Abstract
Crystals of the title compound, {(C6H5C2H4NH3)2[PbBr4]}n, were grown at room temperature from a solution in N,N-dimethylformamide (DMF) using nitromethane as the poor solvent. This perovskite-type organic–inorganic hybrid compound consists of well ordered sheets of corner-sharing disordered PbBr6 octahedra separated by bilayers of phenethylammonium cations. The octahedra are rotated and tilted due to N—H⋯Br hydrogen bonds with the ammonium groups, generating a superstructure in the unit cell similar to that of the tetrachloridoplumbate (C6H5C2H4NH3)2[PbCl4].
Related literature
The title compound has been studied previously and the lattice parameters reported without the complete structure (Mitzi, 1999 ▶). The optical characteristics have been investigated using thin films, see: Cheng et al. (2005 ▶); Kitazawa & Watanabe (2005 ▶). Promising applications have been reported on electroluminescent devices and scintillators, see: Era et al. (1995 ▶); Kishimoto et al. (2008 ▶); van der Eijk et al. (2008 ▶). Structural data of some related materials have been published; for (C6H5C2H4NH3)2PbCl4, see: Mitzi (1999 ▶); for (C6H5C2H4NH3)2CuBr4, see: Willett (1990 ▶); for (C6H5C2H4NH3)2ZnBr4, see: Huh et al. (2006 ▶); for (C6H5C2H4NH3)PbBr3, see: Billing & Lemmerer (2003 ▶). For van der Waals radii, see: Bondi (1964 ▶). For halogen hydrogen bonding, see: Chapuis et al. (1976 ▶). 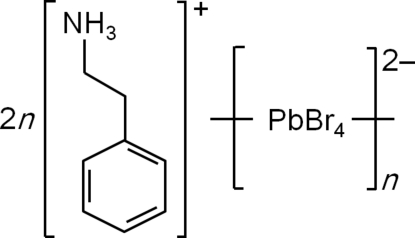
Experimental
Crystal data
(C8H12N)2[PbBr4]
M r = 771.20
Triclinic,
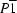
a = 11.6150 (4) Å
b = 11.6275 (5) Å
c = 17.5751 (6) Å
α = 99.5472 (12)°
β = 105.7245 (10)°
γ = 89.9770 (12)°
V = 2250.62 (15) Å3
Z = 4
Mo Kα radiation
μ = 14.63 mm−1
T = 296 K
0.25 × 0.20 × 0.03 mm
Data collection
Rigaku R-AXIS RAPID diffractometer
Absorption correction: numerical (ABSCOR; Higashi, 1999 ▶) T min = 0.106, T max = 0.645
20072 measured reflections
10077 independent reflections
7157 reflections with I > 2σ(I)
R int = 0.053
Refinement
R[F 2 > 2σ(F 2)] = 0.042
wR(F 2) = 0.106
S = 0.97
10077 reflections
416 parameters
H-atom parameters constrained
Δρmax = 3.26 e Å−3
Δρmin = −2.53 e Å−3
Data collection: PROCESS-AUTO (Rigaku Corporation, 1998 ▶); cell refinement: PROCESS-AUTO; data reduction: CrystalStructure (Rigaku Americas & Rigaku Corporation, 2008 ▶); program(s) used to solve structure: SIR2002 (Burla et al., 2003 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: CrystalMaker (Palmer, 2009 ▶); software used to prepare material for publication: publCIF (Westrip, 2009 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S160053680903712X/zq2004sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S160053680903712X/zq2004Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Selected geometric parameters (Å, °).
| Pb1—Br3 | 2.8786 (8) |
| Pb1—Br4 | 2.9927 (7) |
| Pb1—Br1 | 2.9957 (7) |
| Pb1—Br6 | 3.0080 (7) |
| Pb1—Br5 | 3.0095 (7) |
| Pb1—Br2 | 3.1965 (8) |
| Pb2—Br8 | 2.8755 (8) |
| Pb2—Br5i | 2.9935 (6) |
| Pb2—Br6 | 2.9957 (7) |
| Pb2—Br1ii | 3.0082 (7) |
| Pb2—Br4iii | 3.0110 (7) |
| Pb2—Br7 | 3.1982 (8) |
Symmetry codes: (i) 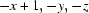 ; (ii)
; (ii) 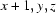 ; (iii)
; (iii) 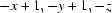 .
.
Table 2. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1⋯Br1 | 0.89 | 3.18 | 3.508 (5) | 104 |
| N1—H3⋯Br2 | 0.89 | 2.54 | 3.411 (5) | 165 |
| N2—H13⋯Br6 | 0.89 | 3.17 | 3.509 (5) | 105 |
| N2—H14⋯Br7 | 0.89 | 2.54 | 3.416 (5) | 167 |
| N3—H26⋯Br7 | 0.89 | 2.71 | 3.448 (6) | 142 |
| N3—H27⋯Br2 | 0.89 | 2.62 | 3.486 (6) | 164 |
| N4—H37⋯Br4 | 0.89 | 2.68 | 3.465 (5) | 148 |
| N4—H39⋯Br2 | 0.89 | 2.73 | 3.462 (6) | 140 |
Acknowledgments
This study was supported financially by CREST from the Japan Science and Technology Agency. The authors thank Professor H. Adachi of Osaka University and Sosho Inc. for careful advice on the refinement. We thank Dr Y. Takeoka of Sophia University for helpful advice on the synthesis.
supplementary crystallographic information
Comment
Recently, much attention has been paid to low-dimensional materials that often exhibit characteristic electronic properties considerably different from those of bulk ones. However, their crystallographic studies are limited because their anisotropic growth nature makes it difficult to obtain a good single crystal. Mitzi reported the structure of the tetrachloroplumbate, (C6H5C2H4NH3)2PbCl4, whose single crystals required approximately one year to be grown up. The present paper is the first report of the detailed structure of the tetrabromoplumbate, whose single crystals were grown up in approximately two months; in order to compare with some related materials: see the tetrachloroplumbate, the tetrabromozincate, (C6H5C2H4NH3)2ZnBr4 (Huh et al., 2006), and the tribromoplumbate, (C6H5C2H4NH3)PbBr3 (Billing & Lemmerer, 2003).
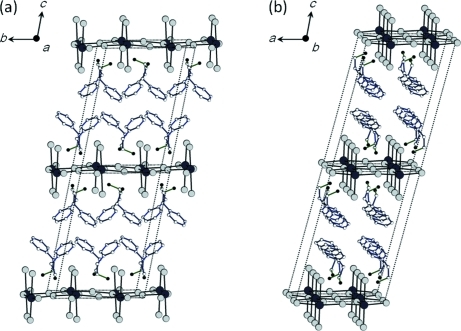
Fig. 1 shows the packing diagram of (C6H5C2H4NH3)2PbBr4, viewed approximately along the c axis. The sheets of corner-sharing PbBr6 octahedra are separated by bilayers of phenethylammonium cations. The corner-sharing PbBr6 octahedra are the common structure among bis-(phenethylammonium) tetrahaloplumbates, (C6H5C2H4NH3)2PbX4 (X = Cl, Br, and I), regardless of the halogen, but are different from face-sharing PbBr6 octahedra of the tribromoplumbate, (C6H5C2H4NH3)PbBr3, and from isolated tetrahedral ZnBr4 of the tetrabromozincate, (C6H5C2H4NH3)2ZnBr4. As the structure of halometalate is notably controlled by surrounding organic molecules, hydrogen bondings between them are discussed later.
Dashed line in Fig. 1 displays the triclinic unit cell, which is similar to the triclinic unit cell of the tetrachloroplumbate, (C6H5C2H4NH3)2PbCl4, but different from the monoclinic unit cell of the tetraiodoplumbate, (C6H5C2H4NH3)2PbI4. The present tetrabromoplumbate possesses two independent but similar Pb atoms with distorted octahedral coordination. The Pb—Br bond lengths range from 2.8755 (8) to 3.1982 (8) Å (average: 3.0136 (7) Å) and Br—Pb—Br bond angles range from 83.44 (2)° to 96.67 (2)° and from 170.97 (2)° to 179.36 (2)°. These angles are somewhat different from those of the perfect octahedron, i.e., 90.0° and 180°, respectively. Furthermore, the bridging Pb—Br—Pb bond angles significantly differ from 180° and range from 150.77 (3)° to 152.15 (3)°. This indicates that adjacent PbBr6 are rotated relative to each other.
