Abstract
We have developed highly efficient ethanologenic E. coli strains that selectively consume pentoses and/or hexoses. Mixed cultures of these strains can be used to selectively adjust the sugar utilization kinetics in ethanol fermentations. Based on the kinetics of sugar utilization, we have designed and implemented an immobilized cell system for the optimized continuous conversion of sugars into ethanol. The results confirm that immobilized mixed cultures support a simultaneous conversion of hexoses and pentoses into ethanol at high yield and at a faster rate than immobilized homogenous cells. Continuous ethanol production has been maintained for several weeks at high productivity with near complete sugar utilization. The control of sugar utilization using immobilized mixed cultures can be adapted to any composition of hexoses and pentoses by adjusting the strain distribution of immobilized cells. The approach, therefore, holds promise for ethanol fermentation from lignocellulosic hydrolysates where the feedstock varies in sugar composition.
Keywords: Immobilized E. coli cells, Continuous ethanol fermentation, Co-utilization of hexoses and pentoses
1. Introduction
Ethanol has emerged as an important renewable and sustainable energy source that can reduce our reliance on fossil resources. Inexpensive, abundant and renewable lignocellulosic biomass has become an attractive feedstock for ethanol fuel production by fermentation (Mielenz, 2001; Ragauskas et al., 2006; Wyman, 2001; Zaldivar et al., 2001). The hydrolysis of lignocellulosic materials releases a mixture of hexoses and pentoses including glucose, galactose, mannose, xylose and arabinose. An economic production of lignocellulose based ethanol would therefore require a simultaneous utilization of these biomass-derived sugars. However, most organisms for the production of ethanol either consume hexoses and pentoses sequentially or are unable to utilize pentoses effectively.
Despite many attempts of genetic engineering an organism for co-fermentation of hexoses and pentoses (Becker and Boles, 2003; Ho et al., 1998; Kotter and Ciriacy, 1993; Wisselink et al., 2007), one sugar is often preferred over another as a result of catabolite repression. This asynchronous consumption of sugar mixtures requires a longer fermentation time to reach a complete conversion of all sugars. In addition, genetically engineered strains can suffer from limited gene expression. For example, Saccharomyces cerevisiae engineered for pentose fermentation yields a high amount of undesired side products like xylitol and arabinitol due to limited enzyme activity in the pentose metabolism pathway (Bruinenberg et al., 1983; Karhumaa et al., 2006; Walfridsson et al., 1995). Furthermore, even if an organism consumes both hexoses and pentoses simultaneously, use of a single organism for the fermentation of sugar mixtures poses a challenge because the organism may not be able to optimally adjust its sugar uptake rate to match fluctuating sugar concentrations in hydrolysates from different sources of biomass. Therefore, for the fermentation of sugar mixtures a process using a co-culture of multiple strains could be preferred over a single strain. Unlike the single-strain culture, the composition of a co-culture of multiple strains can be conveniently adjusted as the relative consumption rate of each sugar in the mixture depends on the ratio of strains used in the immobilization.
We have previously applied a rational strain design in combination with a metabolic evolution step to develop mutant Escherichia coli strains that are capable of efficiently fermenting hexoses and/or pentoses to ethanol and that are robust under technologically challenging conditions. This includes the mutant AFF01/pLOI297 which was designed to ferment both hexoses and pentoses and the mutant CT1101/pLOI297 which was designed specifically for the exclusive fermentation of pentoses (Trinh et al., 2008). In this study, we immobilized these mutant strains for a continuous and efficient ethanol fermentation process from mixtures of hexoses and pentoses. Ethanol fermentation using immobilized cells is attractive since it provides several advantages over freely suspended cells including ease of cell separation and recycling or perfusion (Gilson and Thomas, 1995; Giordano et al., 2008; Nigam, 2000; Takamitsu et al., 1993; Verbelen et al., 2006; Yamada et al., 2002; Zhou et al., 2008). The immobilization technique is simple and low-cost. An easy recovery of immobilized cells helps to reduce process complexity in cell separation. Immobilization is also a suitable process to obtain a high cell density per unit volume which is desirable particularly in a continuous culture. Another benefit of continuous fermentation using immobilized cells is that there is no lag phase and/or turn-around time. Therefore, higher volumetric ethanol production efficiency can be attained.
We demonstrate in this work a continuous co-culture of immobilized AFF01/pLOI297 and CT1101/pLOI297 cells for the fermentation of mixed hexoses and pentoses. A kinetic model was developed and used to design optimal conditions for the co-culture such that the most rapid conversion of hexose and pentose sugars to ethanol is achieved. The process is adaptable to any composition of sugar mixtures available in biomass hydrolysates. The high ethanol productivity of immobilized cells was maintained over several weeks of continuous operation without loss of performance.
2. Materials and methods
2.1. Bacterial strains, plasmids and culture media
Escherichia coli AFF01 and CT1101 expressing plasmid pLOI297 (Alterthum and Ingram, 1989) were used for ethanol fermentation. Both strains are mutant derivatives of TCS083 (ΔzwfΔndhΔsfcAΔmaeBΔldhAΔfrdAΔpoxBΔpta, Trinh et al., 2008). AFF01 was isolated from a chemostat culture of TCS083 after a long-term exposure to high concentration of acetic acid and furfural. CT1101 is a mutant of TCS083 with additional deletions of glucose transporters such that the strain is able to use only pentoses but not glucose (Trinh et al., 2008). Plasmid pLOI297 (ATCC68239) contains the ethanol producing pathway from Zymomonas mobilis consisting of the pyruvate decarboxylase (pdc) and alcohol dehydrogenase genes (adhB) and genes conferring resistance to tetracycline (Alterthum and Ingram, 1989). AFF01/pLOI297 is a hexose/pentose co-utilizing mutant while CT1101/pLOI297 is a pentose-selective mutant. Lurie Bertani medium (10 g/l tryptone; 5 g/l yeast extract; 5 g/l NaCl; 10 µg/ml of tetracycline) was used for preparing cells for inoculation and for immobilization. Culture media used for ethanol fermentation were either LB or semi-defined medium composed of 5 g/l yeast extract, 0.5 g/l NaCl, 1 mM CaCl2, 1 mM MgSO4, 1 mg/l Thiamine-HCl, 1 mM Betaine-HCl, and 1 g/l NH4Cl (Sigma, St. Louis, MO). The medium was supplemented with sugars at concentration as specified. Glucose, xylose and arabinose were sterilized separately and added to the medium before use. Tetracycline (10 µg/ml) was added to the medium when specified.
2.2. Immobilization method
Cells were grown in 2l Erlenmeyer shake flasks with a working volume of 1.5 l LB supplemented with 20 g/l glucose or xylose under aerobic condition at 30°C and 200 rpm. Overnight cultures were used for preparation of immobilized cells. The cells were sedimented by centrifugation at 5000 rpm, 4°C for 15 minutes and were washed once with 1g/l NaCl solution before thoroughly mixing with sterilized agar solution (35 g/l sodium alginate, 1 g/l NaCl) at concentrations as specified. The immobilized cell concentration was determined as the total cell dry weight per liter of alginate agar solution. The cell dry weight was estimated based on the correlation of 1 OD600 = 0.29 g-CDW/l. The suspension at concentrations as specified was extruded through 14 gauge tubing as drops into 40 g/l CaCl2 solution at room temperature. The beads were suspended in CaCl2 solution at 4°C for 1 hour for gelation and then washed twice with sterile distilled water before use. The immobilized mixed cell was prepared by mixing cells at a fraction as specified before immobilization.
2.3. Batch, repeated batch and continuous cultivation
Shake flask batch fermentation was conducted in capped 250 ml Erlenmeyer shake flasks containing 100 ml of culture medium. The flasks were agitated in a Labline incubator shaker (Model 3528, Melrose Park, IC, USA) at 37°C and at a shaking rate of 100 rpm. Bioreactor batch experiments were carried out as fluidized bed reactions in a 2l Braun bioreactor (Biostat MD, B. Braun Biotech International, Melsungen, Germany). The temperature was maintained at 37°C and agitation speed with a rushton impeller was set at 75 rpm. pH was controlled at 7.0 by the addition of 3M NaOH. Nitrogen was sparged into the bioreactor through a 0.2 µ m filter at a volumetric flow rate of 20 ml/min to maintain anaerobic conditions. Batch fermentation was initiated by suspending beads containing immobilized cells into culture medium at a bead concentration of 50% (v/v). Culture samples (1 ml) were withdrawn periodically for measurement of residual sugars and ethanol product. Repeated batch experiments were executed similar to batch fermentations. After each batch run, used medium was drained and immobilized cells were washed with 0.1% CaCl2 solution before re-suspending in fresh medium to start a new batch run. Continuous fermentation was initially conducted as a batch experiment for 24–48 hrs before switching to continuous mode. In the continuous culture, sterile feed medium containing sugar as specified was pumped into the reactor at residence time as described. An outlet tube with a 50 mesh stainless steel screen at the end was set at a level to keep the liquid culture volume constant at 0.7l. Medium was continuously withdrawn through this tube by an outlet pump operated at a higher flow rate than the inlet pump.
