Abstract
Pharmacologic or genetic blockade of metabotropic glutamate mGlu5 receptors (mGluR5) has been shown to attenuate parkinsonian motor deficits and protect nigrostriatal neurons from damage in the acute MPTP model of Parkinson’s disease (PD), suggesting that therapeutically targeting the mGluR5 receptor may offer a novel approach to improving motor symptoms and/or slowing neurodegeneration in PD. This study further explored the neuroprotective potential of targeting mGluR5 receptors. We examined the behavioral and neurochemical effects of receptor elimination on toxicity induced by intra-striatal application of 6-hydroxydopamine (6-OHDA), thought to represent a comparatively progressive model of PD. mGluR5 knockout (KO) mice and wild-type (WT) littermates received unilateral 6-OHDA infusions. Reflecting the imbalance expected following unilateral infusion, WT but not KO mice demonstrated predominantly ipsilateral forepaw use and robust ipsilateral amphetamine-induced rotation. Further, performance on the vertical pole descent task was profoundly impaired in WT mice, while KO mice completed the task significantly faster. Consistent with the behavioral observations, neurochemical analyses of striatal dopamine depletion showed significantly diminished severity in KO mice with only 64% of striatal dopamine lost, compared to 92% in WT mice. The absence of brain mGluR5 receptors in living KO mice was verified using positron emission tomography (PET). Our findings substantiate the key role of mGluR5 receptors in animal models of PD, strengthening the rationale for the development of mGluR5 antagonists for their neuroprotective, as well as symptomatic, benefit.
Keywords: Neuroprotection, mGluR5, dopamine level, behavioral tests, Parkinson’s disease
Parkinson’s disease (PD) patients have markedly reduced levels of striatal dopamine (DA) due to the progressive degeneration of substantia nigra pars compacta (SNpc) neurons, resulting eventually in debilitating motor dysfunction. Although partial relief of motor symptoms can be achieved primarily with ‘DA replacement’ therapies, no approach has been established as a means to alter the underlying neurodegenerative process. Therefore, investigations to identify new treatment strategies that may slow the progression of PD continue to be a priority.
A potentially efficient approach to pursuing protective drug candidates is to target non-dopaminergic transmitter systems that may impact both degenerative and motor pathophysiology of the basal ganglia (for example, [5, 9, 11, 13, 33]). Of interest due to its interaction with the dopaminergic system, metabotropic glutamate mGluR5 receptors are located on striatal neurons [24, 34, 36] and modulate excitatory glutamatergic transmission (for reviews, see [8, 26]), thereby counterbalancing dopaminergic input from the SNpc. The progressive loss of substantia nigra dopaminergic neurons in PD may result in the overstimulation of glutamatergic receptors in striatal output pathways, contributing to PD pathophysiology. Blockade of mGluR5 function with antagonist drugs such as 2-methyl-6-(phenylethynyl)pyridine (MPEP) can attenuate parkinsonian motor deficits [1, 9, 18] and decrease the abnormal involuntary movements (dyskinesias) that develop following chronic levodopa treatment in animal models of PD [14, 17, 20, 29].
Blockade of mGluR5 may also protect against neurodegeneration. Research utilizing in vivo and in vitro models of brain trauma, excitotoxicity, and stroke has indicated that mGluR5 antagonists can be neuroprotective [3, 21, 27]. Further, in animal models of PD, mGluR5 antagonists lessen nigrostriatal degeneration [2, 4, 37, 38]. In addition, Battaglia and colleagues (2004) showed that elimination of the mGluR5 receptor reduced nigrostriatal neuron damage following exposure to MPTP, a toxin that causes an acute, non-progressive loss of DA neurons [4].
The present investigation further explores the protective potential of mGluR5 elimination using a relatively progressive model of PD neurodegeneration, in which striatal 6-OHDA infusion leads to the gradual loss of nigrostriatal DA neurons ([32] for review; [6, 23, 28, 31]). This toxin model allowed us to compare behavioral and neurochemical profiles of mGluR5 receptor knockout (KO) and wild-type (WT) mice over a period of 4 weeks, during which time the retrograde loss of dopaminergic cells in the substantia nigra evolves more slowly than in MPTP models of PD ([32] for review), and thus may be more representative of the degeneration that occurs in humans. We tested the hypothesis that mice lacking the mGluR5 are more resistant to toxin-induced damage, as assessed by DA depletion and motor impairment following intrastriatal 6-OHDA lesioning.
