Abstract
Long-term preservation and easy transportation of human bone marrow-derived mesenchymal stem cells (hBM-MSCs) will facilitate their application in medical treatment and bioengineering. A pilot study on the freeze-drying of hBM-MSCs was carried out. hBM-MSCs were loaded with trehalose. The glass transition temperature of the freeze-drying suspension was measured to provide information for the cooling and primary drying experiment. After freeze-drying, various rehydration processes were tested. The highest recovery rate of hBM-MSCs was (69.33±13.08)%. Possible methods to improve freeze-drying outcomes are discussed. In conclusion, the present study has laid a foundation for the freeze-drying hBM-MSCs.
Keywords: Human bone marrow-derived mesenchymal stem cells (hBM-MSCs), Freeze-drying, Trehalose, Rehydration
1. Introduction
Human bone marrow-derived mesenchymal stem cells (hBM-MSCs) are multipotent stem cells that can proliferate, support the immune system, and differentiate into multiple lineages. They are ideal stem cells for tissue engineering and clinical treatment (Nakamizo et al., 2005; Winer et al., 2009). Resuscitated hBM-MSCs could be used for establishing an abundant hBM-MSC reservoir for further experiment and treatment of various clinical diseases (Xiang et al., 2007). However, the difficulties faced in the transportation and prolonged storage of hBM-MSCs have hindered their use in clinical studies and industrial application. The traditional method for hBM-MSCs storage is cryopreservation. Under standard cryopreservation procedures, cell recovery rates were from 87.67% to 94.76% (Luo et al., 2006; Carvalho et al., 2008). The recovery rate was high and the proliferating ability could be maintained. However, cryopreservation always requires expensive and clumsy equipments, and usually the supply and management of liquid nitrogen, which limits its usage. Gordon et al. (2001) conducted a primary study on the air-drying of hBM-MSCs, and the rehydrated cells maintained high viability and proliferation capacity. Nevertheless, considering the damage of air-drying, including shrinkage of membrane, elevation in intracellular salt concentration, changes in biophysical properties and physiological processes, the air-drying of hBM-MSCs is still not the preferential method (Potts et al., 2005).
Freeze-drying has been used in the preparation of pharmaceuticals and vaccines as one of the most important processes for the preservation of heat-sensitive biological materials. Compared to other techniques, freeze-drying has some well-known advantages, including sample stability at room temperature, defined porous product structure, easy reconstitution by the addition of water or aqueous solution, and easy transportation (Xiao et al., 2004). Freeze-drying of cells used to be limited to prokaryotes. Over recent years, a few types of mammalian cells have been successfully freeze-dried, such as human erythrocytes and human platelets (Crowe et al., 2001; Wolkers et al., 2001; 2002; Han et al., 2005; Li et al., 2005; Török et al., 2005). It has been proven that freeze-dried platelets can be stored at room temperature for several months while their physiological viabilities are remained (Wolkers et al., 2002). Yang et al. (2005) tried different lyoprotectants to lyophilize hBM-MSCs and found 30% (w/v) polyvinylpyrrolidone 40 (PVP40)+20% (w/v) trehalose to be better. However, the recovery rate of rehydrated cells was 16.4%, rather lower than that of cryopreservation. Vacuum-drying was also tried and good results were obtained (Jamil et al., 2005). However, the final water content was 0.3 g H2O/g dry weight, which may influence long-term stability of the products. Obtaining a better recovery rate after freeze-drying still requires further study.