2.4. Analytical methods
Cell concentration was measured via optical density at a wavelength of 600 nm (OD600nm) using a spectrophotometer and 1 cm cuvettes (Hewlett Packard 8453 Diode Array Spectrophotometer, Palo Alto, CA). Residual sugars and ethanol were measured using HPLC (Shimadz10A, Shimadzu, Columbia, MD) equipped with an autosampler (SIL-20AC), an exchange column and a UV–Vis detector (SPD-20AV) in series with a refractive index detector (RID-10A). Concentrations of glucose, xylose, arabinose and ethanol were measured by injecting 10 µl samples into a HPX-87H column (Biorad Labs, Hercules, CA) operated at 65°C and 0.5 ml/min of 5 mM H2SO4 mobile phase. The metabolites were quantified using a standard curve correlating peak area and concentration of standard solutions. Ethanol yield on sugar was determined by dividing total produced ethanol by total sugars consumed. Ethanol production rates were calculated from the slope of ethanol as a function of culture time.
3. Theory
3.1. Kinetic model for the simultaneous utilization of sugar mixtures
A kinetic model describing the specific sugar consumption rate of immobilized AFF01/pLOI297 and CT1101/pLOI297 cells in a sugar mixture was developed. The model is based on the assumption that the sugars are taken up by a transport complex that has a common element for which the sugars compete. One should note that this common element is not necessarily the transport protein itself but rather some other step downstream of the sugar metabolism. The mechanism of sugar uptake can be described by the reaction
| (1) |
where T [1/g-CDW] is the number of transporter complexes per unit cell dry weight, CSi,extand CSi [mmole/l] are extracellular and intracellular concentration of sugar Si and TSi [1/g-CDW] is the number of transporter-sugar complex per unit cell dry weight. The uptake rate of sugar Si is given by
| (2) |
where qSi [mmole/g-CDW-hr] is the specific uptake rate of sugar Si, TSi [1/g-CDW] is the number of transporters bound to sugar per unit cell dry weight and k2,Si [mmole/hr] is the sugar specific rate constant. By applying the quasi-steady state assumption and the conservation of the transporter complex to the balance equation (a detailed account is given in the supplemental file), the sugar uptake rate can be rewritten in a general form as
| (3) |
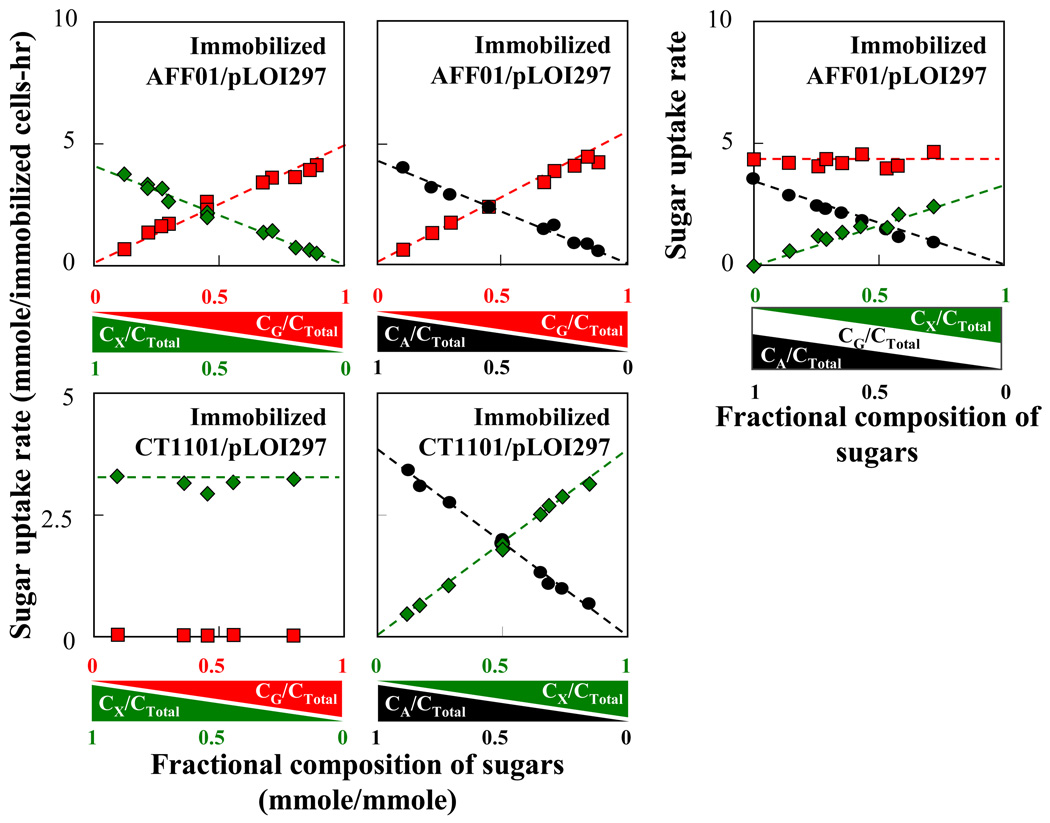
where T0 [1/g-CDW] is the total number of transporter complexes, CSi is the sugar concentration and kSi[mmole/g-CDW-hr] is the overall rate constant. Equ. (3) suggests a linear correlation between the specific rate of sugar consumption and the corresponding sugar mole fraction in the mixture. Thus the overall rate constant can be experimentally determined by measuring the specific consumption rates for various mole fractions of the sugar (see Fig. 3).
Fig. 3.
Rate of sugar consumption in mixed sugar fermentations of immobilized AFF01/pLOI297 and immobilized CT1101/pLOI297. Specific sugar uptake rates (mmole/g-CDW-hr) as a function of sugar composition in the mixture: glucose ( ), xylose (
), xylose ( ), and arabinose (
), and arabinose ( ). The sugar composition is given in mole fraction of total initial sugar concentration [mmole/mmole]. The experiments were conducted in capped shake flasks containing 100 ml of semi-defined medium supplied with sugar mixture and with 35 ml beads containing immobilized cell of 16.5 g-CDW/l-gel. The individual rate constants of each strain in each sugar mixture estimated from the linear regression of these data are summarized in Table 1.
). The sugar composition is given in mole fraction of total initial sugar concentration [mmole/mmole]. The experiments were conducted in capped shake flasks containing 100 ml of semi-defined medium supplied with sugar mixture and with 35 ml beads containing immobilized cell of 16.5 g-CDW/l-gel. The individual rate constants of each strain in each sugar mixture estimated from the linear regression of these data are summarized in Table 1.
In a glucose-xylose mixture and at a constant mole fraction, the sugars are consumed according to a zero order kinetics described by
| (4) |
| (5) |
| (6) |
where Equ. (4) and (5) specify the specific consumption rate by strain AFF01/pLOI297 for glucose and xylose respectively, and Equ. (6) is the specific xylose consumption rate for strain CT1101/pLOI297 that cannot use glucose. The empirical constant kSi,jrepresents a maximum specific consumption capacity of sugar Si by an immobilized cell type j, and CSi,0 is the initial sugar concentration.
3.2. Batch cultures
Because of the zero order consumption kinetics, the glucose and xylose exhaustion times (the times at which the respective sugars are consumed) for cultures containing immobilized AFF01/pLOI297 at a cell concentration XAF can be computed for glucose
| (7) |
and for xylose:
| (8) |
where tglc and txyl [hr] are the exhaustion times for glucose and xylose respectively.