All experiments were performed in accordance with NIH guidelines on the ethical use of animals. As previously described [18], mGluR5 heterozygote KO (+/−) mice were obtained from the Jackson Laboratory (Bar Harbor, ME; B6.129-Grm5tm1Rod/J, stock #003558), back-crossed until in an incipient (N7) congenic C57BL/6 genetic background, and crossed to generate the PCR-genotyped mGluR5 KO, WT, and heterozygote (HZ) littermates used in this study. Male mice from 4–10 months of age were maintained in home cages under a 12h light/dark cycle, with ad libitum access to food and water. Twenty-three mice (12WT/11KO) received infusions of 10μg of freshly prepared 6-OHDA bromide salt into the left striatum at the following coordinates (mm from bregma: 0.5 A, 2.0 L, 2.8 V) as was previously described [15, 40]. HZ littermates were used as unlesioned controls for normal behavioral activity given our prior demonstration that unlesioned homozygous mGluR5 KO mice display normal baseline behaviors indistinguishable from WT littermates (e.g., in open field locomotor testing)[18].
Fourteen days after lesioning surgery, vertical pole and beam traversal tasks were conducted to assess motor coordination and balance. Forepaw asymmetry and amphetamine-induced rotation tests were conducted to assess the extent of motor asymmetry (for review, see [10]) in WT and mGluR5 KO mice compared to unlesioned HZ mice. The asymmetry tasks were conducted at the beginning and end of a 14-day period that began 14 days post-lesion, with both motor coordination assessment tasks performed in the middle of this period. First, we evaluated forepaw asymmetry (10 WT/8 KO), defined as predominate use of the forepaw ipsilateral to the left striatal lesion while rearing. Individual mice were placed in a translucent cylinder and the number of ipsilateral, contralateral, and bilateral (symmetric) forepaw placements were counted for 5 minutes by an experimenter blind to mouse genotype. The number of ipsilateral forepaw placements were divided by the total number of placements and multiplied by 100 to obtain a percentage ipsilateral forepaw preference.
Next, to determine the extent of motor impairment using the vertical pole descent task, individual mice (10 WT and 8 KO lesioned mice, and 4 HZ unlesioned mice) were placed facing upwards along the top of a 50 cm vertical wooden pole (1 cm diameter). Total descent time, comprised of the time to orient downwards plus the time to descend the pole until all 4 paws were on the support block, was measured. Five consecutive trials were run in 1 day. During the beam traversal task, these mice were placed on 1 m long wooden beam that was divided into 4 sections of widths 3.5, 2.5, 1.5, and 0.5 cm. The beam was supported 50 cm above the table surface. The total time to traverse the beam, as well as slips, falls, and experimenter-facilitated restarts, were recorded during 4 consecutive trials in 1 day.
In a final behavioral assessment, to further quantify asymmetry, rotational response of mice (10 WT/8 KO) to DA agonist amphetamine (2.5 mg/kg i.p.) was measured. Contralateral and ipsilateral rotations were recorded for 60 minutes as previously described [40].
Four weeks after unilateral 6-OHDA lesioning and 24-hr after the last behavioral experiment (amphetamine-induced rotation), all mice were euthanized striata were quickly dissected out for analysis of DA and its metabolite DOPAC by high-performance liquid chromatography (HPLC) coupled to electrochemical detection [12].
Using PET to verify the functional depletion of mGluR5 receptors in KO mice, a separate set of KO and WT mice were imaged in vivo with a selective and sensitive mGluR5 antagonist, 3-[18F]Fluoro-5-(2-pyridinylethynyl)benzonitrile ([18F]FPEB), a highly specific PET tracer for mGluR5 [30, 39]. An additional imaging session was also carried out using [11C]CFT ([11C]2-β-carbomethoxy-3-β-(4-fluorophenyl)tropane), a sensitive and selective DA transporter (DAT) ligand, to investigate dopaminergic denervation. A total of 9 mice (4 WT/5 KO) were imaged with [18F]FPEB and 4 mice (2 WT/2 KO) were imaged with [11C]CFT. Imaging studies were performed using a microPET P4 tomograph (Concord Microsystems, Knoxville, TN) as previously described [25]. After imaging was completed, mice were sacrificed, brains were removed and stored at −80 °C. Genotype was confirmed by the presence of appropriately sized mGluR5 immunoreactivity in tissue using western blot analysis (data not shown).
