Abstract
In the title complex, [ZnI2(C14H21BrN2O)]·CH3OH, the asymmetric unit consists of a mononuclear zinc(II) complex molecule and a methanol solvent molecule. The compound was derived from the zwitterionic form of the Schiff base 4-bromo-2-[3-(diethylamino)propyliminomethyl]phenol. The ZnII atom is four-coordinated by the imine N and phenolate O atoms of the Schiff base ligand and by two iodide ions in a distorted tetrahedral coordination. In the crystal structure, the methanol molecules are linked to the Schiff base molecules through N—H⋯O and O—H⋯O hydrogen bonds. One I atom is disordered over two positions in a 0.702 (19):0.298 (19) ratio.
Related literature
For background to the chemistry of Schiff base complexes, see: Ali et al. (2008 ▶); Biswas et al. (2008 ▶); Chen et al. (2008 ▶); Darensbourg & Frantz (2007 ▶); Habibi et al. (2007 ▶); Kawamoto et al. (2008 ▶); Lipscomb & Sträter (1996 ▶); Tomat et al. (2007 ▶); Wu et al. (2008 ▶); Yuan et al. (2007 ▶). For related structures, see: Zhu (2008 ▶); Zhu & Yang (2008a
▶,b
▶,c
▶); Qiu (2006a
▶,b
▶); Wei et al. (2007 ▶); Zhu et al. (2007 ▶). For hydrogen-bond motifs, see: Bernstein et al. (1995 ▶).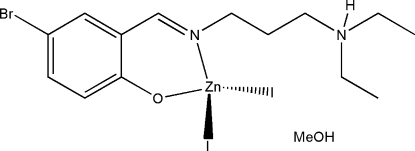
Experimental
Crystal data
[ZnI2(C14H21BrN2O)]·CH4O
M r = 664.45
Monoclinic,
a = 10.869 (2) Å
b = 17.562 (3) Å
c = 11.377 (2) Å
β = 94.358 (3)°
V = 2165.4 (7) Å3
Z = 4
Mo Kα radiation
μ = 5.84 mm−1
T = 298 K
0.20 × 0.20 × 0.17 mm
Data collection
Bruker APEXII CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 2004 ▶) T min = 0.388, T max = 0.437
14106 measured reflections
4664 independent reflections
3499 reflections with I > 2σ(I)
R int = 0.041
Refinement
R[F 2 > 2σ(F 2)] = 0.048
wR(F 2) = 0.105
S = 1.06
4664 reflections
225 parameters
8 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.75 e Å−3
Δρmin = −0.85 e Å−3
Data collection: APEX2 (Bruker, 2004 ▶); cell refinement: SAINT (Bruker, 2004 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809038446/bx2241sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809038446/bx2241Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Selected geometric parameters (Å, °).
| Zn1—O1 | 1.958 (4) |
| Zn1—N1 | 2.032 (5) |
| Zn1—I2′ | 2.545 (6) |
| Zn1—I1 | 2.5627 (9) |
| Zn1—I2 | 2.5768 (18) |
Table 2. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O2—H2⋯O1i | 0.82 | 1.86 | 2.640 (6) | 158 |
| N2—H2A⋯O2 | 0.91 (6) | 1.81 (6) | 2.716 (7) | 173 (8) |
Symmetry code: (i) 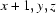 .
.
supplementary crystallographic information
Comment
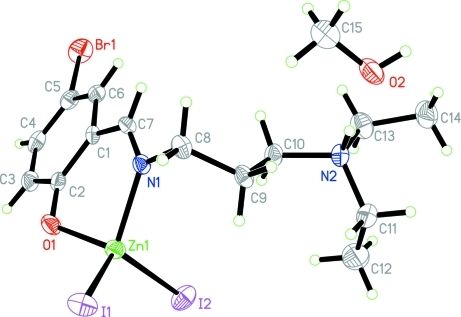
Schiff bases have widely been used as versatile ligands in coordination chemistry (Biswas et al., 2008; Wu et al., 2008; Kawamoto et al., 2008; Ali et al., 2008; Habibi et al., 2007), and their metal complexes are of great interest in many fields (Chen et al., 2008; Yuan et al., 2007; Tomat et al., 2007; Darensbourg & Frantz, 2007). Zinc(II) is an important element in biological systems and functions as the active site of hydrolytic enzymes, such as carboxypeptidase and carbonic anhydrase where it is in a hard-donor coordination environment of nitrogen and oxygen ligands (Lipscomb & Sträter, 1996). Recently, we have reported a few Schiff base zinc complexes (Zhu, 2008; Zhu & Yang, 2008a,b,c). In this paper, the title new zinc(II) complex, Fig. 1, is reported.
