Abstract
The crystal structure of the title compound, sodium strontium orthoarsenate(V) nonahydrate, is isotypic with NaSr(PO4)(H2O)9 and the minerals nabaphite [NaBa(PO4)(H2O)9] and nastrophite [Na(Sr,Ba)(PO4)(H2O)9]. The Na and Sr atoms are located on threefold rotation axes and are in the centres of slightly distorted Na(H2O)6 octahedra and Sr(H2O)9 tricapped trigonal prisms, respectively. A framework structure is established via edge-sharing of these polyhedra. Disordered AsO4 tetrahedra (with threefold symmetry) are situated in the interstitial space of the framework. Although reasonable H-atom positions of the water molecules were not established, close O⋯O contacts between the disordered AsO4 tetrahedra and the water molecules suggest strong O—H⋯O hydrogen bonding.
Related literature
For a previous study of the title compound that revealed cubic symmetry and the lattice parameters, see: Ariguib-Kbir & Guerin (1973 ▶). Isotypic structures have been reported for synthetic NaSr(PO4)(H2O)9 (Takagi et al., 1982 ▶), nabaphite [NaBa(PO4)(H2O)9] (Baturin et al., 1982 ▶) and nastrophite [Na(Sr,Ba)(PO4)(H2O)9] (Baturin et al., 1981 ▶). For crystal structures in the Sr—As—O—(H) system, see: Mihajlovic & Effenberger (2006 ▶); Weil et al. (2009 ▶). As—O bond-length data for tetrahedrally coordinated arsenic were compiled and computed by Baur (1981 ▶) and Schwendtner (2008 ▶). For ionic radii, see: Shannon (1976 ▶).
Experimental
Crystal data
NaSr(AsO4)(H2O)9
M r = 411.67
Cubic,
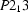
a = 10.6435 (1) Å
V = 1205.74 (2) Å3
Z = 4
Mo Kα radiation
μ = 7.29 mm−1
T = 296 K
0.36 × 0.24 × 0.24 mm
Data collection
Bruker APEXII CCD diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2008 ▶) T min = 0.179, T max = 0.274
41355 measured reflections
3435 independent reflections
3272 reflections with I > 2σ(I)
R int = 0.037
Refinement
R[F 2 > 2σ(F 2)] = 0.023
wR(F 2) = 0.056
S = 1.07
3435 reflections
60 parameters
H-atom parameters not refined
Δρmax = 1.73 e Å−3
Δρmin = −1.66 e Å−3
Absolute structure: Flack (1983 ▶), 1537 Friedel pairs
Flack parameter: −0.005 (6)
Data collection: APEX2 (Bruker, 2008 ▶); cell refinement: SAINT (Bruker, 2008 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ATOMS for Windows (Dowty, 2006 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536809040355/fj2248sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809040355/fj2248Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Selected interatomic distances (Å).
| Sr—O1i | 2.6326 (10) |
| Sr—O2 | 2.6558 (10) |
| Sr—O3ii | 2.6994 (10) |
| Na—O3iii | 2.3926 (12) |
| Na—O2iv | 2.4086 (12) |
| O1⋯O5v | 2.558 (7) |
| O1⋯O5 | 2.612 (8) |
| O1⋯O9vi | 2.623 (8) |
| O1⋯O6vii | 2.6640 (17) |
| O1⋯O4 | 2.7667 (14) |
| O1⋯O7 | 2.829 (5) |
| O1⋯O7vi | 2.919 (5) |
| O2⋯O9vi | 2.515 (9) |
| O2⋯O6vii | 2.7488 (17) |
| O3⋯O8vi | 2.562 (9) |
| O3⋯O6vi | 2.6949 (17) |
| O3⋯O9vi | 2.732 (9) |
| O3⋯O6 | 2.7492 (17) |
| O3⋯O5 | 2.757 (7) |
| O3⋯O7 | 2.776 (5) |
| O3⋯O7vi | 2.930 (5) |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  ; (iv)
; (iv)  ; (v)
; (v)  ; (vi)
; (vi) 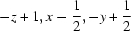 ; (vii)
; (vii)  .