The success of freeze-drying mammalian cells was largely dependent on the usage of trehalose. Anhydrobiotic organisms can tolerate the lack of water because of their ability to synthesize large quantities of trehalose (Alpert, 2006). The major challenge of using trehalose for cell preservation is to increase its intracellular concentration. Many methods have been proposed to load trehalose into the cell across the membrane which is naturally impermeable to this chemical, such as ultrasound, electropermeabilization, transgenes, and microinjection (Chen et al., 2001; Eroglu et al., 2002; Shirakashi et al., 2002; Zhang et al., 2009). Fluid-phase endocytosis is easier to perform compared to other methods and has been employed in the successful loading of trehalose into human platelets (Wolkers et al., 2001). Oliver et al. (2004) have investigated the factors that influenced the loading of trehalose into hBM-MSCs by fluid-phase endocytosis. They found that the uptake process was inhibited below 20 °C, and the quantity of uptake was proportional to the length of incubation and dependent on extracellular trehalose concentration. In this study, the method of fluid-phase endocytosis was also employed to load trehalose into hBM-MSCs.
To reduce the injuries of both desiccation and hypothermia during the freeze-drying process, PVP40 was added as a protectant. It can inhibit sucrose crystallization and stabilize the glassy structure of sugar (Zeng et al., 2001). As to the rehydration process, phosphate buffered saline (PBS) was often used as rehydration solution (Xiao et al., 2004; Li et al., 2005). Both colloidal osmotic pressure and crystalloid pressure of the rehydration solution would affect the recovery of cells, while the colloidal osmotic pressure was more influential on cell survival than crystalloid pressure during rehydration (Han et al., 2004). Based on this finding, rehydration solution containing trehalose and PVP40 will be tried in this study, different formulas were tried in order to obtain the best colloidal osmotic pressure and crystalloid pressure, and the recovery rates were detected.
2. Materials and methods
2.1. Culture of hBM-MSCs
Human bone marrows were collected from three healthy adult donors from the First Affiliated Hospital, School of Medicine, Zhejiang University, China. Mesenchymal stem cells were obtained by density gradient centrifugation. The cells were incubated in Dulbecco’s modified Eagle’s medium-low glucose (DMEM-LG, Gibco, USA) with 10% (w/v) fetal bovine serum (FBS) at 37 °C in 5% CO2 atmosphere. After 48-h incubation, nonadherent cells were discarded, and adherent cells were washed twice with PBS. Fresh culture medium was added every 3 d. In 14 d, the cells grew to 70%–90% confluence. Then the cells were harvested with 0.25% (w/v) trypsin/1 mmol/L ethylenediamine tetraacetic acid (EDTA) (Life Technologies, Gaithersburg, MD, USA) and diluted 1:3 (v/v) for passage. hBM-MSCs of the 3rd passage were used for experiment.
2.2. Trehalose loading and preparation of freeze-drying suspension
In order to load trehalose into hBM-MSCs, the cells were incubated with trehalose (Sigma-Aldrich) solution at 37 °C in 5% CO2 atmosphere for 24 h. The concentrations of trehalose solution tested included 10, 20, 50, 80, 100, and 200 mmol/L. Anthrone-sulfuric acid reaction method (anthrone and sulfuric acid bought from Sigma-Aldrich) was applied to determine intracellular trehalose concentration (Umbreit et al., 1972). The absorbance of the solution was measured at 620 nm on a VIS-723 spectrophotometer (Precision & Scientific Instrument Co., Ltd., Shanghai, China) and compared with a standard curve. Assuming that 14 000 fl of the cell volume is taken up by the cytosol (Oliver et al., 2004), the concentration of intracellular trehalose was calculated as follows:
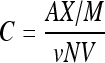 , ,
|
where C (mmol/L) is the concentration of intracellular trehalose, A (ml) is the volume of sample solution, X (μg/ml) is the trehalose concentration of sample solution (derived by the standard curve), M (g/mol) is molecular weight of trehalose, ν (ml) is the volume of the solution used to measure the intracellular trehalose; N (L−1) is the concentration of cells, and V (fl) is the mean cytosol volume.
An appropriate concentration was chosen (20–200 mmol/L) and further experiments were carried out to investigate the impact of incubation duration of 6, 12, and 24 h. To estimate the possible effect of trehalose loading, the growth curve of post-loading hBM-MSCs was compared with non-loading hBM-MSCs. Cell growth was evaluated by counting cell numbers with a counting chamber (n=3). Freeze-drying suspension was composed of 30% PVP40, 100 mmol/L trehalose, and cell suspension at a volume ratio of 2:1:2. The cell concentration in the cell suspension was 107 cells/ml.