The complex consists of a mononuclear zinc(II) complex molecule and a methanol molecule. The ZnII atom is four-coordinated by the imine N and phenolate O atoms of the zwitterionic form of the Schiff base ligand, and by two I- ions, in a distorted tetrahedral coordination. The coordinate bond lengths (Table 1) are typical and comparable to the corresponding values observed in the Schiff base zinc complexes we reported previously and other similar Schiff base zinc complexes (Zhu et al., 2007; Wei et al., 2007; Qiu, 2006a,b). I2 atom is disordered over two positions [0.702(19/0.298 (19)].
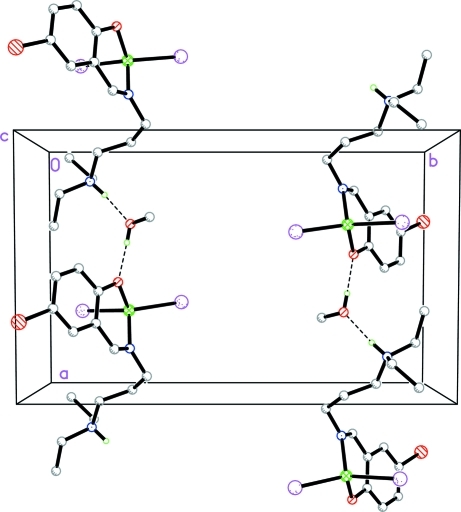
In the crystal structure, the methanol molecules are linked to the Schiff base molecules through O—H···O and N—H···O hydrogen bonds generating a graph-set motif C22(10) chain along [100] direction (Table 2, Fig. 2). (Bernstein et al., 1995)
Experimental
The Schiff base compound was prepared by the condensation of equimolar amounts of 5-bromosalicylaldehyde with N,N-diethylpropane-1,3-diamine in a methanol solution. The complex was prepared by the following method. To an anhydrous methanol solution (5 ml) of ZnI2 (31.9 mg, 0.1 mmol) was added a methanol solution (10 ml) of the Schiff base compound (31.3 mg, 0.1 mmol) with stirring. The mixture was stirred for 30 min at room temperature and filtered. Upon keeping the filtrate in air for a few days, colorless block-shaped crystals were formed.
Refinement
H2A was located from a difference Fourier map and refined isotropically, with N—H distance restrained to 0.90 (1) Å. Other H atoms were placed in geometrically idealized positions and constrained to ride on their parent atoms, with C—H distances in the range 0.93–0.97 Å, O—H distance of 0.82 Å, and with Uiso(H) = 1.2Ueq(C) and 1.5Ueq(methyl C and O). The I2 atom is disordered over two distinct sites with occupancies of 0.702 (2) and 0.298 (2), respectively.
Figures
Fig. 1.
The molecular structure of the title complex, with ellipsoids drawn at the 30% probability level.
Fig. 2.
The crystal packing of the title complex.
