmol/g FW and 0.221 μmol/g FW in the transgenic L1 and L2 plants, respectively, but no betaine accumulation in the wild-type plants (Fig. 5). Therefore, the results suggest that the transgene, codA, expressed well and the gene product, choline oxidase, functioned properly in the transgenic plants.
Fig. 5.
1H-NMR spectra of betaine in the wild-type and transgenic plants
(a) Wild-type plant; (b) Transgenic line 1 (L1) plant; (c) Transgenic line 2 (L2) plant. GB: glycine betaine; tBA: 2-methyl-2-propanol or tert-butyl alcohol, which is similar structurally to betaine and used as an internal standard for the estimation of the content of betaine. The distance between the small peaks was indicated by coupling constants. δ: chemical shift
3.3. Comparison of photosynthesis under low temperature stress
We measured the net photosynthetic rate (P n) at the time point of 1 h after the 6–8-leaf stage plants were exposed to low-temperature (1 °C) for 48 h. The transgenic L1 and L2 plants showed a significantly higher P n (9.61 μmol/(m2∙s) and 9.22 μmol/(m2∙s), respectively) than the wild-type control plants (5.19 μmol/(m2∙s)) (P<0.01), as shown in Table 1.
Table 1.
Comparison of net photosynthetic rate (P n), evaporation rate (E), and stoma resistance (Sr) between the transgenic and wild-type plants under low temperature (1 °C for 48 h) treatment
| Type | Pn (μmol/(m2∙s)) | E (mmol/(m2∙s)) | Sr ((m2∙s)/mol) |
| Wild | 5.19±1.07 | 2.45±0.27 | 28.18±1.41 |
| L1 | 9.61±0.83** | 2.52±0.26 | 13.00±1.61*** |
| L2 | 9.22±0.51** | 2.49±0.21 | 13.08±0.16*** |
Values were expressed as mean±SD (n=3)
Significant differences at P<0.01
Significant differences at P<0.001
L1, L2: transgenic lines 1 and 2, respectively
Under the same stress condition, stoma resistance (Sr) of the transgenic plants was lower than that of wild-type plants, that is, CO2 entering into a mesophyll cell through the stomata is reinforced by increased gradient of CO2 between the external atmosphere and the intercellular space inside the transgenic plant leaves because of the enhanced photosynthesis in the transgenic plants (Table 1). We also measured the chlorophyll contents in the different plants and the results showed that transgenic L2 plants had higher chlorophyll a/b ratio than the wild-type plants (P<0.05) (Table 2).
Table 2.
Comparison of chlorophyll values between the transgenic and wild-type plants under low temperature (1 °C for 48 h) treatment
| Type | Chlorophyll value (mg/g FW) |
|||
| a | b | ab | a/b | |
| Wild | 0.4251±0.0245 | 0.1765±0.0113 | 0.6016±0.0351 | 2.408±0.1134 |
| L1 | 0.4154±0.0211 | 0.1677±0.0089 | 0.5831±0.0255 | 2.477±0.1225 |
| L2 | 0.4469±0.0231 | 0.1487±0.0067 | 0.5956±0.0348 | 3.005±0.1317* |
Values were expressed as mean±SD (n=3)
Significant difference at P<0.05
L1 and L2 are the transgenic lines 1 and 2, respectively
3.4. Comparison of recovery of net photosynthetic rate (P n) under high temperature stress
Under non-stressed condition, the P n in the transgenic line L1 (5.36 μmol/(m2·s)) was almost the same as the wild-type control plants (5.34 μmol/(m2·s)). However, after 4 h exposure to high temperature (45 °C) and 1-h restoration in the phytotron, the P n of the transgenic line L1 dropped to 4.01 μmol/(m2·s), which is significantly higher than that of the wild-type plants (1.87 μmol/(m2·s)) (Table 3). Nevertheless, after 25-h restoration, there is no significant difference for the P n between them (Table 3). The results suggest that the transgenic plants can recover much faster from the stress than the wild-type plants (74.9% vs. 35.0% with 1-h restoration).
Table 3.