2.3. Differential scanning calorimetry (DSC) analysis
The glass transition temperature of the freeze-drying system was measured with DSC-Q100 (TA Instruments, New Castle, DE, USA). An empty sealed aluminum pan was used as reference. Nitrogen was used as carrier gas. Both the cooling rate and the warming rate were 10 °C/min. The Universal Analysis software was used to analyze the DSC curves.
2.4. Residual water content
Residual water content after freeze-drying was measured with the thermogravimetry analysis (WCT-2 A-DTA, Beijing Optical Instrument Factory, China).
2.5. Freeze-drying
The freeze-drying process was carried out on a self-made freeze-dryer as shown in Fig. 1 (Weng et al., 2004). A total of 1 ml freeze-drying system was filled into a sterilized glass bottle covered with a semipermeable film to prevent contamination. The glass bottle’s inner diameter was 1.6 cm (Fig. 6). The bottle was put on the shelf of the freeze-dryer, which had been precooled to −60 °C, so that quick freezing could be realized (Li et al., 2003; Wang and Zhang, 2007). After cooling for 2 h, the primary drying began. The shelf temperature was set at −32 °C, and the vacuum was controlled under 10 Pa. The drying process was conducted similarly to what we did on human platelets (Zhou et al., 2007). The primary drying process lasted for 16 h. Then the shelf was heated up to 20 °C at a rate of 0.2 °C/min and held for 6 h. After freeze-drying, the bottle was sealed and kept at room temperature for 2 h.
Fig. 1.
Self-made freeze-dryer
An auto-cascade refrigeration system was employed. The shelf could be cooled down to −60 °C and the condenser temperature could reach −80 °C. The pressure in the drying chamber could reach 2 Pa (Weng et al., 2004)
Fig. 6.

Freeze-dried sample
2.6. Rehydration
Three kinds of solution were used: (1) PBS buffer (pH 6.8), made of 100 mmol/L NaCl, 9.4 mmol/L Na2HPO4, and 0.6 mmol/L KH2PO4; (2) rehydration solution A, DMEM-LG containing 20% (w/v) FBS; (3) rehydration solution B, composed of 30% PVP40, 100 mmol/L trehalose, and PBS buffer at a volume ratio of 2:1:2. Four different procedures of rehydration were tested: (1) 5 ml rehydration solution A was gradually added into the dried sample; (2) 2 ml PBS was added into the dried sample and held for 5 min, then another 6 ml PBS was added, and the suspension was centrifuged at 900×g for 1 min, then the precipitated sample was resuspended in 5 ml rehydration solution A; (3) the same with (2) except that the dried sample was first equilibrated in 2 ml rehydration solution B for 5 min; and, (4) the dried samples was equilibrated in 0.5 ml rehydration solution B for 5 min, and then added with 4.5 ml rehydration solution A.
2.7. Cell viability evaluation
Cell number and viability were determined by trypan blue (ScienCell, USA) staining. The recovery rate (RR) was defined as the percentage: RR=N 1/N 2×ER×100%, where N 1 is the number of cells after rehydration, N 2 is the number of cells before freeze-drying, and ER is trypan blue excluding rate after rehydration. The recovery rate evaluation was duplicated thrice for each rehydration procedure. The recovered cells were cultured in a 25-cm2 flask with DMEM-LG medium at 37 °C and 5% CO2.
2.8. Statistical analysis
The experimental data were statistically analyzed using two-sample t-test. A level of P≤0.05 was accepted as being statistically significant.
