Abstract
Hamster intrinsic factor (IF) preparations markedly enhanced the uptake of 57cobalt-labeled cyanocobalamin (B12-57Co) by brush borders and microvillous membranes isolated from villous absorptive cells obtained from the distal but not the proximal half of hamster intestine. A similar effect was observed with rat and rabbit IF preparations, but IF preparations obtained from man, dog, and hog were ineffective. After fractionation of hamster IF preparations by gel filtration or ion exchange chromatography, the extent to which each fraction enhanced B12-57Co uptake by brush borders correlated closely with the vitamin B12 binding capacity of the fraction. IF-mediated attachment of B12-57Co to brush borders occurred rapidly, was not diminished by removal of glucose or oxygen from the incubation medium, and was not significantly altered when incubation temperatures were reduced from 37° C to 7° C. Marked reduction in uptake occurred, however, in the absence of divalent cations.
IF enhanced B12-57Co uptake by brush borders isolated from the proximal half of the intestine when these proximal brush borders were preincubated with supernatant fluid obtained after centrifugation of homogenates of distal intestinal mucosa at 28,500 g. The factor in this supernate responsible for the effect on proximal brush borders was shown to be particulate in nature upon centrifugation at speeds of 54,500 g or greater. The resultant pellet contained ribosomes and membranous fragments.
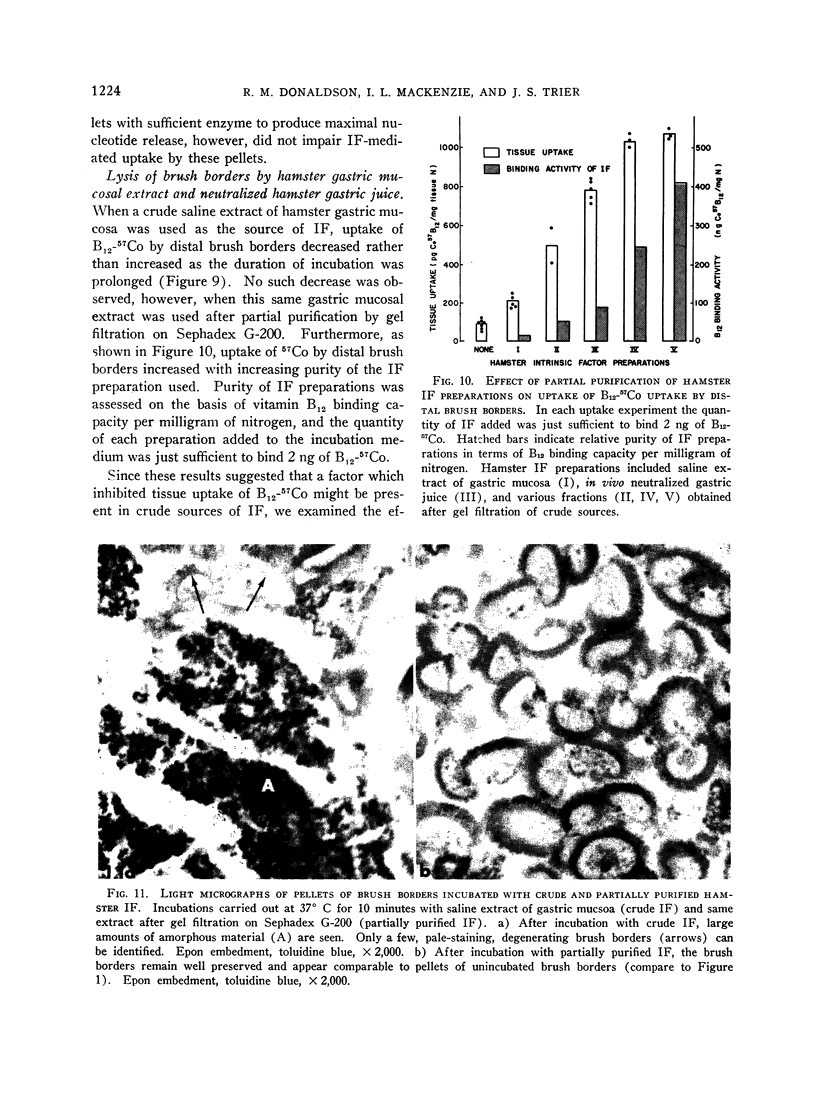
Prolonged incubation of brush borders with crude saline extracts of hamster gastric mucosa resulted in decreased uptake of B12-57Co and marked lysis of brush borders with concomitant release of tissue nitrogen. Neither lysis of brush borders nor decreased uptake of B12-57Co with prolonged incubation was observed when hamster IF was partially purified. Furthermore, uptake of B12-57Co by brush borders increased with increasing purity of the IF preparation used.
