Abstract
Podocyte damage induced by HIV-1 is critical to the pathogenesis of HIV-1 associated nephropathy (HIVAN) and is believed to result from productive replication of the virus. Here we demonstrate that HIV-1 readily enters human podocytes by a dynamin-mediated endocytosis but does not establish productive infection. We provide evidence suggesting that viral nucleic acids and proteins detected in podocytes are delivered by viral particles internalized by the cells. Endocytosed HIV-1 is only transiently harbored by podocytes and is subsequently released to the extracellular milieu as fully infectious virus. Similarly, primary podocytes established from normal human urine do not support productive infection by HIV-1 but sustain replication of VSV-G pseudotyped virus that bypasses HIV-1 entry receptors. Moreover, transfected podocytes expressing CD4 and CXCR4 receptors support productive replication of HIV-1. This further confirms that lack of HIV-1 entry receptors is the major barrier preventing productive infection of podocytes in vitro.
Keywords: HIV-1, podocyte, endocytosis, HIVAN
Introduction
Podocytes are the key components of the glomerular filtration barrier and podocyte injury leads to loss of integrity of the barrier and proteinuria. The pathogenesis of HIV-1 associated nephropathy (HIVAN) is poorly understood. Although HIVAN affects all compartments of the kidney including the tubular epithelium (Bruggeman et al., 2000), infection of podocytes seems to be critical to HIVAN pathogenesis (Yang, Gubler, and Beaufils, 2002). HIV-1 infection of podocytes was shown to stimulate podocyte dedifferentiation, proliferation and loss of the majority of podocyte-specific markers (Lu, He, and Klotman, 2007). It has been reported that the virus remains transcriptionally active in the kidney of patients on highly active antiretroviral therapy with undetectable blood plasma viremia (Bruggeman et al., 2000; Winston et al., 2001), suggesting that the renal epithelium may represent a distinct HIV-1 reservoir. Further support for the replication of HIV-1 in renal epithelium comes from the observation that viral envelope gp120 evolves differently in peripheral blood and the renal compartment (Marras et al., 2002). Since renal epithelial cells do not express classical HIV-1 entry receptors (Huber et al., 2002), the mechanism by which HIV-1 infects the renal epithelium remains unclear. The C-type lectin DEC-2005 was recently proposed to mediate infection of renal HK2 proximal tubular cells, however this entry route also leads to nonproductive infection (Hatsukari et al., 2007; Mikulak et al., 2009).
There is compelling evidence that expression of viral proteins Nef and/or Vpr in immortalized podocytes (Husain et al., 2002; Lu et al., 2008; Sunamoto et al., 2003) or in transgenic animal models of HIVAN (Husain et al., 2005; Rosenstiel et al., 2009; Zuo et al., 2006) is sufficient to induce the podocyte dysfunction observed in HIVAN. Since Nef and Vpr were expressed from transfected HIV-1 constructs, it is unclear whether these proteins would be expressed in podocytes infected with wild-type HIV-1. Although detection of HIV-1 nucleic acids in glomerular podocytes in HIVAN kidney biopsy samples seems to indicate that these cells can be productively infected with HIV-1 in vivo (Bruggeman et al., 2000; Marras et al., 2002), the mechanism of this process remains undetermined.
To gain insights on the permissiveness of podocytes to infection with wild-type HIV-1, we investigated replication of HIV-1 in podocytes in vitro. In our approach, we used both conditionally immortalized podocyte cells AB8/13 (Saleem et al., 2002) and primary podocytes isolated from the urines of healthy donors. We demonstrate that despite HIV-1 particles being readily internalized by podocytes, this process does not lead to productive infection. We further demonstrate that viral nucleic acids and proteins detected in podocytes originate from endocytosed viral particles rather than productive replication of HIV-1 in podocytes. We also show that lack of HIV-1 entry receptors in podocytes is a key barrier to productive infection and can be overcome by the exogenous expression of HIV-1 receptors in podocytes.
Materials and methods
Podocyte culture
Undifferentiated conditionally immortalized human podocytes AB8/13 (Saleem et al., 2002) were cultured at 33°C in RPMI medium supplemented with 10% FCS (RPMI-10% FCS), penicillin and streptomycin, and insulin-transferrin-selenium (ITS) (Invitrogen) on 6-well plastic dishes covered with collagen I (Invitrogen). Podocyte differentiation was achieved by growing the cells for 14 days at 37°C to inactivate temperature-sensitive SV40 large T antigen (U19tsA58). Urine samples were obtained from consented healthy volunteers, after the research protocol was approved by Meharry Medical College’s IRB. A fresh urine sample was centrifuged for 10 min at 700 × g and the urinary pellet was washed twice with RPMI 1640 and centrifuged. The pelleted cells, resuspended in RPMI-10% FCS supplemented with penicillin, streptomycin and ITS were seeded on 60 mm plastic dishes coated with collagen I. The following day, the media with unattached cells and debris were replaced with fresh culture media. After about 7–14 days, the cells were trypsinized for about 1.5 – 2 min and cells that easily detached from culture dishes were transferred to new plates coated with collagen I. After one or two additional subculturing cycles (about 4 weeks in culture), the cells were analyzed by real-time RT-PCR and immunofluorescence to confirm the expression of podocyte-specific mRNAs and proteins.
Virus preparation
Virus stocks were prepared by transfecting 293T cells with HIV-1 plasmid DNA by using PolyFect reagent (Qiagen) as described (Khatua et al., 2009). Purified and concentrated virus was treated with DNase I (300 units/ml) for 30 min at 37°C and for 1 h at room temperature, tittered by p24 ELISA assay or by the reverse transcriptase activity assay (Popik and Pitha, 2000). HIV-1 aliquots were stored at −80°C.
Virus inactivation
Heat inactivation was carried out for 1 h at 65°C as described (Mbisa et al., 2007). For inactivation with aldriothiol-2 (AT-2), a stock solution of AT-2 (100 mM in dimethyl sulfoxide, DMSO) was added to virus to a final concentration of 1 mM (in 300 μl) and incubated for 1 h at 37°C. Control virus (native NL4-3) was exposed to DMSO alone. After the treatment, virus preparation was diluted to 11 ml with RPMI-10%FCS and concentrated by ultracentrifugation. Pelleted virus was resuspended in the original volume of RPMI-10%FCS and used immediately in experiments.
Viral infectivity assay
HIV-1 AD8 was added to differentiated AB8/13 podocytes (AD8 input) and incubated for 3 h. After extensive washing (5 washes with RPMI 1640), the cells were incubated for an additional 3 h, washed again 3 times and virus released from podocytes between 6 and 24 h post-infection (AD8 recovered) was collected and tittered. The same amount of input and recovered viral inoculum (standardized by RT assay) was added in triplicate to TZM-bl indicator cells in a 12-well plate according to the protocol (NIH AIDS Research and Reference Reagent Program; cat no. 1470). After 2 days, cells were fixed and stained for β-galactosidase and positive syncytia (blue) were counted.
Confocal microscopy
Differentiated AB8/13 podocytes were incubated at 37°C for 90 min with HIV-1 AD8 fluorescently labeled with GFP-Vpr (Liu et al., 2002), washed and incubated for 30 min with LysoTracker Red (50 nM) (Invitrogen) or Transferrin-Alexa Fluor 594 (5 μg/ml) (Invitrogen). When indicated, the cells were pretreated for 1h with 200 μM dynasore (Tocris Bioscience) (Macia et al., 2006) in 0.1% DMSO or with 0.1% DMSO alone and subsequently infected with HIV-1 in the presence or absence of dynasore. The cells were washed, fixed in 4% paraformaldehyde for 15 min, washed and mounted with ProLong antifade reagent (Invitrogen) and observed under a laser scanning confocal microscope (Nikon TE2000).