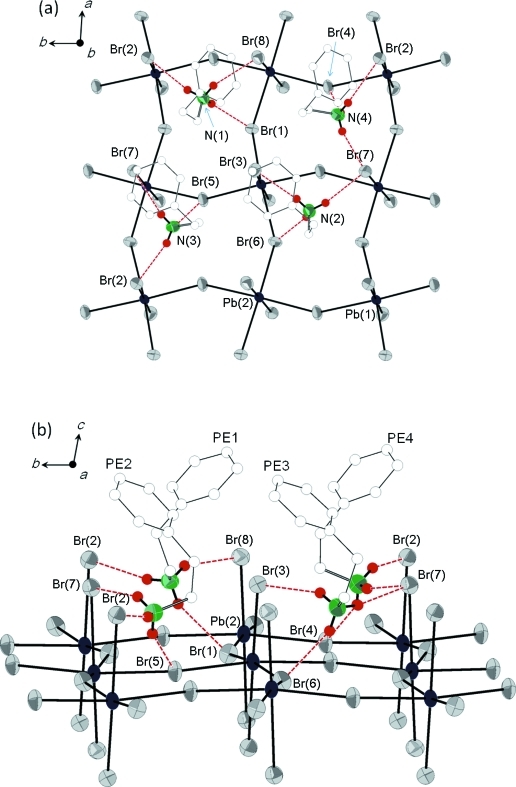
Fig. 2 shows the relative rotation of PbBr6 in the sheet and the hydrogen bondings between the octahedra and ammonium groups. Each ammonium group interacts with three halogen anions through N—H···Br hydrogen bonding in "terminal halogen configuration" involving two terminal halogen anions and one bridging halogen anion (Chapuis et al., 1976). The average hydrogen-bonding distance is 2.630 (2) Å, which is considerably shorter than the sum of the van der Waals radii for H (1.20–1.45 Å) and Br (1.95 Å) (Bondi, 1964). As a result, the opposite sides of the quadrangle, defined by one set of four PbBr6 octahedra, are "pinched-in" or "pushed-out" as shown in Fig. 2. In addition, there are four independent phenethylammonium depicted as PE1, PE2, PE3, and PE4, having similar bond lengths and bond angles. Therefore, two sides of the unit cell along with a and b axes are about twice length of a PbBr6 to have a superstructure in it.
There is no significant π-π interaction found in the organic bilayers because the adjacent aromatic rings are considerably separated by centroid-to-centroid distance of 5.748 (9) Å between PE1 and PE4, and 5.787 (9) Å between PE2 and PE3, respectively. The van der Waals radius for aromatic carbon atoms is about 1.77 Å (Bondi, 1964).
Experimental
Single crystals were obtained in the following three steps. First, phenethylamine bromide, C6H5C2H2NH3Br, as the precursor was synthesized at 10 C° from stoichiometric amount of hydrobromic acid, HBr, and phenethylamine, C6H5C2H2NH2, by their acid-base reaction in a flask. After evaporating the solvent, water, at 70 C°, the white deposition was washed by diethyl ether to remove unreacted reagents and dried in vacuum. Second, the objective compound was synthesized at 25 C° in dry nitrogen atmosphere from stoichiometric amount of the precursor and lead bromide (II), PbBr2, using dehydrated N,N'-dimethylformamide (DMF) as a good solvent. The purity of PbBr2 powder was 4 N, and it was used as delivered from Kojundo Chemical Laboratory Co., Japan. Third, the solution was filtered and contained in a glass bottle for the crystal growth. The bottle was contained in a shaded desiccator where another bottle with nitromethane as a poor solvent was also contained. Then, the vapor of the poor solvent was gradually diffused into the solution to reduce the solubility. Settling it two months grew colorless transparent crystals at the bottom of the former bottle. The crystal size was typically 8 mm × 6 mm × 1 mm, and the one used for the crystallographic study was 0.25 mm × 0.20 mm × 0.03 mm.
Refinement
The structure was solved by direct methods and expanded using Fourier techniques. The non-hydrogen atoms were refined anisotropically. Hydrogen atoms were refined using the riding model. The final cycle of full-matrix least-squares refinement on F2 was based on 10107 observed reflections and 416 variable parameters and converged (largest parameter shift was 0.00 times its e.s.d.) with unweighted and weighted agreement factors of R1 = 0.0460 and wR2 = 0.1483. The standard deviation of an observation of unit weight was 1.06. Unit weights were used. The maximum and minimum peaks on the final difference Fourier map corresponded to 3.95 and -2.77 e-/Å3, respectively.
Figures
Fig. 1.
Packing diagram of (C6H5C2H4NH3)2PbBr4, approximately viewed down (a) the a axis and (b) the b axis. Dashed line shows the outline of two triclinic unit cells along with the c axis. For clarity, the atoms are represented as spheres with each uniform size for the PbBr6 octahedra and the phenethylammonium, respectively. Hydrogen atoms are omitted.
Fig. 2.
The relative rotation of PbBr6 due to hydrogen bonding (dashed lines) between the octahedra and ammonium groups. The structure is approximately viewed down (a) the b axis and (b) the a axis. The thermal ellipsoids are drawn at 50% probability for nitrogen, bromine, and lead atoms. The hydrogen atoms of nothing to do with hydrogen bonding are omitted.
Crystal data
| (C8H12N)2[PbBr4] | Z = 4 |
| Mr = 771.20 | F(000) = 1424.00 |
| Triclinic, P1 | Dx = 2.276 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71075 Å |
| a = 11.6150 (4) Å | Cell parameters from 15239 reflections |
| b = 11.6275 (5) Å | θ = 3.2–27.5° |
| c = 17.5751 (6) Å | µ = 14.63 mm−1 |
| α = 99.5472 (12)° | T = 296 K |
| β = 105.7245 (10)° | Platelet, colourless |
| γ = 89.9770 (12)° | 0.25 × 0.20 × 0.03 mm |
| V = 2250.62 (15) Å3 |
Data collection
| Rigaku R-AXIS RAPID diffractometer | 7157 reflections with I > 2σ(I) |
| Detector resolution: 10.00 pixels mm-1 | Rint = 0.053 |
| ω scans | θmax = 27.5° |
| Absorption correction: numerical see: Higashi (1999) | h = −15→12 |
| Tmin = 0.106, Tmax = 0.645 | k = −15→15 |
| 20072 measured reflections | l = −22→22 |
| 10077 independent reflections |
Refinement
| Refinement on F2 | 0 restraints |
| R[F2 > 2σ(F2)] = 0.042 | H-atom parameters constrained |
| wR(F2) = 0.106 | w = 1/[σ2(Fo2) + (0.0442P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 0.97 | (Δ/σ)max < 0.001 |