Data are expressed as mean ± SEM. Statistical analyses were conducted using two-way ANOVA and Student’s t test where appropriate. Statistical significance was considered reached when p<0.05. Bonferroni/Dunn tests were used for post-hoc analyses.
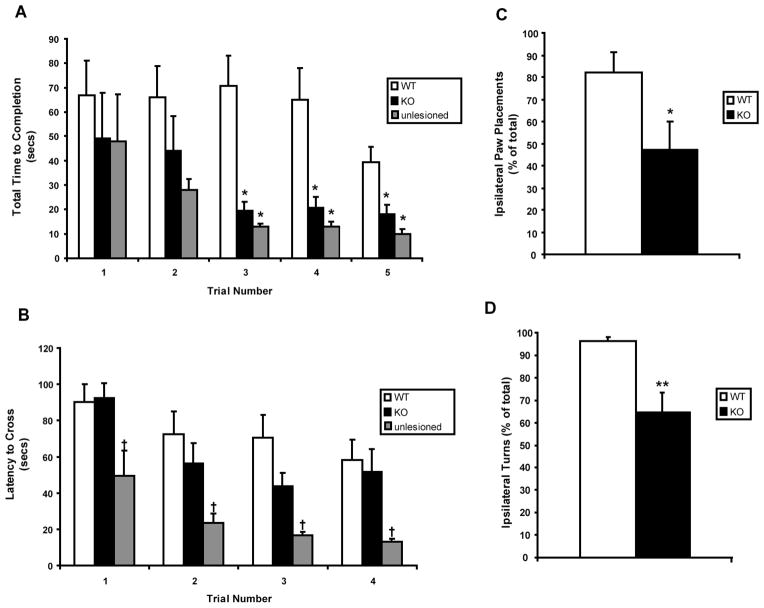
6-OHDA-induced motor impairment was attenuated by mGluR5 receptor depletion. The lesioned WT mice used significantly longer time (turn time + descent time) to complete the vertical pole descent task across all 5 trials than lesioned KO or unlesioned mice (F2,17=6.15, p=0.01; Fig. 1A). Closer observation revealed that all groups took a similar amount of time to orient downwards on the pole, but lesioned WT mice were significantly slower descending the pole (F2,17=6.87, p=.007). Overall, there were no differences between the performance of lesioned KO mice and unlesioned mice on this task (Fig. 1A). On the beam traversal task, lesioned mGluR5 KO and WT mice crossed the beam slower than unlesioned mice (F2,19=4.28, p=0.03; Fig. 1B). Although, lesioned KO mice tended to cross faster compared to WT mice during intermediate trials, no significant difference was appreciated. However, all groups decreased their latency to cross the beam across trials (F3,57=16.38, p<0.0001) and exhibited equivalent numbers of slips, falls, and restarts during the task (data not shown).
Figure 1.
Behavioral analyses of motor impairment and asymmetry following unilateral 6-OHDA lesions of the striatum in WT and mGluR5 KO mice. Values represent the mean (±SEM) for tests of motor impairment: vertical pole descent (A) and beam traversal task (B); and motor asymmetry: cylinder test (C) and amphetamine-induced rotation test (D) in lesioned WT/KO and unlesioned control mice. P values denoting different from lesioned WT mice: † ≤0.05, * ≤0.05, ** ≤0.005.
As expected after unilateral lesions, WT mice demonstrated profound asymmetry when rearing and making forepaw contact with the cylinder wall. Unilaterally lesioned WT mice used only the ipsilateral forepaw on 82 ±9% of cylinder wall touches (Fig. 1C; total touch rate of 6.5 ±1.1 per 5 min), with the remainder being bilateral forepaw touches because no contacts (0.0 ±0.0%) were made with just their contralateral forepaw. Lesioned mGluR5 KO mice demonstrated less forepaw use asymmetry, touching exclusively with the ipsilateral or contralateral forepaw on 47 ±12% or 10 ±4%, respectively, of all wall contacts. Their total touch rate was 10.3 ±3.0 contacts per 5 min, which was not significantly greater than for their lesioned WT littermates. Likewise, amphetamine induced a greater ipsilateral turning bias (96 ±2% of 162 ±37 total turns) in lesioned WT mice, in contrast to a more symmetric response (65 ±9% of 94 ±25 total turns) in lesioned mGluR5 mice (Fig. 1D). Thus the asymmetry was significantly greater in lesioned WT compared to mGluR5 KO mice on both the forepaw asymmetry (t14=2.33; p=0.035, Student’s t test and p=0.0155, nonparametric Mann-Whitney test) and amphetamine rotation (t16=3.93; p=0.001) tests.