Immunoblot analysis
Cellular lysates were prepared from uninfected or HIV-1 infected differentiated podocytes growing in 6-well plates. Cell culture supernatants from 3 wells (6 ml) were collected at different time points, filtered through 0.45-μm filter and viral particles were sedimented by ultracentrifugation for 1 h at 100,000 × g and lysed in SDS lysis buffer. Proteins (30 μg/lane) were separated on SDS-10% polyacrylamide gels and analyzed as described (Khatua et al., 2009). Monoclonal p24 and rabbit Nef antibodies were obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. Rabbit anti-actin antibody was from Sigma.
Quantitative analyses of HIV-1 reverse transcription products
Differentiated AB8/13 podocytes or urinary podocytes growing in 6-well plates were infected for 3 h at 37°C with DNase I treated viruses (total 100 ng p24/plate), washed 5 times with PBS, and total DNA was isolated immediately after infection (3h) or after the indicated times. Total DNA was purified using a DNeasy kit (Qiagen). In experiments with reverse transcription inhibitors (RTI), the cells were pretreated for 1 h with 50 μM zidovudine (AZT) and 50 μM lamivudine (3TC). Infection and subsequent cell cultivation was performed in the presence of RTI. The levels of early (ERT) and late (LRT) reverse transcripts and 2-LTR circles were quantified by real-time PCR and normalized against GAPDH. PCR conditions and oligonucleotide primers were described (Khatua et al., 2009). Standard curves were generated using plasmids containing HIV-1 DNA (pNL4-3-deltaE-EGFP, NIH AIDS Research and Reference Reagent Program #11100) and GAPDH cDNA (OriGene).
RNA preparation, cDNA synthesis and real-time RT-PCR
Total RNA was isolated using total RNA purification kit (Norgen). RNA was treated with Turbo DNase (Ambion) and 1 μg RNA was reverse transcribed using iScript cDNA synthesis kit (Bio-Rad) and subjected to real-time-PCR using conditions and primers for podocyte-specific and non-specific genes (Rahmoune et al., 2005; Sakairi et al., 2009; Schmid et al., 2003). Amplified PCR products were resolved on 2% agarose gel and visualized with ethidium bromide. Real-time PCR conditions and primers to detect HIV-1 unspliced genomic RNA and multiply spliced RNA (Pasternak et al., 2008) and Nef2 mRNA (Dowling et al., 2008) are described elsewhere, respectively.
Statistical analysis
Unless stated otherwise, all experiments were performed at least three times. The variation in one experiment was expressed by calculating standard deviation (SD) from the triplicates and presented as the mean value ± SD.
Results
HIV-1 AD8 is internalized by human AB8/13 podocytes
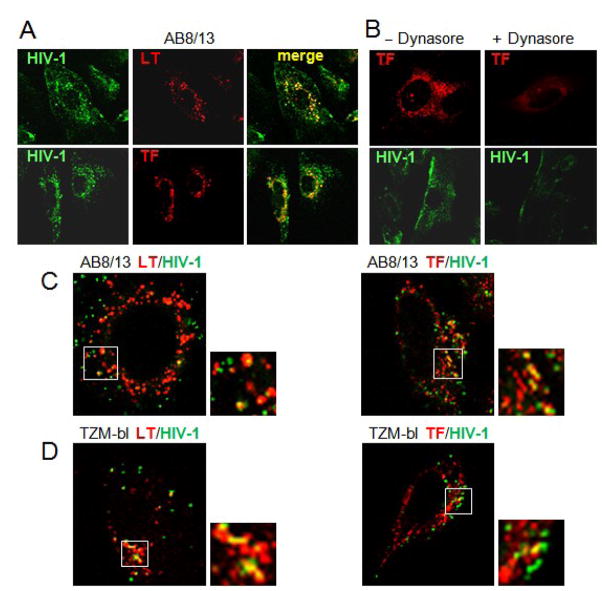
The major objective of this study was to establish whether wild-type HIV-1 can establish productive infection in human podocytes in vitro. HIV-1 entry into conditionally immortalized and differentiated human podocytes AB8/13 (Saleem et al., 2002) was investigated by confocal microscopy using macrophage-tropic (R5) HIV-1 AD8. To enable the monitoring of viral particles that entered cells, HIV-1 was labeled with GFP-Vpr fusion protein that localizes to the virus core (Campbell et al., 2007). Confocal microscopy images (Fig. 1A) show that fluorescently labeled HIV-1 efficiently entered AB8/13 podocytes and partially colocalized with markers specific for late endosomes/lysosomes (LysoTracker, LT) and early/recycling endosomes (Transferrin, TF). This suggests that a fraction of endocytosed virus may be degraded in podocytes or recycled back to the cell surface and released to the cellular environment. To investigate virus entry in more detail, we used a 100-fold lower input of HIV-1 AD8. Our results confirmed that fluorescent virus particles partially colocalized with LysoTracker and Transferrin in podocytes (Fig. 1C) as well as in TZM-bl cells that express HIV-1 receptors and support productive infection (Fig. 1D). A specific dynamin inhibitor, dynasore (Macia et al., 2006; Miyauchi et al., 2009), potently reduced virus entry and transferrin uptake into podocytes (Fig. 1B), indicating that dynamin-mediated endocytosis plays a key role in virus entry into podocytes.
Fig. 1. HIV-1 enters podocytes by a dynamin-dependent endocytosis.
(A) Differentiated AB 8/13 podocytes were incubated for 90 min with fluorescently labeled HIV-1 AD8, washed and incubated for 30 min with LysoTracker Red (LT, 50 nM) or Transferrin-Alexa Fluor 594 (TF, 5 μg/ml). Separate and merged confocal microscopy images are shown. (B) Podocytes were pretreated for 1 h with 200 μM dynasore in 0.1% DMSO (+Dynasore) or with 0.1% DMSO alone (−Dynasore) and infected with AD8 as described in (A). Confocal images of AD8 entry and Transferrin-Alexa Fluor 594 uptake in the absence or presence of dynasore are shown. (C, D) Colocalization of HIV-1 particles (multiplicity of infection 100-fold lower than in Fig. 1A and B) with LysoTracker Red (LT) and Transferrin-Alexa Fluor 594 (TF) in podocytes (C) and TZM-bl cells susceptible to HIV-1 infection (D).
