Abstract
In the crystal structure of the title compound, C10H8N4·H2O, the organic molecules are approximately planar [maximum deviation from the least-squares plane = 0.041 (2) Å]. Two molecules are connected by two water molecules via O—H⋯N hydrogen bonding into dimers, which are located around centres of inversion. In the crystal, molecules are stacked in the a-axis direction, with mean distances between the π systems of 3.43 (1) and 3.46 (1) Å [centroid–centroid distances are 3.604 (2) and 3.591 (2) Å].
Related literature
For general background to phthalazines, see: Cheng et al. (1999 ▶); Coates (1999 ▶); De Stevens (1981 ▶); Shubin et al. (2004 ▶); Tarzia et al. (1989 ▶); Yatani et al. (2001 ▶). For related structures, see: Boulanger et al. (1991 ▶); Burton-Pye et al. (2005 ▶); Zimmer et al. (1995 ▶). For a description of the Cambridge Structural Database, see: Allen (2002 ▶). 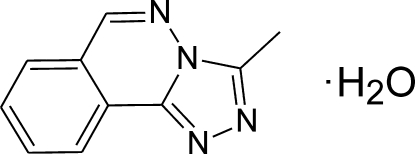
Experimental
Crystal data
C10H8N4·H2O
M r = 202.22
Triclinic,

a = 7.3009 (9) Å
b = 7.9253 (9) Å
c = 9.2755 (10) Å
α = 109.663 (10)°
β = 104.91 (1)°
γ = 95.830 (9)°
V = 477.83 (10) Å3
Z = 2
Cu Kα radiation
μ = 0.80 mm−1
T = 295 K
0.4 × 0.2 × 0.1 mm
Data collection
Oxford Diffraction SuperNova (single source at offset) Atlas diffractometer
Absorption correction: multi-scan (CrysAlis Pro; Oxford Diffraction, 2009 ▶) T min = 0.831, T max = 0.932
2893 measured reflections
1772 independent reflections
1615 reflections with I > 2σ(I)
R int = 0.024
Refinement
R[F 2 > 2σ(F 2)] = 0.057
wR(F 2) = 0.147
S = 1.12
1772 reflections
164 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.20 e Å−3
Δρmin = −0.17 e Å−3
Data collection: CrysAlis Pro (Oxford Diffraction, 2009 ▶); cell refinement: CrysAlis Pro; data reduction: CrysAlis Pro; program(s) used to solve structure: SIR92 (Altomare et al., 1993 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: XP (Siemens, 1989 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536809040677/nc2160sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809040677/nc2160Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1W—H1W2⋯N1 | 0.92 (5) | 2.08 (5) | 2.987 (2) | 168 (4) |
| O1W—H1W1⋯N2i | 0.83 (3) | 2.21 (3) | 3.043 (2) | 177 (3) |
Symmetry code: (i)  .
.
Acknowledgments
CSC thanks the University of Mysore for research facilities.
supplementary crystallographic information
Comment
The practical interest upon phthalazine derivatives is based on their widespread applications (Coates, 1999). They are commonly used as ligands in transition metal catalysis (e.g., Yatani et al., 2001), as chemiluminescent materials (Shubin et al., 2004) and for optical applications (Cheng et al., 1999). The chemistry of phthalazine derivatives has been of increasing interest since many of these compounds have found chemotherapeutic applications, especially as antihypertensive agents (De Stevens, 1981). 3-substituted 1,2,4-triazolo[3,4-a]phthalazines have been described as high affinity ligands for benzodiazepine receptor site (e.g., Tarzia et al., 1989).
In the Cambridge Database (Allen, 2002; ver. 5.30, November 2008) there are only four structures with 1,2,4-triazolo[3,4-a]phthalazine units. This includes 3-chloromethyl-1,2,4-triazolophthalazine (Burton-Pye et al., 2005), 3-(p-methoxyphenyl)triazolo(4,3 - a)phthalazine (Boulanger et al., 1991), 3-(p-methoxyphenyl)-6-(N,N-bis(2-methoxyethyl)amino)triazolo(4,3 - a)phthalazine (Boulanger et al., 1991), and 3-butyl-s-triazolo(3,4 - a)phthalazine (Zimmer et al., 1995). Here we present the X-ray structural analysis of the hydrate of 3-methyl[1,2,4]triazolo[3,4-a]phthalazine.
