Abstract
In previous studies it was found that renal cortical slices from rats with induced metabolic acidosis have an increased capacity to produce glucose, whereas cortical slices from rats with metabolic alkalosis manifest decreased gluconeogenesis. To evaluate the relative influence of extracellular fluid pH, [HCO3-], and carbon dioxide tension on renal gluconeogenesis, we observed glucose production by cortex from rats with induced respiratory acidosis, and by cortex taken from normal animals and incubated in acid and alkaline media.
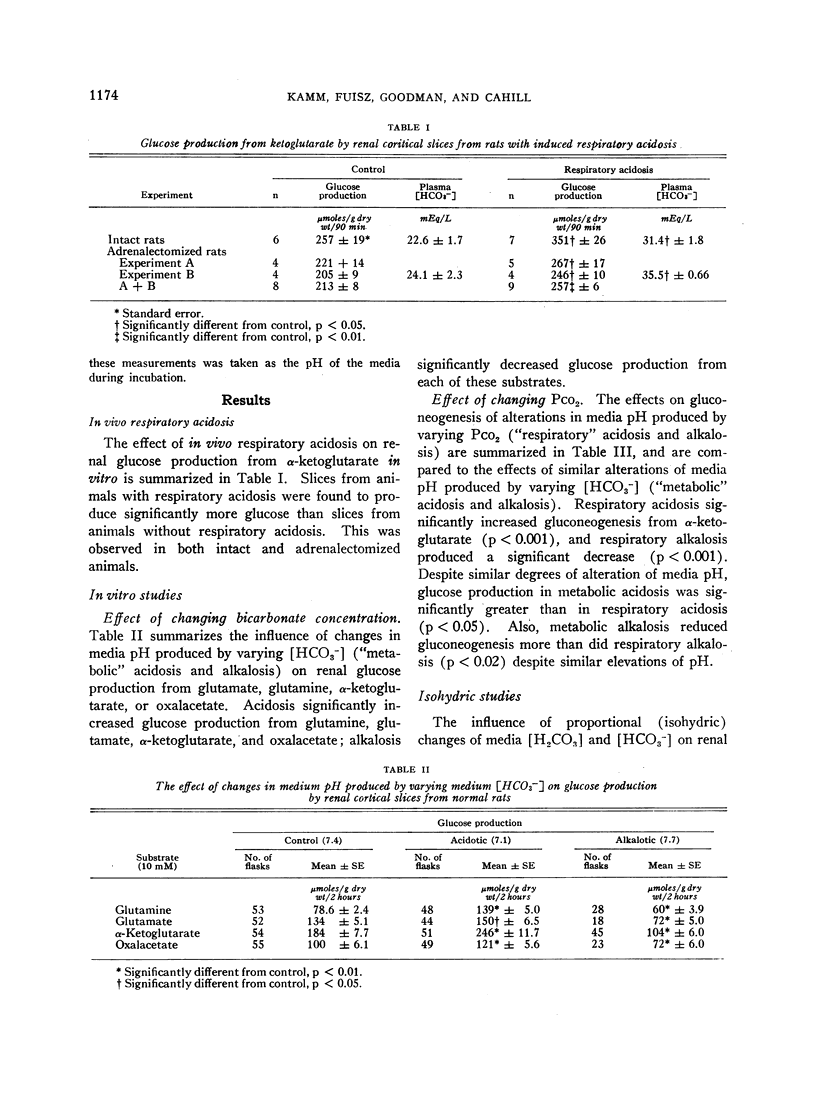
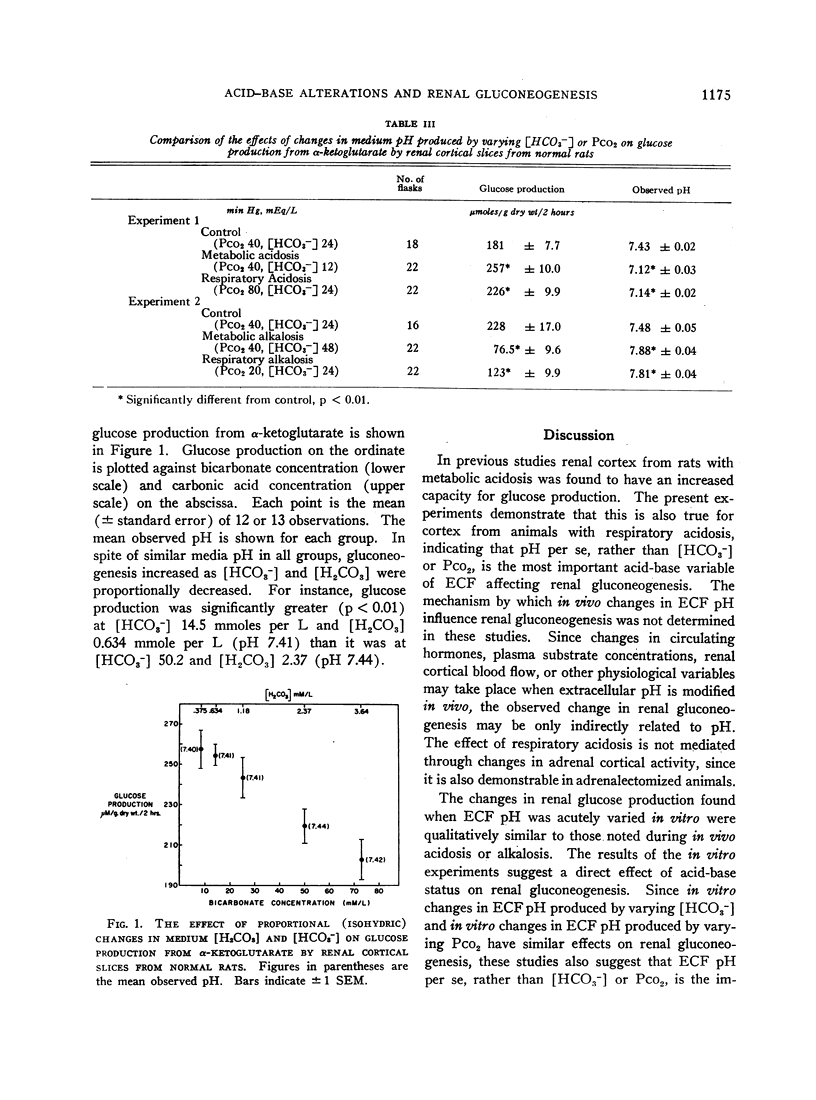
We found glucose production to be increased in cortex from rats with respiratory acidosis, as is the case in metabolic acidosis. Glucose production by slices from normal rats was increased in media made acidic by reducing [HCO3-], and decreased in media made alkaline by raising [HCO3-]. These effects were evident whether the gluconeogenic substrate employed was glutamine, glutamate, α-ketoglutarate, or oxalacetate. Glucose production was also increased in media made acidic by raising CO2 tension and decreased in media made alkaline by reducing CO2 tension. These data indicate that both in vivo and in vitro, pH, rather than CO2 tension or [HCO3-], is the most important acid-base variable affecting renal gluconeogenesis.
The findings suggest that a decrease in extracellular fluid pH enhances renal gluconeogenesis through direct stimulation of one of the rate-limiting reactions involved in the conversion of oxalacetate to glucose. We hypothesize that the resultant increase in the rate of removal of glutamate, a precursor of oxalacetate, may constitute an important step in the mechanism by which acidosis increases renal ammonia production.