HIV-1 DNA accumulating in podocytes is delivered by endocytosed viral particles
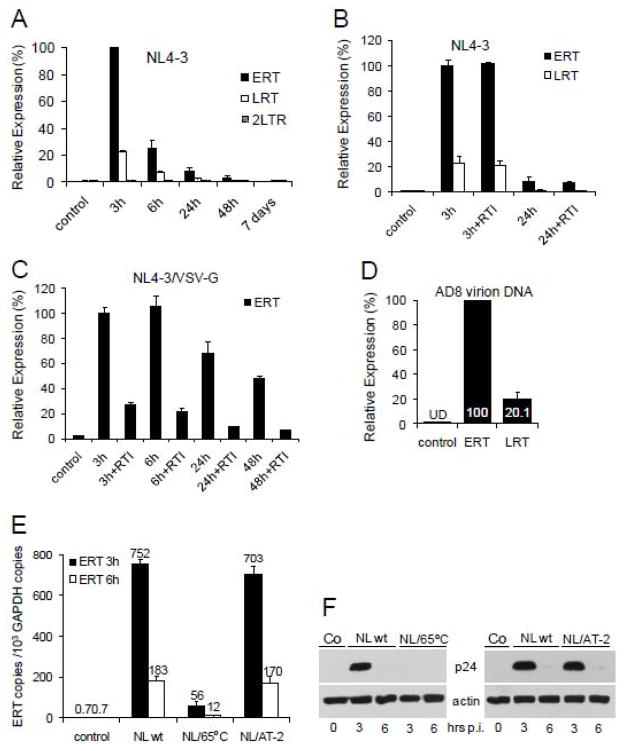
Virus endocytosis often leads to non-productive infection of CD4-negative cells (Dezzutti et al., 2001; Vacharaksa et al., 2008). However, coexistence of endocytic entry with productive infection was also observed (Vidricaire and Tremblay, 2007). Thus, to determine whether HIV-1 replicates productively in podocytes, we analyzed accumulation of HIV-1 reverse transcription DNA products in podocytes infected with T-tropic (X4) HIV-1 NL4-3 (Fig. 2A). HIV-1 early reverse transcription (ERT) and late (LRT) DNA products peaked at 3 h post-infection (the earliest time point analyzed) and rapidly diminished thereafter. Expression of ERT, LRT and 2-LTR circles was undetectable between 2 and 7 days post-infection (last time point analyzed). A similar pattern of ERT and LRT expression was also observed for AD8 (not shown). To investigate whether ERT and LRT were de novo synthesized in podocytes, we used a mix of two reverse transcription (RT) inhibitors, AZT and 3TC. The expression of ERT and LRT in the presence of RT inhibitors was unchanged, indicating that ERT and LRT were not synthesized de novo but rather were delivered by viral particles captured by podocytes (Fig. 2B). Indeed, we have detected both ERT and LRT in purified and DNase I-treated HIV-1 virions (Fig. 2D). These results support previous observations demonstrating the presence of HIV-1 DNA species inside HIV-1 virions (Houzet, Morichaud, and Mougel, 2007; Houzet et al., 2007). As expected, replication of NL4-3 pseudotyped with VSV-G envelope that directs the delivery of the viral core into the cytoplasm (a prerequisite for productive infection) was sensitive to RT inhibitors (Fig. 2C), suggesting that AZT and 3TC were functional in podocytes. To investigate whether virus entry is receptor-mediated, the virus was inactivated by incubation for 1h at 65°C or treatment with aldrithiol-2 (AT-2), known to preserve conformational integrity of virion surface proteins (Rossio et al., 1998) (Fig. 2E). Results demonstrate that accumulation of ERT in podocytes at 3 h and 6 h post-infection was severely reduced (13–15-fold) in heat-treated virions but only marginally reduced in AT-2-treated virions. Western blot analysis (Fig. 2F) confirmed results obtained using real-time PCR. Together, these results suggest that non-productive entry of HIV-1 into podocytes is specific.
Fig. 2. Accumulations of HIV-1 reverse transcription products in infected podocytes and HIV-1 virions.
(A) Differentiated AB8/13 podocytes were infected for 3 h with DNase I-treated NL4-3, washed, and total DNA isolated immediately after infection (3 h) and again after the indicated times. The levels of ERT, LRT and 2-LTR circles were quantified by real-time PCR and normalized against GAPDH. PCR conditions and oligonucleotide primers are described (Khatua et al., 2009). Relative expression levels were compared by setting the level of ERT at 3 h post-infection to 100%. Control, uninfected podocytes. (B, C) The cells were pretreated for 1 h with a mixture of AZT (50 μM) and 3TC (50 μM) (RTI). Infection with NL4-3 (B) or VSV-G pseudotyped NL4-3 (C) was performed in the presence of RTI. The level of ERT at 3 h post-infection without RTI was set to 100%. (D) Total DNA isolated from AD8 virions was analyzed for the expression of ERT and LRT. The expression of ERT was set to 100%. The levels of ERT and LRT DNA in uninfected podocytes (control) were undetectable (UD).
(E, F) Heat-inactivated HIV-1 does not enter podocytes. Native NL4-3 (NL wt) or NL4-3 inactivated for 1 h at 65°C (Mbisa et al., 2007) or treated with aldrithiol-2 (AT-2) that preserves conformational and functional integrity of virion surface proteins (Rossio et al., 1998) were exposed to podocytes for 3 h. At indicated times, expression of ERT (E) or viral p24 (F) were analyzed by real-time RT-PCR or Western blotting, respectively. ERT and GAPDH copy numbers were calculated using standard curves generated from 1 to 1 × 107 copies of respective plasmid DNAs. Error bars, means ± SDs of triplicate samples from one experiment.
HIV-1 Nef2 mRNA accumulates transiently in infected podocytes
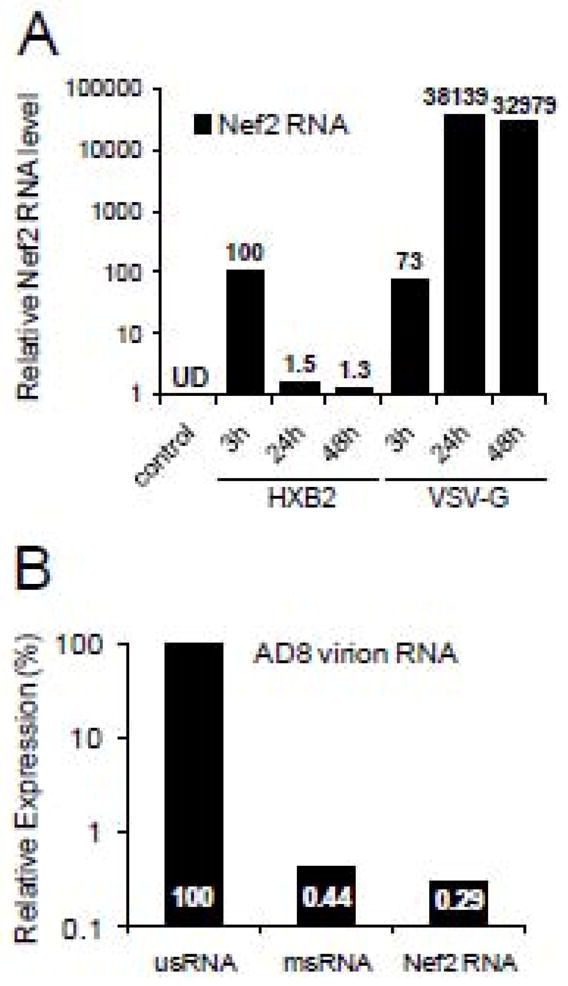
Active replication of HIV-1 can also be assessed by the accumulation of viral transcripts in infected cells. Since doubly spliced Nef2 mRNA is produced at high levels early in infection, we have quantified accumulation of Nef2 mRNA using real-time RT-PCR (Dowling et al., 2008). We have found that in podocytes infected with X4 HXB2-pseudotyped virus, Nef2 mRNA accumulated transiently followed by about 70-fold reduction at 24 h post-infection. In contrast, in podocytes infected with VSV-G-pseudotyped HIV-1, Nef2 mRNA levels at 24 h post-infection increased 522-fold over the level detected at 3 h post-infection (Fig. 3A). These results suggest that HIV-1 pseudotyped with VSV-G, but not with HXB2 envelope, replicated in infected podocytes. Similar levels of Nef2 mRNA detected at 3 h post-infection with VSV-G- or HXB2-pseudotyped HIV-1 indicate that endocytosed virions were the source of Nef2 mRNA accumulated in podocytes. In line with earlier observations showing that HIV-1 virions incorporate detectable amounts of singly and fully spliced mRNAs coding for Tat, Rev and Nef (Houzet, Morichaud, and Mougel, 2007; Houzet et al., 2007; Liang et al., 2004; Pasternak et al., 2008), we have detected low levels of Nef2 mRNA and multiply spliced RNA (msRNA) in AD8 virions (Fig. 3B).