These times are generally not the same. One can see that for kglc,AF > kxyl,AF the following inequality holds for the sugar consumption times
| (9) |
In a mixed cell culture of immobilized CT1101/pLOI297 and AFF01/pLOI297, the cell concentration of each strain is represented by
| (10) |
| (11) |
where XTOT [g-CDW/l] is the total cell concentration and f is the fraction of the total cells consisting of strain CT1101/pLOI297 that can utilize only pentoses.
The exhaustion time for glucose and xylose in a mixture of both sugars by immobilized mixed cultures of the two cell types becomes
| (12) |
| (13) |
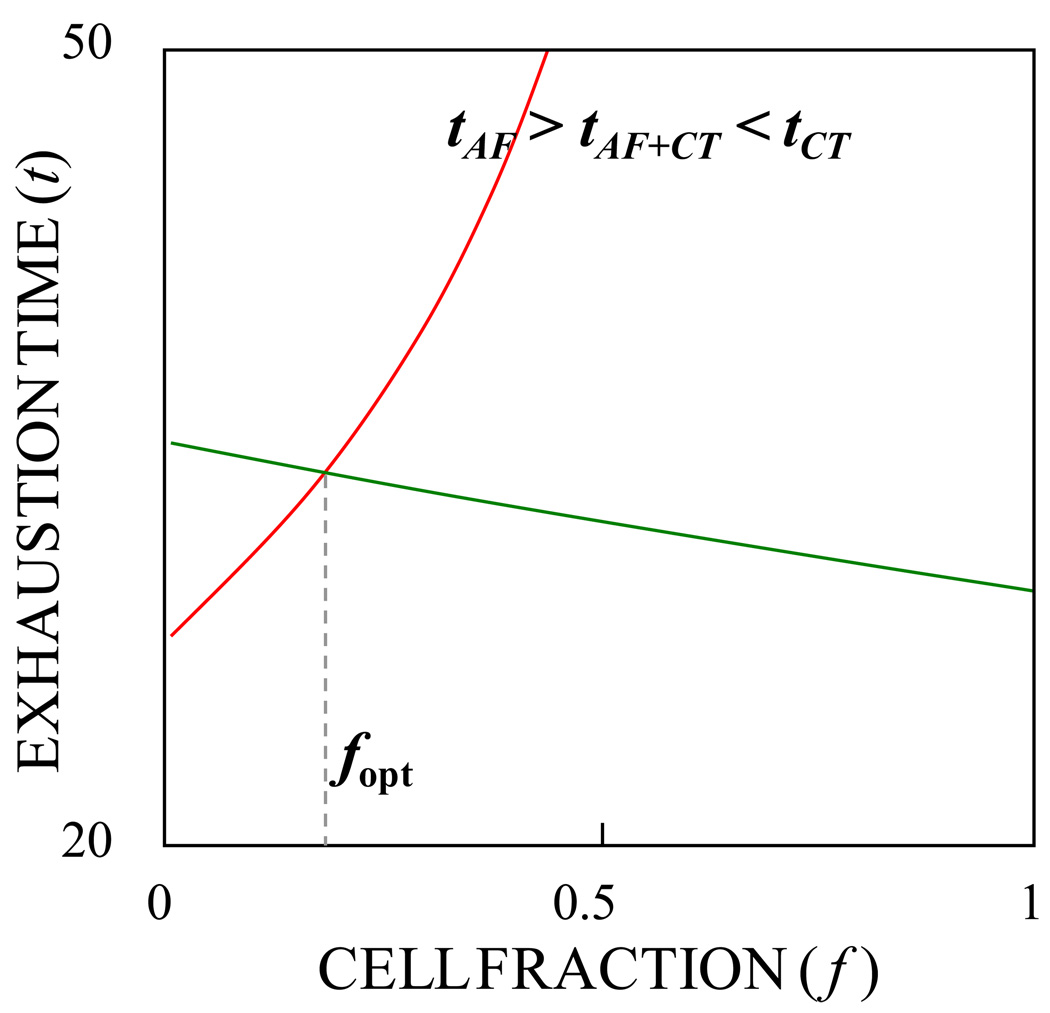
Fig. 1 shows the exhaustion times for the individual sugars as a function of the cell fraction f. One can see that the exhaustion times can be adjusted with the cell composition. A practical process should completely consume both sugars in the shortest possible time. This is achieved when both sugars are exhausted at the same time (tglc = txyl= topt) which occurs at an optimal cell fraction that can be computed from
| (14) |
Fig. 1.
Exhaustion time in hours of glucose (red) and xylose (green) as a function of the fraction of CT1101/pLOI297 cells used in the immobilization (see Equ. (12) and ((13)). Parameters used for the model are Cglc,0 = 111 mmole/l; Cxyl,0 = 400 mmole/l; XTOT = 3.62 g-CDW/l. At cell fraction fopt = 0.21, both sugars are consumed at the same time.
With the optimal cell fraction, the exhaustion time of each sugar becomes
| (15) |
Fig. 1 also illustrates that the time required for exhausting a sugar mixture is shorter in the mixed cell culture than in the single cell culture.
3.3. Continuous cultures
The previous result can be readily extended to a continuous culture. In a continuous fermentation of sugar mixtures using immobilized cells of multiple strains, the balance of sugar Si is
| (16) |
where DL is dilution rate based on liquid volume of the culture, CSi,F is the sugar concentration in the feed medium, Xj is the cell concentration of strain j, and qSi,j represents a specific uptake rate of sugar Si by the strain j.
For a complete conversion of each sugar at the same exhaustion time, the optimal residence time τL,opt becomes
| (17) |
For a steady state with complete utilization of a glucose-xylose mixture by immobilized cells of AFF01/pLI297 and CT1101/pLOI297, the optimal residence time and cell composition are
| (18) |
| (19) |
which are the same expressions as for the batch culture. Thus, an optimal residence time and cell fraction for a continuous fermentation of mixed sugars by immobilized mixed cells of AFF01/pLI297 and CT1101/pLOI297 can be determined from the known concentration of sugars in the feed.
4. Results
4.1. Batch fermentation of hexoses and pentoses by immobilized cells
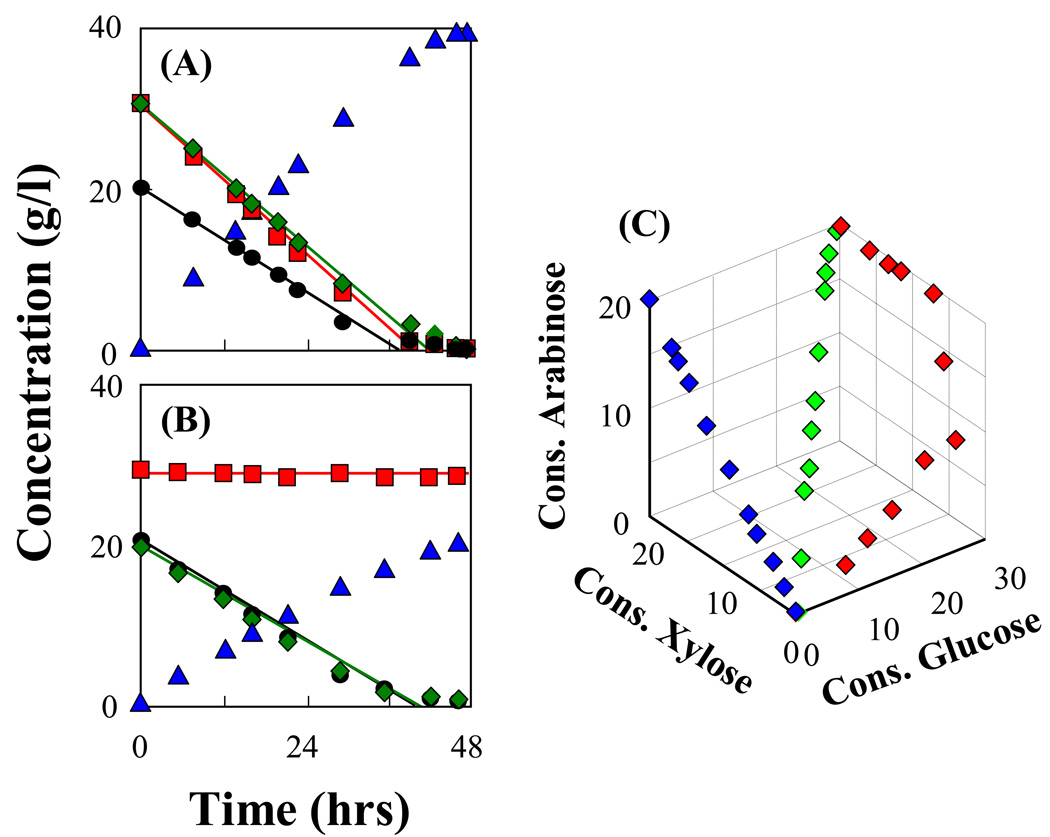
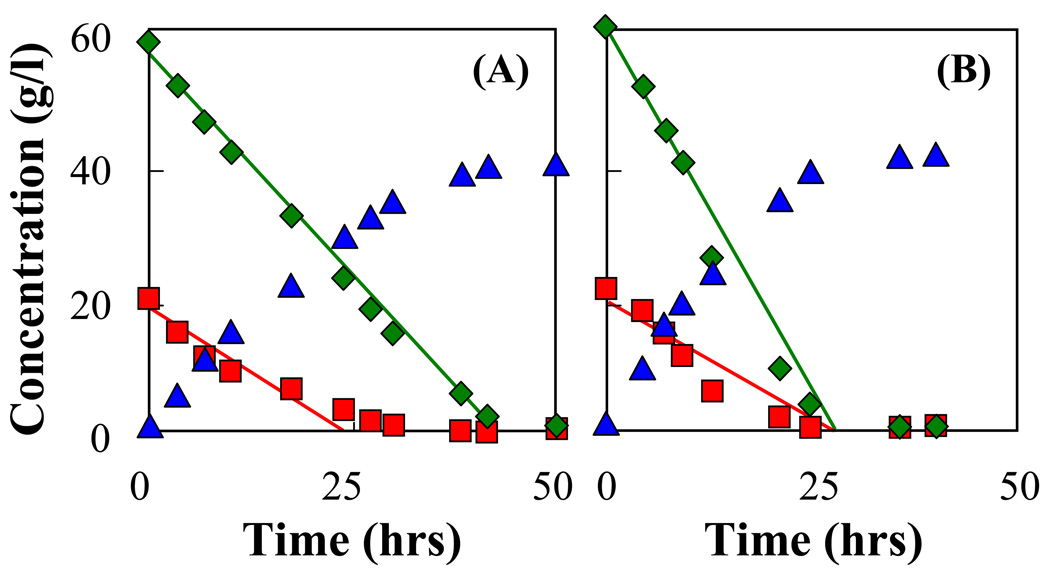
Fermentation of hexose and pentose sugars by immobilized cells of AFF01/pLOI297 and CT1101/pLOI297 was examined as shown in Fig. 2. The relation of consumed sugars shows that glucose, xylose and arabinose were utilized simultaneously by the mutant AFF01/pLOI297 compared to a sequential consumption of sugars by its parent MG1655/pLOI297. The mutant CT1101/pLOI297 also shows co-utilization of xylose and arabinose but it does not consume glucose. Ethanol yields by immobilized AFF01/pLOI297 and immobilized CT1101/pLOI297 in these mixed sugar fermentations exceeded 94% of the theoretical yield.
Fig. 2.