The molecules are almost planar with maximum deviation from the least-squares plane through all 13 ring atoms of 0.041 (2) Å (Fig. 1). The dihedral angle between the planes of the phenyl and the 1,2,4-triazole rings amount to 2.84 (7)°.
In the crystal the principal motif is built of two molecules of 3-methyl[1,2,4]triazolo[3,4-a]phthalazine and two water molecules, which are connected by means of O—H···N hydrogen bonds into centrosymmetric dimers which can be described according to the graph set notation as R44(10), cf. (Fig. 2). The planar molecules are stacked in a head-to-tail manner in the direction of the crystallographic a-axis with distances between the subsequent mean planes of 3.43 (1)Å and 3.46 (1)Å.
Experimental
1-Hydrazinophthalazine (2 g, 12.5 mmol) in 10 ml acetic acid was refluxed for 4 h. The reaction mixture was quenched to ice cold water and the solid separated was collected by filteration. The solid obtained was crystallized from methanol. Crystals for x-ray measurements were grown by a slow evaporation of ethyl acetate solution (m.p.: 421–423 K).
Refinement
Hydrogen atoms from the methyl group were placed in idealized positions and were refined as riding model with Uiso values set at 1.5 times Ueq of their carrier carbon atom. All other hydrogen atoms, including those from water molecule, were freely refined.
Figures
Fig. 1.
View of the dimers in the crystal structure of the title compound with labelling and displacement ellipsoids drawn at 50% probability level. Hydrogen atoms are depicted as spheres with arbitrary radii and hydrogen bonding is shown as dashed lines. Symmetry code: (i) -x,1 - y,-z.
Fig. 2.
Two mutually perpendicular views of the stacking in the crystal structure of the title compound.
Crystal data
| C10H8N4·H2O | Z = 2 |
| Mr = 202.22 | F(000) = 212 |
| Triclinic, P1 | Dx = 1.405 Mg m−3 |
| Hall symbol: -P 1 | Cu Kα radiation, λ = 1.5418 Å |
| a = 7.3009 (9) Å | Cell parameters from 2647 reflections |
| b = 7.9253 (9) Å | θ = 6.4–75.6° |
| c = 9.2755 (10) Å | µ = 0.80 mm−1 |
| α = 109.663 (10)° | T = 295 K |
| β = 104.91 (1)° | Block, colourless |
| γ = 95.830 (9)° | 0.4 × 0.2 × 0.1 mm |
| V = 477.83 (10) Å3 |
Data collection
| Oxford Diffraction SuperNova (single source at offset) Atlas diffractometer | 1772 independent reflections |
| Radiation source: Nova (Cu) X-ray Source | 1615 reflections with I > 2σ(I) |
| mirror | Rint = 0.024 |
| Detector resolution: 5.2679 pixels mm-1 | θmax = 70.0°, θmin = 6.4° |
| ω scans | h = −7→8 |
| Absorption correction: multi-scan (CrysAlis PRO; Oxford Diffraction, 2009) | k = −9→9 |