| 10077 reflections | Δρmax = 3.26 e Å−3 |
| 416 parameters | Δρmin = −2.53 e Å−3 |
Special details
| Geometry. ENTER SPECIAL DETAILS OF THE MOLECULAR GEOMETRY |
| Refinement. Refinement was performed using all reflections. The weighted R-factor (wR) and goodness of fit (S) are based on F2. R-factor (gt) are based on F. The threshold expression of F2 > 2.0 σ(F2) is used only for calculating R-factor (gt). |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Pb1 | 0.24494 (2) | 0.239633 (18) | −0.011473 (16) | 0.03270 (15) | |
| Pb2 | 0.74492 (2) | 0.254601 (18) | −0.011604 (16) | 0.03274 (15) | |
| Br1 | −0.00101 (6) | 0.18802 (6) | 0.00154 (5) | 0.04426 (17) | |
| Br2 | 0.31464 (7) | 0.27662 (6) | 0.18009 (5) | 0.04282 (16) | |
| Br3 | 0.18198 (7) | 0.20941 (6) | −0.18389 (5) | 0.04710 (18) | |
| Br4 | 0.18722 (7) | 0.49165 (5) | −0.00100 (5) | 0.04628 (18) | |
| Br5 | 0.31258 (7) | −0.00779 (5) | 0.00106 (5) | 0.04622 (18) | |
| Br6 | 0.49894 (6) | 0.31257 (6) | 0.00149 (5) | 0.04407 (18) | |
| Br7 | 0.81414 (7) | 0.31351 (6) | 0.18005 (5) | 0.04330 (17) | |
| Br8 | 0.68260 (7) | 0.19889 (6) | −0.18383 (5) | 0.04685 (17) | |
| N1 | 0.1391 (5) | 0.0296 (4) | 0.1519 (3) | 0.0467 (14) | |
| N2 | 0.6387 (5) | 0.5470 (4) | 0.1515 (3) | 0.0488 (15) | |
| N3 | 0.5666 (5) | 0.1314 (4) | 0.1523 (3) | 0.0452 (14) | |
| N4 | 0.0652 (5) | 0.4444 (4) | 0.1512 (3) | 0.0488 (15) | |
| C1 | 0.0360 (7) | 0.0491 (7) | 0.1886 (5) | 0.057 (2) | |
| C2 | 0.0764 (7) | 0.1128 (6) | 0.2729 (5) | 0.057 (2) | |
| C3 | 0.1601 (7) | 0.0445 (6) | 0.3284 (4) | 0.0496 (19) | |
| C4 | 0.2817 (8) | 0.0695 (7) | 0.3537 (5) | 0.061 (2) | |
| C5 | 0.3586 (10) | 0.0075 (9) | 0.4031 (6) | 0.083 (3) | |
| C6 | 0.3178 (12) | −0.0828 (9) | 0.4286 (5) | 0.092 (3) | |
| C7 | 0.1951 (13) | −0.1109 (8) | 0.4068 (6) | 0.093 (3) | |
| C8 | 0.1187 (9) | −0.0466 (7) | 0.3569 (5) | 0.069 (2) | |
| C9 | 0.5357 (7) | 0.5449 (7) | 0.1881 (5) | 0.056 (2) | |
| C10 | 0.5782 (7) | 0.5256 (6) | 0.2735 (4) | 0.055 (2) | |
| C11 | 0.6609 (8) | 0.6222 (6) | 0.3296 (4) | 0.052 (2) | |
| C12 | 0.6160 (9) | 0.7258 (7) | 0.3569 (5) | 0.068 (2) | |
| C13 | 0.6902 (13) | 0.8155 (8) | 0.4068 (6) | 0.090 (3) | |
| C14 | 0.8131 (12) | 0.8005 (9) | 0.4275 (5) | 0.092 (3) | |
| C15 | 0.8591 (10) | 0.6986 (9) | 0.4008 (5) | 0.080 (3) | |
| C16 | 0.7830 (9) | 0.6098 (8) | 0.3539 (5) | 0.064 (2) | |
| C17 | 0.5851 (7) | 0.0204 (6) | 0.1850 (4) | 0.057 (2) | |
| C18 | 0.5733 (8) | 0.0381 (6) | 0.2699 (4) | 0.058 (2) | |
| C19 | 0.6595 (7) | 0.1290 (6) | 0.3289 (4) | 0.0483 (19) | |
| C20 | 0.6198 (8) | 0.2342 (7) | 0.3589 (5) | 0.063 (2) | |
| C21 | 0.6982 (12) | 0.3185 (8) | 0.4121 (6) | 0.084 (3) | |
| C22 | 0.8178 (11) | 0.2988 (9) | 0.4339 (5) | 0.085 (3) | |
| C23 | 0.8600 (9) | 0.1948 (9) | 0.4045 (5) | 0.079 (2) | |
| C24 | 0.7809 (8) | 0.1109 (8) | 0.3529 (5) | 0.063 (2) | |
| C25 | 0.0873 (7) | 0.5722 (5) | 0.1864 (5) | 0.055 (2) | |
| C26 | 0.0736 (8) | 0.5965 (6) | 0.2690 (5) | 0.061 (2) | |
| C27 | 0.1587 (8) | 0.5336 (6) | 0.3276 (4) | 0.053 (2) | |
| C28 | 0.2787 (8) | 0.5620 (8) | 0.3514 (5) | 0.067 (2) | |
| C29 | 0.3581 (9) | 0.5032 (9) | 0.4037 (6) | 0.083 (3) | |
| C30 | 0.3166 (12) | 0.4151 (9) | 0.4318 (5) | 0.094 (3) | |
| C31 | 0.1934 (12) | 0.3866 (8) | 0.4114 (6) | 0.088 (3) | |
| C32 | 0.1190 (9) | 0.4465 (7) | 0.3589 (5) | 0.071 (2) | |
| H1 | 0.1122 | −0.0084 | 0.1017 | 0.056* | |
| H2 | 0.1928 | −0.0124 | 0.1804 | 0.056* | |
| H3 | 0.1730 | 0.0983 | 0.1519 | 0.056* | |
| H4 | −0.0232 | 0.0936 | 0.1568 | 0.069* | |
| H5 | −0.0017 | −0.0257 | 0.1878 | 0.069* | |
| H6 | 0.0068 | 0.1303 | 0.2925 | 0.068* | |
| H7 | 0.1164 | 0.1863 | 0.2737 | 0.068* | |
| H8 | 0.3126 | 0.1310 | 0.3363 | 0.073* | |
| H9 | 0.4401 | 0.0280 | 0.4193 | 0.100* | |
| H10 | 0.3710 | −0.1266 | 0.4606 | 0.110* | |
| H11 | 0.1653 | −0.1716 | 0.4255 | 0.111* | |
| H12 | 0.0369 | −0.0653 | 0.3419 | 0.082* | |
| H13 | 0.6115 | 0.5585 | 0.1010 | 0.059* | |
| H14 | 0.6741 | 0.4792 | 0.1523 | 0.059* | |
| H15 | 0.6913 | 0.6046 | 0.1795 | 0.059* | |
| H16 | 0.4964 | 0.6184 | 0.1865 | 0.068* | |
| H17 | 0.4778 | 0.4830 | 0.1570 | 0.068* | |
| H18 | 0.6194 | 0.4531 | 0.2745 | 0.066* | |
| H19 | 0.5090 | 0.5168 | 0.2932 | 0.066* | |
| H20 | 0.5339 | 0.7351 | 0.3414 | 0.082* | |
| H21 | 0.6592 | 0.8845 | 0.4262 | 0.108* | |
| H22 | 0.8648 | 0.8611 | 0.4602 | 0.111* | |
| H23 | 0.9414 | 0.6899 | 0.4145 | 0.096* | |
| H24 | 0.8139 | 0.5390 | 0.3377 | 0.077* | |
| H25 | 0.5738 | 0.1194 | 0.1024 | 0.054* | |
| H26 | 0.6213 | 0.1856 | 0.1829 | 0.054* | |
| H27 | 0.4938 | 0.1555 | 0.1519 | 0.054* | |
| H28 | 0.6641 | −0.0065 | 0.1848 | 0.068* | |
| H29 | 0.5263 | −0.0390 | 0.1512 | 0.068* | |
| H30 | 0.4923 | 0.0601 | 0.2687 | 0.070* | |
| H31 | 0.5849 | −0.0357 | 0.2891 | 0.070* | |
| H32 | 0.5387 | 0.2485 | 0.3430 | 0.075* | |
| H33 | 0.6700 | 0.3882 | 0.4331 | 0.101* | |
| H34 | 0.8713 | 0.3562 | 0.4688 | 0.102* | |
| H35 | 0.9414 | 0.1816 | 0.4196 | 0.094* | |
| H36 | 0.8092 | 0.0403 | 0.3336 | 0.076* | |
| H37 | 0.0738 | 0.4324 | 0.1018 | 0.059* | |