Fig. 3. Expression of Nef2 mRNA in infected podocytes and HIV-1 virions.
(A) Differentiated podocytes were infected with NL4-3 ΔEnv virions pseudotyped with HXB2 or VSV-G envelopes. At indicated times, cellular RNA was isolated, reverse transcribed and analyzed by real-time RT-PCR. Expression of Nef2 mRNA was normalized to GAPDH and the level of Nef2 mRNA at 3 h post-infection with HXB2 pseudotyped HIV-1 was set to 100%. (B) Relative expression of genomic unspliced (usRNA) RNA, multiply spliced RNA (msRNA) and Nef2 mRNA in AD8 virions were quantitated and normalized against 7SL RNA (Onafuwa-Nuga, Telesnitsky, and King, 2006). Expression of virion usRNA was set to 100%.
HIV-1 Gag accumulates transiently in HIV-1 infected podocytes
Synthesis of viral structural and accessory proteins is another hallmark of productive HIV-1 infection. Therefore, we have investigated whether podocytes infected with different tropism viruses express HIV-1 Gag and accessory Nef protein implicated in podocyte injury (Atta, Deray, and Lucas, 2008; He et al., 2004; Husain et al., 2005; Husain et al., 2002; Sunamoto et al., 2003; Zuo et al., 2006). HIV-1 Gag p24 was detected at 3 h post-infection in podocytes infected with all analyzed viruses (Fig. 4). However, at 6 h post-infection, the levels of intracellular p24 were markedly reduced (10–20-fold). This correlated with release of pelletable p24 (viral particles) into the supernatant by cells infected with NL4-3, AD8 or HXB2-pseudotyped virus. In contrast, virus-like particles (VLPs) secreted by cells infected with VSV-G pseudotyped HIV-1 were detectable in the supernatant at 48 h post-infection. Analysis of Gag p24 in podocytes infected with X4 or R5 HIV-1 showed no accumulation of Gag p24 between 2 and 8 days post-infection (not shown). Intracellular expression of Nef protein was detected only in podocytes infected with VSV-G pseudotyped virus at 48 h post-infection and coincided with the release of VLPs. In summary, viral Gag proteins detected early in infection did not reflect productive infection but originated from virions captured by podocytes.
Fig. 4. Immunoblot analysis of HIV-1 proteins expressed in podocytes and released virions.
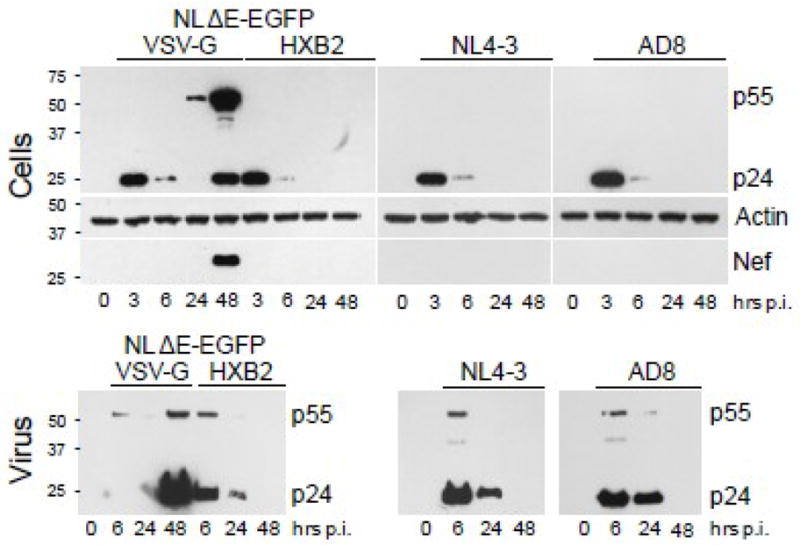
Podocytes were infected for 3 h with NL4-3, AD8 or NLΔE-EGFP pseudotyped with HXB2 or VSV-G envelopes. Cellular lysates (Cells) were prepared at indicated times and analyzed by Western blotting for the expression of HIV-1 Gag (p24, p55), Nef, and actin. After 3 h infection, the cells were washed 5 times and virus particles released from infected cells were sedimented from the same volume of media (6 ml) collected between 3 h and 6 h (denoted as 6 h), between 6 h and 24 h (24 h), and between 24 h and 48 h post-infection (48 h) (Virus). Pelleted virions were analyzed by immunoblotting for the expression of HIV-1 Gag.
HIV-1 released from podocytes is fully infectious
We have next analyzed the infectivity of the virus released from infected podocytes. Input HIV-1 and virus recovered from podocytes between 6 and 24 h post-infection were normalized by RT assay and the same amount of virus was tested on TZM-bl reporter cells (Fig. 5). We demonstrate that AD8 virus released from podocytes (AD8 recovered) retained infectivity similar to the input virus. Thus, virus retained in podocytes could potentially disseminate HIV-1 to susceptible cells.
Fig. 5. HIV-1 released from infected podocytes is infectious.
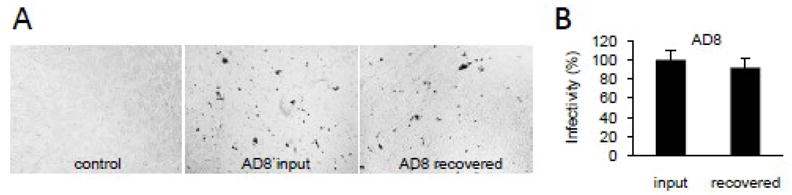
(A) AD8 added to differentiated AB8/13 podocytes (AD8 input) and released from podocytes between 6 and 24 h post-infection (AD8 recovered) were tittered and the same amount of viral inoculum (RT assay standardized) was added in triplicate to TZM-bl indicator cells in a 12-well plate. Three dilutions of viral inoculum were applied: 105, 104, and 103 RT cpm. After 2 days, cells were fixed and stained for β-galactosidase and positive syncytia were counted (B). Viral inoculum (105 RT cpm) applied to indicator cells is shown. Control, uninfected TZM-bl cells. (B) Percent infectivity is presented relative to infectivity of input virus (100%). Error bars, means ± SDs of triplicate samples from one experiment.
Podocytes expressing CD4 and CXCR4 become susceptible to productive infection
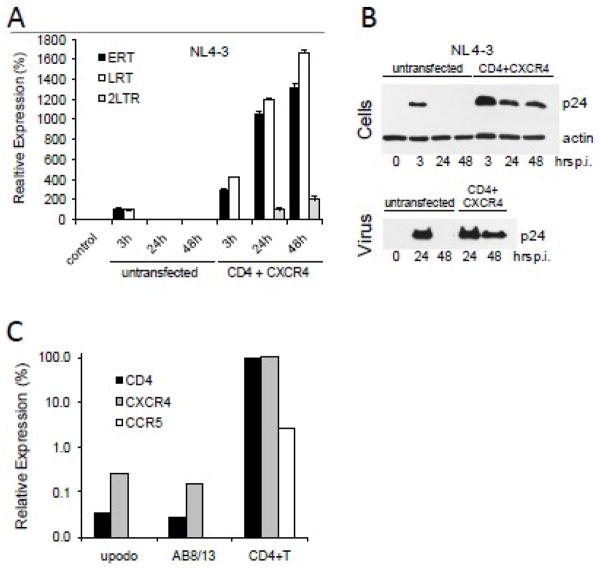
Lack of expression of HIV-1 entry receptors by podocytes (Eitner et al., 2000; Huber et al., 2002) is likely to be the major barrier that prevents productive replication of HIV-1 in these cells. Therefore, we have tested whether expression of CD4 and CXCR4 in podocytes would lead to productive infection. In this experiment, we used undifferentiated podocytes that can be transfected more efficiently than differentiated cells. We have found that undifferentiated podocytes transfected with CD4 and CXCR4 expression vectors supported productive replication of NL4-3, as demonstrated by cellular accumulation of ERT, LRT, 2-LTR (Fig. 6A) and p24 Gag, and release of viral particles (Fig. 6B). In contrast, untransfected and undifferentiated podocytes did not support productive NL4-3 replication and only transiently accumulated viral DNA and Gag.