Fermentation kinetics of immobilized cultures. The experiments were conducted in anaerobic batch bioreactors containing 0.7 l semi-defined medium supplemented with sugars and with 0.35 l of beads. pH was controlled at 7. The immobilized cell concentration was 11.4 g-CDW/l-gel (see Materials and Methods). (A) Immobilized AFF01/pLOI297 with an initial concentration of 30 g/l glucose ( ), 30 g/l xylose (
), 30 g/l xylose ( ), and 20 g/l arabinose (
), and 20 g/l arabinose ( ) converted into ethanol (
) converted into ethanol ( ). (B) Immobilized CT1101/pLOI297 with an initial concentration of 30 g/l glucose, 20 g/l xylose and 20 g/l arabinose. (C) Relation of consumed sugars from a sugar mixture of glucose, xylose, and arabinose for immobilized cells of AFF01/pLOI297 (
). (B) Immobilized CT1101/pLOI297 with an initial concentration of 30 g/l glucose, 20 g/l xylose and 20 g/l arabinose. (C) Relation of consumed sugars from a sugar mixture of glucose, xylose, and arabinose for immobilized cells of AFF01/pLOI297 ( ), CT1101/pLOI297 (
), CT1101/pLOI297 ( ) and wild-type MG1655/pLOI297 (
) and wild-type MG1655/pLOI297 ( ).
).
4.2. Kinetics of hexose and pentose utilization by immobilized cells
The kinetics of sugar utilization was measured in more detail in individual batch experiments. Specifically, it was of interest to find out how the consumption rates depend on the initial sugar composition in the medium. These experiments were not conducted under strict anaerobic conditions but in closed shake flasks. However, due to the high cell density the oxygen concentration in the medium was essentially zero. The results are summarized in Table 1 and Fig. 3. The data confirm the validity of Equ. (3) that predicts on the basis of the assumed mechanisms that the zeroth order consumption rates depend on the initial mole fractions of the sugars.
Table 1.
Individual rate constants kSi (mmole/g-CDW-hr) of immobilized AFF01/pLOI297 and immobilized CT1101/pLOI297 in sugar mixtures. The rate constants are estimated from the linear regression of mixed sugar batch fermentation data shown in Fig.3. The regression coefficient (R2) for all plots are greater than 0.9.
| Immobilized AFF01/pLOI297 in mixture of |
kglu,AF | kxyl,AF | kara,AF |
|---|---|---|---|
| glucose-xylose | 5.1± 0.1 | 4.1 ± 0.1 | n.a. |
| glucose-arabinose | 5.7 ± 0.1 | n.a. | 4.2 ± 0.3 |
| glucose-xylose-arabinose | 4.0 ± 0.3 | 3.0 ± 0.1 | 3.7 ± 0.1 |
|
Immobilized CT1101/pLOI297 in mixture of |
kglu,CT | kxyl,CT | kara,CT |
| glucose-xylose | 0.0 ± 0.0 | 3.4 ± 0.2 | n.a. |
| xylose-arabinose | n.a. | 3.8 ± 0.1 | 3.9 ± 0.1 |
4.3. Immobilized mixed cell culture for fermentation of hexoses and pentoses
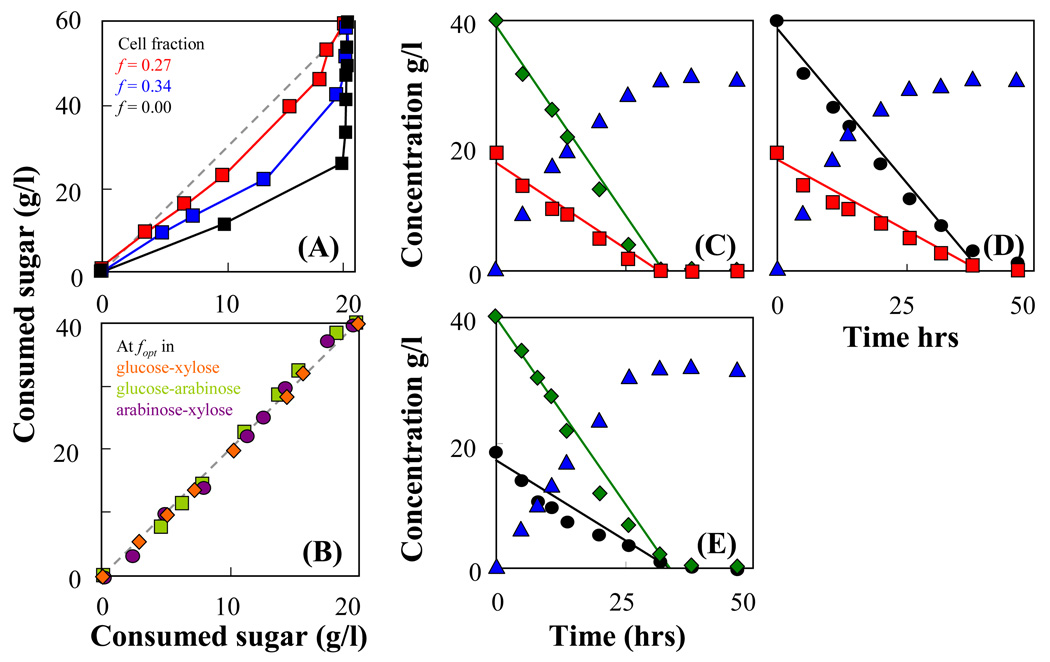
Fig. 4A presents the relation of consumed glucose (20 g/l) and xylose (60 g/l) by immobilized homogenous cells and immobilized mixed cultures. The concentration of glucose and xylose chosen reflects the sugar concentrations typically found in lignocellulosic hydrolysates. Due to unequal amounts of glucose and xylose, the two sugars were not consumed at the same rate by the immobilized cell culture of AFF01/pLOI297 and glucose was exhausted first. The use of an immobilized mixed culture of AFF01/pLOI297 and CT1101/pLOI297 permits the amount of each sugar consumed to be precisely adjusted by varying the composition of the strain mixture. As demonstrated in Fig. 4A, synchronous consumption of both sugars was achieved when the cell fraction of CT1101/pLOI297 in the immobilized mixed culture was approximately at f = 0.27.