| H38 | −0.0089 | 0.4220 | 0.1496 | 0.059* | |
| H39 | 0.1175 | 0.4033 | 0.1816 | 0.059* | |
| H40 | 0.1676 | 0.5967 | 0.1874 | 0.066* | |
| H41 | 0.0312 | 0.6171 | 0.1526 | 0.066* | |
| H42 | −0.0078 | 0.5744 | 0.2670 | 0.073* | |
| H43 | 0.0858 | 0.6798 | 0.2886 | 0.073* | |
| H44 | 0.3076 | 0.6224 | 0.3321 | 0.080* | |
| H45 | 0.4395 | 0.5242 | 0.4194 | 0.099* | |
| H46 | 0.3702 | 0.3729 | 0.4649 | 0.113* | |
| H47 | 0.1636 | 0.3291 | 0.4329 | 0.105* | |
| H48 | 0.0373 | 0.4270 | 0.3438 | 0.086* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Pb1 | 0.027 (12) | 0.02690 (13) | 0.04648 (18) | 0.00509 (10) | 0.01243 (12) | 0.01035 (11) |
| Pb2 | 0.027 (12) | 0.02591 (13) | 0.04690 (18) | 0.00448 (10) | 0.01214 (12) | 0.00715 (11) |
| Br1 | 0.0289 (3) | 0.0460 (4) | 0.0576 (5) | 0.0041 (2) | 0.0130 (3) | 0.0062 (3) |
| Br2 | 0.0381 (4) | 0.0421 (3) | 0.0472 (4) | 0.0046 (3) | 0.0104 (3) | 0.0071 (3) |
| Br3 | 0.0522 (4) | 0.0434 (4) | 0.0430 (4) | −0.0007 (3) | 0.0087 (3) | 0.0074 (3) |
| Br4 | 0.0558 (4) | 0.0270 (3) | 0.0602 (4) | 0.0065 (3) | 0.0206 (4) | 0.0115 (3) |
| Br5 | 0.0557 (4) | 0.0268 (3) | 0.0604 (4) | 0.0064 (3) | 0.0212 (4) | 0.0105 (3) |
| Br6 | 0.0276 (3) | 0.0502 (4) | 0.0577 (5) | 0.0072 (3) | 0.0131 (3) | 0.0163 (3) |
| Br7 | 0.0384 (4) | 0.0435 (3) | 0.0482 (4) | 0.0037 (3) | 0.0105 (3) | 0.0109 (3) |
| Br8 | 0.0509 (4) | 0.0438 (4) | 0.0432 (4) | 0.0082 (3) | 0.0077 (3) | 0.0086 (3) |
| N1 | 0.053 (4) | 0.043 (3) | 0.041 (3) | 0.010 (2) | 0.006 (3) | 0.007 (2) |
| N2 | 0.052 (4) | 0.043 (3) | 0.050 (4) | 0.003 (2) | 0.009 (3) | 0.012 (2) |
| N3 | 0.036 (3) | 0.053 (3) | 0.048 (3) | −0.001 (2) | 0.015 (3) | 0.008 (2) |
| N4 | 0.044 (3) | 0.051 (3) | 0.054 (4) | 0.009 (2) | 0.016 (3) | 0.011 (3) |
| C1 | 0.046 (4) | 0.067 (5) | 0.063 (5) | 0.010 (4) | 0.014 (4) | 0.024 (4) |
| C2 | 0.060 (5) | 0.062 (5) | 0.056 (5) | 0.013 (4) | 0.027 (4) | 0.012 (4) |
| C3 | 0.060 (5) | 0.049 (4) | 0.041 (4) | 0.006 (3) | 0.020 (4) | 0.001 (3) |
| C4 | 0.068 (6) | 0.064 (5) | 0.051 (5) | 0.000 (4) | 0.016 (4) | 0.009 (4) |
| C5 | 0.072 (7) | 0.108 (8) | 0.058 (6) | 0.003 (6) | 0.000 (5) | 0.014 (5) |
| C6 | 0.118 (11) | 0.090 (8) | 0.042 (5) | 0.026 (7) | −0.016 (6) | 0.004 (5) |
| C7 | 0.163 (13) | 0.055 (6) | 0.056 (6) | −0.003 (6) | 0.019 (7) | 0.019 (4) |
| C8 | 0.085 (7) | 0.067 (5) | 0.055 (5) | −0.010 (4) | 0.020 (5) | 0.011 (4) |
| C9 | 0.040 (4) | 0.061 (5) | 0.060 (5) | 0.005 (3) | 0.008 (4) | −0.003 (4) |
| C10 | 0.052 (5) | 0.066 (5) | 0.050 (5) | −0.004 (4) | 0.021 (4) | 0.005 (4) |
| C11 | 0.064 (5) | 0.054 (4) | 0.047 (5) | 0.010 (4) | 0.024 (4) | 0.018 (3) |
| C12 | 0.076 (7) | 0.074 (6) | 0.059 (6) | 0.019 (5) | 0.022 (5) | 0.018 (4) |
| C13 | 0.145 (11) | 0.058 (6) | 0.062 (7) | 0.020 (6) | 0.023 (7) | 0.005 (5) |
| C14 | 0.129 (12) | 0.079 (7) | 0.045 (5) | −0.028 (7) | −0.009 (6) | −0.002 (5) |
| C15 | 0.081 (8) | 0.106 (8) | 0.049 (6) | −0.006 (6) | 0.016 (5) | 0.011 (5) |
| C16 | 0.075 (7) | 0.073 (6) | 0.051 (5) | 0.020 (5) | 0.026 (5) | 0.011 (4) |
| C17 | 0.066 (5) | 0.041 (4) | 0.060 (5) | 0.013 (3) | 0.007 (4) | 0.015 (3) |
| C18 | 0.068 (6) | 0.059 (5) | 0.050 (5) | −0.003 (4) | 0.011 (4) | 0.024 (4) |
| C19 | 0.057 (5) | 0.052 (4) | 0.039 (4) | 0.002 (3) | 0.012 (4) | 0.018 (3) |
| C20 | 0.066 (6) | 0.070 (5) | 0.054 (5) | 0.014 (4) | 0.017 (4) | 0.017 (4) |
| C21 | 0.138 (11) | 0.057 (6) | 0.054 (6) | 0.011 (6) | 0.024 (7) | 0.003 (4) |
| C22 | 0.108 (10) | 0.087 (7) | 0.043 (5) | −0.021 (6) | −0.006 (6) | 0.006 (5) |
| C23 | 0.055 (6) | 0.113 (8) | 0.060 (6) | −0.007 (6) | 0.007 (5) | 0.007 (6) |
| C24 | 0.062 (6) | 0.077 (6) | 0.049 (5) | 0.014 (4) | 0.014 (4) | 0.009 (4) |
| C25 | 0.057 (5) | 0.037 (4) | 0.064 (5) | −0.002 (3) | 0.008 (4) | 0.003 (3) |
| C26 | 0.067 (6) | 0.048 (4) | 0.061 (5) | 0.017 (4) | 0.012 (4) | −0.005 (4) |
| C27 | 0.068 (6) | 0.047 (4) | 0.048 (5) | 0.005 (3) | 0.029 (4) | 0.000 (3) |
| C28 | 0.061 (6) | 0.086 (6) | 0.058 (5) | 0.004 (4) | 0.019 (5) | 0.020 (4) |
| C29 | 0.065 (7) | 0.113 (8) | 0.059 (6) | 0.003 (6) | 0.007 (5) | 0.003 (6) |
| C30 | 0.135 (11) | 0.083 (7) | 0.045 (6) | 0.043 (7) | −0.002 (7) | 0.004 (5) |
| C31 | 0.136 (11) | 0.063 (6) | 0.057 (6) | −0.005 (6) | 0.014 (7) | 0.011 (5) |
| C32 | 0.077 (7) | 0.069 (6) | 0.064 (6) | −0.012 (5) | 0.017 (5) | 0.004 (4) |
Geometric parameters (Å, °)
| Pb1—Br3 | 2.8786 (8) | C31—C32 | 1.365 (14) |
| Pb1—Br4 | 2.9927 (7) | N1—H1 | 0.890 |
| Pb1—Br1 | 2.9957 (7) | N1—H2 | 0.890 |
| Pb1—Br6 | 3.0080 (7) | N1—H3 | 0.890 |
| Pb1—Br5 | 3.0095 (7) | N2—H13 | 0.890 |
| Pb1—Br2 | 3.1965 (8) | N2—H14 | 0.890 |
| Pb2—Br8 | 2.8755 (8) | N2—H15 | 0.890 |
| Pb2—Br5i | 2.9935 (6) | N3—H25 | 0.890 |
| Pb2—Br6 | 2.9957 (7) | N3—H26 | 0.890 |
| Pb2—Br1ii | 3.0082 (7) | N3—H27 | 0.890 |
| Pb2—Br4iii | 3.0110 (7) | N4—H37 | 0.890 |
| Pb2—Br7 | 3.1982 (8) | N4—H38 | 0.890 |
| Br1—Pb2iv | 3.0082 (7) | N4—H39 | 0.890 |
| Br4—Pb2iii | 3.0110 (7) | C1—H4 | 0.970 |
| Br5—Pb2i | 2.9935 (6) | C1—H5 | 0.970 |
| N1—C1 | 1.507 (9) | C2—H6 | 0.970 |
