Abstract
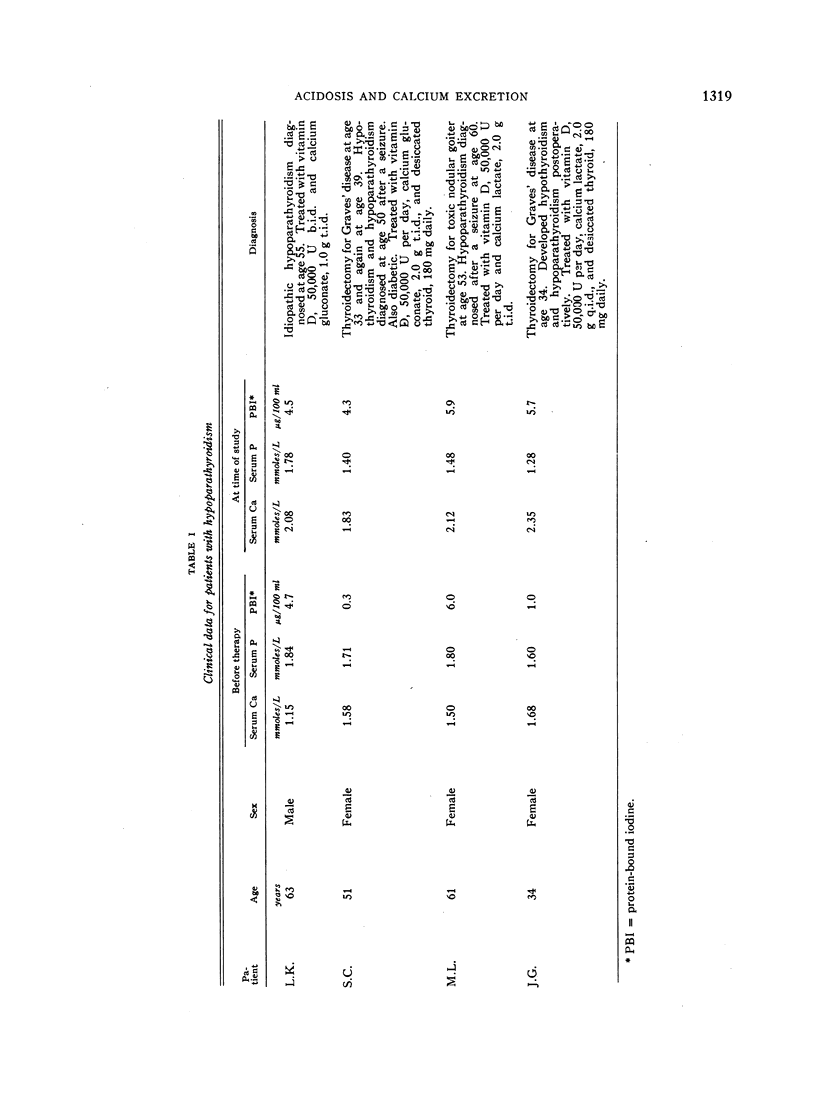
We carried out clearance studies in nine healthy adults and four patients with hypoparathyroidism before and after inducing stable metabolic acidosis with either NH4Cl or acetazolamide. Clearances were repeated in seven normal subjects and three of the patients 3 days after stopping these agents.