3. Results
3.1. Trehalose uptake by hBM-MSCs
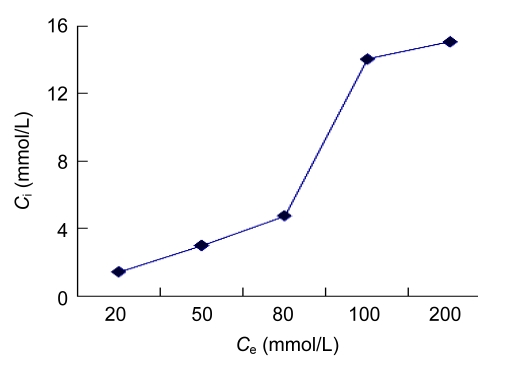
Intracellular trehalose concentration of hBM-MSCs increased with increasing extracellular trehalose concentration up to 100 mmol/L. While the extracellular trehalose concentration increased from 100 mmol/L to 200 mmol/L, the magnitude of the increasing of intracellular trehalose concentration was very limited (Fig. 2).
Fig. 2.
Trehalose uptake by hBM-MSCs as a function of extracellular trehalose concentration
Loading experiments were conducted at 37 °C in the presence of 20–200 mmol/L trehalose solutions for 24 h. Data were derived in triplicates. Intracellular trehalose concentration (C i) increased with increasing extracellular trehalose concentration (C e)
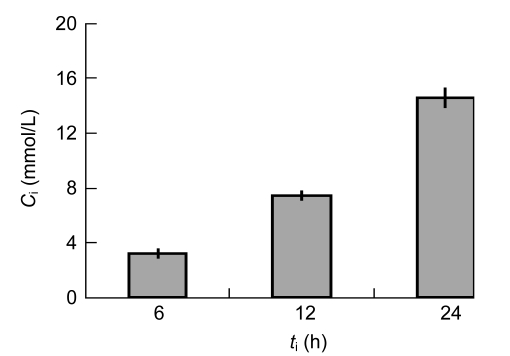
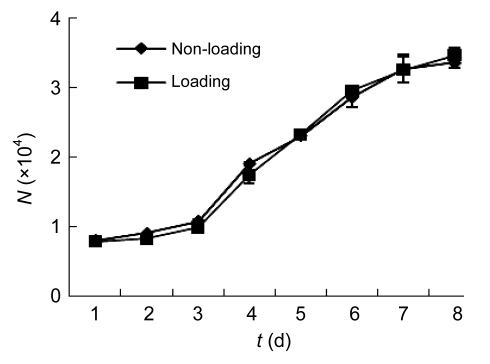
Intracellular trehalose concentration increased linearly (r=0.99) with incubation time when incubated with 100 mmol/L trehalose solution. After 24 h of incubation, the intracellular trehalose concentration reached (14.57±0.74) mmol/L (Fig. 3). This value was a little lower than former reports as 19 mmol/L (Oliver et al., 2004). It may be caused by different cell conditions. Twenty-four hours and 100 mmol/L were chosen as the best conditions for trehalose loading. No significant difference (P>0.05) was observed between the two growth curves of post-loading and non-loading cells (Fig. 4).
Fig. 3.
Uptake of trehalose by hBM-MSCs as a function of incubation time
hBM-MSCs were incubated in 100 mmol/L trehalose solution for 6, 12, and 24 h. Data were derived in triplicates and the error bars represent standard deviations. Intracellular trehalose concentration (C i) increased linearly (r=0.99) with incubation time (t i)
Fig. 4.
Growth curves of trehalose loading and non-loading hBM-MSCs at passage 3
No significant difference was found between two growth curves (P>0.05). N: cell number; t: incubation time
3.2. Glass transition temperature
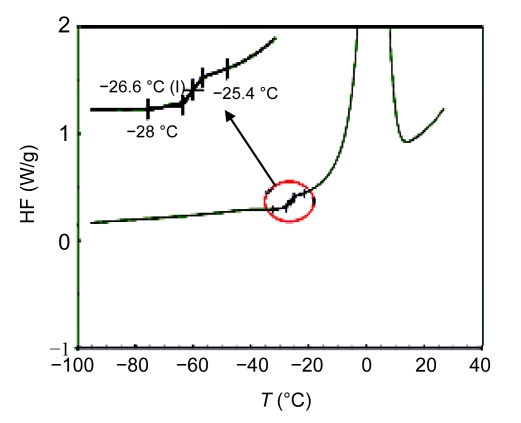
The mid-point of the transition was considered to be the glass transition temperature T g. The T g of the freeze-drying suspension was determined to be −26.6 °C (Fig. 5). During the primary drying process, the sample temperature should be lower than this temperature; thus, the shelf temperature was set at −32 °C.