| N2—C9 | 1.505 (9) | C2—H7 | 0.970 |
| N3—C17 | 1.491 (8) | C4—H8 | 0.930 |
| N4—C25 | 1.505 (8) | C5—H9 | 0.930 |
| C1—C2 | 1.491 (11) | C6—H10 | 0.930 |
| C2—C3 | 1.506 (11) | C7—H11 | 0.930 |
| C3—C4 | 1.377 (12) | C8—H12 | 0.930 |
| C3—C8 | 1.383 (10) | C9—H16 | 0.970 |
| C4—C5 | 1.364 (13) | C9—H17 | 0.970 |
| C5—C6 | 1.342 (13) | C10—H18 | 0.970 |
| C6—C7 | 1.395 (15) | C10—H19 | 0.970 |
| C7—C8 | 1.382 (14) | C12—H20 | 0.930 |
| C9—C10 | 1.502 (11) | C13—H21 | 0.930 |
| C10—C11 | 1.509 (11) | C14—H22 | 0.930 |
| C11—C12 | 1.377 (11) | C15—H23 | 0.930 |
| C11—C16 | 1.381 (12) | C16—H24 | 0.930 |
| C12—C13 | 1.374 (14) | C17—H28 | 0.970 |
| C13—C14 | 1.393 (16) | C17—H29 | 0.970 |
| C14—C15 | 1.364 (14) | C18—H30 | 0.970 |
| C15—C16 | 1.361 (13) | C18—H31 | 0.970 |
| C17—C18 | 1.516 (11) | C20—H32 | 0.930 |
| C18—C19 | 1.506 (11) | C21—H33 | 0.930 |
| C19—C20 | 1.380 (11) | C22—H34 | 0.930 |
| C19—C24 | 1.385 (12) | C23—H35 | 0.930 |
| C20—C21 | 1.379 (13) | C24—H36 | 0.930 |
| C21—C22 | 1.368 (15) | C25—H40 | 0.970 |
| C22—C23 | 1.378 (13) | C25—H41 | 0.970 |
| C23—C24 | 1.369 (12) | C26—H42 | 0.970 |
| C25—C26 | 1.483 (11) | C26—H43 | 0.970 |
| C26—C27 | 1.507 (12) | C28—H44 | 0.930 |
| C27—C28 | 1.366 (12) | C29—H45 | 0.930 |
| C27—C32 | 1.365 (10) | C30—H46 | 0.930 |
| C28—C29 | 1.382 (13) | C31—H47 | 0.930 |
| C29—C30 | 1.350 (13) | C32—H48 | 0.930 |
| C30—C31 | 1.403 (15) | ||
| Br1···N1 | 3.507 (5) | H10···H44ix | 3.328 |
| Br1···N1v | 3.412 (5) | H11···C21vii | 3.569 |
| Br1···N4 | 3.564 (5) | H11···C22vii | 3.047 |
| Br2···N1 | 3.411 (5) | H11···C23vii | 3.135 |
| Br2···N3 | 3.486 (6) | H11···C26ix | 3.454 |
| Br2···N4 | 3.462 (5) | H11···C27ix | 3.563 |
| Br3···N2iii | 3.393 (5) | H11···C28ix | 3.551 |
| Br4···N4 | 3.466 (7) | H11···H23xii | 3.005 |
| Br4···N4vi | 3.546 (5) | H11···H34vii | 3.161 |
| Br5···N3 | 3.566 (5) | H11···H35vii | 3.301 |
| Br5···N3i | 3.473 (6) | H11···H43ix | 2.654 |
| Br6···N2 | 3.510 (5) | H11···H44ix | 3.366 |
| Br6···N2iii | 3.402 (6) | H12···Br3v | 3.408 |
| Br6···N3 | 3.577 (6) | H12···H22xii | 3.448 |
| Br7···N2 | 3.416 (5) | H12···H23xii | 3.596 |
| Br7···N3 | 3.448 (5) | H12···H35iv | 3.295 |
| Br7···N4ii | 3.483 (6) | H12···H36iv | 2.893 |
| Br8···N1i | 3.395 (5) | H12···H43ix | 3.057 |
| N1···Br1 | 3.507 (5) | H13···Br3iii | 3.459 |
| N1···Br1v | 3.412 (5) | H13···Br4iii | 3.284 |
| N1···Br2 | 3.411 (5) | H13···Br6 | 3.171 |
| N1···Br8i | 3.395 (5) | H13···Br6iii | 2.604 |
| N2···Br3iii | 3.393 (5) | H14···Br4iii | 3.522 |
| N2···Br6 | 3.510 (5) | H14···Br6 | 3.209 |
| N2···Br6iii | 3.402 (6) | H14···Br7 | 2.544 |
| N2···Br7 | 3.416 (5) | H15···Br3iii | 2.596 |
| N3···Br2 | 3.486 (6) | H15···H42ii | 3.459 |
| N3···Br5 | 3.566 (5) | H16···Br6iii | 3.540 |
| N3···Br5i | 3.473 (6) | H16···Br8iii | 2.968 |
| N3···Br6 | 3.577 (6) | H17···Br2 | 3.203 |
| N3···Br7 | 3.448 (5) | H17···Br6 | 3.166 |
| N4···Br1 | 3.564 (5) | H18···Br7 | 3.412 |
| N4···Br2 | 3.462 (5) | H18···C20 | 3.150 |
| N4···Br4 | 3.466 (7) | H18···C21 | 3.031 |
| N4···Br4vi | 3.546 (5) | H18···H26 | 3.259 |
| N4···Br7iv | 3.483 (6) | H18···H32 | 3.090 |
| Br1···H1 | 3.181 | H18···H33 | 2.914 |
| Br1···H1v | 2.608 | H19···Br2 | 3.547 |
| Br1···H3 | 3.189 | H19···C28 | 3.128 |
| Br1···H4 | 3.177 | H19···C29 | 2.968 |
| Br1···H5v | 3.550 | H19···H32 | 3.371 |
| Br1···H37 | 3.073 | H19···H33 | 3.240 |
| Br1···H38 | 3.459 | H19···H44 | 2.833 |
| Br2···H3 | 2.544 | H19···H45 | 2.545 |
| Br2···H7 | 3.426 | H20···Br8iii | 3.392 |
| Br2···H8 | 3.462 | H20···H10x | 3.403 |
| Br2···H17 | 3.203 | H20···H31x | 3.066 |
| Br2···H19 | 3.547 | H20···H44 | 2.889 |
| Br2···H27 | 2.623 | H20···H45 | 3.321 |
| Br2···H30 | 3.547 | H21···C5viii | 3.116 |
| Br2···H32 | 3.368 | H21···C6viii | 3.096 |
| Br2···H37 | 3.426 | H21···C18x | 3.445 |
| Br2···H39 | 2.726 | H21···C19x | 3.553 |
| Br3···H5v | 2.971 | H21···C24x | 3.564 |
| Br3···H12v | 3.408 | H21···H9x | 3.028 |
| Br3···H13iii | 3.459 | H21···H9viii | 3.243 |
| Br3···H15iii | 2.596 | H21···H10x | 3.564 |
| Br3···H28i | 2.961 | H21···H10viii | 3.244 |
| Br3···H41vi | 3.296 | H21···H31x | 2.649 |
| Br3···H42vi | 3.486 | H21···H36x | 3.389 |
| Br3···H43vi | 3.521 | H22···C8viii | 3.521 |
| Br4···H13iii | 3.284 | H22···C23xi | 3.562 |
| Br4···H14iii | 3.522 | H22···H12xiii | 3.448 |
| Br4···H37 | 2.679 | H22···H35xi | 2.747 |
| Br4···H37vi | 3.273 | H22···H36x | 3.236 |
| Br4···H38vi | 3.171 | H22···H47viii | 3.200 |
| Br4···H40 | 3.403 | H23···C22xi | 3.283 |
| Br4···H41vi | 3.218 | H23···C23xi | 3.442 |
| Br5···H1 | 3.283 | H23···C26ii | 3.360 |
| Br5···H3 | 3.524 | H23···H11xiii | 3.005 |
| Br5···H25 | 3.281 | H23···H12xiii | 3.596 |
| Br5···H25i | 2.693 | H23···H34xi | 2.678 |
| Br5···H27 | 3.221 | H23···H35xi | 2.998 |
| Br5···H28i | 3.380 | H23···H42ii | 2.924 |
| Br5···H29 | 3.166 | H23···H43ii | 3.107 |
| Br6···H13 | 3.171 | H23···H47viii | 3.269 |
| Br6···H13iii | 2.604 | H23···H48ii | 3.409 |
| Br6···H14 | 3.209 | H24···Br7 | 3.485 |
| Br6···H16iii | 3.540 | H24···C21 | 3.486 |
| Br6···H17 | 3.166 | H24···C22 | 3.490 |