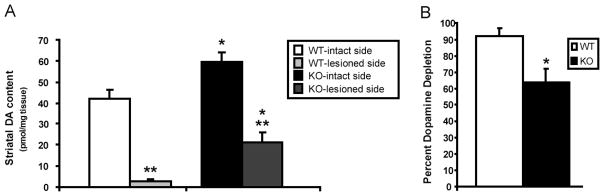
These data were substantiated by findings from post-mortem HPLC analyses of striatal DA and DOPAC. By comparing residual striatal DA/DOPAC content from the 6-OHDA-lesioned left hemisphere to the unlesioned right hemisphere within each animal, each mouse served as its own control. The results from biochemical analyses of striatal tissue indicated substantial reductions in residual DA and DOPAC content in the 6-OHDA lesioned hemisphere when compared to the unlesioned hemisphere in WT (DA: t9=−8.73, p<0.0001; DOPAC: t9=−7.48, p<0.0001) and KO (DA: t7=−7.26, p<0.0002; DOPAC: t7=−5.85, p<0.0001) mice (Fig. 2A). A higher baseline DA levels (intact side) was observed in the striata of KO mice compared to wild type, different with that in MPTP model of parkinsonism [4], which might be due to compensatory mechanism specific to 6-OHDA model. Though evidence of dopaminergic neuron denervation (DA loss) was exhibited in mice of both genotypes, lesioned WT mice had a substantially larger percent DA (and DOPAC) depletion than lesioned KO mice, 92% (and 80%) versus 64% (and 52%) respectively, suggesting attenuated DA depletion and a possible neuroprotective phenotype in mGluR5 KO mice (Fig. 2B).
Figure 2.
Neurochemical comparison of striatal dopamine content in 6-OHDA lesioned vs intact striata of WT and mGluR5 KO mice, showing attenuated dopamine depletion in mGluR5 KO. Values represent the mean (±SEM). P values denoting different from corresponding WT value: * ≤0.01; P values denoting different from intact side: ** ≤ 0.0005.
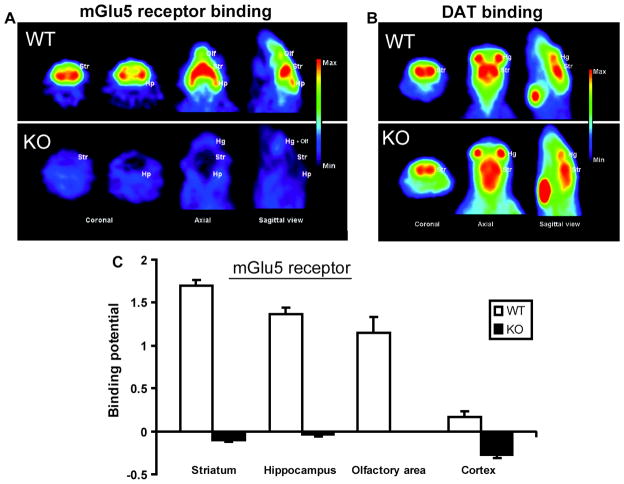
As verification of receptor function, PET imaging of a separate group of unlesioned WT and mGluR5 KO mice revealed that the accumulation of [18F]FPEB, an mGluR5 receptor ligand, is prominent in the striatum, hippocampus and olfactory bulb in WT mouse brain sections, but absent in those of mGluR5 KO mice (Fig. 3A,C). Similar high accumulation of [11C]CFT, a selective DAT ligand, is observed in the striatum of a WT and an mGluR5 KO mouse, suggesting that the CNS dopaminergic pathways are intact in the absence of the mGluR5 receptor (Fig. 3B). The binding potentials (mean ± SEM) of [18F]FPEB were calculated by a graphical analyzing method using the cerebellum as reference tissue (Fig. 3C).
Figure 3.