| Tmin = 0.831, Tmax = 0.932 | l = −10→11 |
| 2893 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.057 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.147 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.12 | w = 1/[σ2(Fo2) + (0.07P)2 + 0.128P] where P = (Fo2 + 2Fc2)/3 |
| 1772 reflections | (Δ/σ)max < 0.001 |
| 164 parameters | Δρmax = 0.20 e Å−3 |
| 0 restraints | Δρmin = −0.16 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N1 | 0.0820 (2) | 0.4413 (2) | 0.25290 (18) | 0.0576 (5) | |
| N2 | −0.0116 (2) | 0.2599 (2) | 0.16838 (18) | 0.0585 (5) | |
| C3 | 0.0258 (2) | 0.1699 (3) | 0.2642 (2) | 0.0504 (4) | |
| C31 | −0.0377 (3) | −0.0275 (3) | 0.2252 (2) | 0.0629 (5) | |
| H31A | −0.1301 | −0.0825 | 0.1193 | 0.094* | |
| H31B | −0.0966 | −0.0436 | 0.3024 | 0.094* | |
| H31C | 0.0722 | −0.0847 | 0.2287 | 0.094* | |
| N4 | 0.1420 (2) | 0.2902 (2) | 0.41193 (16) | 0.0455 (4) | |
| N5 | 0.2127 (2) | 0.2489 (2) | 0.54647 (17) | 0.0530 (4) | |
| C6 | 0.3218 (3) | 0.3879 (3) | 0.6698 (2) | 0.0533 (5) | |
| H6 | 0.372 (3) | 0.360 (3) | 0.765 (3) | 0.070 (6)* | |
| C7 | 0.3736 (2) | 0.5716 (2) | 0.6764 (2) | 0.0477 (4) | |
| C8 | 0.4993 (3) | 0.7118 (3) | 0.8151 (2) | 0.0562 (5) | |
| H8 | 0.557 (4) | 0.683 (3) | 0.913 (3) | 0.080 (7)* | |
| C9 | 0.5466 (3) | 0.8835 (3) | 0.8153 (3) | 0.0600 (5) | |
| H9 | 0.636 (3) | 0.978 (3) | 0.909 (3) | 0.067 (6)* | |
| C10 | 0.4682 (3) | 0.9207 (3) | 0.6786 (3) | 0.0613 (5) | |
| H10 | 0.506 (4) | 1.043 (4) | 0.685 (3) | 0.080 (7)* | |
| C11 | 0.3434 (3) | 0.7866 (3) | 0.5412 (2) | 0.0573 (5) | |
| H11 | 0.283 (3) | 0.805 (3) | 0.447 (3) | 0.074 (7)* | |
| C12 | 0.2970 (2) | 0.6096 (2) | 0.5382 (2) | 0.0467 (4) | |
| C13 | 0.1741 (2) | 0.4575 (3) | 0.4000 (2) | 0.0466 (4) | |
| O1W | 0.2725 (3) | 0.6362 (3) | 0.0877 (2) | 0.0876 (6) | |
| H1W2 | 0.198 (6) | 0.571 (6) | 0.125 (5) | 0.143 (14)* | |
| H1W1 | 0.197 (4) | 0.662 (4) | 0.018 (4) | 0.092 (9)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0568 (9) | 0.0734 (11) | 0.0449 (8) | 0.0125 (8) | 0.0080 (7) | 0.0313 (8) |
| N2 | 0.0558 (9) | 0.0759 (11) | 0.0431 (8) | 0.0143 (8) | 0.0092 (6) | 0.0263 (8) |
| C3 | 0.0470 (9) | 0.0637 (11) | 0.0399 (9) | 0.0137 (8) | 0.0129 (7) | 0.0185 (8) |
| C31 | 0.0655 (12) | 0.0629 (12) | 0.0510 (10) | 0.0102 (9) | 0.0132 (9) | 0.0145 (9) |
| N4 | 0.0483 (8) | 0.0565 (8) | 0.0363 (7) | 0.0158 (6) | 0.0120 (6) | 0.0228 (6) |
| N5 | 0.0653 (9) | 0.0569 (9) | 0.0416 (8) | 0.0159 (7) | 0.0124 (7) | 0.0265 (7) |
| C6 | 0.0634 (11) | 0.0610 (11) | 0.0382 (9) | 0.0159 (8) | 0.0103 (8) | 0.0253 (8) |