.
supplementary crystallographic information
Comment
A previous study of NaSr(AsO4)(H2O)9 reports cubic symmetry and the lattice parameter as a = 10.70 Å (Ariguib-Kbir & Guerin, 1973). Moreover, isotypism with NaSr(PO4)(H2O)9 (Takagi et al., 1982) was also revealed. Besides the synthetic phosphate analogue, NaSr(AsO4)(H2O)9 is also isotypic with the minerals nabaphite [NaBa(PO4)(H2O)9] (Baturin et al., 1982) and nastrophite [Na(Sr,Ba)(PO4)(H2O)9] (Baturin et al., 1981).
The crystal structures consist of slightly distorted Na(OH2)6 octahedra (3 symmetry) and M(OH2)9 tricapped trigonal prisms (3 symmetry), where M = Sr, Ba. These polyhedra share edges and establish a framework structure. Disordered XO4 tetrahedra (X = P, As; 3 symmetry) are situated in the interstitial space of the framework (Fig. 1).
The Na(H2O)6 octahedron is slightly distorted. The corresponding Na—O bond lenghts are in the usual range with an average of 2.40 Å, conform with the values in the isotypic compounds (NaSr(PO4)(H2O)9: 2.41 Å; nabaphite: 2.42 Å; nastrophite: 2.42 Å) and the sum of the ionic radii of 2.41 Å, as calculated for six-coordinated Na (Shannon, 1976).
The Sr2+ ion is surrounded by 9 oxygen atoms with an average Sr—O distance of 2.663 Å, in good agreement with the phosphate analogue (2.668 Å; Takagi et al., 1982) and the sum of the ionic radii of 2.67 Å, as calculated for nine-coordinated Sr (Shannon, 1976).
The environment of the disordered AsO4 group consists of 16 water molecules with donor (D) — acceptor (A) distances between 2.5 and 3.0 Å (see Table 2). The overcrowding of water molecules (only 12 surrounding O atoms are expected, considering an ordered tetrahedral configuration for the arsenate unit with three donator atoms) may be the reason for the disorder of the AsO4 group. The average As—O bond length for the disordered AsO4 group is 1.685 Å, a value in very good agreement with those of 1.682 Å (Baur, 1981) and 1.686 Å (Schwendtner, 2008).
Experimental
Crystals of the title compound were obtained during phase formation studies in the system Sr—As—O (Weil et al., 2009) from hydrous solutions. All chemicals used were of analytical grade and employed without further purification. To a saturated Sr(OH)2.8H2O solution a diluted arsenic acid solution was dropwise added which resulted in a flocculent white precipitate (pH ca. 9). A concentrated NaOH solution was then added until a pH of 12 was reached. The resulting suspension was boiled for an hour. Then the precipitate was filtered off and the remaining solution was left to stand for several days. Besides few crystals of SrHAsO4 (Mihajlovic & Effenberger, 2006), colourless columnar crystals of the title compound up to several mm in length were obtained after complete evaporation of water.
Refinement
The oxygen atoms O4 and O6 of the arsenate group were clearly discernible from Fourier maps. Consideration of full occupancy of these sites resulted in high electron densities of ca 4 e Å3 at a distance of ca 1.7 Å from As, indicating other disordered oxygen atoms. Therefore four additional O atoms were considered in the final model. Free refinement of the site occupancy factors (s.o.f.) of all six O atoms attached to arsenic resulted in a composition very close to the theoretical value. In the last refinement cycles the s.o.f.'s were constrained to meet the criterion for electroneutrality. The six disordered O atoms were finally refined with isotropic displacement parameters. No reasonable positions of the H atoms attached to the water molecules (O1, O2, O3) could be found in difference Fourier maps which may be due to the disorder of the AsO4 tetrahedron and the resulting complex hydrogen bonding scheme. Therefore all H atoms were excluded from the refinement. The highest remaining peak in the final difference Fourier map is 0.49 Å from As and the deepest hole is 0.43 Å from the same atom.
Figures
Fig. 1.
The crystal structure of NaSr(PO4)(H2O)9 in a projection approximately along [001]. Sr atoms are represented as blue spheres and O atoms as white spheres; NaO6 octahedra are given in yellow and AsO4 tetrahedra in red. For clarity, Sr—O bonds are omitted and only one orientation of the disordered AsO4 group is given. Ellipsoids are drawn at the 90% probability level.