| Br6···H25 | 3.071 | H24···H33 | 3.342 |
| Br6···H27 | 3.466 | H24···H34 | 3.326 |
| Br7···H4ii | 3.216 | H24···H42ii | 2.751 |
| Br7···H6ii | 3.529 | H24···H48ii | 2.887 |
| Br7···H14 | 2.544 | H25···Br5 | 3.281 |
| Br7···H18 | 3.412 | H25···Br5i | 2.693 |
| Br7···H24 | 3.485 | H25···Br6 | 3.071 |
| Br7···H25 | 3.416 | H25···Br7 | 3.416 |
| Br7···H26 | 2.706 | H26···Br7 | 2.706 |
| Br7···H38ii | 2.632 | H26···H18 | 3.259 |
| Br7···H42ii | 3.545 | H27···Br2 | 2.623 |
| Br7···H48ii | 3.382 | H27···Br5 | 3.221 |
| Br8···H1i | 3.444 | H27···Br6 | 3.466 |
| Br8···H2i | 2.606 | H28···Br3i | 2.961 |
| Br8···H16iii | 2.968 | H28···Br5i | 3.380 |
| Br8···H20iii | 3.392 | H29···Br5 | 3.166 |
| Br8···H29i | 3.284 | H29···Br8i | 3.284 |
| Br8···H30i | 3.506 | H30···Br2 | 3.547 |
| Br8···H31i | 3.521 | H30···Br8i | 3.506 |
| Br8···H40iii | 2.965 | H30···C4 | 3.185 |
| C2···H35iv | 3.354 | H30···C5 | 3.291 |
| C2···H47 | 3.372 | H30···H2 | 3.444 |
| C3···H35iv | 3.594 | H30···H8 | 2.732 |
| C3···H47 | 3.502 | H30···H9 | 2.951 |
| C4···H30 | 3.185 | H31···Br8i | 3.521 |
| C4···H47 | 3.545 | H31···C12ix | 3.178 |
| C5···H9vii | 3.442 | H31···C13ix | 2.928 |
| C5···H10vii | 3.513 | H31···H9 | 3.184 |
| C5···H21viii | 3.116 | H31···H20ix | 3.066 |
| C5···H30 | 3.291 | H31···H21ix | 2.649 |
| C6···H9vii | 3.290 | H32···Br2 | 3.368 |
| C6···H21viii | 3.096 | H32···H8 | 2.925 |
| C6···H44ix | 3.550 | H32···H9 | 3.415 |
| C7···H43ix | 2.957 | H32···H18 | 3.090 |
| C7···H44ix | 3.554 | H32···H19 | 3.371 |
| C8···H22viii | 3.521 | H32···H45 | 3.580 |
| C8···H35iv | 3.572 | H32···H46 | 3.429 |
| C8···H43ix | 3.192 | H33···C10 | 3.380 |
| C10···H33 | 3.380 | H33···C11 | 3.501 |
| C10···H45 | 3.380 | H33···C16 | 3.533 |
| C11···H33 | 3.501 | H33···C29viii | 3.039 |
| C12···H31x | 3.178 | H33···C30viii | 2.982 |
| C12···H46viii | 3.473 | H33···C31viii | 3.481 |
| C13···H31x | 2.928 | H33···H18 | 2.914 |
| C13···H36x | 3.533 | H33···H19 | 3.240 |
| C13···H46viii | 3.588 | H33···H24 | 3.342 |
| C13···H47viii | 3.553 | H33···H45 | 3.082 |
| C14···H35xi | 3.325 | H33···H45viii | 3.208 |
| C14···H36x | 3.463 | H33···H46viii | 3.154 |
| C14···H47viii | 3.037 | H34···C15xi | 3.472 |
| C15···H34xi | 3.472 | H34···C31viii | 3.564 |
| C15···H35xi | 3.443 | H34···C32viii | 3.457 |
| C15···H42ii | 3.294 | H34···H11vii | 3.161 |
| C15···H47viii | 3.072 | H34···H23xi | 2.678 |
| C16···H33 | 3.533 | H34···H24 | 3.326 |
| C16···H42ii | 3.195 | H34···H48ii | 3.483 |
| C16···H47viii | 3.576 | H35···C2ii | 3.354 |
| C18···H9 | 3.407 | H35···C3ii | 3.594 |
| C18···H21ix | 3.445 | H35···C8ii | 3.572 |
| C19···H21ix | 3.553 | H35···C14xi | 3.325 |
| C20···H10vii | 3.574 | H35···C15xi | 3.443 |
| C20···H18 | 3.150 | H35···H6ii | 2.529 |
| C21···H11vii | 3.569 | H35···H11vii | 3.301 |
| C21···H18 | 3.031 | H35···H12ii | 3.295 |
| C21···H24 | 3.486 | H35···H22xi | 2.747 |
| C22···H11vii | 3.047 | H35···H23xi | 2.998 |
| C22···H23xi | 3.283 | H35···H47ii | 3.033 |
| C22···H24 | 3.490 | H36···C13ix | 3.533 |
| C23···H6ii | 2.944 | H36···C14ix | 3.463 |
| C23···H11vii | 3.135 | H36···H6ii | 2.833 |
| C23···H22xi | 3.562 | H36···H12ii | 2.893 |
| C23···H23xi | 3.442 | H36···H21ix | 3.389 |
| C24···H6ii | 3.106 | H36···H22ix | 3.236 |
| C24···H21ix | 3.564 | H37···Br1 | 3.073 |
| C26···H11x | 3.454 | H37···Br2 | 3.426 |
| C26···H23iv | 3.360 | H37···Br4 | 2.679 |
| C27···H11x | 3.563 | H37···Br4vi | 3.273 |
| C28···H11x | 3.551 | H38···Br1 | 3.459 |
| C28···H19 | 3.128 | H38···Br4vi | 3.171 |
| C29···H19 | 2.968 | H38···Br7iv | 2.632 |
| C29···H33viii | 3.039 | H39···Br2 | 2.726 |
| C29···H45viii | 3.416 | H39···H3 | 3.582 |
| C29···H46viii | 3.509 | H39···H7 | 3.217 |
| C30···H8 | 3.442 | H40···Br4 | 3.403 |
| C30···H33viii | 2.982 | H40···Br8iii | 2.965 |
| C30···H45viii | 3.277 | H41···Br3vi | 3.296 |
| C31···H7 | 3.007 | H41···Br4vi | 3.218 |
| C31···H8 | 3.489 | H42···Br3vi | 3.486 |
| C31···H33viii | 3.481 | H42···Br7iv | 3.545 |
| C31···H34viii | 3.564 | H42···C15iv | 3.294 |
| C32···H7 | 3.142 | H42···C16iv | 3.195 |
| C32···H34viii | 3.457 | H42···H15iv | 3.459 |
| H1···Br1 | 3.181 | H42···H23iv | 2.924 |
| H1···Br1v | 2.608 | H42···H24iv | 2.751 |
| H1···Br5 | 3.283 | H43···Br3vi | 3.521 |
| H1···Br8i | 3.444 | H43···C7x | 2.957 |
| H2···Br8i | 2.606 | H43···C8x | 3.192 |
| H2···H30 | 3.444 | H43···H11x | 2.654 |
| H3···Br1 | 3.189 | H43···H12x | 3.057 |
| H3···Br2 | 2.544 | H43···H23iv | 3.107 |
| H3···Br5 | 3.524 | H44···C6x | 3.550 |
| H3···H39 | 3.582 | H44···C7x | 3.554 |
| H4···Br1 | 3.177 | H44···H10x | 3.328 |
| H4···Br7iv | 3.216 | H44···H11x | 3.366 |
| H5···Br1v | 3.550 | H44···H19 | 2.833 |
| H5···Br3v | 2.971 | H44···H20 | 2.889 |
| H6···Br7iv | 3.529 | H45···C10 | 3.380 |
| H6···C23iv | 2.944 | H45···C29viii | 3.416 |
| H6···C24iv | 3.106 | H45···C30viii | 3.277 |
| H6···H35iv | 2.529 | H45···H19 | 2.545 |
| H6···H36iv | 2.833 | H45···H20 | 3.321 |
| H6···H47 | 3.229 | H45···H32 | 3.580 |
| H6···H48 | 3.411 | H45···H33 | 3.082 |
| H7···Br2 | 3.426 | H45···H33viii | 3.208 |
| H7···C31 | 3.007 | H45···H45viii | 2.949 |