Fig. 6. Productive infection of podocytes expressing CD4 and CXCR4 receptors.
(A) Undifferentiated AB8/13 podocytes were transfected with CD4 and CXCR4 expression vectors using PolyFect (Qiagen). Twenty-four hours later, the cells were infected with NL4-3 for 3 h. Cellular DNA was analyzed by real-time PCR for the expression of ERT, LRT and 2-LTR circles. Expression was normalized against GAPDH. Relative expression levels were compared by individually setting the levels of ERT and LRT at 3 h post-infection of untransfected cells to 100%. Uninfected cells (control) show undetectable levels of HIV-1 DNA. (B) Lysates from infected podocytes (Cells) and pelleted virions (Virus), prepared as described in Fig. 4 legend, were analyzed for the expression of HIV-1 Gag p24. Time “0” represents uninfected cells. (C) Differentiated AB8/13 podocytes and primary urinary podocytes (upodo) show negligible expression of CD4, CXCR4 and CCR5 mRNAs. Human blood-derived PHA/IL-2 activated CD4+ T cells (Khatua et al., 2009) express CD4, CXCR4 and CCR5 mRNAs and served as a positive control.
To investigate whether differentiated podocytes express HIV-1 entry receptors, we analyzed by real-time RT-PCR the expression of CD4, CXCR4 and CCR5 mRNAs by AB8/13 and primary urinary podocytes (see Fig. 7) and compared it with the expression of the receptor mRNAs by activated blood-derived CD4+ T cells (Khatua et al., 2009). Results (Fig. 6C) show that in contrast to CD4+ T cells, differentiated AB8/13 and urinary podocytes have undetectable expression of CCR5 mRNA and negligible expression of CD4 and CXCR4 mRNAs.
Fig. 7. Urinary podocytes do not support replication of HIV-1.
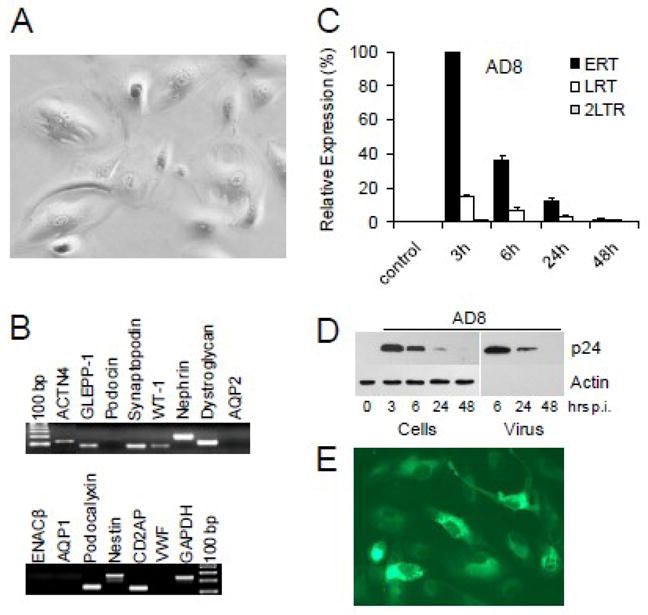
(A) Urinary cells from a healthy volunteer cultured for about 4 weeks display morphology of differentiated podocytes and (B) express podocyte-specific mRNAs detected in real-time RT-PCR (Rahmoune et al., 2005; Sakairi et al., 2009; Schmid et al., 2003). Amplified PCR products were resolved on 2% agarose gels and visualized with ethidium bromide. (C) Urinary podocytes were infected with AD8 for 3 h, extensively washed and cultured for the indicated times. Relative expression of ERT, LRT and 2-LTR circles (normalized against GAPDH) were compared by setting the level of ERT at 3 h post-infection to 100%. (D) Transient expression of HIV-1 Gag proteins in urinary podocytes coincides with a transient release of HIV-1 virions (pelletable p24) into culture medium. (E) Accumulation of truncated Env-EGFP fusion protein indicates replication of VSV-G pseudotyped NL4-3 ΔE-EGFP (Zhang et al., 2004) in urinary podocytes.
Urinary podocytes do not support HIV-1 replication
Recently, viable podocytes have been isolated from human (Sakairi et al., 2009; Vogelmann et al., 2003) and rat (Petermann et al., 2003; Yu et al., 2005) urines. Interestingly, human urinary podocytes may potentially represent a novel model of renal podocyte diseases that can be established without need for invasive renal biopsy. We have thus investigated whether urinary podocytes from normal donors support HIV-1 replication. The cells were isolated from the urinary pellets and cultured for about 4 weeks to allow for differentiation and expression of markers characteristic for differentiated podocytes. Urinary cells cultured on collagen I coated dishes showed morphology typical for differentiated podocytes (Fig. 7A). Using real-time RT-PCR, we have detected expression of several podocyte-specific genes (Fig. 7B), however the expression of podocin mRNA was undetectable (Sakairi et al., 2009). Expression of aquaporin-1 (AQP1), AQP2, epithelial sodium channel β (ENACβ) and von Willebrand factor (VWF) mRNAs were not detected, confirming that these cells were not detectably contaminated with proximal tubular cells, collecting duct or endothelial cells. Immunofluorescent staining of vimentin, podocalyxin, synaptopodin, CD35, and WT-1 (not shown) further verified that urinary cells had characteristics of differentiated podocytes.
As observed with glomerular AB 8/13 podocytes, urinary podocytes infected with HIV-1 AD8 showed only a transient accumulation of HIV-1 ERT, LRT and Gag p24 (Fig. 7C,D). This suggests that HIV-1 infection was non-productive and detected HIV-1 DNA and p24 were delivered to urinary podocytes by internalized virions. To rule out the possibility that cultured urinary podocytes do not support virus replication for reasons other than lack of HIV-1 entry receptors (Fig. 6C), the cells were infected with VSV-G pseudotyped NL4-3 ΔEnv-EGFP (Zhang et al., 2004). Results show that HIV-1 pseudotyped with VSV-G envelope, which bypasses HIV-1 entry receptors, replicates in urinary podocytes, as demonstrated by the accumulation of truncated Env-EGFP fusion protein (Fig. 7E).
Discussion
The mechanism of HIV-1 entry and podocyte infection is critical to HIVAN pathogenesis and for the development of novel therapies preventing infection or damage of podocytes. Although existing data suggest the possibility of infection of podocytes by HIV-1 in vivo, there is no data from in vitro studies that could confirm these observations and provide insights on the mechanism of podocyte infection. Therefore, in this study we investigated whether podocytes in vitro can be productively infected with wild-type HIV-1. We have demonstrated that podocytes efficiently internalize fluorescently labeled HIV-1 particles through a dynamin-dependent process independent of virus tropism. It is likely that to internalize HIV-1, podocytes use an existing and highly efficient endocytic activity (Eyre et al., 2007; Ina et al., 2002; Rastaldi et al., 2006) that normally plays an essential role in podocyte physiology and maintenance of the healthy glomerular filter (Akilesh et al., 2008). Further, we have shown that HIV-1 internalization requires intact virion surface proteins, suggesting that HIV-1 internalization is mediated by the interaction of virus with a specific receptor expressed by podocytes. Indeed, while our manuscript was under review, a report addressing the replication of HIV-1 in podocytes was published (Mikulak et al.). The authors confirmed our observations that HIV-1 enters podocytes by endocytosis without establishing a productive infection. Furthermore, the authors demonstrated that HIV-1 internalization was mediated by DC-SIGN, a receptor that functions as an attachment factor of HIV-1 and facilitates dendritic cell-mediated viral transmission (Geijtenbeek et al., 2000a; Geijtenbeek et al., 2000b).