Crystal data
| NaSr(AsO4)(H2O)9 | Dx = 2.268 Mg m−3 |
| Mr = 411.67 | Mo Kα radiation, λ = 0.71073 Å |
| Cubic, P213 | Cell parameters from 9782 reflections |
| Hall symbol: P 2ac 2ab 3 | θ = 5.8–45.2° |
| a = 10.6435 (1) Å | µ = 7.29 mm−1 |
| V = 1205.74 (2) Å3 | T = 296 K |
| Z = 4 | Fragment, colourless |
| F(000) = 816 | 0.36 × 0.24 × 0.24 mm |
Data collection
| Bruker APEXII CCD diffractometer | 3435 independent reflections |
| Radiation source: fine-focus sealed tube | 3272 reflections with I > 2σ(I) |
| graphite | Rint = 0.037 |
| ω and φ scans | θmax = 45.7°, θmin = 5.4° |
| Absorption correction: multi-scan (SADABS; Bruker, 2008) | h = −21→21 |
| Tmin = 0.179, Tmax = 0.274 | k = −20→21 |
| 41355 measured reflections | l = −17→17 |
Refinement
| Refinement on F2 | H-atom parameters not refined |
| Least-squares matrix: full | w = 1/[σ2(Fo2) + (0.0274P)2 + 0.5628P] where P = (Fo2 + 2Fc2)/3 |
| R[F2 > 2σ(F2)] = 0.023 | (Δ/σ)max = 0.001 |
| wR(F2) = 0.056 | Δρmax = 1.73 e Å−3 |
| S = 1.07 | Δρmin = −1.66 e Å−3 |
| 3435 reflections | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 60 parameters | Extinction coefficient: 0.0147 (10) |
| 0 restraints | Absolute structure: Flack (1983), 1537 Friedel pairs |
| Primary atom site location: structure-invariant direct methods | Flack parameter: −0.005 (6) |
| Secondary atom site location: difference Fourier map |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| Sr | 0.055001 (8) | 0.055001 (8) | 0.055001 (8) | 0.00459 (3) | |
| As | 0.421839 (11) | 0.421839 (11) | 0.421839 (11) | 0.00709 (4) | |
| Na | 0.67077 (6) | 0.67077 (6) | 0.67077 (6) | 0.01193 (15) | |
| O1 | 0.39368 (12) | 0.07909 (10) | 0.35359 (10) | 0.01687 (18) | |
| O2 | 0.29270 (9) | 0.13067 (11) | 0.04892 (10) | 0.01519 (16) | |
| O3 | 0.60854 (10) | 0.29633 (9) | 0.14338 (10) | 0.01243 (14) | |
| O4 | 0.33000 (13) | 0.33000 (13) | 0.33000 (13) | 0.0077 (3)* | 0.60 |
| O5 | 0.4046 (7) | 0.3186 (7) | 0.3016 (7) | 0.0117 (10)* | 0.13 |
| O6 | 0.56151 (12) | 0.44234 (13) | 0.35120 (12) | 0.00759 (17)* | 0.60 |
| O7 | 0.5256 (5) | 0.3069 (5) | 0.3904 (5) | 0.0115 (7)* | 0.20 |
| O8 | 0.5623 (8) | 0.3833 (8) | 0.4407 (8) | 0.0088 (11)* | 0.10 |
| O9 | 0.5627 (8) | 0.3668 (8) | 0.4973 (8) | 0.0084 (11)* | 0.10 |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Sr | 0.00459 (3) | 0.00459 (3) | 0.00459 (3) | 0.00016 (2) | 0.00016 (2) | 0.00016 (2) |
| As | 0.00709 (4) | 0.00709 (4) | 0.00709 (4) | −0.00024 (3) | −0.00024 (3) | −0.00024 (3) |
| Na | 0.01193 (15) | 0.01193 (15) | 0.01193 (15) | 0.00126 (17) | 0.00126 (17) | 0.00126 (17) |