Crystal data
| [ZnI2(C14H21BrN2O)]·CH4O | F(000) = 1264 |
| Mr = 664.45 | Dx = 2.038 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2yn | Cell parameters from 3253 reflections |
| a = 10.869 (2) Å | θ = 2.2–24.5° |
| b = 17.562 (3) Å | µ = 5.84 mm−1 |
| c = 11.377 (2) Å | T = 298 K |
| β = 94.358 (3)° | Block, colorless |
| V = 2165.4 (7) Å3 | 0.20 × 0.20 × 0.17 mm |
| Z = 4 |
Data collection
| Bruker APEXII CCD area-detector diffractometer | 4664 independent reflections |
| Radiation source: fine-focus sealed tube | 3499 reflections with I > 2σ(I) |
| graphite | Rint = 0.041 |
| ω scans | θmax = 27.0°, θmin = 2.1° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 2004) | h = −13→13 |
| Tmin = 0.388, Tmax = 0.437 | k = −22→22 |
| 14106 measured reflections | l = −14→13 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.048 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.105 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.06 | w = 1/[σ2(Fo2) + (0.0298P)2 + 7.4538P] where P = (Fo2 + 2Fc2)/3 |
| 4664 reflections | (Δ/σ)max = 0.001 |
| 225 parameters | Δρmax = 0.75 e Å−3 |
| 8 restraints | Δρmin = −0.85 e Å−3 |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2sigma(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| Zn1 | 0.17105 (6) | 0.26943 (4) | −0.06556 (7) | 0.0380 (2) | |
| I1 | 0.13989 (4) | 0.14098 (3) | −0.17051 (4) | 0.05239 (16) | |
| I2 | 0.1766 (2) | 0.39343 (11) | −0.1861 (2) | 0.0541 (8) | 0.702 (19) |
| I2' | 0.1771 (8) | 0.3793 (9) | −0.2109 (16) | 0.118 (2) | 0.298 (19) |
| Br1 | 0.19879 (8) | 0.49547 (5) | 0.46762 (7) | 0.0630 (2) | |
| O1 | 0.0560 (4) | 0.2904 (3) | 0.0548 (4) | 0.0443 (11) | |
| O2 | 0.8239 (4) | 0.2628 (3) | −0.0180 (5) | 0.0546 (13) | |
| H2 | 0.8982 | 0.2714 | −0.0142 | 0.082* | |
| N1 | 0.3188 (4) | 0.2626 (3) | 0.0547 (4) | 0.0311 (11) | |
| N2 | 0.6505 (5) | 0.3665 (3) | −0.0945 (5) | 0.0383 (12) | |
| C1 | 0.2134 (5) | 0.3368 (3) | 0.1971 (5) | 0.0301 (13) | |
| C2 | 0.0899 (5) | 0.3330 (4) | 0.1466 (5) | 0.0338 (14) | |
| C3 | 0.0011 (6) | 0.3764 (4) | 0.2014 (6) | 0.0414 (16) | |
| H3 | −0.0810 | 0.3733 | 0.1723 | 0.050* | |
| C4 | 0.0311 (6) | 0.4231 (4) | 0.2955 (6) | 0.0431 (16) | |
| H4 | −0.0297 | 0.4516 | 0.3284 | 0.052* | |
| C5 | 0.1523 (6) | 0.4276 (4) | 0.3417 (6) | 0.0400 (15) | |
| C6 | 0.2417 (6) | 0.3841 (3) | 0.2958 (5) | 0.0355 (14) | |
| H6 | 0.3220 | 0.3857 | 0.3302 | 0.043* | |
| C7 | 0.3161 (5) | 0.2950 (3) | 0.1550 (5) | 0.0340 (14) | |
| H7 | 0.3870 | 0.2914 | 0.2059 | 0.041* | |
| C8 | 0.4322 (5) | 0.2223 (3) | 0.0264 (6) | 0.0369 (14) | |
| H8A | 0.4115 | 0.1707 | 0.0018 | 0.044* | |