| H7···C32 | 3.142 | H45···H46viii | 2.686 |
| H7···H39 | 3.217 | H46···C12viii | 3.473 |
| H7···H47 | 2.919 | H46···C13viii | 3.588 |
| H7···H48 | 3.108 | H46···C29viii | 3.509 |
| H8···Br2 | 3.462 | H46···H8 | 3.246 |
| H8···C30 | 3.442 | H46···H32 | 3.429 |
| H8···C31 | 3.489 | H46···H33viii | 3.154 |
| H8···H30 | 2.732 | H46···H45viii | 2.686 |
| H8···H32 | 2.925 | H47···C2 | 3.372 |
| H8···H46 | 3.246 | H47···C3 | 3.502 |
| H8···H47 | 3.387 | H47···C4 | 3.545 |
| H9···C5vii | 3.442 | H47···C13viii | 3.553 |
| H9···C6vii | 3.290 | H47···C14viii | 3.037 |
| H9···C18 | 3.407 | H47···C15viii | 3.072 |
| H9···H9vii | 2.979 | H47···C16viii | 3.576 |
| H9···H10vii | 2.696 | H47···H6 | 3.229 |
| H9···H21ix | 3.028 | H47···H7 | 2.919 |
| H9···H21viii | 3.243 | H47···H8 | 3.387 |
| H9···H30 | 2.951 | H47···H22viii | 3.200 |
| H9···H31 | 3.184 | H47···H23viii | 3.269 |
| H9···H32 | 3.415 | H47···H35iv | 3.033 |
| H10···C5vii | 3.513 | H48···Br7iv | 3.382 |
| H10···C20vii | 3.574 | H48···H6 | 3.411 |
| H10···H9vii | 2.696 | H48···H7 | 3.108 |
| H10···H20ix | 3.403 | H48···H23iv | 3.409 |
| H10···H21ix | 3.564 | H48···H24iv | 2.887 |
| H10···H21viii | 3.244 | H48···H34iv | 3.483 |
| Br3—Pb1—Br4 | 90.86 (2) | C17—N3—H27 | 109.5 |
| Br3—Pb1—Br1 | 96.54 (2) | H25—N3—H27 | 109.5 |
| Br4—Pb1—Br1 | 88.17 (2) | H26—N3—H27 | 109.5 |
| Br3—Pb1—Br6 | 91.58 (2) | C25—N4—H37 | 109.5 |
| Br4—Pb1—Br6 | 88.03 (2) | C25—N4—H38 | 109.5 |
| Br1—Pb1—Br6 | 171.08 (2) | H37—N4—H38 | 109.5 |
| Br3—Pb1—Br5 | 96.46 (2) | C25—N4—H39 | 109.5 |
| Br4—Pb1—Br5 | 172.68 (2) | H37—N4—H39 | 109.5 |
| Br1—Pb1—Br5 | 91.34 (2) | H38—N4—H39 | 109.5 |
| Br6—Pb1—Br5 | 91.41 (2) | C2—C1—H4 | 109.3 |
| Br3—Pb1—Br2 | 179.289 (18) | N1—C1—H4 | 109.3 |
| Br4—Pb1—Br2 | 88.44 (2) | C2—C1—H5 | 109.3 |
| Br1—Pb1—Br2 | 83.56 (2) | N1—C1—H5 | 109.3 |
| Br6—Pb1—Br2 | 88.28 (2) | H4—C1—H5 | 108.0 |
| Br5—Pb1—Br2 | 84.24 (2) | C1—C2—H6 | 109.0 |
| Br8—Pb2—Br5i | 91.00 (2) | C3—C2—H6 | 109.0 |
| Br8—Pb2—Br6 | 96.67 (2) | C1—C2—H7 | 109.0 |
| Br5i—Pb2—Br6 | 88.12 (2) | C3—C2—H7 | 109.0 |
| Br8—Pb2—Br1ii | 91.54 (2) | H6—C2—H7 | 107.8 |
| Br5i—Pb2—Br1ii | 87.98 (2) | C5—C4—H8 | 118.7 |
| Br6—Pb2—Br1ii | 170.97 (2) | C3—C4—H8 | 118.7 |
| Br8—Pb2—Br4iii | 96.35 (2) | C6—C5—H9 | 119.7 |
| Br5i—Pb2—Br4iii | 172.63 (2) | C4—C5—H9 | 119.7 |
| Br6—Pb2—Br4iii | 91.46 (2) | C5—C6—H10 | 120.1 |
| Br1ii—Pb2—Br4iii | 91.36 (2) | C7—C6—H10 | 120.1 |
| Br8—Pb2—Br7 | 179.356 (18) | C8—C7—H11 | 120.6 |
| Br5i—Pb2—Br7 | 88.37 (2) | C6—C7—H11 | 120.6 |
| Br6—Pb2—Br7 | 83.44 (2) | C7—C8—H12 | 119.0 |
| Br1ii—Pb2—Br7 | 88.31 (2) | C3—C8—H12 | 119.0 |
| Br4iii—Pb2—Br7 | 84.28 (2) | C10—C9—H16 | 109.4 |
| Pb1—Br1—Pb2iv | 150.77 (3) | N2—C9—H16 | 109.4 |
| Pb1—Br4—Pb2iii | 152.07 (3) | C10—C9—H17 | 109.4 |
| Pb2i—Br5—Pb1 | 152.15 (3) | N2—C9—H17 | 109.4 |
| Pb2—Br6—Pb1 | 150.86 (3) | H16—C9—H17 | 108.0 |
| C2—C1—N1 | 111.6 (7) | C9—C10—H18 | 108.7 |
| C1—C2—C3 | 112.9 (7) | C11—C10—H18 | 108.7 |
| C4—C3—C8 | 116.4 (8) | C9—C10—H19 | 108.7 |
| C4—C3—C2 | 121.9 (7) | C11—C10—H19 | 108.7 |
| C8—C3—C2 | 121.7 (8) | H18—C10—H19 | 107.6 |
| C5—C4—C3 | 122.5 (9) | C13—C12—H20 | 119.4 |
| C6—C5—C4 | 120.6 (11) | C11—C12—H20 | 119.4 |
| C5—C6—C7 | 119.8 (10) | C12—C13—H21 | 120.9 |
| C8—C7—C6 | 118.7 (9) | C14—C13—H21 | 120.9 |
| C7—C8—C3 | 122.0 (10) | C15—C14—H22 | 119.4 |
| C10—C9—N2 | 111.0 (7) | C13—C14—H22 | 119.4 |
| C9—C10—C11 | 114.4 (6) | C16—C15—H23 | 120.4 |
| C12—C11—C16 | 118.4 (9) | C14—C15—H23 | 120.4 |
| C12—C11—C10 | 120.5 (9) | C15—C16—H24 | 119.2 |
| C16—C11—C10 | 121.1 (8) | C11—C16—H24 | 119.2 |
| C13—C12—C11 | 121.3 (10) | N3—C17—H28 | 109.5 |
| C12—C13—C14 | 118.3 (10) | C18—C17—H28 | 109.5 |
| C15—C14—C13 | 121.2 (10) | N3—C17—H29 | 109.5 |
| C16—C15—C14 | 119.1 (11) | C18—C17—H29 | 109.5 |
| C15—C16—C11 | 121.6 (9) | H28—C17—H29 | 108.1 |
| N3—C17—C18 | 110.7 (6) | C19—C18—H30 | 108.5 |
| C19—C18—C17 | 114.9 (6) | C17—C18—H30 | 108.5 |
| C20—C19—C24 | 118.1 (8) | C19—C18—H31 | 108.5 |
| C20—C19—C18 | 120.7 (8) | C17—C18—H31 | 108.5 |
| C24—C19—C18 | 121.1 (8) | H30—C18—H31 | 107.5 |
| C21—C20—C19 | 121.1 (10) | C21—C20—H32 | 119.4 |
| C22—C21—C20 | 119.4 (10) | C19—C20—H32 | 119.4 |
| C21—C22—C23 | 120.6 (10) | C22—C21—H33 | 120.3 |
| C24—C23—C22 | 119.4 (10) | C20—C21—H33 | 120.3 |
| C23—C24—C19 | 121.3 (9) | C21—C22—H34 | 119.7 |
| C26—C25—N4 | 111.4 (6) | C23—C22—H34 | 119.7 |
| C25—C26—C27 | 114.2 (7) | C24—C23—H35 | 120.3 |
| C28—C27—C32 | 117.6 (9) | C22—C23—H35 | 120.3 |
| C28—C27—C26 | 121.0 (7) | C23—C24—H36 | 119.3 |
| C32—C27—C26 | 121.5 (8) | C19—C24—H36 | 119.3 |
| C27—C28—C29 | 121.6 (9) | C26—C25—H40 | 109.4 |
| C30—C29—C28 | 119.4 (11) | N4—C25—H40 | 109.4 |
| C29—C30—C31 | 120.6 (10) | C26—C25—H41 | 109.4 |
| C32—C31—C30 | 117.5 (10) | N4—C25—H41 | 109.4 |
| C27—C32—C31 | 123.1 (10) | H40—C25—H41 | 108.0 |
| C1—N1—H1 | 109.5 | C25—C26—H42 | 108.7 |
| C1—N1—H2 | 109.5 | C27—C26—H42 | 108.7 |
| H1—N1—H2 | 109.5 | C25—C26—H43 | 108.7 |
| C1—N1—H3 | 109.5 | C27—C26—H43 | 108.7 |
| H1—N1—H3 | 109.5 | H42—C26—H43 | 107.6 |