Comparison of recoveries of net photosynthetic rates (P n) of 1-h and 25-h restoration after exposure to high temperature (45 °C for 4 h) stress
| Type |
Pn (μmol/(m2∙s)) |
Recovery of Pn (%) |
|||
| Pre-exposure | 1 h after exposure | 25 h after exposure | 1 h after exposure | 25 h after exposure | |
| Wild | 5.34±0.37 | 1.87±0.1 | 4.02±0.38 | 35.0±1.0 | 75.2±12.8 |
| L1 | 5.36±0.42 | 4.01±0.30** | 4.85±0.55 | 74.9±11.0** | 90.5±3.7* |
Values were expressed as mean±SD (n=3); A total of 15 plants per treatment were tested
Significant difference at P<0.05
Significant difference at P<0.01
L1: transgenic line 1
3.5. Comparison of survival rate and photosynthesis under salt stress
The survival rate for the transgenic L1 plants was higher than that of wild-type plants under all the three levels of NaCl concentration (Table 4). For example, under 100 mmol/L NaCl, 94.4% of L1 plants vs. 65.4% of wild-type plants survived (Fig. 6). While most of the wild-type plants (16.7% survivals) died under 200 mmol/L NaCl, more than half (53.3%) of the transgenic L1 plants were still alive. Though most died among both types of plants under 300 mmol/L NaCl, there were still 27.8% transgenic L1 plants that survived.
Table 4.
Comparison of some photosynthetic characteristics and survival rate between the transgenic L1 and wild-type plants under the stress of different NaCl concentrations
| NaCl (mmol/L) | Types | Pn (μmol/(m2∙s))a | E (mmol/(m2∙s)) | Sr ((m2∙s)/mol) | Su (%) |
| 0 | Wild | 5.71±0.67 | 1.28±0.11 | 27.72±2.74 | 100±0 |
| L1 | 5.75±0.68 | 0.95±0.14 | 29.71±3.01 | 100±0 | |
| 100 | Wild | 1.50±0.53 | 0.61±0.12 | 61.66±3.87 | 65.4±8.9 |
| L1 | 4.12±0.75** | 0.87±0.13 | 42.21±3.65* | 94.4±8.0** | |
| 200 | Wild | 0.27±0.41 | 0.40±0.09 | 84.11±3.23 | 16.7±7.8 |
| L1 | 2.66±0.52*** | 0.74±0.13* | 47.75±3.90* | 53.3±9.1*** | |
| 300 | Wild | −0.10±0.12 | 0.37±0.08 | 90.93±4.22 | 13.6±6.0 |
| L1 | 1.27±0.21*** | 0.58±0.11* | 72.45±4.43* | 27.8±5.7*** |
Values were expressed as mean±SD (n=3)
Significant difference at P<0.05
Significant difference at P<0.01
Significant difference at P<0.001
P n: net photosynthetic rate; E: evaporation rate; Sr: stoma resistance; Su: survival rate
Fig. 6.
Comparison between the transgenic L1 and the wild-type plants grown under salt stress of 100 mmol/L
(a) Transgenic L1 plants; (b) Wild-type plants; (c) Transgenic plants with flowering
Similar P n were observed between the transgenic line L1 plants (5.75 μmol/(m2∙s)) and wild-type plants (5.71 μmol/(m2∙s)) under non-stress condition. However, like the situation discussed above in high temperature stress, less reduction of P n was also observed in the transgenic L1 plants than in the wild-type plants under all the three NaCl concentrations (Table 4). For example, under 100 mmol/L NaCl, the P n of the transgenic L1 plants was close to three-fourths of that observed under non-stress condition, but that was only one-fourth for the wild-type control plants (1.50 μmol/(m2∙s)).
As for the evaporation rate (E), no differences were observed between the transgenic and wild-type plants under non-stress condition. However, the evaporation rate was higher in the transgenic plants than in the wild-type plants under both 200 and 300 mmol/L NaCl (P<0.05) (Table 4).
Under high salinity stress, the transgenic plants showed a lower stoma resistance (Sr) than the wild-type plants (Table 4), indicating that the high photosynthesis for transgenic plants is due to less reduced stomata opening from stress, i.e., the stomata opening of transgenic plants is less affected by salt stress than that of wild-type plants, which facilitates CO2 entering the leaf and increases photosynthesis. Therefore increased photosynthesis is the result of more CO2 entering inside the leaf through stomata.