| O1 | 0.0319 (5) | 0.0088 (3) | 0.0098 (3) | 0.0043 (3) | −0.0045 (3) | −0.0005 (3) |
| O2 | 0.0109 (3) | 0.0229 (4) | 0.0118 (3) | −0.0039 (3) | 0.0002 (3) | 0.0007 (3) |
| O3 | 0.0130 (3) | 0.0111 (3) | 0.0132 (3) | 0.0024 (3) | −0.0005 (3) | 0.0015 (3) |
Geometric parameters (Å, °)
| Sr—O1i | 2.6326 (10) | As—O9iv | 1.799 (9) |
| Sr—O1ii | 2.6326 (10) | As—O9v | 1.799 (9) |
| Sr—O1iii | 2.6326 (10) | Na—O3ix | 2.3926 (12) |
| Sr—O2iv | 2.6558 (10) | Na—O3x | 2.3926 (12) |
| Sr—O2 | 2.6558 (10) | Na—O3xi | 2.3926 (12) |
| Sr—O2v | 2.6558 (10) | Na—O2xii | 2.4086 (12) |
| Sr—O3vi | 2.6994 (10) | Na—O2xiii | 2.4086 (12) |
| Sr—O3vii | 2.6994 (10) | Na—O2xiv | 2.4086 (12) |
| Sr—O3viii | 2.6994 (10) | O4—O5iv | 0.858 (7) |
| As—O8iv | 1.563 (8) | O4—O5 | 0.858 (7) |
| As—O8 | 1.563 (8) | O4—O5v | 0.858 (7) |
| As—O8v | 1.563 (8) | O5—O5v | 1.440 (13) |
| As—O6 | 1.6801 (13) | O5—O5iv | 1.440 (13) |
| As—O6v | 1.6801 (13) | O5—O7 | 1.602 (9) |
| As—O6iv | 1.6801 (13) | O6—O8 | 1.141 (9) |
| As—O7iv | 1.682 (5) | O6—O9v | 1.462 (9) |
| As—O7 | 1.682 (5) | O6—O7 | 1.549 (5) |
| As—O7v | 1.682 (5) | O6—O9 | 1.750 (8) |
| As—O4 | 1.693 (2) | O7—O8 | 1.049 (10) |
| As—O5iv | 1.697 (7) | O7—O9 | 1.362 (9) |
| As—O5 | 1.697 (7) | O8—O9 | 0.627 (11) |
| As—O5v | 1.697 (7) | O9—O6iv | 1.462 (9) |
| As—O9 | 1.799 (9) | ||
| O1···O5iv | 2.558 (7) | O2···O6xvi | 2.7488 (17) |
| O1···O5 | 2.612 (8) | O3···O8xv | 2.562 (9) |
| O1···O9xv | 2.623 (8) | O3···O6xv | 2.6949 (17) |
| O1···O6xvi | 2.6640 (17) | O3···O9xv | 2.732 (9) |
| O1···O4 | 2.7667 (14) | O3···O6 | 2.7492 (17) |
| O1···O7 | 2.829 (5) | O3···O5 | 2.757 (7) |
| O1···O7xv | 2.919 (5) | O3···O7 | 2.776 (5) |
| O2···O9xv | 2.515 (9) | O3···O7xv | 2.930 (5) |
| O1i—Sr—O1ii | 80.79 (4) | O8v—As—O5iv | 140.9 (4) |
| O1i—Sr—O1iii | 80.79 (4) | O6—As—O5iv | 128.5 (3) |
| O1ii—Sr—O1iii | 80.79 (4) | O6v—As—O5iv | 113.1 (3) |
| O1i—Sr—O2iv | 86.96 (4) | O6iv—As—O5iv | 80.9 (3) |
| O1ii—Sr—O2iv | 134.39 (3) | O7iv—As—O5iv | 56.6 (3) |
| O1iii—Sr—O2iv | 140.21 (3) | O7—As—O5iv | 81.6 (3) |
| O1i—Sr—O2 | 140.21 (3) | O7v—As—O5iv | 106.7 (3) |
| O1ii—Sr—O2 | 86.96 (4) | O8iv—As—O5 | 140.9 (4) |
| O1iii—Sr—O2 | 134.39 (3) | O8—As—O5 | 91.7 (4) |
| O2iv—Sr—O2 | 75.03 (4) | O8v—As—O5 | 115.8 (4) |
| O1i—Sr—O2v | 134.39 (3) | O6—As—O5 | 80.9 (3) |
| O1ii—Sr—O2v | 140.21 (3) | O6v—As—O5 | 128.5 (3) |
| O1iii—Sr—O2v | 86.96 (4) | O6iv—As—O5 | 113.1 (3) |
| O2iv—Sr—O2v | 75.03 (4) | O7iv—As—O5 | 106.7 (3) |
| O2—Sr—O2v | 75.03 (4) | O7—As—O5 | 56.6 (3) |