Distribution of (A, C) mGluR5 receptor ligand [18F]FPEB binding and (B) dopamine transporter (DAT) ligand [11C]CFT binding in WT and mGluR5 KO mouse brain. Accumulation of [18F]FPEB is prominent in the striatum (Str), hippocampus (Hp), Harderian glands (Hg) and olfactory area (Olf) in WT mouse brain sections (A, upper row), but absent in those of mGluR5 KO mice (A, lower row). Similar high accumulation of [11C]CFT in the striatum of WT and KO (B) suggests that the CNS dopaminergic projection pathways are intact in the absence of the mGluR5.
The neurochemical and behavioral findings of the present study indicate that mGluR5 receptors play a substantial facilitative role in the dopaminergic neuron dysfunction of the 6-OHDA model of PD. Our demonstration of reduced DA loss in mGluR5 KO mice in this progressive model of nigrostriatal toxicity complements previous findings of reduced dopaminergic neuron loss in SNpc in MPTP-treated mGluR5 KO mice [4]. The attenuated 6-OHDA-induced loss of striatal DA likely reflects attenuated nigrostriatal neuron degeneration per se. Protection against dopaminergic neuron loss in the SNpc of MPTP-treated mGluR5 KO mice closely correlated with protection against striatal DA depletion in these mice [4]. Similarly, mGluR5 antagonist treatment attenuated both the losses of nigral dopaminergic neurons and striatal DA induced by 6-OHDA [38]. However, the ability of mGluR5 antagonists to confer protection in neuronal cultures derived from mGluR5 KO as well as WT mice [19] highlights the limitation of non-specificity with pharmacological studies, and the utility of complementary KO studies in understanding receptor function. Thus these pharmacological and KO data together comprise a consistent body of preclinical evidence for mGluR5 receptor involvement in the pathophysiology of PD.
How the receptor contributes to dopaminergic neuron degeneration is uncertain. However because the mGluR5 receptor is expressed on SNpc dopaminergic neurons themselves [16, 35], it may facilitate their degeneration directly (for review see [22]), for example by mediating the excitotoxic influence of glutamatergic innervation from the subthalamic nucleus [2, 7].
Similarly, our behavioral findings support a neuroprotective phenotype of mGluR5 KO mice, which performed better than WT mice in the pole and motor asymmetry tests. By contrast we found no improvement of beam walk performance, a test for motor balance and coordination, in these mice. The difference may be due to a greater performance incentive in the pole test (descending to the security of pole’s home base) compared to that of our modified version of the commonly used beam walk task, (e.g. review by [10]), in which there was no home platform at the end of the beam to serve as a motivational cue. It also remains unclear whether the improved motor abnormalities observed in 6-OHDA-lesioned mGluR5 KO mice reflect a true neuroprotective phenotype (as suggested by our and others’ [4] characterization of these mice in toxin models of PD), or an antiparkinsonian ‘symptomatic’ effect of normalized basal ganglia activity [9, 18] as shown with mGluR5 antagonism in a similar 6-OHDA model [1], or both.
In conclusion, our data support roles for mGluR5 in neurodegeneration and for its blockade in neuroprotection. These findings strengthen the rationale for mGluR5 antagonism as a potential strategy to slow the underlying neurodegeneration as well as the motor dysfunction in PD.
Highlights.
Dopamine loss by 6-OHDA lesioning is attenuated by a gene knockout of the mGluR5.
Motor dysfunction in the 6-OHDA model is also reduced in these mGluR5 KO mice.