To investigate whether internalization of the virus leads to productive infection of podocytes, we performed a time-course analysis of cellular accumulation of viral nucleic acids and proteins. We have shown that RT inhibitors had no effect on the levels of ERT and LRT in podocytes, indicating that it is unlikely that these transcripts were synthesized in infected podocytes. Indeed, we have detected ERT and LRT in viral particles, suggesting that HIV-1 DNA could be delivered by endocytosed particles. Detection of several viral DNA species inside HIV-1 virions (Houzet, Morichaud, and Mougel, 2007; Houzet et al., 2007) further supports this conclusion. In addition, HIV-1 virions were shown to accumulate also singly and fully spliced mRNAs coding for Tat, Rev and Nef (Houzet, Morichaud, and Mougel, 2007; Houzet et al., 2007; Liang et al., 2004; Pasternak et al., 2008). Indeed, we have detected Nef2 mRNA in AD8 virions and in infected cells. However, in contrast to podocytes productively infected with VSV-G pseudotyped HIV-1, Nef2 mRNA quickly disappeared from cells infected with HIV-1 expressing HXB2 envelope.
Immunoblot analysis of HIV-1 Gag in cell lysates and culture supernatants from podocytes infected with wild-type HIV-1 indicates that a transient accumulation of Gag resulted from endocytic uptake of the virus that was subsequently released into the culture medium. Moreover, the virus released into the extracellular milieu was fully infectious, suggesting that podocytes in vivo may transiently accumulate HIV-1 and disseminate it to susceptible cells (Hatsukari et al., 2007; Mikulak et al., 2009).
To expand our observations beyond conditionally immortalized glomerular podocytes, we have also examined HIV-1 infection of primary podocytes isolated from the urines of normal donors. Recently, viable podocytes have been isolated from human (Sakairi et al., 2009; Vogelmann et al., 2003) and rat (Petermann et al., 2003; Yu et al., 2005) urines. Urinary podocytes show originally a dedifferentiated phenotype and express very few markers typical for podocytes. However, after about 3–4 weeks in culture, podocytes expressed the majority of protein and mRNA markers typical for differentiated podocytes. As reported earlier, podocin mRNA was usually undetectable in urinary podocytes (Sakairi et al., 2009). Using this model, we have confirmed that urinary podocytes did not support HIV-1 replication, although the cells were susceptible to infection with VSV-G pseudotyped HIV-1.
Several reports (Husain et al., 2005; Husain et al., 2002; Lu et al., 2008; Sunamoto et al., 2003; Zuo et al., 2006) convincingly showed that expression of viral proteins Nef or Vpr in podocytes leads to podocyte injury, supporting a view that expression of viral proteins is crucial for podocyte injury. Although these approaches are informative, expression of viral proteins in podocytes infected with viruses pseudotyped with VSV-G or transfected with HIV-1 constructs may lead to overexpression of viral proteins and non-specific effects.
To reconcile our in vitro results, obtained using two different sources of podocytes, with observations in vivo suggesting productive infection of podocytes, we hypothesize the existence of mechanism(s) in vivo that could overcome the lack of HIV-1 entry receptors in podocytes and help to establish productive infection. One of the possible mechanisms is transfer of HIV-1 receptors via exosomes secreted by CD4-positive cells. In the present study, we have shown that expression of CD4 and CXCR4 in podocytes confers susceptibility to HIV-1 infection. Our previous results demonstrate that cells expressing CD4 and CXCR4 incorporate these receptors into secreted exosomes (Khatua et al., 2009). Similarly, transfer of CCR5 (Mack et al., 2000) or CXCR4 (Rozmyslowicz et al., 2003) by microvesicles to CCR5- or CXCR4-deficient cells, respectively, was shown to allow for virus replication in these cells. Another possibility is that viral proteins that damage podocytes may be transferred to podocytes by exosomes released from infected cells. Indeed, accumulation of functional Nef in exosomes released from infected T cells has been documented (Ali et al.; Lenassi et al.; Muratori et al., 2009). This suggests that limited replication of HIV-1 in podocytes combined with toxic effects of exosomes carrying Nef could produce extensive damage to podocytes. Thus, it is conceivable that exosomes may play an essential role in infection of podocytes by HIV-1 in vivo and may provide new insights into the pathogenesis of HIVAN.
Acknowledgments
This work was supported by NIH grants from NCRR (U54RR019192), NIAID (P30AI054999), NIDDK (R21 DK089980) and in part by the Vanderbilt CTSA grant (UL1RR024975) from NCRR.
We thank Dr. Moin A. Saleem for providing human AB8/13 podocytes. Antibodies against p24 and Nef, expression vectors for CD4, CXCR4 and NL4-3-ΔE-EGFP, AZT and 3TC were obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. Confocal microscopy experiments were performed in the MMC Morphology Core. We thank Mr. Jared Elzey for his assistance in manuscript editing and preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akilesh S, Huber TB, Wu H, Wang G, Hartleben B, Kopp JB, Miner JH, Roopenian DC, Unanue ER, Shaw AS. Podocytes use FcRn to clear IgG from the glomerular basement membrane. Proc Natl Acad Sci U S A. 2008;105(3):967–72. doi: 10.1073/pnas.0711515105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali SA, Huang MB, Campbell PE, Roth WW, Campbell T, Khan M, Newman G, Villinger F, Powell MD, Bond VC. Genetic characterization of HIV type 1 Nef-induced vesicle secretion. AIDS Res Hum Retroviruses. 26(2):173–92. doi: 10.1089/aid.2009.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atta MG, Deray G, Lucas GM. Antiretroviral nephrotoxicities. Semin Nephrol. 2008;28(6):563–75. doi: 10.1016/j.semnephrol.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Bruggeman LA, Ross MD, Tanji N, Cara A, Dikman S, Gordon RE, Burns GC, D’Agati VD, Winston JA, Klotman ME, Klotman PE. Renal epithelium is a previously unrecognized site of HIV-1 infection. J Am Soc Nephrol. 2000;11(11):2079–87. doi: 10.1681/ASN.V11112079. [DOI] [PubMed] [Google Scholar]
- Campbell EM, Perez O, Melar M, Hope TJ. Labeling HIV-1 virions with two fluorescent proteins allows identification of virions that have productively entered the target cell. Virology. 2007;360(2):286–93. doi: 10.1016/j.virol.2006.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezzutti CS, Guenthner PC, Cummins JE, Jr, Cabrera T, Marshall JH, Dillberger A, Lal RB. Cervical and prostate primary epithelial cells are not productively infected but sequester human immunodeficiency virus type 1. J Infect Dis. 2001;183(8):1204–13. doi: 10.1086/319676. [DOI] [PubMed] [Google Scholar]