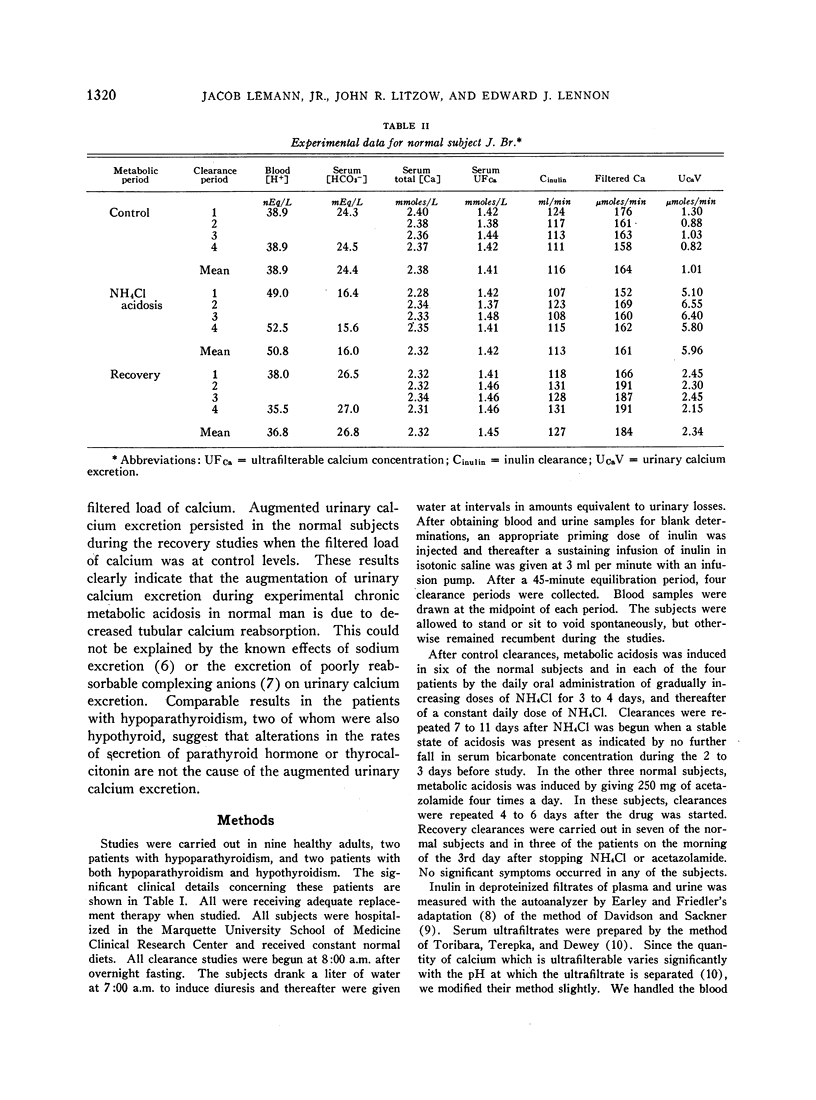
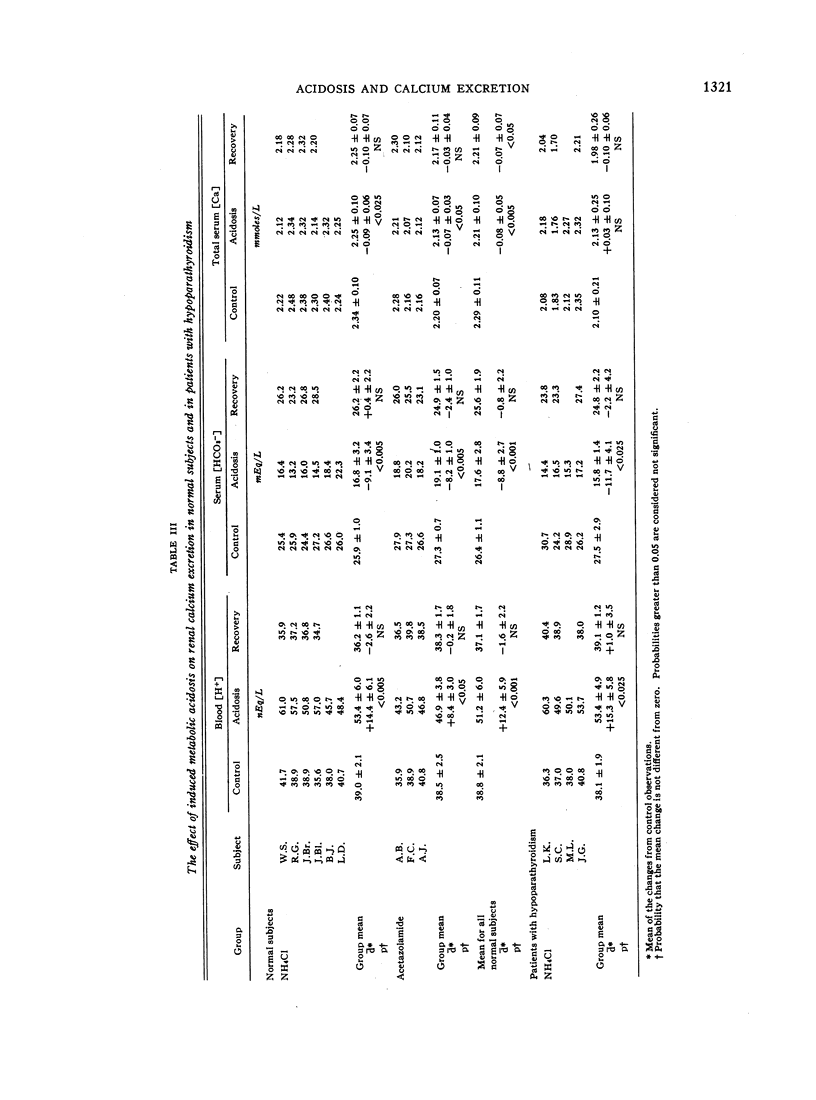
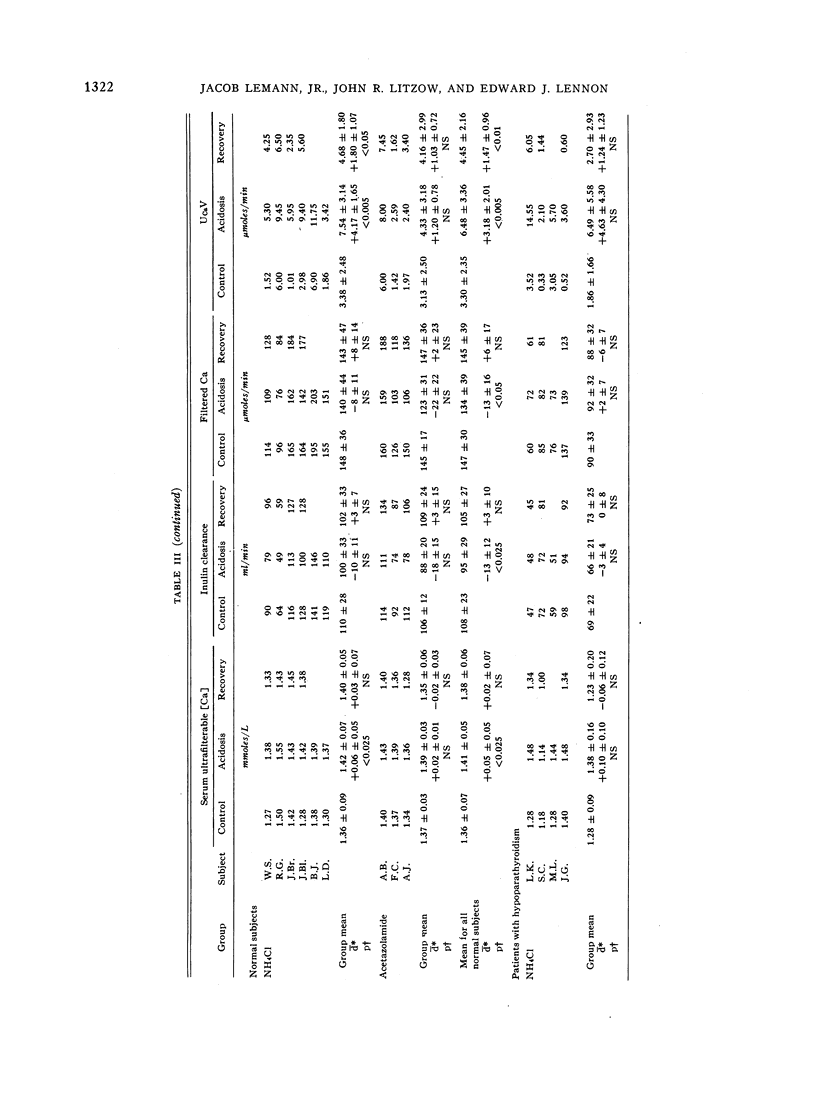
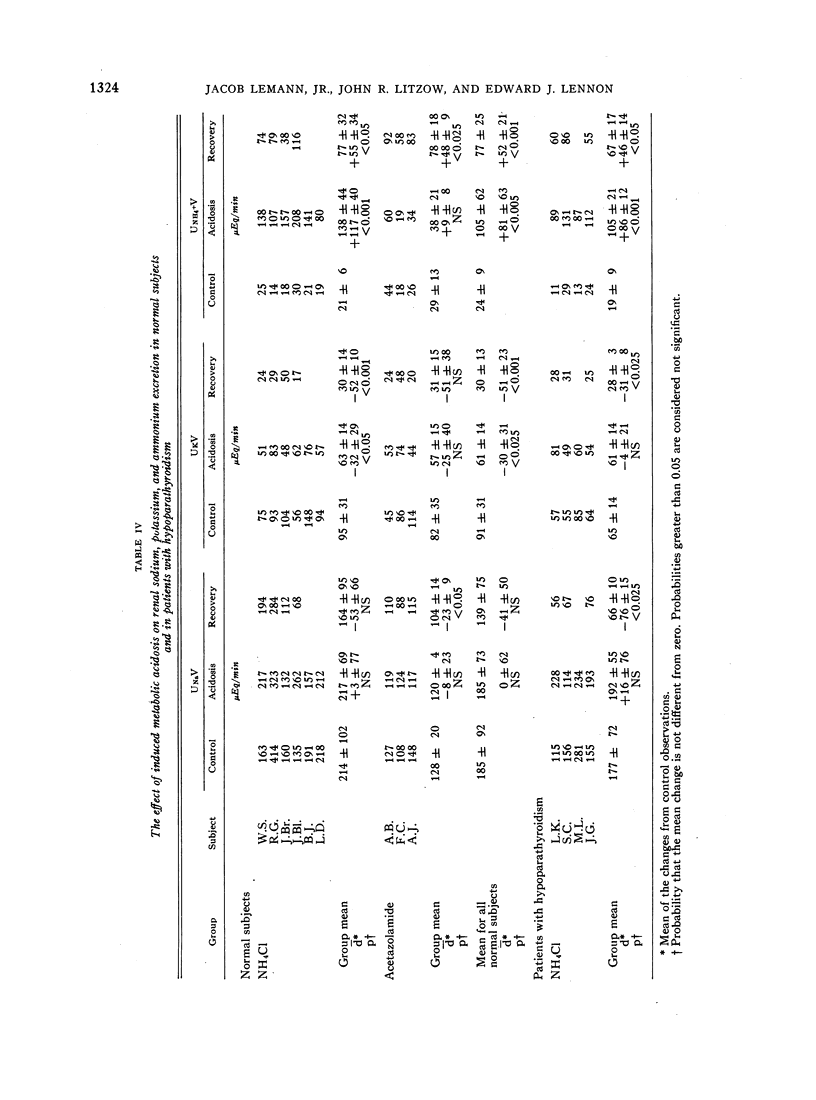
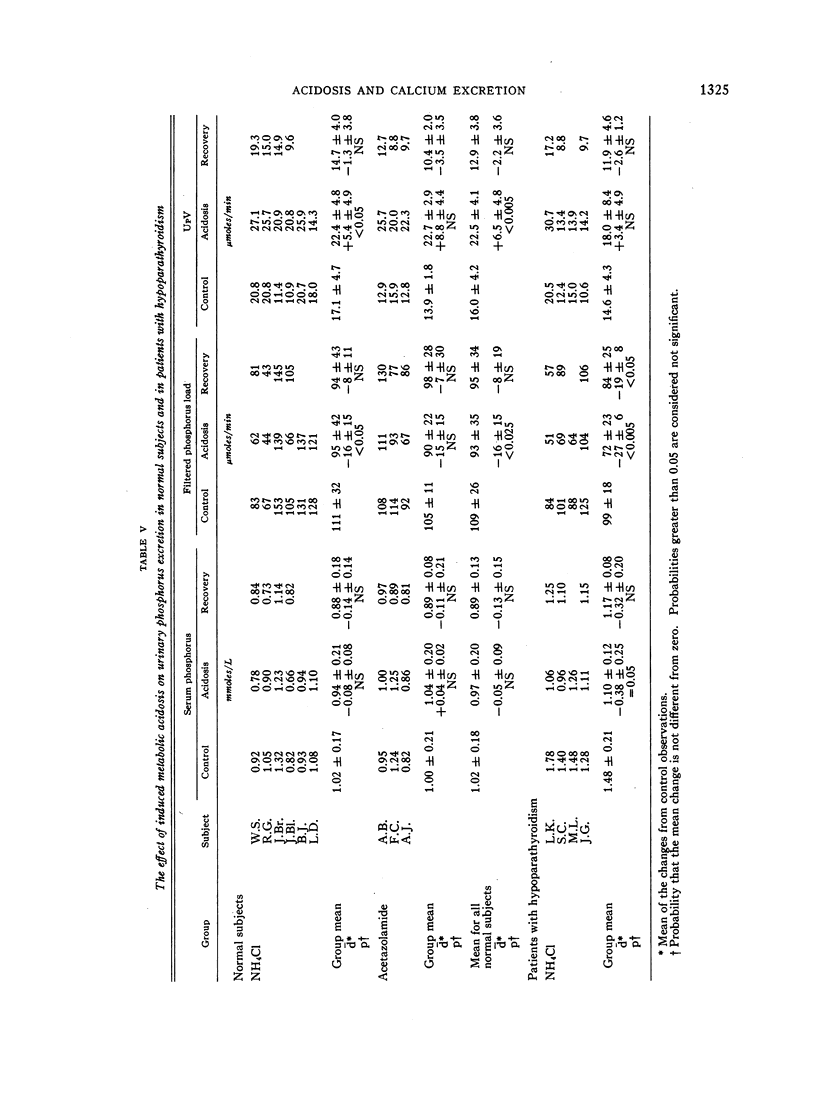
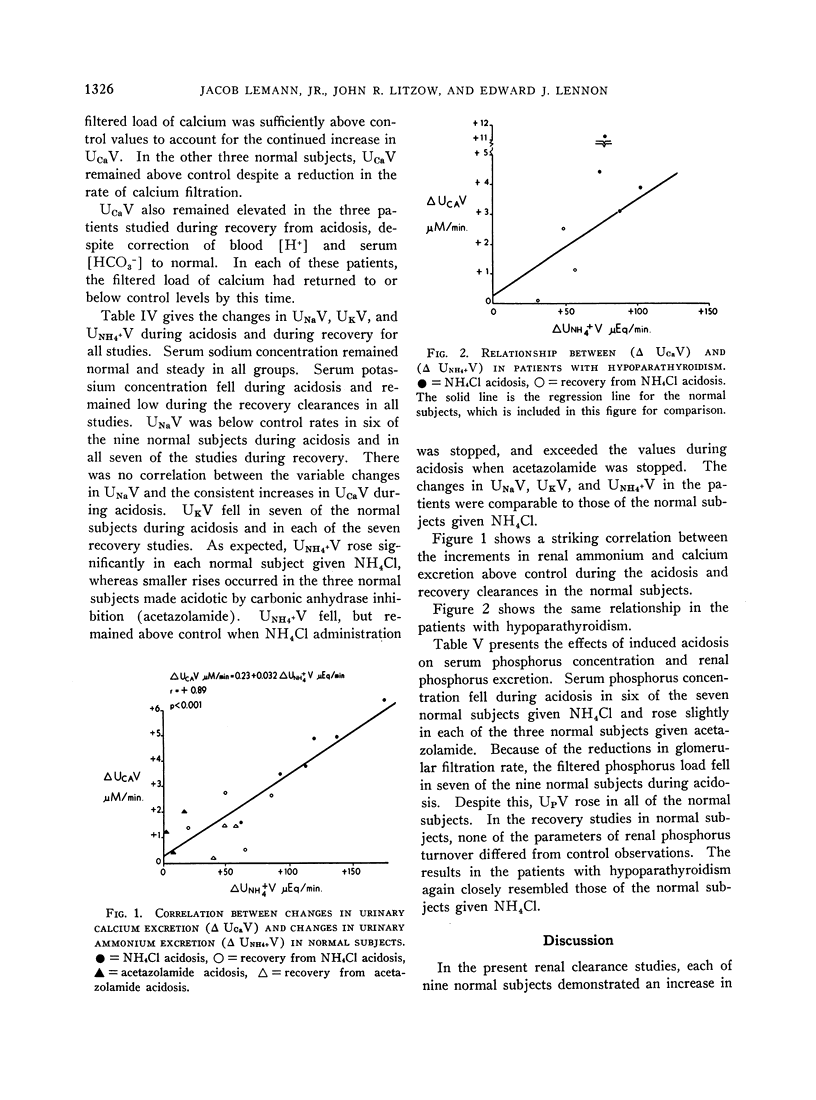
During acidosis in the normal subjects, serum ultrafilterable calcium concentration rose significantly, but inulin clearance fell to a greater extent, so that the calculated filtered load of calcium fell significantly. Despite this, urinary calcium excretion rose. Urinary calcium excretion remained elevated in the recovery studies when the serum ultrafilterable calcium concentration and filtered load of calcium had returned to control levels. Evidence is presented indicating that the increased calcium excretion which occurred during acidosis and recovery clearances was not due to natriuresis or to increased excretion of complexing anions. The comparable results in the four patients with hypoparathyroidism, two of whom also had hypothyroidism, suggest that the capacity to alter secretion rates of parathyroid hormone, thyrocalcitonin or both is not a critical determinant of the augmented rates of calcium excretion during acidosis.