Neuroimaging demonstrated complete absence of receptor function in mGluR5 KO mice.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants NS054978 and NS060991, NS60232, DoD grant W81XWH-04-1-0881.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ambrosi G, Armentero MT, Levandis G, Bramanti P, Nappi G, Blandini F. Effects of early and delayed treatment with an mGluR5 antagonist on motor impairment, nigrostriatal damage and neuroinflammation in a rodent model of Parkinson’s disease. Brain Res Bull. 2010;82:29–38. doi: 10.1016/j.brainresbull.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 2.Armentero MT, Fancellu R, Nappi G, Bramanti P, Blandini F. Prolonged blockade of NMDA or mGluR5 glutamate receptors reduces nigrostriatal degeneration while inducing selective metabolic changes in the basal ganglia circuitry in a rodent model of Parkinson’s disease. Neurobiol Dis. 2006;22:1–9. doi: 10.1016/j.nbd.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Bao WL, Williams AJ, Faden AI, Tortella FC. Selective mGluR5 receptor antagonist or agonist provides neuroprotection in a rat model of focal cerebral ischemia. Brain Res. 2001;922:173–9. doi: 10.1016/s0006-8993(01)03062-1. [DOI] [PubMed] [Google Scholar]
- 4.Battaglia G, Busceti CL, Molinaro G, Biagioni F, Storto M, Fornai F, Nicoletti F, Bruno V. Endogenous activation of mGlu5 metabotropic glutamate receptors contributes to the development of nigro-striatal damage induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. J Neurosci. 2004;24:828–35. doi: 10.1523/JNEUROSCI.3831-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Battaglia G, Busceti CL, Molinaro G, Biagioni F, Traficante A, Nicoletti F, Bruno V. Pharmacological activation of mGlu4 metabotropic glutamate receptors reduces nigrostriatal degeneration in mice treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. J Neurosci. 2006;26:7222–9. doi: 10.1523/JNEUROSCI.1595-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger K, Przedborski S, Cadet JL. Retrograde degeneration of nigrostriatal neurons induced by intrastriatal 6-hydroxydopamine injection in rats. Brain Res Bull. 1991;26:301–7. doi: 10.1016/0361-9230(91)90242-c. [DOI] [PubMed] [Google Scholar]
- 7.Blandini F, Nappi G, Greenamyre JT. Subthalamic infusion of an NMDA antagonist prevents basal ganglia metabolic changes and nigral degeneration in a rodent model of Parkinson’s disease. Ann Neurol. 2001;49:525–9. [PubMed] [Google Scholar]
- 8.Bonsi P, Platania P, Martella G, Madeo G, Vita D, Tassone A, Bernardi G, Pisani A. Distinct roles of group I mGlu receptors in striatal function. Neuropharmacology. 2008;55:392–5. doi: 10.1016/j.neuropharm.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 9.Breysse N, Baunez C, Spooren W, Gasparini F, Amalric M. Chronic but not acute treatment with a metabotropic glutamate 5 receptor antagonist reverses the akinetic deficits in a rat model of parkinsonism. J Neurosci. 2002;22:5669–78. doi: 10.1523/JNEUROSCI.22-13-05669.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brooks SP, Dunnett SB. Tests to assess motor phenotype in mice: a user’s guide. Nat Rev Neurosci. 2009;10:519–29. doi: 10.1038/nrn2652. [DOI] [PubMed] [Google Scholar]
- 11.Chen JF. The adenosine A(2A) receptor as an attractive target for Parkinson’s disease treatment. Drug News Perspect. 2003;16:597–604. doi: 10.1358/dnp.2003.16.9.829342. [DOI] [PubMed] [Google Scholar]
- 12.Chen JF, Xu K, Petzer JP, Staal R, Xu YH, Beilstein M, Sonsalla PK, Castagnoli K, Castagnoli N, Jr, Schwarzschild MA. Neuroprotection by caffeine and A(2A) adenosine receptor inactivation in a model of Parkinson’s disease. J Neurosci. 2001;21:RC143. doi: 10.1523/JNEUROSCI.21-10-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coccurello R, Breysse N, Amalric M. Simultaneous blockade of adenosine A2A and metabotropic glutamate mGlu5 receptors increase their efficacy in reversing Parkinsonian deficits in rats. Neuropsychopharmacology. 2004;29:1451–61. doi: 10.1038/sj.npp.1300444. [DOI] [PubMed] [Google Scholar]