- Dowling D, Nasr-Esfahani S, Tan CH, O’Brien K, Howard JL, Jans DA, Purcell DF, Stoltzfus CM, Sonza S. HIV-1 infection induces changes in expression of cellular splicing factors that regulate alternative viral splicing and virus production in macrophages. Retrovirology. 2008;5:18. doi: 10.1186/1742-4690-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitner F, Cui Y, Hudkins KL, Stokes MB, Segerer S, Mack M, Lewis PL, Abraham AA, Schlondorff D, Gallo G, Kimmel PL, Alpers CE. Chemokine receptor CCR5 and CXCR4 expression in HIV-associated kidney disease. J Am Soc Nephrol. 2000;11(5):856–67. doi: 10.1681/ASN.V115856. [DOI] [PubMed] [Google Scholar]
- Eyre J, Ioannou K, Grubb BD, Saleem MA, Mathieson PW, Brunskill NJ, Christensen EI, Topham PS. Statin-sensitive endocytosis of albumin by glomerular podocytes. Am J Physiol Renal Physiol. 2007;292(2):F674–81. doi: 10.1152/ajprenal.00272.2006. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, Cornelissen IL, Nottet HS, KewalRamani VN, Littman DR, Figdor CG, van Kooyk Y. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000a;100(5):587–97. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, Torensma R, van Vliet SJ, van Duijnhoven GC, Adema GJ, van Kooyk Y, Figdor CG. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000b;100(5):575–85. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- Hatsukari I, Singh P, Hitosugi N, Messmer D, Valderrama E, Teichberg S, Chaung W, Gross E, Schmidtmayerova H, Singhal PC. DEC-205-mediated internalization of HIV-1 results in the establishment of silent infection in renal tubular cells. J Am Soc Nephrol. 2007;18(3):780–7. doi: 10.1681/ASN.2006121307. [DOI] [PubMed] [Google Scholar]
- He JC, Husain M, Sunamoto M, D’Agati VD, Klotman ME, Iyengar R, Klotman PE. Nef stimulates proliferation of glomerular podocytes through activation of Src-dependent Stat3 and MAPK1,2 pathways. J Clin Invest. 2004;114(5):643–51. doi: 10.1172/JCI21004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houzet L, Morichaud Z, Mougel M. Fully-spliced HIV-1 RNAs are reverse transcribed with similar efficiencies as the genomic RNA in virions and cells, but more efficiently in AZT-treated cells. Retrovirology. 2007;4:30. doi: 10.1186/1742-4690-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houzet L, Paillart JC, Smagulova F, Maurel S, Morichaud Z, Marquet R, Mougel M. HIV controls the selective packaging of genomic, spliced viral and cellular RNAs into virions through different mechanisms. Nucleic Acids Res. 2007;35(8):2695–704. doi: 10.1093/nar/gkm153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber TB, Reinhardt HC, Exner M, Burger JA, Kerjaschki D, Saleem MA, Pavenstadt H. Expression of functional CCR and CXCR chemokine receptors in podocytes. J Immunol. 2002;168(12):6244–52. doi: 10.4049/jimmunol.168.12.6244. [DOI] [PubMed] [Google Scholar]
- Husain M, D’Agati VD, He JC, Klotman ME, Klotman PE. HIV-1 Nef induces dedifferentiation of podocytes in vivo: a characteristic feature of HIVAN. AIDS. 2005;19(17):1975–80. doi: 10.1097/01.aids.0000191918.42110.27. [DOI] [PubMed] [Google Scholar]
- Husain M, Gusella GL, Klotman ME, Gelman IH, Ross MD, Schwartz EJ, Cara A, Klotman PE. HIV-1 Nef induces proliferation and anchorage-independent growth in podocytes. J Am Soc Nephrol. 2002;13(7):1806–15. doi: 10.1097/01.asn.0000019642.55998.69. [DOI] [PubMed] [Google Scholar]
- Ina K, Kitamura H, Tatsukawa S, Takayama T, Fujikura Y. Glomerular podocyte endocytosis of the diabetic rat. J Electron Microsc (Tokyo) 2002;51(4):275–9. doi: 10.1093/jmicro/51.4.275. [DOI] [PubMed] [Google Scholar]
- Khatua AK, Taylor HE, Hildreth JE, Popik W. Exosomes packaging APOBEC3G confer human immunodeficiency virus resistance to recipient cells. J Virol. 2009;83(2):512–21. doi: 10.1128/JVI.01658-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenassi M, Cagney G, Liao M, Vaupotic T, Bartholomeeusen K, Cheng Y, Krogan NJ, Plemenitas A, Peterlin BM. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic. 11(1):110–22. doi: 10.1111/j.1600-0854.2009.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C, Hu J, Russell RS, Kameoka M, Wainberg MA. Spliced human immunodeficiency virus type 1 RNA is reverse transcribed into cDNA within infected cells. AIDS Res Hum Retroviruses. 2004;20(2):203–11. doi: 10.1089/088922204773004923. [DOI] [PubMed] [Google Scholar]
- Liu NQ, Lossinsky AS, Popik W, Li X, Gujuluva C, Kriederman B, Roberts J, Pushkarsky T, Bukrinsky M, Witte M, Weinand M, Fiala M. Human immunodeficiency virus type 1 enters brain microvascular endothelia by macropinocytosis dependent on lipid rafts and the mitogen-activated protein kinase signaling pathway. J Virol. 2002;76(13):6689–700. doi: 10.1128/JVI.76.13.6689-6700.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu TC, He JC, Klotman PE. Podocytes in HIV-associated nephropathy. Nephron Clin Pract. 2007;106(2):c67–71. doi: 10.1159/000101800. [DOI] [PubMed] [Google Scholar]
- Lu TC, He JC, Wang ZH, Feng X, Fukumi-Tominaga T, Chen N, Xu J, Iyengar R, Klotman PE. HIV-1 Nef disrupts the podocyte actin cytoskeleton by interacting with diaphanous interacting protein. J Biol Chem. 2008;283(13):8173–82. doi: 10.1074/jbc.M708920200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10(6):839–50. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Mack M, Kleinschmidt A, Bruhl H, Klier C, Nelson PJ, Cihak J, Plachy J, Stangassinger M, Erfle V, Schlondorff D. Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: a mechanism for cellular human immunodeficiency virus 1 infection. Nat Med. 2000;6(7):769–75. doi: 10.1038/77498. [DOI] [PubMed] [Google Scholar]
- Marras D, Bruggeman LA, Gao F, Tanji N, Mansukhani MM, Cara A, Ross MD, Gusella GL, Benson G, D’Agati VD, Hahn BH, Klotman ME, Klotman PE. Replication and compartmentalization of HIV-1 in kidney epithelium of patients with HIV-associated nephropathy. Nat Med. 2002;8(5):522–6. doi: 10.1038/nm0502-522. [DOI] [PubMed] [Google Scholar]
- Mbisa JL, Barr R, Thomas JA, Vandegraaff N, Dorweiler IJ, Svarovskaia ES, Brown WL, Mansky LM, Gorelick RJ, Harris RS, Engelman A, Pathak VK. Human immunodeficiency virus type 1 cDNAs produced in the presence of APOBEC3G exhibit defects in plus-strand DNA transfer and integration. J Virol. 2007;81(13):7099–110. doi: 10.1128/JVI.00272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikulak J, Teichberg S, Arora S, Kumar D, Yadav A, Salhan D, Pullagura S, Mathieson PW, Saleem MA, Singhal PC. DC-specific ICAM-3-Grabbing Nonintegrin Mediates Internalization of HIV-1 into Human Podocytes. Am J Physiol Renal Physiol. doi: 10.1152/ajprenal.00629.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikulak J, Teichberg S, Faust T, Schmidtmayerova H, Singhal PC. HIV-1 harboring renal tubular epithelial cell interaction with T cells results in T cell trans-infection. Virology. 2009;385(1):105–14. doi: 10.1016/j.virol.2008.11.029. [DOI] [PubMed] [Google Scholar]