We conclude that metabolic acidosis produces increased urinary calcium excretion by causing decreased renal tubular calcium reabsorption. Evidence is presented which suggests that this is a direct effect of metabolic acidosis on metabolic processes within renal tubular cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atchley D. W., Loeb R. F., Richards D. W., Benedict E. M., Driscoll M. E. ON DIABETIC ACIDOSIS: A Detailed Study of Electrolyte Balances Following the Withdrawal and Reestablishment of Insulin Therapy. J Clin Invest. 1933 Mar;12(2):297–326. doi: 10.1172/JCI100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIDSON W. D., SACKNER M. A. SIMPLIFICATION OF THE ANTHRONE METHOD FOR THE DETERMINATION OF INULIN IN CLEARANCE STUDIES. J Lab Clin Med. 1963 Aug;62:351–356. [PubMed] [Google Scholar]
- Earley L. E., Friedler R. M. Studies on the mechanism of natriuresis accompanying increased renal blood flow and its role in the renal response to extracellular volume expansion. J Clin Invest. 1965 Nov;44(11):1857–1865. doi: 10.1172/JCI105293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquharson R. F., Salter W. T., Tibbetts D. M., Aub J. C. STUDIES OF CALCIUM AND PHOSPHORUS METABOLISM: XII. The Effect of the Ingestion of Acid-producing Substances. J Clin Invest. 1931 Jun;10(2):221–249. doi: 10.1172/JCI100347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman A. D., Fuisz R. E., Cahill G. F., Jr Renal gluconeogenesis in acidosis, alkalosis, and potassium deficiency: its possible role in regulation of renal ammonia production. J Clin Invest. 1966 Apr;45(4):612–619. doi: 10.1172/JCI105375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemann J., Jr, Litzow J. R., Lennon E. J. The effects of chronic acid loads in normal man: further evidence for the participation of bone mineral in the defense against chronic metabolic acidosis. J Clin Invest. 1966 Oct;45(10):1608–1614. doi: 10.1172/JCI105467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon E. J., Lemann J., Jr, Litzow J. R. The effects of diet and stool composition on the net external acid balance of normal subjects. J Clin Invest. 1966 Oct;45(10):1601–1607. doi: 10.1172/JCI105466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OWEN E. E., TYOR M. P., FLANAGAN J. F., BERRY J. N. The kidney as a source of blood ammonia in patients with liver disease: the effect of acetazolamide. J Clin Invest. 1960 Feb;39:288–294. doi: 10.1172/JCI104039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson C. J., Martin T. J., MacIntyre I. Phosphaturic effect of thyrocalcitonin. Lancet. 1966 Jul 9;2(7454):83–84. doi: 10.1016/s0140-6736(66)91807-1. [DOI] [PubMed] [Google Scholar]
- Sartorius O. W., Roemmelt J. C., Pitts R. F., Calhoon D., Miner P. THE RENAL REGULATION OF ACID-BASE BALANCE IN MAN. IV. THE NATURE OF THE RENAL COMPENSATIONS IN AMMONIUM CHLORIDE ACIDOSIS. J Clin Invest. 1949 May;28(3):423–439. doi: 10.1172/JCI102087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TORIBARA T. Y., TEREPKA A. R., DEWEY P. A. The ultrafiltrable calcium of human serum. I. Ultrafiltration methods and normal values. J Clin Invest. 1957 May;36(5):738–748. doi: 10.1172/JCI103477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALSER M. Calcium clearance as a function of sodium clearance in the dog. Am J Physiol. 1961 May;200:1099–1104. doi: 10.1152/ajplegacy.1961.200.5.1099. [DOI] [PubMed] [Google Scholar]
- WALSER M. Ion association. VII. Dependence of calciuresis on natriuresis during sulfate infusion. Am J Physiol. 1961 Nov;201:769–773. doi: 10.1152/ajplegacy.1961.201.5.769. [DOI] [PubMed] [Google Scholar]
- WIDROW S. H., LEVINSKY N. G. The effect of parathyroid extract on renal tubular calcium reabsorption in the dog. J Clin Invest. 1962 Dec;41:2151–2159. doi: 10.1172/JCI104673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON B. J., FREEMAN S. Effects of acute changes in acid-base balance on renal calcium excretion in dogs. Am J Physiol. 1957 Nov;191(2):384–387. doi: 10.1152/ajplegacy.1957.191.2.384. [DOI] [PubMed] [Google Scholar]