| C7 | 0.0495 (9) | 0.0572 (10) | 0.0413 (9) | 0.0157 (7) | 0.0159 (7) | 0.0218 (8) |
| C8 | 0.0594 (11) | 0.0645 (11) | 0.0429 (9) | 0.0142 (9) | 0.0142 (8) | 0.0191 (8) |
| C9 | 0.0565 (11) | 0.0608 (11) | 0.0555 (11) | 0.0067 (9) | 0.0189 (9) | 0.0136 (9) |
| C10 | 0.0616 (11) | 0.0558 (11) | 0.0717 (13) | 0.0122 (9) | 0.0251 (10) | 0.0274 (10) |
| C11 | 0.0586 (11) | 0.0634 (11) | 0.0599 (11) | 0.0164 (8) | 0.0184 (9) | 0.0345 (9) |
| C12 | 0.0444 (8) | 0.0585 (10) | 0.0451 (9) | 0.0165 (7) | 0.0168 (7) | 0.0255 (8) |
| C13 | 0.0454 (8) | 0.0595 (10) | 0.0435 (9) | 0.0164 (7) | 0.0150 (7) | 0.0275 (8) |
| O1W | 0.0682 (10) | 0.1226 (15) | 0.0838 (12) | 0.0115 (9) | 0.0052 (8) | 0.0695 (12) |
Geometric parameters (Å, °)
| N1—C13 | 1.312 (2) | C7—C8 | 1.398 (3) |
| N1—N2 | 1.384 (2) | C7—C12 | 1.404 (2) |
| N2—C3 | 1.309 (2) | C8—C9 | 1.369 (3) |
| C3—N4 | 1.365 (2) | C8—H8 | 1.01 (3) |
| C3—C31 | 1.476 (3) | C9—C10 | 1.391 (3) |
| C31—H31A | 0.9600 | C9—H9 | 0.96 (2) |
| C31—H31B | 0.9600 | C10—C11 | 1.370 (3) |
| C31—H31C | 0.9600 | C10—H10 | 0.96 (3) |
| N4—C13 | 1.369 (2) | C11—C12 | 1.399 (3) |
| N4—N5 | 1.3818 (18) | C11—H11 | 0.94 (3) |
| N5—C6 | 1.289 (2) | C12—C13 | 1.432 (3) |
| C6—C7 | 1.443 (3) | O1W—H1W2 | 0.92 (5) |
| C6—H6 | 0.97 (2) | O1W—H1W1 | 0.83 (3) |
| C13—N1—N2 | 107.24 (15) | C12—C7—C6 | 118.75 (16) |
| C3—N2—N1 | 108.84 (14) | C9—C8—C7 | 120.13 (18) |
| N2—C3—N4 | 108.18 (16) | C9—C8—H8 | 121.1 (14) |
| N2—C3—C31 | 127.98 (17) | C7—C8—H8 | 118.7 (14) |
| N4—C3—C31 | 123.82 (16) | C8—C9—C10 | 120.41 (19) |
| C3—C31—H31A | 109.5 | C8—C9—H9 | 119.8 (14) |
| C3—C31—H31B | 109.5 | C10—C9—H9 | 119.8 (14) |
| H31A—C31—H31B | 109.5 | C11—C10—C9 | 120.85 (19) |
| C3—C31—H31C | 109.5 | C11—C10—H10 | 122.1 (15) |
| H31A—C31—H31C | 109.5 | C9—C10—H10 | 117.0 (15) |
| H31B—C31—H31C | 109.5 | C10—C11—C12 | 119.35 (18) |
| C3—N4—C13 | 106.81 (14) | C10—C11—H11 | 124.5 (15) |
| C3—N4—N5 | 126.01 (15) | C12—C11—H11 | 116.2 (15) |
| C13—N4—N5 | 127.18 (15) | C11—C12—C7 | 120.10 (18) |
| C6—N5—N4 | 113.22 (14) | C11—C12—C13 | 124.20 (16) |
| N5—C6—C7 | 126.56 (16) | C7—C12—C13 | 115.70 (16) |
| N5—C6—H6 | 113.6 (14) | N1—C13—N4 | 108.93 (16) |
| C7—C6—H6 | 119.8 (14) | N1—C13—C12 | 132.48 (17) |
| C8—C7—C12 | 119.14 (17) | N4—C13—C12 | 118.57 (15) |
| C8—C7—C6 | 122.10 (16) | H1W2—O1W—H1W1 | 107 (3) |
| C13—N1—N2—C3 | −0.4 (2) | C10—C11—C12—C7 | 1.7 (3) |
| N1—N2—C3—N4 | 0.5 (2) | C10—C11—C12—C13 | −177.70 (16) |
| N1—N2—C3—C31 | −177.91 (17) | C8—C7—C12—C11 | −1.3 (3) |