4. Discussion
Brassica compestris L. spp. chinensis is a very important vegetable crop, commonly grown in South China. It is not able to synthesize endogenously betaine, and therefore is very sensitive to salt, drought, high temperature, and other environmental stresses. In this study, we obtained transgenic plants expressing codA gene from Arthrobacter globiformis and demonstrated enhanced tolerance to extreme temperature and high salinity in these transgenic plants.
The content of betaine in the transgenic plant is influenced directly by its subcellular location. In tomato, there are reports that the transcript of codA could be targeted to the chloroplasts (Chl-codA), cytosol (Cyt-codA) or both compartments simultaneously (ChlCyt-codA). A comparison between these three types of transgenic plants showed that Chl-codA plants with the lowest amounts of betaine exhibited equal or higher degrees of enhanced tolerance to various abiotic stresses, suggesting more effectiveness of chloroplastic betaine in protecting plant cells (Park et al., 2007). Our results showed that the codA gene product in Brassica compestris L. spp. chinensis was targeted to chloroplasts, which agreed with the results from both A. thaliana (Alia et al., 1998b) and Brassica juncea (Prasad et al., 2000). The contents of betaine in chloroplasts of the codA transgenic plants varied generally due to the different plant species, such as 0.30 μmol/g FW in transgenic Diospyros kaki and Lycopersicon esculentum, and 1.43 μmol/g FW in transgenic Solanum tuberosum (Chen and Murata, 2008). Moreover, the expression level of codA is affected by the cultivation conditions and the tested tissues or organs for the transgenic plants. In our experiment, all the plants were cultured in water, and thus had much higher water content (~90%) than that cultured in soil. Therefore, our transgenic plants had low measured values of betaine (~0.22 μmol/g FW). However, they still showed high tolerance to extreme temperature and high salinity.
Environmental stresses such as salt and extreme temperature will eventually cause yield losses for plant production. During adaptation, plant may develop tolerance to environment stresses by accumulating some small organic solutes, including the polyhydroxylated compounds, carbohydrate, amino acid, betaine, and related compounds (Bohnert and Jensen, 1996; Hayashi et al., 1998) known as compatible materials (Bohnert et al., 1995). In plants, the major compatible osmoprotectant solutes include betaine, proline, and polyols (Rontein et al., 2002). In this study, transgenic plants expressing the codA gene were able to accumulate betaine in vivo, which may have possible protective effects on the biological macromolecules (Schobert, 1977). In the chloroplast of spinach and sugar beet plants, betaine could be synthesized and accumulated naturally, but not in that of most other plant species. However, we could engineer theses plants to accumulate betaine through a transgenic approach (Sakamoto and Murata, 2001; Prasad and Saradhi, 2004). The strategy for engineering betaine synthesis was employed by transformation with the codA gene which encodes choline oxidase and offers an attractive conversion from choline to betaine under the enzymatic catalysis. Transgenic A. thaliana plants with the codA gene significantly enhanced the tolerance to low temperature and high-salt stress (Hayashi et al., 1998). Under low temperature, the transgenic A. thaliana with codA gene had an obviously higher biological output than the wild-type control (Hayashi et al., 1997; Alia et al., 1999).
The codA-mediated tolerance to salt stress has been reported in other field crops, including corn, (Saneoka et al., 1995), rice (Mohanty et al., 2002; Sawahel, 2003), Brassica juncea (Prasad et al., 2000), and tobacco (He et al., 2001). Here we reported that transgenic Brassica compestris L. spp. chinensis seedlings expressing a codA gene from A. globiformis could accumulate betaine in vivo and showed significant tolerance to high and low temperatures and high salinity stresses, compared with wild-type plants. Moreover, the wild-type plants had more difficulty surviving compared to the transgenic plants under the 300 mmol/L NaCl condition, suggesting that betaine may be important for osmotic adjustment under salinity stress in the transgenic plants.