These results demonstrate IF-mediated attachment of B12-57Co to brush borders and microvillous membranes of hamster intestinal cells and provide further support for the presence of a specific receptor for IF-bound vitamin B12 at the microvillous surface of the intestinal cell. IF-mediated attachment to the intestinal cell surface appears to be facilitated by divalent cations and to result from adsorption rather than an energy-requiring enzymatic reaction. Crude sources of hamster IF contain a factor which causes lysis of brush borders in vitro and which may explain in part the inhibitory effects of IF excess previously observed in vitro.
Full text
PDF


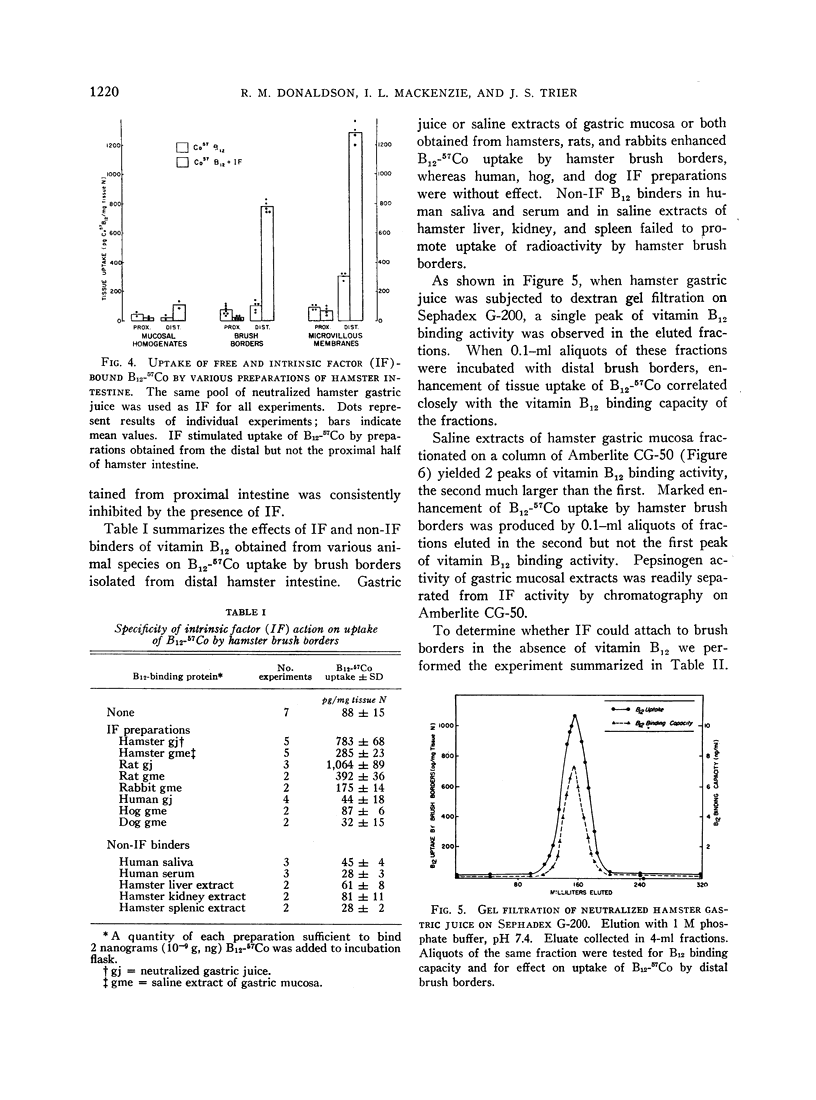
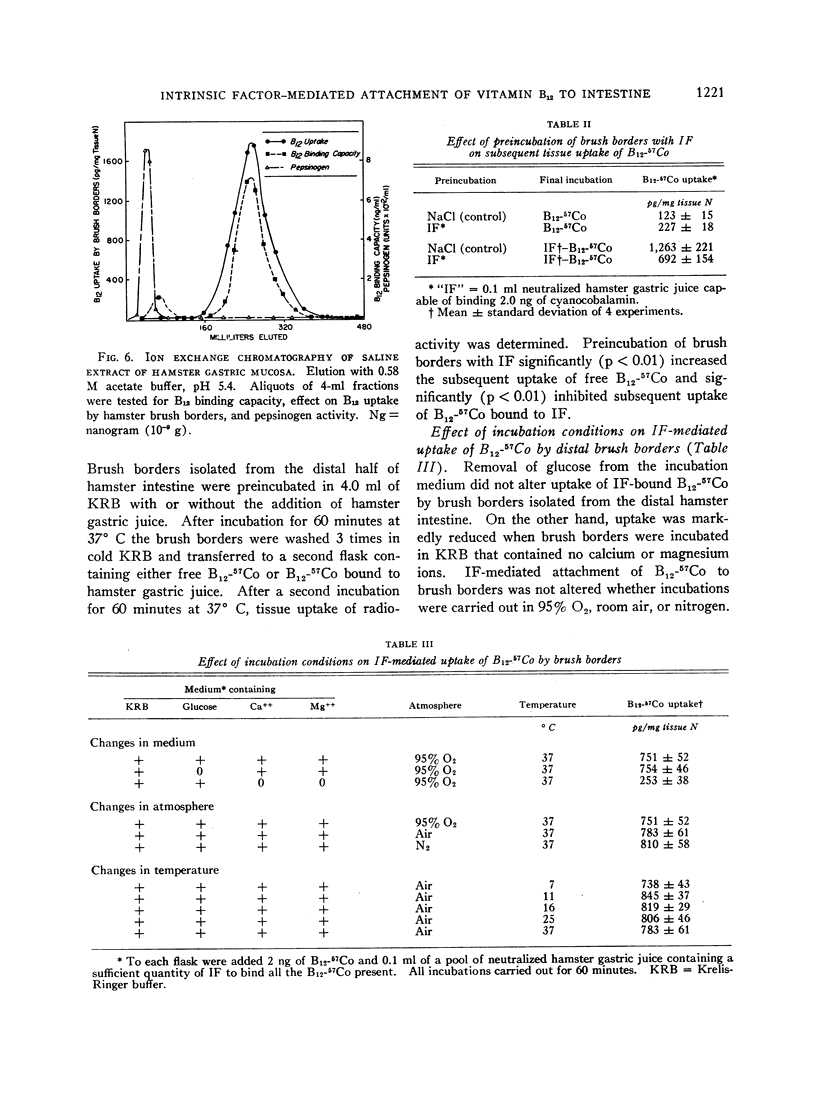
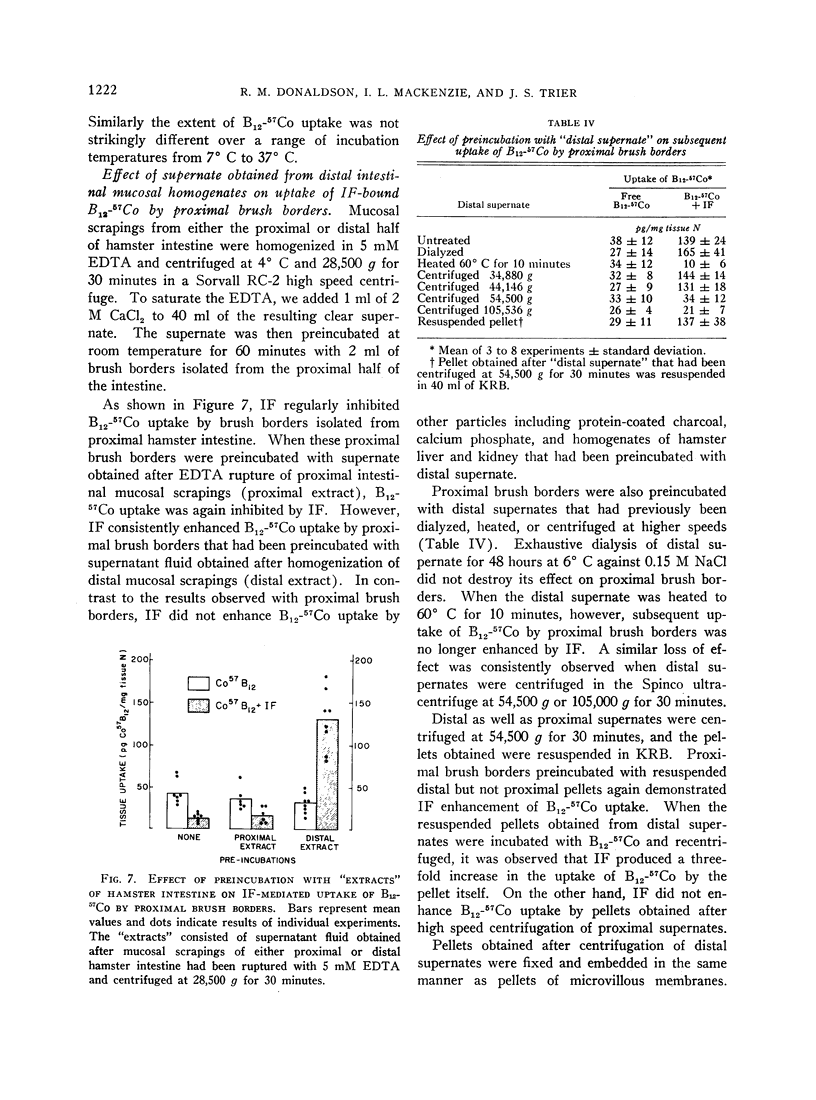
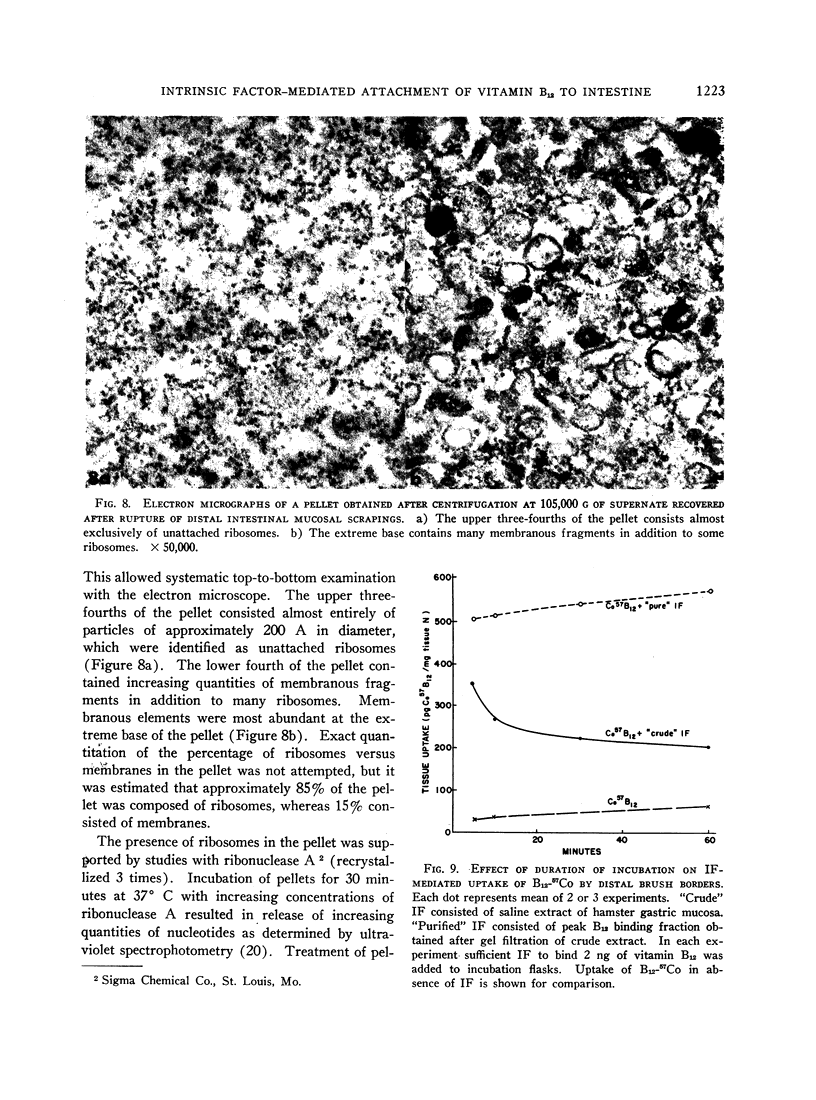




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOASS A., WILSON T. H. An assay for gastric intrinsic factor. Am J Physiol. 1963 Jan;204:97–100. doi: 10.1152/ajplegacy.1963.204.1.97. [DOI] [PubMed] [Google Scholar]