| N2—C3—N4—C13 | −0.45 (19) | C6—C7—C12—C11 | 179.58 (15) |
| C31—C3—N4—C13 | 178.04 (16) | C8—C7—C12—C13 | 178.19 (14) |
| N2—C3—N4—N5 | 179.64 (14) | C6—C7—C12—C13 | −0.9 (2) |
| C31—C3—N4—N5 | −1.9 (3) | N2—N1—C13—N4 | 0.06 (19) |
| C3—N4—N5—C6 | 178.98 (16) | N2—N1—C13—C12 | 178.52 (17) |
| C13—N4—N5—C6 | −0.9 (2) | C3—N4—C13—N1 | 0.23 (19) |
| N4—N5—C6—C7 | −0.6 (3) | N5—N4—C13—N1 | −179.86 (14) |
| N5—C6—C7—C8 | −177.53 (18) | C3—N4—C13—C12 | −178.47 (13) |
| N5—C6—C7—C12 | 1.6 (3) | N5—N4—C13—C12 | 1.4 (3) |
| C12—C7—C8—C9 | 0.0 (3) | C11—C12—C13—N1 | 0.7 (3) |
| C6—C7—C8—C9 | 179.10 (17) | C7—C12—C13—N1 | −178.71 (17) |
| C7—C8—C9—C10 | 0.8 (3) | C11—C12—C13—N4 | 179.08 (15) |
| C8—C9—C10—C11 | −0.4 (3) | C7—C12—C13—N4 | −0.4 (2) |
| C9—C10—C11—C12 | −0.9 (3) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1W—H1W2···N1 | 0.92 (5) | 2.08 (5) | 2.987 (2) | 168 (4) |
| O1W—H1W1···N2i | 0.83 (3) | 2.21 (3) | 3.043 (2) | 177 (3) |
Symmetry codes: (i) −x, −y+1, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: NC2160).
References
- Allen, F. H. (2002). Acta Cryst. B58, 380–388. [DOI] [PubMed]
- Altomare, A., Cascarano, G., Giacovazzo, C. & Guagliardi, A. (1993). J. Appl. Cryst 26, 343–350.
- Boulanger, T., Evrard, C., Vercauteren, D. P., Evrard, G. & Durant, F. (1991). J. Crystallogr. Spectrosc. Res.21, 287–295.
- Burton-Pye, B. P., Heath, S. L. & Faulkner, S. (2005). Dalton Trans. pp. 146–149. [DOI] [PubMed]
- Cheng, Y., Ma, B. & Wuld, F. (1999). J. Mater. Chem 9, 2183–2188.
- Coates, W. J. (1999). In Comprehensive Heterocyclic Chemistry II, Vol. 6, edited by A. R. Katritzky, C. W. Rees & E. F. V. Scriven. Oxford: Pergamon Press.
- De Stevens, G. (1981). Medicinal Research Reviews, Vol. 1, p 73. New York: Wiley-Interscience.
- Oxford Diffraction (2009). CrysAlis Pro Oxford Diffraction Ltd, Yarnton, England.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Shubin, K. M., Kuznestsov, V. A. & Galishev, V. A. (2004). Tetrahedron Lett.45, 1407–1408.
- Siemens (1989). XP Operations Manual Siemens Analytical X-ray Instruments Inc., Madison, Wisconsin, USA.
- Tarzia, G., Occelli, E. & Barone, D. (1989). Farmaco, 44, 3–16. [DOI] [PubMed]
- Yatani, A., Fuji, M., Nakao, Y., Kashino, S., Kinoshita, M., Mori, W. & Suzuki, S. (2001). Inorg. Chim. Acta, 316, 127–131.
- Zimmer, H., Safwat, A. R., Ho, D., Amer, A. & Badawi, M. (1995). J. Org. Chem.60, 1908–1910.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536809040677/nc2160sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809040677/nc2160Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