Fig. 5.
DSC curve of the freeze-drying suspension
The cooling rate and the warming rate were 10 °C/min. The glass transition temperature (T g) of the freeze-drying suspension was determined to be −26.6 °C. The enlargement of the glass transition is shown on the left-top corner. HF: heat flow; T: temperature; I: intermediate
3.3. Residual water content
After freeze-drying, a dried and sterile sample was obtained (Fig. 6). The residual water content of the sample was determined to be 2.9%.
3.4. Cell recovery after rehydration
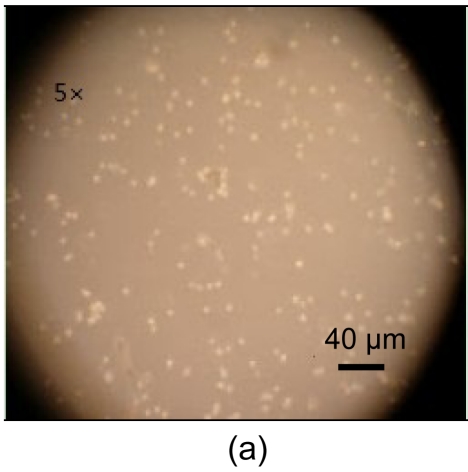
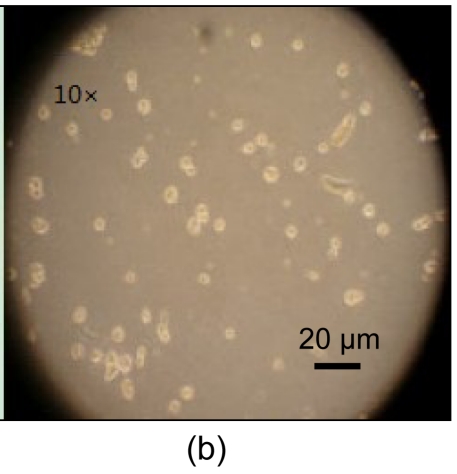
Freeze-dried samples were rehydrated with four procedures mentioned above. The recovery rates after 12 h planting for procedures 1, 2, 3, and 4 were (61.75±15.64)%, (41.17±7.56)%, (48.56±18.10)%, and (69.33±13.08)%, respectively. Procedures 1 and 4 showed higher viability than procedures 2 and 3 (P<0.05), but there was no statistical differences between procedures 1 and 4 (P>0.05). The morphologies of recovered cells immediately after rehydration and after 12-h incubation are shown in Fig. 7. The recovery rate was higher than former reports by Yang et al. (2005). However, recovered hBM-MSCs had impaired adhere and proliferation abilities and died within a week.
Fig. 7.
Cell morphologies of hBM-MSCs
(a) Immediately after rehydration; (b) After 12-h incubation
Compared to procedures 1 and 4, procedures 2 and 3 tended to have lower concentrations of extracellular trehalose and more physical disturbance, which could lead to more susceptibility to physical and chemical stresses during rehydration. It seemed that the rehydration solution containing trehalose was beneficial for cell survival, which might be due to its higher colloidal osmotic pressure than that of other solutions without trehalose.
4. Conclusions
In this study, hBM-MSCs undergoing freeze-drying had a recovery rate ranging from (41.17±7.56)% to (69.33±13.08)% at 12 h after recovering. However, the proliferation ability of rehydrated hBM-MSCs was not shown. Nevertheless, the present study has laid a foundation for the freeze-drying of hBM-MSCs. The attractive prospects of freeze-drying hBM-MSCs prompt us to put more effort into designing better protocols in future studies.