- Miyauchi K, Kim Y, Latinovic O, Morozov V, Melikyan GB. HIV enters cells via endocytosis and dynamin-dependent fusion with endosomes. Cell. 2009;137(3):433–44. doi: 10.1016/j.cell.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muratori C, Cavallin LE, Kratzel K, Tinari A, De Milito A, Fais S, D’Aloja P, Federico M, Vullo V, Fomina A, Mesri EA, Superti F, Baur AS. Massive secretion by T cells is caused by HIV Nef in infected cells and by Nef transfer to bystander cells. Cell Host Microbe. 2009;6(3):218–30. doi: 10.1016/j.chom.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Onafuwa-Nuga AA, Telesnitsky A, King SR. 7SL RNA, but not the 54-kd signal recognition particle protein, is an abundant component of both infectious HIV-1 and minimal virus-like particles. RNA. 2006;12(4):542–6. doi: 10.1261/rna.2306306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak AO, Adema KW, Bakker M, Jurriaans S, Berkhout B, Cornelissen M, Lukashov VV. Highly sensitive methods based on seminested real-time reverse transcription-PCR for quantitation of human immunodeficiency virus type 1 unspliced and multiply spliced RNA and proviral DNA. J Clin Microbiol. 2008;46(7):2206–11. doi: 10.1128/JCM.00055-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petermann AT, Krofft R, Blonski M, Hiromura K, Vaughn M, Pichler R, Griffin S, Wada T, Pippin J, Durvasula R, Shankland SJ. Podocytes that detach in experimental membranous nephropathy are viable. Kidney Int. 2003;64(4):1222–31. doi: 10.1046/j.1523-1755.2003.00217.x. [DOI] [PubMed] [Google Scholar]
- Popik W, Pitha PM. Inhibition of CD3/CD28-mediated activation of the MEK/ERK signaling pathway represses replication of X4 but not R5 human immunodeficiency virus type 1 in peripheral blood CD4(+) T lymphocytes. J Virol. 2000;74(6):2558–66. doi: 10.1128/jvi.74.6.2558-2566.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmoune H, Thompson PW, Ward JM, Smith CD, Hong G, Brown J. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes. 2005;54(12):3427–34. doi: 10.2337/diabetes.54.12.3427. [DOI] [PubMed] [Google Scholar]
- Rastaldi MP, Armelloni S, Berra S, Calvaresi N, Corbelli A, Giardino LA, Li M, Wang GQ, Fornasieri A, Villa A, Heikkila E, Soliymani R, Boucherot A, Cohen CD, Kretzler M, Nitsche A, Ripamonti M, Malgaroli A, Pesaresi M, Forloni GL, Schlondorff D, Holthofer H, D’Amico G. Glomerular podocytes contain neuron-like functional synaptic vesicles. FASEB J. 2006;20(7):976–8. doi: 10.1096/fj.05-4962fje. [DOI] [PubMed] [Google Scholar]
- Rosenstiel P, Gharavi A, D’Agati V, Klotman P. Transgenic and infectious animal models of HIV-associated nephropathy. J Am Soc Nephrol. 2009;20(11):2296–304. doi: 10.1681/ASN.2008121230. [DOI] [PubMed] [Google Scholar]
- Rossio JL, Esser MT, Suryanarayana K, Schneider DK, Bess JW, Jr, Vasquez GM, Wiltrout TA, Chertova E, Grimes MK, Sattentau Q, Arthur LO, Henderson LE, Lifson JD. Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J Virol. 1998;72(10):7992–8001. doi: 10.1128/jvi.72.10.7992-8001.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozmyslowicz T, Majka M, Kijowski J, Murphy SL, Conover DO, Poncz M, Ratajczak J, Gaulton GN, Ratajczak MZ. Platelet- and megakaryocyte-derived microparticles transfer CXCR4 receptor to CXCR4-null cells and make them susceptible to infection by X4-HIV. AIDS. 2003;17(1):33–42. doi: 10.1097/00002030-200301030-00006. [DOI] [PubMed] [Google Scholar]
- Sakairi T, Abe Y, Kajiyama H, Bartlett LD, Howard LV, Jat PS, Kopp JB. Conditionally immortalized human podocyte cell lines established from urine. Am J Physiol Renal Physiol. 2009 doi: 10.1152/ajprenal.00509.2009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Saleem MA, O’Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol. 2002;13(3):630–8. doi: 10.1681/ASN.V133630. [DOI] [PubMed] [Google Scholar]
- Schmid H, Henger A, Cohen CD, Frach K, Grone HJ, Schlondorff D, Kretzler M. Gene expression profiles of podocyte-associated molecules as diagnostic markers in acquired proteinuric diseases. J Am Soc Nephrol. 2003;14(11):2958–66. doi: 10.1097/01.asn.0000090745.85482.06. [DOI] [PubMed] [Google Scholar]
- Sunamoto M, Husain M, He JC, Schwartz EJ, Klotman PE. Critical role for Nef in HIV-1-induced podocyte dedifferentiation. Kidney Int. 2003;64(5):1695–701. doi: 10.1046/j.1523-1755.2003.00283.x. [DOI] [PubMed] [Google Scholar]
- Vacharaksa A, Asrani AC, Gebhard KH, Fasching CE, Giacaman RA, Janoff EN, Ross KF, Herzberg MC. Oral keratinocytes support non-replicative infection and transfer of harbored HIV-1 to permissive cells. Retrovirology. 2008;5:66. doi: 10.1186/1742-4690-5-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidricaire G, Tremblay MJ. A clathrin, caveolae, and dynamin-independent endocytic pathway requiring free membrane cholesterol drives HIV-1 internalization and infection in polarized trophoblastic cells. J Mol Biol. 2007;368(5):1267–83. doi: 10.1016/j.jmb.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Vogelmann SU, Nelson WJ, Myers BD, Lemley KV. Urinary excretion of viable podocytes in health and renal disease. Am J Physiol Renal Physiol. 2003;285(1):F40–8. doi: 10.1152/ajprenal.00404.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston JA, Bruggeman LA, Ross MD, Jacobson J, Ross L, D’Agati VD, Klotman PE, Klotman ME. Nephropathy and establishment of a renal reservoir of HIV type 1 during primary infection. N Engl J Med. 2001;344(26):1979–84. doi: 10.1056/NEJM200106283442604. [DOI] [PubMed] [Google Scholar]
- Yang Y, Gubler MC, Beaufils H. Dysregulation of podocyte phenotype in idiopathic collapsing glomerulopathy and HIV-associated nephropathy. Nephron. 2002;91(3):416–23. doi: 10.1159/000064281. [DOI] [PubMed] [Google Scholar]
- Yu D, Petermann A, Kunter U, Rong S, Shankland SJ, Floege J. Urinary podocyte loss is a more specific marker of ongoing glomerular damage than proteinuria. J Am Soc Nephrol. 2005;16(6):1733–41. doi: 10.1681/ASN.2005020159. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhou Y, Alcock C, Kiefer T, Monie D, Siliciano J, Li Q, Pham P, Cofrancesco J, Persaud D, Siliciano RF. Novel single-cell-level phenotypic assay for residual drug susceptibility and reduced replication capacity of drug-resistant human immunodeficiency virus type 1. J Virol. 2004;78(4):1718–29. doi: 10.1128/JVI.78.4.1718-1729.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y, Matsusaka T, Zhong J, Ma J, Ma LJ, Hanna Z, Jolicoeur P, Fogo AB, Ichikawa I. HIV-1 genes vpr and nef synergistically damage podocytes, leading to glomerulosclerosis. J Am Soc Nephrol. 2006;17(10):2832–43. doi: 10.1681/ASN.2005080878. [DOI] [PubMed] [Google Scholar]