- BOOTH C. C., MOLLIN D. L. The site of absorption of vitamin B12 in man. Lancet. 1959 Jan 3;1(7062):18–21. doi: 10.1016/s0140-6736(59)90979-1. [DOI] [PubMed] [Google Scholar]
- CASTLE W. B. Factors involved in the absorption of vitamin B12. Gastroenterology. 1959 Oct;37:377–384. [PubMed] [Google Scholar]
- CHOSY J. J., SCHILLING R. F. Intrinsic factor studies. VII. The use of ion exchange chromatography, gel filtration, and ultrafiltration to purify the intrinsic factor of human gastric juice. J Lab Clin Med. 1963 Jun;61:907–916. [PubMed] [Google Scholar]
- GRASBECK R. Physiology and pathology of vitamin B12 absorption, distribution, and excretion. Adv Clin Chem. 1960;3:299–366. [PubMed] [Google Scholar]
- HERBERT V. Mechanism of intrinsic factor action in everted sacs of rat small intestine. J Clin Invest. 1959 Jan 1;38(1 Pt 1):102–109. doi: 10.1172/JCI103779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERBERT V. Studies of the mechanism of the effect of hog intrinsic factor concentrate on the uptake of vitamin B12 by rat liver slices. J Clin Invest. 1958 May;37(5):646–650. doi: 10.1172/JCI103648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S. The enteric surface coat on cat intestinal microvilli. J Cell Biol. 1965 Dec;27(3):475–491. doi: 10.1083/jcb.27.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALNITSKY G., HUMMEL J. P., DIERKS C. [Some factors which affect the enzymatic digestion of ribonucleic acid]. J Biol Chem. 1959 Jun;234(6):1512–1516. [PubMed] [Google Scholar]
- MILLER D., CRANE R. K. A procedure for the isolation of the epithelial brush border membrane of hamster small intestine. Anal Biochem. 1961 Jun;2:284–286. doi: 10.1016/s0003-2697(61)80014-6. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRAUSS E. W., WILSON T. H. Effect of intrinsic factor on vitamin B12 uptake by rat intestine in vitro. Proc Soc Exp Biol Med. 1958 Oct;99(1):224–226. doi: 10.3181/00379727-99-24303. [DOI] [PubMed] [Google Scholar]
- STRAUSS E. W., WILSON T. H. Factors controlling B12 uptake by intestinal sacs in vitro. Am J Physiol. 1960 Jan;198:103–107. doi: 10.1152/ajplegacy.1960.198.1.103. [DOI] [PubMed] [Google Scholar]
- SULLIVAN L. W., HERBERT V., CASTLE W. B. IN VITRO ASSAY FOR HUMAN INTRINSIC FACTOR. J Clin Invest. 1963 Sep;42:1443–1458. doi: 10.1172/JCI104829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRUMP B. F., SMUCKLER E. A., BENDITT E. P. A method for staining epoxy sections for light microscopy. J Ultrastruct Res. 1961 Aug;5:343–348. doi: 10.1016/s0022-5320(61)80011-7. [DOI] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. II. Application of solutions containing lead and barium. J Biophys Biochem Cytol. 1958 Nov 25;4(6):727–730. doi: 10.1083/jcb.4.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON T. H., STRAUSS E. W. Some species differences in the intrinsic factor stimulation of B12 uptake by small intestine in vitro. Am J Physiol. 1959 Oct;197:926–928. doi: 10.1152/ajplegacy.1959.197.4.926. [DOI] [PubMed] [Google Scholar]