Fig. 4.
Fermentation kinetics by immobilized mixed culture of AFF01/pLOI297 and CT1101/pLOI297. The experiments were performed in capped shake flasks containing 100 ml of semi-defined medium supplied with sugar mixtures and with 35 ml beads containing cell concentration of 10.2 g-CDW/l-gel. Optimal cell fraction fopt was determined from the kinetic model (see Equ. (14)). (A) Relation of consumed xylose with an initial concentration of 60 g/l and consumed glucose with an initial concentration of 20 g/l at the fraction of CT1101/pLOI297 cells used in the immobilization: f = 0.27 ( ), f = 0.34 (
), f = 0.34 ( ), f = 0 (
), f = 0 ( ). (B) Relation of consumed sugars in the mixture: glucose-xylose at cell fraction fopt = 0.41 (
). (B) Relation of consumed sugars in the mixture: glucose-xylose at cell fraction fopt = 0.41 ( ), glucose-arabinose at cell fraction fopt = 0.47 (
), glucose-arabinose at cell fraction fopt = 0.47 ( ), and arabinose-xylose at cell fraction fopt = 0.58 (
), and arabinose-xylose at cell fraction fopt = 0.58 ( ). (C) Immobilized mixed culture at the optimal cell fraction in a sugar mixture of glucose (
). (C) Immobilized mixed culture at the optimal cell fraction in a sugar mixture of glucose ( ) and xylose (
) and xylose ( ) converted into ethanol (
) converted into ethanol ( ). (D) Immobilized mixed cultures at the optimal cell fraction in a sugar mixture of glucose and arabinose (
). (D) Immobilized mixed cultures at the optimal cell fraction in a sugar mixture of glucose and arabinose ( ). (E) Immobilized mixed cultures at the optimal cell fraction in a sugar mixture of xylose and arabinose.
). (E) Immobilized mixed cultures at the optimal cell fraction in a sugar mixture of xylose and arabinose.
Using the kinetic model, we can predict with Equ. (14) an optimal cell fraction fopt of the two immobilized strains that will result in a simultaneous consumption of each sugar in a mixture. Batch fermentation kinetics of immobilized mixed cells of AFF01/pLOI297 and CT1101/pLOI297 operated at the predicted optimal cell fractions in a mixture of glucose-xylose, glucose-arabinose and arabinose-xylose are shown in Fig. 4C, 4D and 4E, respectively. In these immobilized mixed cell cultures, each sugar was consumed from the mixture at a rate proportional to the initial sugar composition in the mixture resulting in the exhaustion of all sugars at an equal fermentation time as predicted by Equ. (14). The relation of consumed sugars by immobilized mixed cells at the optimal cell fraction in glucose-xylose, glucose-arabinose and arabinose-xylose mixtures is shown in Fig. 4B. The ethanol yield achieved by immobilized mixed cells exceeded 96% of the theoretical yield.
4.4. Immobilized single cells vs. immobilized cell mixtures
The performance of immobilized homogeneous cells and immobilized mixed cells in glucose-xylose fermentations was tested and compared in Fig. 5. As expected, the immobilized cells of AFF01/pLOI297 show a complete consumption of glucose and xylose at different times and all sugars are completely consumed within about 48 hrs. On the other hand, the immobilized mixed cultures of strains AFF01/pLOI297 and CT1101/pLOI297 simultaneously consumed glucose and xylose with both sugars exhausted at an equal fermentation time of approximately 40 hrs. The experimental results are in agreement with the model prediction in Fig. 1 which shows that the immobilized mixed cells consume sugar mixtures more quickly resulting in a higher ethanol productivity than the immobilized single strain culture.
Fig. 5.
Mixed sugar fermentations by immobilized pure culture and immobilized mixed culture. The experiments were conducted in anaerobic batch bioreactors containing 0.7 l semi-defined medium supplied with sugars and with 0.35 l of beads at cell concentrations of 14.8 g-CDW/l-gel. pH was controlled at 7. (A) Immobilized pure culture of AFF01/pLOI297 with a sugar mixture of glucose ( ) and xylose (
) and xylose ( ) converted to ethanol (
) converted to ethanol ( ). (B) Immobilized mixed culture of AFF01/pLOI297 and CT1101/pLOI297 at a CT1101/pLOI297 cell fraction of f = 0.27 with a sugar mixture of glucose and xylose.
). (B) Immobilized mixed culture of AFF01/pLOI297 and CT1101/pLOI297 at a CT1101/pLOI297 cell fraction of f = 0.27 with a sugar mixture of glucose and xylose.
4.5. Continuous ethanol fermentation by immobilized mixed cells
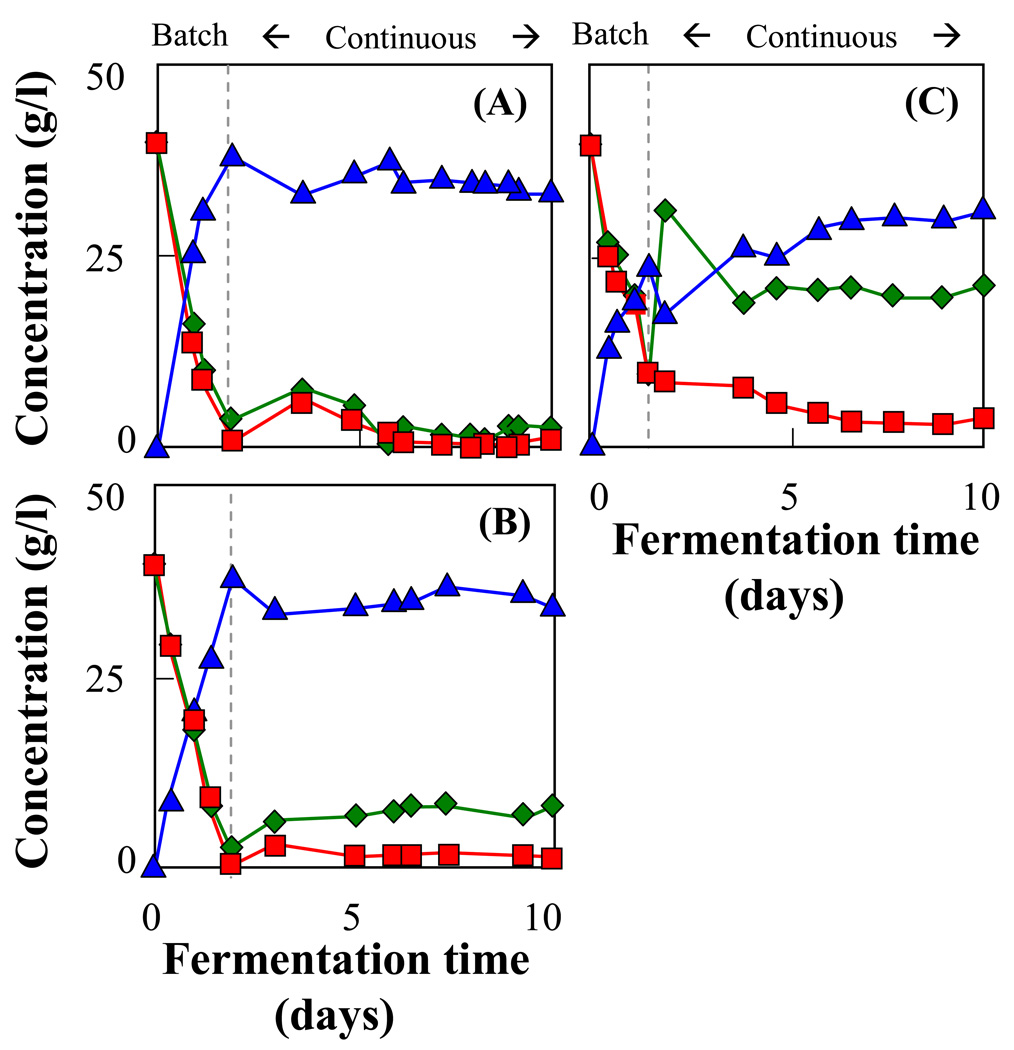
The kinetic model was also applied to design an immobilized mixed culture of AFF01/pLOI297 and CT1101/pLOI297 for an optimized continuous ethanol fermentation from a mixture of glucose and xylose. The continuous culture of immobilized mixed cells was operated at an optimal residence time and cell fraction based on Equ. (18) and (19). The continuous cultures using immobilized mixed strains (Fig. 6A and 6B) resulted in a simultaneous conversion of glucose and xylose into ethanol with a steady-state ethanol titer of approximately 34 g/l and ethanol yield of about 90% of the theoretical maximum. Immobilized pure cultures of strain AFF01/pLOI297 (Fig. 6C) utilized the same initial sugar mixture less efficiently and at a slower overall rate than the immobilized mixed culture of AFF01/pLOI297 and CT1101/pLOI297. At steady state, only 72.3% of the total sugar was consumed by the immobilized AFF01/pLOI297, compared to 91.7% of the total sugar utilized by the immobilized mixed strains of AFF01/pLOI297 and CT1101/pLOI297. The ethanol productivity achieved by the immobilized pure culture and by the immobilized mixed culture was 1.6 g/l-hr and 2.2 g/l-hr, respectively. The immobilized mixed cell approach is adaptable to any given feed composition of sugar mixtures by varying the operating residence time and the cells fraction of each immobilized strain. The kinetic parameters of continuous ethanol fermentation by immobilized single cells and cell mixtures are summarized in Table 2. The results are also compared to published performance data reporting the conversion of a single sugar into ethanol (Table 3).