| O1i—Sr—O3vi | 68.72 (3) | O7v—As—O5 | 81.6 (3) |
| O1ii—Sr—O3vi | 68.05 (3) | O5iv—As—O5 | 50.2 (4) |
| O1iii—Sr—O3vi | 138.94 (4) | O8iv—As—O9 | 80.4 (4) |
| O2iv—Sr—O3vi | 66.55 (3) | O8v—As—O9 | 108.7 (4) |
| O2—Sr—O3vi | 71.58 (3) | O8—As—O9 | 19.9 (4) |
| O2v—Sr—O3vi | 134.06 (3) | O6v—As—O9 | 127.1 (3) |
| O1i—Sr—O3vii | 68.05 (3) | O6—As—O9 | 60.3 (3) |
| O1ii—Sr—O3vii | 138.94 (4) | O6iv—As—O9 | 49.6 (3) |
| O1iii—Sr—O3vii | 68.72 (3) | O7iv—As—O9 | 104.4 (3) |
| O2iv—Sr—O3vii | 71.58 (3) | O7—As—O9 | 45.9 (3) |
| O2—Sr—O3vii | 134.06 (3) | O7v—As—O9 | 134.8 (3) |
| O2v—Sr—O3vii | 66.55 (3) | O4—As—O9 | 123.4 (3) |
| O3vi—Sr—O3vii | 119.992 (1) | O5iv—As—O9 | 110.0 (4) |
| O1i—Sr—O3viii | 138.94 (4) | O5—As—O9 | 102.5 (4) |
| O1ii—Sr—O3viii | 68.72 (3) | O5v—As—O9 | 152.0 (4) |
| O1iii—Sr—O3viii | 68.05 (3) | O8iv—As—O9iv | 19.9 (4) |
| O2iv—Sr—O3viii | 134.06 (3) | O8v—As—O9iv | 80.4 (4) |
| O2—Sr—O3viii | 66.55 (3) | O8—As—O9iv | 108.7 (4) |
| O2v—Sr—O3viii | 71.58 (3) | O6v—As—O9iv | 49.6 (3) |
| O3vi—Sr—O3viii | 119.992 (1) | O6—As—O9iv | 127.1 (3) |
| O3vii—Sr—O3viii | 119.992 (1) | O6iv—As—O9iv | 60.3 (3) |
| O8iv—As—O8 | 99.3 (4) | O7iv—As—O9iv | 45.9 (3) |
| O8iv—As—O8v | 99.3 (4) | O7—As—O9iv | 134.8 (3) |
| O8—As—O8v | 99.3 (4) | O7v—As—O9iv | 104.4 (3) |
| O8iv—As—O6 | 130.1 (3) | O4—As—O9iv | 123.4 (3) |
| O8v—As—O6 | 69.2 (3) | O5iv—As—O9iv | 102.5 (4) |
| O8iv—As—O6v | 69.2 (3) | O5—As—O9iv | 152.0 (4) |
| O8—As—O6v | 130.1 (3) | O5v—As—O9iv | 110.0 (4) |
| O6—As—O6v | 109.83 (4) | O9—As—O9iv | 92.6 (4) |
| O8—As—O6iv | 69.2 (3) | O8iv—As—O9v | 108.7 (4) |
| O8v—As—O6iv | 130.1 (3) | O8v—As—O9v | 19.9 (4) |
| O6—As—O6iv | 109.83 (4) | O8—As—O9v | 80.4 (4) |
| O6v—As—O6iv | 109.83 (4) | O6v—As—O9v | 60.3 (3) |
| O8—As—O7iv | 124.0 (4) | O6—As—O9v | 49.6 (3) |
| O8v—As—O7iv | 117.1 (3) | O6iv—As—O9v | 127.1 (3) |
| O6—As—O7iv | 164.45 (18) | O7iv—As—O9v | 134.8 (3) |
| O6v—As—O7iv | 76.41 (17) | O7—As—O9v | 104.4 (3) |
| O6iv—As—O7iv | 54.87 (18) | O7v—As—O9v | 45.9 (3) |
| O8iv—As—O7 | 117.1 (3) | O4—As—O9v | 123.4 (3) |
| O8v—As—O7 | 124.0 (4) | O5iv—As—O9v | 152.0 (4) |
| O6—As—O7 | 54.87 (18) | O5—As—O9v | 110.0 (4) |
| O6v—As—O7 | 164.45 (18) | O5v—As—O9v | 102.5 (4) |
| O6iv—As—O7 | 76.41 (17) | O9—As—O9v | 92.6 (4) |
| O7iv—As—O7 | 117.62 (9) | O9iv—As—O9v | 92.6 (4) |
| O8iv—As—O7v | 124.0 (4) | O3ix—Na—O3x | 89.64 (5) |
| O8—As—O7v | 117.1 (3) | O3ix—Na—O3xi | 89.65 (5) |
| O6—As—O7v | 76.41 (17) | O3x—Na—O3xi | 89.64 (5) |
| O6v—As—O7v | 54.87 (18) | O3ix—Na—O2xii | 75.47 (4) |
| O6iv—As—O7v | 164.45 (18) | O3x—Na—O2xii | 155.21 (3) |