It has been demonstrated that the biosynthesis of betaine is stress-inducible (Sakamoto and Murata, 2002). The direct protective effects of betaine on macromolecules and membranes may be not only osmotic, but also via the mechanism of compatible solutes and oxygen radical scavenging (Blumwald and Grover, 2006). This point may be supported by our results that the transgenic seedlings of Brassica compestris L. spp. chinensis showed a higher P n after the low temperature treatment and a higher recovery rate of photosynthesis after exposure to high temperature (45 °C for 4 h). The role of betaine in stress tolerance could be protecting the oxygen-evolving PSII complex, stabilizing the protein structure of PSII complex, and maintaining ATP synthesis under stress conditions (Sakamoto and Murata, 2001; Rahman et al., 2002).
In this study, we found that the transgenic plants with codA gene showed higher P n and lower stoma resistance accompanying with higher evaporation rate than the wild-type plants under the salt stress condition. In contrast, under temperature stress, an insignificant difference of evaporation rate but higher P n was observed in the transgenic plants, compared with the wild-type plants. This may indicate that the action mechanisms for increasing P n were different in the transgenic plants under the two different stress conditions. The stomatal resistance may be involved in the salt stress, but not in the temperature stress. Our results are consistent with the experiment of Mäkelä et al. (1998), who showed that application of exogenous betaine can significantly increase stomatal conductance of plants grown in saline conditions. An increase in stomatal conductance was related to the maintenance of higher turgor pressure or water potential in plant leaf cells (Cushman et al., 1989). We found that the transgenic plants with codA did not show significantly higher transpiration rates than the wild-type plants in the temperature stress experiments, indicating a less or insignificant effect of stomatal opening on the P n. Actually, the P n can be affected by a number of factors, including a variety of antioxidant enzymes in vivo, such as superoxide dismutase, catalase, peroxidase, ascorbic acid oxidase, and glutathione reductase. The activity of those enzymes may be well conserved by the presence of betaine. Some experiments showed that the increase of the above antioxidant enzymes could efficiently eliminate active oxygen and oxygen free radicals, and thus maintain the structural stability and integrity of the cell membrane and chloroplast membrane under stress conditions (Hayashi et al., 1997). It appears that the remaining higher stomatal conductance under salt stress and protecting integrity of photosynthetic apparatus under temperature stress might be two reasonable explanations for increased photosynthesis observed in our transgenic plants.
Acknowledgments
We thank Dr. Norio MURATA (National Institute for Basic Biology, Okazaki, Japan) for his kind gift of the binary vector pGAH/codA and are grateful to Mr. Fang-zheng WANG, Prof. Da-quan XU, Ms. Ya-fang ZHU, and Mr. Ji-hu SU (Shanghai Institute of Plant Physiology and Ecology, Chinese Academy of Sciences, China) for technical assistance. We also thank Dr. Neng-yi ZHANG (Cornell University, USA) for English language correction.
Footnotes
Project supported by the National Science Foundation of China (No. 30571146) and the National Key Basic Research Special Foundation of China (No. G1999011700)
References
- 1.Alia , Hayashi H, Sakamoto A, Murata N. Enhancement of the tolerance of Arabidopsis to high temperatures by genetic engineering of the synthesis of glycinebetaine. Plant J. 1998;16(2):155–161. doi: 10.1046/j.1365-313x.1998.00284.x. [DOI] [PubMed] [Google Scholar]
- 2.Alia , Hayashi H, Chen THH, Murata N. Transformation with a gene for choline oxidase enhances the cold tolerance of Arabidopsis during germination and early growth. Plant Cell Environ. 1998;21(2):232–239. doi: 10.1046/j.1365-3040.1998.00264.x. [DOI] [Google Scholar]