Fig. 6.
Continuous fermentation by immobilized pure culture and immobilized mixed cultures. The experiments were carried out in anaerobic batch bioreactor containing 0.7 l semi-defined medium supplemented with sugars and with 0.35 l of beads. Immobilized cell concentration was 11.4 g-CDW/l-gel. pH was controlled at 7. The immobilized mixed cultures were operated at optimal dilution rate and optimal CT1101/pLOI297 cell fraction (see Equ. (17) and (18)). (A) Immobilized mixed culture of AFF01/pLOI297 and CT1101/pLOI297 at a cell fraction f = 0.32 with a feed sugar mixture of 40 g/l glucose ( ) and 40 g/l xylose (
) and 40 g/l xylose ( ) converted to ethanol (
) converted to ethanol ( ) at a dilution rate D = 0.096 hr−1. (B) Immobilized mixed culture of AFF01/pLOI297 and CT1101/pLOI297 at a cell fraction f = 0.40 with a feed sugar mixture of 25 g/l glucose and 65 g/l xylose converted to ethanol at a dilution rate D = 0.059 hr−1. (C) Immobilized pure culture of AFF01/pLOI297 with a feed sugar mixture of 25 g/l glucose and 65 g/l xylose converted to ethanol at a dilution rate D = 0.056 hr−1.
) at a dilution rate D = 0.096 hr−1. (B) Immobilized mixed culture of AFF01/pLOI297 and CT1101/pLOI297 at a cell fraction f = 0.40 with a feed sugar mixture of 25 g/l glucose and 65 g/l xylose converted to ethanol at a dilution rate D = 0.059 hr−1. (C) Immobilized pure culture of AFF01/pLOI297 with a feed sugar mixture of 25 g/l glucose and 65 g/l xylose converted to ethanol at a dilution rate D = 0.056 hr−1.
Table 2.
Performance summary of continuous ethanol production by immobilized cells of AFF01/pLOI297 and CT1101/pLOI297. The immobilization resulted in beads of approximately 4 mm diameter containing cells at a density of 11.4 g-CDW/l-gel. Sugar concentration in the feed medium is given in parenthesis. Cell fraction f represents a fraction of strain CT1101/pLOI297.
| Strain/ pLOI297 |
Feed Medium g/l |
DL hr−1 |
% Sugar utilization |
[Ethanol] g/l |
YETOH (%) |
Cell fraction f |
|---|---|---|---|---|---|---|
| AFF01 | Xylose (65); Glucose (25) |
0.056 | 72.3 | 30.1 | 90.2 | - |
| CT1101+ AFF01 |
Xylose (65); Glucose (25) |
0.059 | 91.7 | 36.7 | 87.2 | 0.40 |
| CT1101+ AFF01 |
Xylose (40); Glucose (40) |
0.096 | 96.0 | 34.0 | 86.6 | 0.32 |
Table 3.
Comparison of kinetic parameters of immobilized ethanologenic E. coli strains in continuous ethanol fermentation. The immobilization yielded beads of approximately 4 mm diameter at cell concentration of 11.4 g-CDW/l-gel. The feed sugar concentration is shown in parenthesis.
| Strain/ pLOI297 |
Feed Medium g/l |
DL hr−1 |
[Ethanol] g/l |
QETOH g/l-hr |
YETOH (%) |
% Sugar utilization |
Sources/ References |
|---|---|---|---|---|---|---|---|
| AFF01 | Xylose (65); Glucose (25) |
0.06 | 30.1 | 1.6 | 90 | 72 | This study |
| CT1101+ AFF01 |
Xylose (65); Glucose (25) |
0.06 | 36.7 | 2.2 | 87 | 91 | This study |
| KO11 | Xylose(50) | 0.05 | 15.5 | 0.8 | 87 | 70 | Zhou et al., 2008 |
| FBR5 | Xylose(50) | 0.05 | 19 | 1.0 | 76 | 98 | Martin et al., 2006 |
| FBR5 | Xylose (100) | 0.02 | 37.1 | 0.8 | 98 | 74 | Qureshi et al., 2005 |
| FBR5 | Xylose(100) | 0.24 | 18.9 | 4.5 | 98 | 38 | Qureshi et al., 2005 |
4.6. Operational stability of immobilized cells
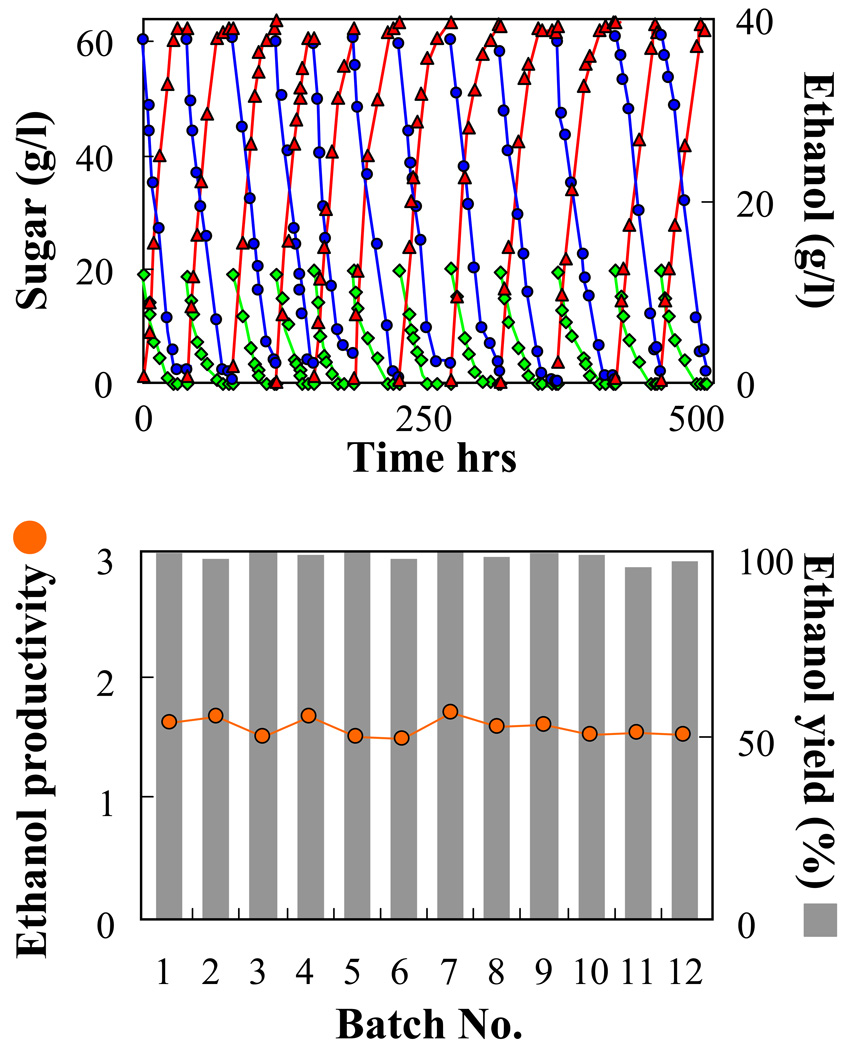
For a viable industrial application a long term process should have a stable productivity at the highest possible conversion efficiency. Several recombinant ethanologenic E. coli strains have shown loss or diminishing production of ethanol during long-term cultivation (Dumsday et al., 1999; Martin et al., 2006). The stability and the efficiency of conversion of immobilized mixed cultures of AFF01/pLOI297 and CT1101/pLOI297 were tested in a long-term process of repeated batch fermentations. The repeated batch process was operated as an anaerobic bioreactor containing LB medium supplied with 20 g/l glucose and 60 g/l xylose. The time profile of the pH during each batch process was monitored. A sudden rise of the pH (data not shown) indicated a depletion of sugars in the culture marking the end of each batch cycle. After each cycle the culture medium was harvested, the immobilized cells were washed with 0.1% CaCl2 solution and resuspended in fresh medium. The kinetics of sugar consumption is shown in Fig. 7. The system was stable for 12 batch cycles performed over 3 weeks. It did not show any significant loss in yield or productivity over this time period and could have been continued still longer.