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aber G. M., Morris L. O., Housley E., Harris A. M. Bidirectional release of ammonium by the kidneys in patients with respiratory failure. Effect of increasing the concentration of inspired oxygen. Nephron. 1965;2(3):148–158. doi: 10.1159/000179400. [DOI] [PubMed] [Google Scholar]
- CARTER N. W., SELDIN D. W., TENG H. C. Tissue and renal response to chronic respiratory acidosis. J Clin Invest. 1959 Jun;38(6):949–960. doi: 10.1172/JCI103878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIES B. M. A., YUDKIN J. Studies in biochemical adaptation; the origin or urinary ammonia as indicated by the effect of chronic acidosis and alkalosis on some renal enzymes in the rat. Biochem J. 1952 Nov;52(3):407–412. doi: 10.1042/bj0520407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDSTEIN L. RELATION OF RENAL GLUTSMINE TRANSEMINASE-OMEGA-AMIDASE ACTIVITY TO AMMONIA EXCRETION IN THE RAT. Nature. 1964 Mar 21;201:1229–1230. doi: 10.1038/2011229a0. [DOI] [PubMed] [Google Scholar]
- Goodman A. D., Fuisz R. E., Cahill G. F., Jr Renal gluconeogenesis in acidosis, alkalosis, and potassium deficiency: its possible role in regulation of renal ammonia production. J Clin Invest. 1966 Apr;45(4):612–619. doi: 10.1172/JCI105375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RECTOR F. C., Jr, SELDIN D. W., COPENHAVER J. H. The mechanism of ammonia excretion during ammonium chloride acidosis. J Clin Invest. 1955 Jan;34(1):20–26. doi: 10.1172/JCI103058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARTZ W. B., BRACKETT N. C., Jr, COHEN J. J. THE RESPONSE OF EXTRACELLULAR HYDROGEN ION CONCENTRATION TO GRADED DEGREES OF CHRONIC HYPERCAPNIA: THE PHYSIOLOGIC LIMITS OF THE DEFENSE OF PH. J Clin Invest. 1965 Feb;44:291–301. doi: 10.1172/JCI105143. [DOI] [PMC free article] [PubMed] [Google Scholar]