- 14.Dekundy A, Pietraszek M, Schaefer D, Cenci MA, Danysz W. Effects of group I metabotropic glutamate receptors blockade in experimental models of Parkinson’s disease. Brain Res Bull. 2006;69:318–26. doi: 10.1016/j.brainresbull.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Fredduzzi S, Moratalla R, Monopoli A, Cuellar B, Xu K, Ongini E, Impagnatiello F, Schwarzschild MA, Chen JF. Persistent behavioral sensitization to chronic L-DOPA requires A2A adenosine receptors. Journal of Neuroscience. 2002;22:1054–62. doi: 10.1523/JNEUROSCI.22-03-01054.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hubert GW, Paquet M, Smith Y. Differential subcellular localization of mGluR1a and mGluR5 in the rat and monkey Substantia nigra. J Neurosci. 2001;21:1838–47. doi: 10.1523/JNEUROSCI.21-06-01838.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnston TH, Fox SH, McIldowie MJ, Piggott MJ, Brotchie JM. Reduction of L-DOPA-induced dyskinesia by the selective metabotropic glutamate receptor 5 (mGlu5) antagonist MTEP in the MPTP-lesioned macaque model of Parkinson’s disease. J Pharmacol Exp Ther. 2010 doi: 10.1124/jpet.110.166629. [DOI] [PubMed] [Google Scholar]
- 18.Kachroo A, Orlando LR, Grandy DK, Chen JF, Young AB, Schwarzschild MA. Interactions between metabotropic glutamate 5 and adenosine A2A receptors in normal and parkinsonian mice. J Neurosci. 2005;25:10414–9. doi: 10.1523/JNEUROSCI.3660-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lea PMt, Movsesyan VA, Faden AI. Neuroprotective activity of the mGluR5 antagonists MPEP and MTEP against acute excitotoxicity differs and does not reflect actions at mGluR5 receptors. Br J Pharmacol. 2005;145:527–34. doi: 10.1038/sj.bjp.0706219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mela F, Marti M, Dekundy A, Danysz W, Morari M, Cenci MA. Antagonism of metabotropic glutamate receptor type 5 attenuates l-DOPA-induced dyskinesia and its molecular and neurochemical correlates in a rat model of Parkinson’s disease. J Neurochem. 2007;101:483–97. doi: 10.1111/j.1471-4159.2007.04456.x. [DOI] [PubMed] [Google Scholar]
- 21.Movsesyan VA, O’Leary DM, Fan L, Bao W, Mullins PG, Knoblach SM, Faden AI. mGluR5 antagonists 2-methyl-6-(phenylethynyl)-pyridine and (E)-2-methyl-6-(2-phenylethenyl)-pyridine reduce traumatic neuronal injury in vitro and in vivo by antagonizing N-methyl-D-aspartate receptors. J Pharmacol Exp Ther. 2001;296:41–7. [PubMed] [Google Scholar]
- 22.Obeso JA, Rodriguez-Oroz MC, Benitez-Temino B, Blesa FJ, Guridi J, Marin C, Rodriguez M. Functional organization of the basal ganglia: therapeutic implications for Parkinson’s disease. Mov Disord. 2008;23(Suppl 3):S548–59. doi: 10.1002/mds.22062. [DOI] [PubMed] [Google Scholar]
- 23.Oiwa Y, Sanchez-Pernaute R, Harvey-White J, Bankiewicz KS. Progressive and extensive dopaminergic degeneration induced by convection-enhanced delivery of 6-hydroxydopamine into the rat striatum: a novel rodent model of Parkinson disease. J Neurosurg. 2003;98:136–44. doi: 10.3171/jns.2003.98.1.0136. [DOI] [PubMed] [Google Scholar]
- 24.Ouattara B, Gasparini F, Morissette M, Gregoire L, Samadi P, Gomez-Mancilla B, Di Paolo T. Effect of L-Dopa on metabotropic glutamate receptor 5 in the brain of parkinsonian monkeys. J Neurochem. 2010;113:715–24. doi: 10.1111/j.1471-4159.2010.06635.x. [DOI] [PubMed] [Google Scholar]
- 25.Pellegrino D, Cicchetti F, Wang X, Zhu A, Yu M, Saint-Pierre M, Brownell AL. Modulation of dopaminergic and glutamatergic brain function: PET studies on parkinsonian rats. J Nucl Med. 2007;48:1147–53. doi: 10.2967/jnumed.106.037796. [DOI] [PubMed] [Google Scholar]