Fig. 7.
Immobilized mixed culture of AFF01/pLOI297 and CT1101/pLOI297 in a repeated batch fermentation. The experiment was conducted in anaerobic batch bioreactor containing 0.7 l of LB medium supplied with an initial concentration of 20 g/l glucose and 60 g/l xylose and with 0.35 l of beads. Immobilized cell contained 11.4 g-CDW/l-gel and a CT1101/pLOI297 cell fraction f = 0.27. (Top) Time profiles of residual glucose ( ), xylose (
), xylose ( ), and produced ethanol (
), and produced ethanol ( ). (Bottom) Ethanol yield in % of theoretical and ethanol productivity in g/l-hr obtained in each batch run.
). (Bottom) Ethanol yield in % of theoretical and ethanol productivity in g/l-hr obtained in each batch run.
5. Discussion
We have previously designed and constructed two mutant E. coli strains where each strain is selective in the consumption of hexose and/or pentose for ethanol production at yields close to the theoretical maximum (Trinh et al., 2008). In this study, we have implemented an immobilized cell system for the conversion of sugar mixtures into ethanol using the two strains AFF01/pLOI297 and CT1101/pLOI297. Immobilization of mixed cultures of the two strains has proven to be an ideal condition for an efficient and simultaneous conversion of sugar mixtures into ethanol. The advantage of this process is that the two strains can act in concert in consuming mixed sugars resulting in a faster utilization of a sugar mixture than a single strain could convert. The process is adjustable to any sugar composition in the medium by varying the cell fraction of each strain in the immobilization process. This was confirmed in both batch and continuous fermentations of hexose and pentose mixtures where we achieved high ethanol yield and productivity.
The developed kinetic model of mixed sugar utilization and the experimental data shows that individual rates of sugar uptake in a sugar mixture is dependent on the mole fraction of the sugar in the medium. This dependence is a consequence of multiple sugars competing for a common step in the initial metabolism. A sugar with higher mole fraction has a higher chance of binding with the transport complex leading to a higher uptake rate than a sugar with lower mole fraction.
The uptake rate of each sugar follows a zero-order kinetic since the high sugar concentration used in the experiment (400–500 mM) is much higher than the Km value for glucose (1–2 µM) of the transporter protein (Natarajan and Srienc, 1999). The zero-order kinetic results in a linear relationship between the specific consumption rate of each sugar and its mole fraction in the mixture which is in good agreement with the batch experiments of immobilized AFF01/pLOI297 and immobilized CT1101/pLOI297 as shown in Fig. 3. It was also observed that the sugar mole fraction in a sugar mixture remained relatively constant throughout the course of fermentation. Thus, the rates of uptake can be estimated from the mole fraction of the initial sugar concentration. The validity of the kinetic model is demonstrated by the fact that it can be used to accurately predict an optimal cell fraction of immobilized mixed cells for synchronous consumption of sugar mixtures.
The use of minimal media compared to complex media could offer both practical and economic advantages in a continuous ethanol production process. Although we have conducted the repeated batch experiments in LB media supplemented with the individual sugars, we have conducted tests with simple M9 medium without phosphate buffer that can be used in the immobilized cell culture process. The phosphate buffer has been omitted since it causes destruction of alginate gel beads. We observed similar high ethanol yields as with complex LB medium. However, the ethanol productivity is significantly reduced when minimal medium is used. The productivity can be recovered with the addition of 5 g/l yeast extract which is the semi-defined medium we used in the continuous culture experiments.
Controlling the uptake rate of sugar mixtures in a freely-suspended mixed cell culture could be challenging since the cell distribution could change over time as each strain grows at a different rate. In contrast, the cell distribution of an immobilized mixed cell culture is expected to remain relatively constant since the growth of immobilized cells is restricted. As a result, the process of an immobilized mixed cell culture permits a better control of the consumption rate of each sugar in a mixture than a freely-suspended mixed cell culture. In the conducted experiments the two strains were mixed before immobilization. Thus, each gel bead contained a mixture of both strains. In principle, one could also immobilize pure cultures of the respective strains and then mix desired proportions of the beads containing homogeneous cell populations for use in the conversion reactor. In the latter case one should expect higher local conversion rates in individual beads that could result in mass transfer limitations of individual sugars into the beads.
We have evaluated the internal mass transfer effects through computation of the effectiveness factor (Shuler and Kargi, 1992) which measures the degree of internal mass transfer resistance relative to the reaction rate. It was found that under the condition of immobilization examined in this work (bead size of about 4mm in diameter and cell loading of approximately up to 20 g-CDW/l-gel), the effectiveness factor is close to 1. Thus, internal mass transfer limitation is negligible in our case and the immobilization of pure cultures with subsequent mixing of beads is expected to result in the same performance as the immobilization of cultures that are mixed at the desired proportions.
The gel bead inoculum of 50% (v/v) used in this work represents an optimized condition where high cell loading has been achieved with minimal splitting of gel beads during mixing. During the course of fermentation, little cell leakage was observed. The suspended cell concentration was below OD600 ≤ 0.5 (equivalent to about 0.1 g-CDW/l) suggesting that most of the cells remain entrapped inside the alginate gel beads. The extent of cell leakage increased if a higher cell loading was used. We observed that the maximum cell loading with minimal cell leakage is approximately 15 g-CDW/l-gel volume. In addition to the fluidized bed reactor, we tested also a packed bed reactor. But this resulted in problems because the evolving CO2 could not be efficiently removed.
In the continuous operation a lower ethanol yield was observed (about 90% of theoretical yield) compared to the repeated batch operation (> 95% of theoretical yield). This is likely caused by the continuous exposure of immobilized cells to high concentrations of ethanol during the continuous operation. In contrast, the immobilized cells during repeated batch operation are exposed to high ethanol concentrations only towards the end of each cycle when the sugar source is exhausted. In addition, it is likely that cells can regenerate during the initial time during a repeated batch cycle when cells are exposed to fresh medium. Inhibitory effects of ethanol could also explain the incomplete utilization of sugar mixtures (Fig. 6) in the continuous culture of the immobilized mixed cell cultures. The immobilized mixed cell cultures exhibited a high degree of operational stability when they were repeatedly used for several weeks without any loss of viability or ethanologenicity. The observed stability of immobilized cells may be contributed by the slowly- or non-growing state of cells when cells are immobilized (Barbotin et al., 1998; Chau et al., 2000; Dincbas et al., 1993; Najafpour, 1990). With the restricted growth of immobilized cells, the loss of genes or intracellular enzyme activity is minimized.