| O7iv—As—O7v | 117.62 (9) | O3xi—Na—O2xii | 109.62 (4) |
| O7—As—O7v | 117.62 (9) | O3ix—Na—O2xiii | 109.62 (4) |
| O8iv—As—O4 | 118.4 (3) | O3x—Na—O2xiii | 75.47 (4) |
| O8—As—O4 | 118.4 (3) | O3xi—Na—O2xiii | 155.21 (3) |
| O8v—As—O4 | 118.4 (3) | O2xii—Na—O2xiii | 90.76 (5) |
| O6—As—O4 | 109.11 (5) | O3ix—Na—O2xiv | 155.21 (3) |
| O6v—As—O4 | 109.11 (5) | O3x—Na—O2xiv | 109.62 (4) |
| O6iv—As—O4 | 109.11 (5) | O3xi—Na—O2xiv | 75.47 (4) |
| O7iv—As—O4 | 81.04 (17) | O2xii—Na—O2xiv | 90.76 (5) |
| O7—As—O4 | 81.04 (17) | O2xiii—Na—O2xiv | 90.76 (5) |
| O7v—As—O4 | 81.04 (17) | Naxvi—O2—Sr | 103.37 (4) |
| O8iv—As—O5iv | 91.7 (4) | Naxvii—O3—Srxviii | 102.52 (4) |
| O8—As—O5iv | 115.8 (4) |
Symmetry codes: (i) −y, z−1/2, −x+1/2; (ii) −x+1/2, −y, z−1/2; (iii) z−1/2, −x+1/2, −y; (iv) y, z, x; (v) z, x, y; (vi) −y+1/2, −z, x−1/2; (vii) −z, x−1/2, −y+1/2; (viii) x−1/2, −y+1/2, −z; (ix) −y+1, z+1/2, −x+3/2; (x) z+1/2, −x+3/2, −y+1; (xi) −x+3/2, −y+1, z+1/2; (xii) y+1/2, −z+1/2, −x+1; (xiii) −z+1/2, −x+1, y+1/2; (xiv) −x+1, y+1/2, −z+1/2; (xv) −z+1, x−1/2, −y+1/2; (xvi) −x+1, y−1/2, −z+1/2; (xvii) −x+3/2, −y+1, z−1/2; (xviii) x+1/2, −y+1/2, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: FJ2248).
References
- Ariguib-Kbir, N. & Guerin, H. (1973). C. R. Acad. Sci. Ser. D., 276, 67–70.
- Baturin, S. V., Malinovskii, Yu. A. & Belov, N. V. (1981). Dokl. Akad. Nauk SSSR, 261, 619–623.
- Baturin, S. V., Malinovskii, Yu. A. & Belov, N. V. (1982). Dokl. Akad. Nauk SSSR, 266, 624–627.
- Baur, W. (1981). Interatomic distance predictions for computer simulations of crystal structures, in Structure and bonding in crystals II, edited by M. O’Keeffe & A. Navrotsky, pp. 31–52. New York: Academic Press.
- Bruker (2008). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Dowty, E. (2006). ATOMS for Windows Shape Software, Kingsport, Tennessee, USA.
- Flack, H. D. (1983). Acta Cryst. A39, 876–881.
- Mihajlovic, T. & Effenberger, H. (2006). Z. Kristallogr.221, 770–781.
- Schwendtner, K. (2008). PhD thesis, University of Vienna, Austria.
- Shannon, R. D. (1976). Acta Cryst. A32, 751–767.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Takagi, S., Mathew, M. & Brown, W. E. (1982). Acta Cryst. B38, 1408–1413.
- Weil, M., Đorđević, T., Lengauer, C. L. & Kolitsch, U. (2009). http://dx.doi.org/10.1016/j.solidstatesciences.2009.08.019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536809040355/fj2248sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809040355/fj2248Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report