| H2—N1—H3 | 109.5 | C27—C28—H44 | 119.2 |
| C9—N2—H13 | 109.5 | C29—C28—H44 | 119.2 |
| C9—N2—H14 | 109.5 | C30—C29—H45 | 120.3 |
| H13—N2—H14 | 109.5 | C28—C29—H45 | 120.3 |
| C9—N2—H15 | 109.5 | C29—C30—H46 | 119.7 |
| H13—N2—H15 | 109.5 | C31—C30—H46 | 119.7 |
| H14—N2—H15 | 109.5 | C32—C31—H47 | 121.2 |
| C17—N3—H25 | 109.5 | C30—C31—H47 | 121.2 |
| C17—N3—H26 | 109.5 | C27—C32—H48 | 118.4 |
| H25—N3—H26 | 109.5 | C31—C32—H48 | 118.4 |
| Br3—Pb1—Br1—Pb2iv | −71.22 (6) | C9—C10—C11—C12 | 76.7 (9) |
| Br4—Pb1—Br1—Pb2iv | 19.43 (6) | C9—C10—C11—C16 | −102.4 (9) |
| Br5—Pb1—Br1—Pb2iv | −167.88 (6) | C16—C11—C12—C13 | 0.5 (13) |
| Br2—Pb1—Br1—Pb2iv | 108.07 (6) | C10—C11—C12—C13 | −178.7 (8) |
| Br3—Pb1—Br4—Pb2iii | −89.77 (6) | C11—C12—C13—C14 | 1.7 (14) |
| Br1—Pb1—Br4—Pb2iii | 173.72 (6) | C12—C13—C14—C15 | −1.5 (16) |
| Br6—Pb1—Br4—Pb2iii | 1.78 (6) | C13—C14—C15—C16 | −0.8 (15) |
| Br2—Pb1—Br4—Pb2iii | 90.11 (6) | C14—C15—C16—C11 | 3.0 (14) |
| Br3—Pb1—Br5—Pb2i | −92.15 (6) | C12—C11—C16—C15 | −2.9 (13) |
| Br1—Pb1—Br5—Pb2i | 4.58 (7) | C10—C11—C16—C15 | 176.3 (7) |
| Br6—Pb1—Br5—Pb2i | 176.10 (6) | N3—C17—C18—C19 | −58.9 (9) |
| Br2—Pb1—Br5—Pb2i | 87.97 (6) | C17—C18—C19—C20 | 109.3 (9) |
| Br8—Pb2—Br6—Pb1 | 71.35 (6) | C17—C18—C19—C24 | −69.1 (9) |
| Br5i—Pb2—Br6—Pb1 | −19.43 (6) | C24—C19—C20—C21 | −0.8 (12) |
| Br4iii—Pb2—Br6—Pb1 | 167.92 (6) | C18—C19—C20—C21 | −179.2 (7) |
| Br7—Pb2—Br6—Pb1 | −108.01 (6) | C19—C20—C21—C22 | 1.8 (13) |
| Br3—Pb1—Br6—Pb2 | −76.20 (6) | C20—C21—C22—C23 | −1.5 (15) |
| Br4—Pb1—Br6—Pb2 | −167.00 (6) | C21—C22—C23—C24 | 0.2 (15) |
| Br5—Pb1—Br6—Pb2 | 20.30 (6) | C22—C23—C24—C19 | 0.8 (14) |
| Br2—Pb1—Br6—Pb2 | 104.50 (6) | C20—C19—C24—C23 | −0.5 (12) |
| N1—C1—C2—C3 | −64.3 (9) | C18—C19—C24—C23 | 177.9 (7) |
| C1—C2—C3—C4 | 103.1 (9) | N4—C25—C26—C27 | 60.4 (10) |
| C1—C2—C3—C8 | −77.0 (10) | C25—C26—C27—C28 | 68.7 (11) |
| C8—C3—C4—C5 | 0.9 (13) | C25—C26—C27—C32 | −111.4 (9) |
| C2—C3—C4—C5 | −179.2 (9) | C32—C27—C28—C29 | 1.7 (14) |
| C3—C4—C5—C6 | 0.9 (16) | C26—C27—C28—C29 | −178.4 (9) |
| C4—C5—C6—C7 | −2.5 (17) | C27—C28—C29—C30 | 0.3 (16) |
| C5—C6—C7—C8 | 2.2 (17) | C28—C29—C30—C31 | −3.0 (17) |
| C6—C7—C8—C3 | −0.4 (16) | C29—C30—C31—C32 | 3.7 (16) |
| C4—C3—C8—C7 | −1.1 (13) | C28—C27—C32—C31 | −0.9 (14) |
| C2—C3—C8—C7 | 179.0 (9) | C26—C27—C32—C31 | 179.2 (9) |
| N2—C9—C10—C11 | 64.1 (9) | C30—C31—C32—C27 | −1.8 (16) |
Symmetry codes: (i) −x+1, −y, −z; (ii) x+1, y, z; (iii) −x+1, −y+1, −z; (iv) x−1, y, z; (v) −x, −y, −z; (vi) −x, −y+1, −z; (vii) −x+1, −y, −z+1; (viii) −x+1, −y+1, −z+1; (ix) x, y−1, z; (x) x, y+1, z; (xi) −x+2, −y+1, −z+1; (xii) x−1, y−1, z; (xiii) x+1, y+1, z.
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1···Br1 | 0.89 | 3.18 | 3.508 (5) | 104 |
| N1—H3···Br2 | 0.89 | 2.54 | 3.411 (5) | 165 |
| N2—H13···Br6 | 0.89 | 3.17 | 3.509 (5) | 105 |
| N2—H14···Br7 | 0.89 | 2.54 | 3.416 (5) | 167 |
| N3—H26···Br7 | 0.89 | 2.71 | 3.448 (6) | 142 |
| N3—H27···Br2 | 0.89 | 2.62 | 3.486 (6) | 164 |
| N4—H37···Br4 | 0.89 | 2.68 | 3.465 (5) | 148 |
| N4—H39···Br2 | 0.89 | 2.73 | 3.462 (6) | 140 |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: ZQ2004).
References
- Billing, D. G. & Lemmerer, A. (2003). Acta Cryst. E59, m381–m383.
- Bondi, A. (1964). J. Phys. Chem.68, 441–451.
- Burla, M. C., Camalli, M., Carrozzini, B., Cascarano, G. L., Giacovazzo, C., Polidori, G. & Spagna, R. (2003). J. Appl. Cryst.36, 1103.
- Chapuis, G., Kind, R. & Arend, H. (1976). Phys. Status Solidi A36, 285–295.
- Cheng, Z. Y., Wang, H. F., Quan, Z. W., Lin, C. K., Lin, J. & Han, Y. C. (2005). J. Cryst. Growth285, 352–357.
- Eijk, C. W. E. van der, de Haas, J. T. M., Rodnyi, P. A., Khodyuk, I. V., Shibuya, K., Nishikido, F. & Koshimizu, M. (2008). Conf. Rec. IEEE 2008 Nucl. Sci. Symp. Med. Img. Conf., Dresden, Germany, Oct. 19–25, N69-3.
- Era, M., Morimoto, S., Tsutsui, T. & Saito, S. (1995). Synth. Met.71, 2013–2014.
- Higashi, T. (1999). ABSCOR Rigaku Corporation, Japan.
- Huh, Y.-D., Kim, J.-H., Kweon, S. S., Kuk, W.-K., Hwang, C.-S., Hyun, J.-W., Kim, Y.-J. & Park, Y.-B. (2006). Curr. Appl. Phys.6, 219–223.
- Kishimoto, S., Shibuya, K., Nishikido, F., Koshimizu, M., Haruki, R. & Yoda, Y. (2008). Appl. Phys. Lett.93, 261901 1–3.
- Kitazawa, N. & Watanabe, Y. (2005). Surf. Coat. Technol.198, 9–13.
- Mitzi, D. B. (1999). J. Solid State Chem.145, 694–704.
- Palmer, D. (2009). CrystalMaker CrystalMaker Software Ltd, Yarnton, Oxfordshire, England.
- Rigaku Americas & Rigaku Corporation (2008). CrystalStructure Rigaku Americas, Texas, USA, and Rigaku Corporation, Tokyo, Japan.
- Rigaku Corporation (1998). PROCESS-AUTO Rigaku Corporation, Tokyo, Japan.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Westrip, S. P. (2009). publCIF In preparation.
- Willett, R. D. (1990). Acta Cryst. C46, 565–568.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S160053680903712X/zq2004sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S160053680903712X/zq2004Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report