In summary, ethanol production from hexoses and pentoses by immobilized mixed cultures of E. coli presented in this study represents an example of a case where a cell consortium results in a superior process performance than what can be achieved with homogeneous cell populations. The approach permits a precise adjustment of sugar consumption times which could be useful in the fermentation of sugar mixtures derived from lignocellulosic biomass which is known to fluctuate in the sugar contents. In addition, immobilization offers an effective means for a robust and stable continuous ethanol production process over extended time periods.
Supplementary Material
Acknowledgements
This work has been supported in part by a grant from NIH (GM077529) and by Mascoma Corp.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alterthum F, Ingram LO. Efficient ethanol production from glucose, lactose, and xylose by recombinant Escherichia coli. Appl. Environ. Microbiol. 1989;55(8):1943–1948. doi: 10.1128/aem.55.8.1943-1948.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbotin JN, Mater D, Craynest M, Saucedo JN, Truffaut N, Thomas D. Immobilized cells: Plasmid stability and plasmid transfer. Biotechnol. Prog. 1998;15:591–602. [Google Scholar]
- Becker J, Boles E. A modified saccharomyces cerevisiae strain that consumes L-arabinose and produces ethanol. Appl. Environ. Microbiol. 2003;69(7):4144–4150. doi: 10.1128/AEM.69.7.4144-4150.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruinenberg PM, Peter HM, van Dijken JP, Scheffers WA. The role of redox balances in the anaerobic fermentation of xylose by yeasts. Eur. J. Appl. Microb.Biotech. 1983;18:287–292. [Google Scholar]
- Chau TL, Guillan A, Roca E, Nunez MJ, Lema JM. Enhancement of plasmid stability and enzymatic expression by immobilizing recombinant Saccharomyces cerevisiae. Biotechnol. Lett. 2000;22:1247–1250. [Google Scholar]
- Dincbas V, Hortacsu A, Camurdan A. Plasmid stability in immobilized mixed cultures of recombinant escherichia coli. Biotechnol. Prog. 1993;9(2):218–220. doi: 10.1021/bp00020a017. [DOI] [PubMed] [Google Scholar]
- Dumsday GJ, Zhou B, Yaqin W, Stanley GA, Pamment NB. Comparative stability of ethanol production by Escherichia coli KO11 in batch and chemostat culture. J. Ind. Microbiol. Biotechnol. 1999;23(1):701–708. doi: 10.1038/sj.jim.2900690. [DOI] [PubMed] [Google Scholar]
- Gilson CD, Thomas A. Ethanol production by alginate immobilized yeast in a fluidized bed bioreactor. J. Chem. Technol. Biotechnol. 1995;62:38–45. [Google Scholar]
- Giordano RL, Trovati J, Schmidell W. Continuous production of ethanol from starch using glucoamylase and yeast co-immobilized in pectin gel. Appl. Biochem. Biotechnol. 2008;147(1–3):47–61. doi: 10.1007/s12010-007-8067-1. [DOI] [PubMed] [Google Scholar]
- Ho NW, Chen Z, Brainard AP. Genetically engineered saccharomyces yeast capable of effective cofermentation of glucose and xylose. Appl. Environ. Microbiol. 1998;64(5):1852–1859. doi: 10.1128/aem.64.5.1852-1859.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karhumaa K, Wiedemann B, Hahn-Hagerdal B, Boles E, Gorwa-Grauslund MF. Co-utilization of L-arabinose and D-xylose by laboratory and industrial Saccharomyces cerevisiae strains. Microb. Cell Fact. 2006;5:18. doi: 10.1186/1475-2859-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotter P, Ciriacy M. Xylose fermentation by Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 1993;38:776–783. [Google Scholar]
- Martin GJ, Knepper A, Zhou B, Pamment NB. Performance and stability of ethanologenic Escherichia coli strain FBR5 during continuous culture on xylose and glucose. J. Ind. Microbiol. Biotechnol. 2006;33(10):834–844. doi: 10.1007/s10295-006-0129-9. [DOI] [PubMed] [Google Scholar]
- Mielenz JR. Ethanol production from biomass: Technology and commercialization status. Curr. Opin. Microbiol. 2001;4(3):324–329. doi: 10.1016/s1369-5274(00)00211-3. [DOI] [PubMed] [Google Scholar]
- Najafpour GD. Immobilization of microbial cells for the production of organic acids. J. Sci. I. R. Iran. 1990;1:172–176. [Google Scholar]
- Natarajan A, Srienc F. Dynamics of glucose uptake by single Escherichia coli cells. Metab. Eng. 1999;1(4):320–333. doi: 10.1006/mben.1999.0125. [DOI] [PubMed] [Google Scholar]
- Nigam JN. Continuous ethanol production from pineapple cannery waste using immobilized yeast cells. J. Biotechnol. 2000;80(2):189–193. doi: 10.1016/s0168-1656(00)00246-7. [DOI] [PubMed] [Google Scholar]
- Qureshi N, Dien BS, Nichols NN, Liu S, Hughes SR, Iten LB. Continuous production of ethanol in high productivity bioreactors using genetically engineered Escherichia coli FBR5: Membrane and fixed cell reactors American Institute of Chemical Engineers, Paper No. 589g. 2005 [Google Scholar]
- Ragauskas AJ, Williams CK, Davison BH, Britovsek G, Cairney J, Eckert CA, et al. The path forward for biofuels and biomaterials. Science. 2006;311(5760):484–489. doi: 10.1126/science.1114736. [DOI] [PubMed] [Google Scholar]
- Shuler M, Kargi F. Bioprocess Engineering Basic concepts. New Jersey: Prentice Hall; 1992. pp. 248–261. [Google Scholar]
- Takamitsu I, Izumida H, Akagi Y, Sakamoto M. Continuous ethanol fermentation in molasses medium using Zymomonas mobilis immobilized in photo-cross linkable resin gels. J. Ferment. Bioeng. 1993;75:32–35. [Google Scholar]
- Trinh CT, Unrean P, Srienc F. Minimal Escherichia coli cell for the most efficient production of ethanol from hexoses and pentoses. Appl. Environ. Microbiol. 2008;74(12):3634–3643. doi: 10.1128/AEM.02708-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbelen PJ, De Schutter DP, Delvaux F, Verstrepen KJ, Delvaux FR. Immobilized yeast cell systems for continuous fermentation applications. Biotechnol. Lett. 2006;28(19):1515–1525. doi: 10.1007/s10529-006-9132-5. [DOI] [PubMed] [Google Scholar]
- Walfridsson M, Hallborn J, Penttila M, Keranen S, Hahn-Hagerdal B. Xylose-metabolizing Saccharomyces cerevisiae strains overexpressing the TKL1 and TAL1 genes encoding the pentose phosphate pathway enzymes transketolase and transaldolase. Appl. Environ. Microbiol. 1995;61(12):4184–4190. doi: 10.1128/aem.61.12.4184-4190.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisselink HW, Toirkens MJ, del Rosario Franco Berriel M, Winkler AA, van Dijken JP, Pronk JT. Engineering of Saccharomyces cerevisiae for efficient anaerobic alcoholic fermentation of L-arabinose. Appl. Environ. Microbiol. 2007;73(15):4881–4891. doi: 10.1128/AEM.00177-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyman CE. Twenty years of trials, tribulations, and research progress in bioethanol technology: Selected key events along the way. Appl. Biochem. Biotechnol. 2001;91–93:5–21. doi: 10.1385/abab:91-93:1-9:5. [DOI] [PubMed] [Google Scholar]
- Yamada T, Fatigati MA, Zhang M. Performance of immobilized Zymomonas mobilis 31821 (pZB5) on actual hydrolysates produced by arkenol technology. Appl. Biochem. Biotechnol. 2002;98–100:899–907. doi: 10.1385/abab:98-100:1-9:899. [DOI] [PubMed] [Google Scholar]
- Zaldivar J, Nielsen J, Olsson L. Fuel ethanol production from lignocellulose: A challenge for metabolic engineering and process integration. Appl. Microbiol. Biotechnol. 2001;56(1–2):17–34. doi: 10.1007/s002530100624. [DOI] [PubMed] [Google Scholar]
- Zhou B, Martin GJ, Pamment NB. Increased phenotypic stability and ethanol tolerance of recombinant Escherichia coli KO11 when immobilized in continuous fluidized bed culture. Biotechnol. Bioeng. 2008;100(4):627–633. doi: 10.1002/bit.21800. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.