- 3.Alia , Kondo Y, Sakamoto A, Nonaka H, Hayashi H, Saradhi PP, Chen THH, Murata N. Enhanced tolerance to light stress of transgenic Arabidopsis plants that express the codA gene for a bacterial choline oxidase. Plant Mol Biol. 1999;40(2):279–288. doi: 10.1023/A:1006121821883. [DOI] [PubMed] [Google Scholar]
- 4.Blumwald E, Grover A. Salt Tolerance. In: Halford N, editor. Plant Biotechnology, Current and Future Applications of Genetically Modified Crops. Chichester, UK: John Wiley & Sons, Ltd; 2006. pp. 206–224. [DOI] [Google Scholar]
- 5.Bohnert HJ, Jensen RG. Strategies for engineering water-stress tolerance in plants. Trends Biotechnol. 1996;14(3):89–97. doi: 10.1016/0167-7799(96)80929-2. [DOI] [Google Scholar]
- 6.Bohnert HJ, Nelson DE, Jensen RG. Adaptations to environmental stresses. Plant Cell. 1995;7(7):1099–1111. doi: 10.1105/tpc.7.7.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen HHT, Murata N. Glycinebetaine: an effective protectant against abiotic stress in plants. Trends Plant Sci. 2008;13(9):499–505. doi: 10.1016/j.tplants.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Cushman JC, Meyer G, Michalowski CB, Schmitt JM, Bohnert HJ. Salt stress leads to differential expression of two isogenes of phosphoenolpyruvate carboxylase during Crassulacean acid metabolism induction in the common ice plant. The Plant Cell. 1989;1(7):715–725. doi: 10.1105/tpc.1.7.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorham J. Betaines in Higher Plants—Biosynthesis and Role in Stress Metabolism. In: Wallsgrove RM, editor. Amino Acids and Their Derivatives in Higher Plants. Cambridge: Cambridge University Press; 1995. pp. 171–203. [Google Scholar]
- 10.Hayashi H, Alia , Mustardy L, Deshnium P, Ida M, Murata N. Transformation of Arabidopsis thaliana with the codA gene for choline oxidase: accumulation of glycinebetaine and enhanced tolerance to salt and cold stress. Plant J. 1997;12(1):133–142. doi: 10.1046/j.1365-313X.1997.12010133.x. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi H, Alia , Sakamoto A, Nonaka H, Chen THH, Murata N. Enhanced germination under high-salt conditions of seeds of transgenic Arabidopsis with a bacterial gene (codA) for choline oxidase. J Plant Res. 1998;111(2):357–362. doi: 10.1007/BF02512197. [DOI] [Google Scholar]
- 12.He PM, Zhang DB, Liang WQ, Yao QH, Zhang RX. Expression of choline oxidase (codA) enhances salt tolerance of the tobacco. Acta Biochem Biophys Sin. 2001;33(5):519–524. (in Chinese) [PubMed] [Google Scholar]
- 13.Holmström KO, Somersalo S, Mandal A, Palva TE, Welin B. Improved tolerance to salinity and low temperature in transgenic tobacco producing glycine betaine. J Exp Bot. 2000;51(343):177–185. doi: 10.1093/jexbot/51.343.177. [DOI] [PubMed] [Google Scholar]
- 14.Huang J, Hirji R, Adam L, Rozwadowski KL, Hammerlindl JK, Keller WA, Selvaraj G. Genetic engineering of glycinebetaine production toward enhancing stress tolerance in plants: metabolic limitations. Plant Physiol. 2000;122(3):747–756. doi: 10.1104/pp.122.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramer HJ, Schmidt R, Günthe RA, Becker G, Suzuki Y, Seeger W. ELISA technique for quantification of surfactant protein B (SP-B) in bronchoalveolar lavage fluid. Am J Respir Crit Care Med. 1995;152(5):1540–1544. doi: 10.1164/ajrccm.152.5.7582290. [DOI] [PubMed] [Google Scholar]
- 16.Mäkelä P, Munns R, Colmer TD, Condon AG, Peltonen-Sainio P. Effect of foliar applications of glycinebetaine on stomatal conductance, abscisic acid and solute concentrations in leaves of salt- or drought-stressed tomato. Aust J Plant Physiol. 1998;25(6):655–663. doi: 10.1071/PP98024. [DOI] [Google Scholar]