Footnotes
Project (Nos. 30600256 and 50606032) supported by the National Natural Science Foundation of China
References
- 1.Alpert P. Constraints of tolerance: why are desiccation-tolerant organisms so small or rare? J Exp Biol. 2006;209(9):1575–1584. doi: 10.1242/jeb.02179. [DOI] [PubMed] [Google Scholar]
- 2.Carvalho KA, Cury CC, Oliveira L, et al. Evaluation of bone marrow mesenchymal stem cell standard cryopreservation procedure efficiency. Transp. Proc. 2008;40(3):839–841. doi: 10.1016/j.transproceed.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Chen T, Acker JP, Eroglu A. Beneficial effect of intracellular trehalose on the membrane integrity of dried mammalian cells. Cryobiology. 2001;43(2):168–181. doi: 10.1006/cryo.2001.2360. [DOI] [PubMed] [Google Scholar]
- 4.Crowe JH, Crowe LM, Oliver AE. The trehalose myth revisited: introduction to a symposium on stabilization of cells in the dry state. Cryobiology. 2001;43(2):89–105. doi: 10.1006/cryo.2001.2353. [DOI] [PubMed] [Google Scholar]
- 5.Eroglu A, Toner M, Toth TL. Beneficial effect of microinjected trehalose on the cryosurvival of human oocytes. Fertil Steril. 2002;77(1):152–158. doi: 10.1016/S0015-0282(01)02959-4. [DOI] [PubMed] [Google Scholar]
- 6.Gordon SL, Oppenheimer SR, Mackay AM. Recovery of human mesenchymal stem cells following dehydration and rehydration. Cryobiology. 2001;43(2):182–187. doi: 10.1006/cryo.2001.2361. [DOI] [PubMed] [Google Scholar]
- 7.Han Y, Quan GB, Liu XZ, Liu A, Jin P, Cao W. Effect of reconstitution solutions on the recovery of lyophilized red blood cells. Chin J Blood Transfus. 2004;17(1):308–311. [Google Scholar]
- 8.Han Y, Quan GB, Liu XZ. Improved preservation of human red blood cells by lyophilization. Cryobiology. 2005;51(2):152–164. doi: 10.1016/j.cryobiol.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Jamil K, Crowe JH, Tablin F, Oliver AE. Arbutin enhances recovery and osteogenic differentiation in dried and rehydrated human mesenchymal stem cells. Cell Preserv Technol. 2005;3(4):244–255. doi: 10.1089/cpt.2005.3.244. [DOI] [Google Scholar]
- 10.Li J, Hua TC, Gu XL. Morphology study of freeze-drying mononuclear cells of human cord blood. CryoLetters. 2005;26(3):193–200. [PubMed] [Google Scholar]
- 11.Li XS, Fan HT, Yuan Y, Hou CM, Mao N. Modification of in situ cryopreservation of human bone marrow mesenchymal stem cells. J Exp Hematol. 2003;11(5):530–533. (in Chinese) [PubMed] [Google Scholar]
- 12.Luo Y, Huang H, Yu J, Liang B. Effect of cryopreservation on the biological characteristics of adult mesenchymal stem cells derived from bone marrow. Chin J Pathophysiol. 2006;2(2):384–387. (in Chinese) [Google Scholar]
- 13.Nakamizo F, Marini T, Amano T. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;64(4):3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 14.Oliver A, Jamil K, Crowe JH, Tablin F. Loading human mesenchymal stem cells with trehalose by fluid-phase endocytosis. Cell Preserv Technol. 2004;2(1):35–49. doi: 10.1089/153834404322708745. [DOI] [Google Scholar]
- 15.Potts M, Slaughter SM, Hunneke FU. Desiccation tolerance of prokaryotes: application of principles to human cells. Integr Comp Biol. 2005;45(5):800–809. doi: 10.1093/icb/45.5.800. [DOI] [PubMed] [Google Scholar]