- 26.Pilc A, Chaki S, Nowak G, Witkin JM. Mood disorders: regulation by metabotropic glutamate receptors. Biochem Pharmacol. 2008;75:997–1006. doi: 10.1016/j.bcp.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 27.Popoli P, Pintor A, Tebano MT, Frank C, Pepponi R, Nazzicone V, Grieco R, Pezzola A, Reggio R, Minghetti L, De Berardinis MA, Martire A, Potenza RL, Domenici MR, Massotti M. Neuroprotective effects of the mGlu5R antagonist MPEP towards quinolinic acid-induced striatal toxicity: involvement of pre- and post-synaptic mechanisms and lack of direct NMDA blocking activity. J Neurochem. 2004;89:1479–89. doi: 10.1111/j.1471-4159.2004.02448.x. [DOI] [PubMed] [Google Scholar]
- 28.Przedborski S, Levivier M, Jiang H, Ferreira M, Jackson-Lewis V, Donaldson D, Togasaki DM. Dose-dependent lesions of the dopaminergic nigrostriatal pathway induced by intrastriatal injection of 6-hydroxydopamine. Neuroscience. 1995;67:631–47. doi: 10.1016/0306-4522(95)00066-r. [DOI] [PubMed] [Google Scholar]
- 29.Rylander D, Iderberg H, Li Q, Dekundy A, Zhang J, Li H, Baishen R, Danysz W, Bezard E, Cenci MA. A mGluR5 antagonist under clinical development improves L-DOPA-induced dyskinesia in parkinsonian rats and monkeys. Neurobiol Dis. 2010 doi: 10.1016/j.nbd.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez-Pernaute R, Wang JQ, Kuruppu D, Cao L, Tueckmantel W, Kozikowski A, Isacson O, Brownell AL. Enhanced binding of metabotropic glutamate receptor type 5 (mGluR5) PET tracers in the brain of parkinsonian primates. Neuroimage. 2008;42:248–51. doi: 10.1016/j.neuroimage.2008.04.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sauer H, Oertel WH. Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: a combined retrograde tracing and immunocytochemical study in the rat. Neuroscience. 1994;59:401–15. doi: 10.1016/0306-4522(94)90605-x. [DOI] [PubMed] [Google Scholar]
- 32.Schober A. Classic toxin-induced animal models of Parkinson’s disease: 6-OHDA and MPTP. Cell Tissue Res. 2004;318:215–24. doi: 10.1007/s00441-004-0938-y. [DOI] [PubMed] [Google Scholar]
- 33.Schwarzschild MA, Agnati L, Fuxe K, Chen JF, Morelli M. Targeting adenosine A2A receptors in Parkinson’s disease. Trends Neurosci. 2006;29:647–54. doi: 10.1016/j.tins.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci Lett. 1993;163:53–7. doi: 10.1016/0304-3940(93)90227-c. [DOI] [PubMed] [Google Scholar]
- 35.Smith Y, Charara A, Paquet M, Kieval JZ, Pare JF, Hanson JE, Hubert GW, Kuwajima M, Levey AI. Ionotropic and metabotropic GABA and glutamate receptors in primate basal ganglia. J Chem Neuroanat. 2001;22:13–42. doi: 10.1016/s0891-0618(01)00098-9. [DOI] [PubMed] [Google Scholar]
- 36.Testa CM, Standaert DG, Landwehrmeyer GB, Penney JB, Jr, Young AB. Differential expression of mGluR5 metabotropic glutamate receptor mRNA by rat striatal neurons. J Comp Neurol. 1995;354:241–52. doi: 10.1002/cne.903540207. [DOI] [PubMed] [Google Scholar]
- 37.Vernon AC, Palmer S, Datla KP, Zbarsky V, Croucher MJ, Dexter DT. Neuroprotective effects of metabotropic glutamate receptor ligands in a 6-hydroxydopamine rodent model of Parkinson’s disease. Eur J Neurosci. 2005;22:1799–806. doi: 10.1111/j.1460-9568.2005.04362.x. [DOI] [PubMed] [Google Scholar]
- 38.Vernon AC, Zbarsky V, Datla KP, Croucher MJ, Dexter DT. Subtype selective antagonism of substantia nigra pars compacta Group I metabotropic glutamate receptors protects the nigrostriatal system against 6-hydroxydopamine toxicity in vivo. J Neurochem. 2007;103:1075–91. doi: 10.1111/j.1471-4159.2007.04860.x. [DOI] [PubMed] [Google Scholar]
- 39.Wang JQ, Tueckmantel W, Zhu A, Pellegrino D, Brownell AL. Synthesis and preliminary biological evaluation of 3-[(18)F]fluoro-5-(2-pyridinylethynyl)benzonitrile as a PET radiotracer for imaging metabotropic glutamate receptor subtype 5. Synapse. 2007;61:951–61. doi: 10.1002/syn.20445. [DOI] [PubMed] [Google Scholar]
- 40.Xiao D, Bastia E, Xu YH, Benn CL, Cha JH, Peterson TS, Chen JF, Schwarzschild MA. Forebrain adenosine A2A receptors contribute to L-3,4-dihydroxyphenylalanine-induced dyskinesia in hemiparkinsonian mice. J Neurosci. 2006;26:13548–55. doi: 10.1523/JNEUROSCI.3554-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]