- 17.Mohanty A, Kathuria H, Ferjani A, Sakamoto A, Mohanty P, Murata N, Tyagi AK. Transgenics of an elite indica rice variety Pusa Basmati 1 harboring the codA gene are highly tolerant to salt stress. Theor Appl Genet. 2002;106(1):51–57. doi: 10.1007/s00122-002-1063-5. [DOI] [PubMed] [Google Scholar]
- 18.Mustardy L, Cunningham FXJr, Gantt E. Localization and quantitation of chloroplast enzymes and light-harvesting components using immunocytochemical methods. Plant Physiol. 1990;94(1):334–340. doi: 10.1104/pp.94.1.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park EJ, Jeknic Z, Pino MT, Murata N, Chen THH. Glycinebetaine accumulation in chloroplasts is more effective than that in cytosol in protecting transgenic tomato plants against abiotic stress. Plant Cell Environ. 2007;30(8):994–1005. doi: 10.1111/j.1365-3040.2007.01694.x. [DOI] [PubMed] [Google Scholar]
- 20.Parvanova D, Ivanov S, Konstantinova T, Karanov E, Atanassov A, Tsvetkov T, Alexieva V, Djilianov D. Transgenic tobacco plants accumulating osmolytes show reduced oxidative damage under freezing stress. Plant Physiol Biochem. 2004;42(1):57–63. doi: 10.1016/j.plaphy.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Prasad KVSK, Saradhi PP. Enhanced tolerance to photoinhibition in transgenic plants through targeting of glycinebetaine biosynthesis into the chloroplasts. Plant Sci. 2004;166(5):1197–1212. doi: 10.1016/j.plantsci.2003.12.031. [DOI] [Google Scholar]
- 22.Prasad KVSK, Sharmila P, Kumar PA, Saradhi PP. Transformation of Brassica juncea (L) Czern with bacterial codA gene enhances its tolerance to salt stress. Mol Breed. 2000;6(5):489–499. doi: 10.1023/A:1026542109965. [DOI] [Google Scholar]
- 23.Rahman MS, Miyake H, Takeoka Y. Effects of exogenous glycinebetaine on growth and ultrastructure of salt-stressed rice seedlings (Oryza sativa L.) Plant Prod Sci. 2002;5(1):33–44. doi: 10.1626/pps.5.33. [DOI] [Google Scholar]
- 24.Rontein D, Basset G, Hanson AD. Metabolic engineering of osmoprotectant accumulation in plants. Met Eng. 2002;4(1):49–56. doi: 10.1006/mben.2001.0208. [DOI] [PubMed] [Google Scholar]
- 25.Sakamoto A, Murata N. Genetic engineering of glycinebetaine synthesis in plants: current status and implications for enhancement of stress tolerance. J Exp Bot. 2000;51(342):81–88. doi: 10.1093/jexbot/51.342.81. [DOI] [PubMed] [Google Scholar]
- 26.Sakamoto A, Murata N. The use of bacterial choline oxidase, a glycinebetaine synthesizing enzyme, to create stress-resistant transgenic plants. Plant Physiol. 2001;125(1):180–188. doi: 10.1104/pp.125.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakamoto A, Murata N. The role of glycinebetaine in the protection of plants from stress: clues from transgenic plants. Plant Cell Environ. 2002;25(2):163–171. doi: 10.1046/j.0016-8025.2001.00790.x. [DOI] [PubMed] [Google Scholar]
- 28.Sakamoto A, Alia , Murata N. Metabolic engineering of rice leading to biosynthesis of glycinebetaine and tolerance to salt and cold. Plant Mol Biol. 1998;38(6):1011–1019. doi: 10.1023/A:1006095015717. [DOI] [PubMed] [Google Scholar]
- 29.Saneoka H, Nagasaka C, Hahn DT, Yang WJ, Premachandra GS, Joly RJ, Rhodes D. Salt tolerance of glycinebetaine deficient and containing maize lines. Plant Physiol. 1995;107(2):631–638. doi: 10.1104/pp.107.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sawahel W. Improved performance of transgenic glycinebetaine-accumulating rice plants under drought stress. Biologia Plantarum. 2003;47(1):39–44. doi: 10.1023/A:1027372629612. [DOI] [Google Scholar]
- 31.Schobert B. Is there an osmotic regulatory mechanism in algae and higher plants? J Theor Biol. 1977;68(1):17–26. doi: 10.1016/0022-5193(77)90224-7. [DOI] [PubMed] [Google Scholar]
- 32.Xu W, Sun MH, Zhu YF, Su WA. Protective effects of glycibetaine on Brassica chinensis under salt stress. Acta Bot Sin. 2001;43(8):809–814. [Google Scholar]
- 33.Yang SY. Determination of Chlorophyll Content. In: Tang ZC, editor. Modern Experiment Protocol in Plant Physiology. Beijing, China: Science Press; 1999. pp. 95–96. (in Chinese) [Google Scholar]