- 16.Shirakashi R, Kostner CM, Muller KJ. Intracellular delivery of trehalose into mammalian cells by electropermeabilization. J Membr Boil. 2002;189(1):45–54. doi: 10.1007/s00232-002-1003-y. [DOI] [PubMed] [Google Scholar]
- 17.Török Z, Satpathy GR, Banerjee M, Bali R, Little E, Novaes R, Ly HV, Dwyre DM, Kheirolomoom A, Tablin F, et al. Preservation of trehalose-loaded red blood cells by lyophilization. Cell Preserv Technol. 2005;3(2):96–111. doi: 10.1089/cpt.2005.3.96. [DOI] [Google Scholar]
- 18.Umbreit WW, Burris RH, Stauffer JF. Manometric and Biochemical Techniques. 5th Ed. Minneapolis, USA: Burgess Publishing Company; 1972. p. 262. [Google Scholar]
- 19.Wang J, Zhang H. The influence of one-step −80 °C cryopreservation on the osteogenesis differentiation ability of human bone marrow-derived mesenchymal stem cells. Guangdong Med. 2007;28(3):365–367. (in Chinese) [Google Scholar]
- 20.Weng Y, Zhou XL, Chen GM. The development of a freeze-dryer. Low Temp Technol. 2004;44(3):12–16. (in Chinese) [Google Scholar]
- 21.Winer JP, Janmey PA, McCormick ME, Funaki M. Bone marrow-derived human mesenchymal stem cells become quiescent on soft substrates but remain responsive to chemical or mechanical stimuli. Tissue Eng Part A. 2009;15(1):147–154. doi: 10.1089/ten.tea.2007.0388. [DOI] [PubMed] [Google Scholar]
- 22.Wolkers WF, Walker NJ, Tablin F, Crowe JH. Human platelets loaded with trehalose survive freeze-drying. Cryobiology. 2001;42(2):79–87. doi: 10.1006/cryo.2001.2306. [DOI] [PubMed] [Google Scholar]
- 23.Wolkers WF, Walker NJ, Tamari Y. Towards a clinical application of freeze-dried human platelets. Cell Preserv Technol. 2002;1(3):175–188. doi: 10.1089/153834402765035617. [DOI] [Google Scholar]
- 24.Xiang Y, Zheng Q, Jia B, Huang G, Xie C, Pan J, Wang J. Ex vivo expansion, adipogenesis and neurogenesis of cryopreserved human bone marrow mesenchymal stem cells. J Zhejiang Univ-Sci B. 2007;8(2):136–146. doi: 10.1631/jzus.2007.B0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao HH, Hua TC, Li J. Freeze drying of mononuclear cells and whole blood of human cord blood. CryoLetters. 2004;25(2):111–120. [PubMed] [Google Scholar]
- 26.Yang F, Cheng QK, Wang X, Hua ZZ, Chen FF, Shu CF, Cui L, Cao YL. Research on freeze-drying of human bone mesenchymal stem cells. J Refriger. 2005;26(1):19–23. (in Chinese) [Google Scholar]
- 27.Zeng XM, Martin GP, Marriott C. Effects of molecular weight of polyvinylpyrrolidone on the glass transition and crystallization of co-lyophilized sucrose. Int J Pharm. 2001;218(1-2):63–73. doi: 10.1016/S0378-5173(01)00613-5. [DOI] [PubMed] [Google Scholar]
- 28.Zhang SZ, Fan JL, Xu XG. An experimental study of the use of ultrasound to facilitate the loading of trehalose into platelets. Cryobiology. 2009;59(2):135–140. doi: 10.1016/j.cryobiol.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Zhou XL, Zhu H, Zhang SZ, Zhu FM, Chen GM, Yan LX. Freeze-drying of human platelets: influence of saccharide, freezing rate and cell concentration. CryoLetters. 2007;28(3):187–196. [PubMed] [Google Scholar]