| H8B | 0.4888 | 0.2195 | 0.0965 | 0.044* | |
| C9 | 0.4942 (5) | 0.2625 (3) | −0.0704 (6) | 0.0349 (14) | |
| H9A | 0.4367 | 0.2659 | −0.1397 | 0.042* | |
| H9B | 0.5644 | 0.2326 | −0.0911 | 0.042* | |
| C10 | 0.5383 (5) | 0.3429 (4) | −0.0348 (6) | 0.0395 (15) | |
| H10A | 0.4722 | 0.3789 | −0.0547 | 0.047* | |
| H10B | 0.5568 | 0.3446 | 0.0499 | 0.047* | |
| C11 | 0.6305 (6) | 0.3689 (4) | −0.2268 (6) | 0.0506 (18) | |
| H11A | 0.5991 | 0.3198 | −0.2544 | 0.061* | |
| H11B | 0.7096 | 0.3766 | −0.2591 | 0.061* | |
| C12 | 0.5423 (8) | 0.4303 (5) | −0.2747 (8) | 0.074 (3) | |
| H12A | 0.4639 | 0.4237 | −0.2425 | 0.111* | |
| H12B | 0.5320 | 0.4265 | −0.3590 | 0.111* | |
| H12C | 0.5753 | 0.4794 | −0.2528 | 0.111* | |
| C13 | 0.7034 (6) | 0.4385 (4) | −0.0402 (7) | 0.0545 (19) | |
| H13A | 0.7097 | 0.4334 | 0.0450 | 0.065* | |
| H13B | 0.6478 | 0.4804 | −0.0608 | 0.065* | |
| C14 | 0.8299 (7) | 0.4567 (5) | −0.0810 (8) | 0.064 (2) | |
| H14A | 0.8834 | 0.4137 | −0.0666 | 0.096* | |
| H14B | 0.8637 | 0.5000 | −0.0384 | 0.096* | |
| H14C | 0.8225 | 0.4679 | −0.1638 | 0.096* | |
| C15 | 0.7989 (8) | 0.2122 (6) | 0.0666 (8) | 0.075 (3) | |
| H15A | 0.8652 | 0.1762 | 0.0773 | 0.113* | |
| H15B | 0.7236 | 0.1858 | 0.0433 | 0.113* | |
| H15C | 0.7900 | 0.2387 | 0.1393 | 0.113* | |
| H2A | 0.705 (6) | 0.329 (3) | −0.073 (7) | 0.080* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Zn1 | 0.0302 (4) | 0.0493 (4) | 0.0342 (4) | 0.0016 (3) | 0.0001 (3) | −0.0028 (3) |
| I1 | 0.0523 (3) | 0.0513 (3) | 0.0513 (3) | 0.0029 (2) | −0.0108 (2) | −0.0073 (2) |
| I2 | 0.0678 (10) | 0.0487 (8) | 0.0474 (9) | 0.0168 (6) | 0.0150 (8) | 0.0091 (5) |
| I2' | 0.130 (4) | 0.100 (4) | 0.115 (5) | −0.016 (3) | −0.036 (3) | 0.035 (4) |
| Br1 | 0.0832 (6) | 0.0568 (5) | 0.0503 (5) | 0.0002 (4) | 0.0141 (4) | −0.0192 (4) |
| O1 | 0.026 (2) | 0.065 (3) | 0.042 (3) | −0.005 (2) | 0.0035 (19) | −0.009 (2) |
| O2 | 0.032 (2) | 0.059 (3) | 0.071 (4) | −0.006 (2) | −0.006 (2) | 0.006 (3) |
| N1 | 0.024 (2) | 0.035 (3) | 0.035 (3) | 0.0020 (19) | 0.005 (2) | −0.002 (2) |
| N2 | 0.034 (3) | 0.040 (3) | 0.042 (3) | −0.003 (2) | 0.009 (2) | 0.002 (3) |
| C1 | 0.025 (3) | 0.032 (3) | 0.033 (3) | −0.003 (2) | 0.009 (2) | 0.003 (3) |
| C2 | 0.029 (3) | 0.041 (4) | 0.032 (3) | −0.002 (2) | 0.010 (3) | 0.005 (3) |
| C3 | 0.030 (3) | 0.048 (4) | 0.047 (4) | 0.005 (3) | 0.010 (3) | 0.009 (3) |
| C4 | 0.051 (4) | 0.040 (4) | 0.041 (4) | 0.008 (3) | 0.020 (3) | 0.008 (3) |
| C5 | 0.055 (4) | 0.034 (3) | 0.032 (4) | 0.000 (3) | 0.011 (3) | −0.003 (3) |
| C6 | 0.038 (3) | 0.042 (4) | 0.027 (3) | −0.003 (3) | 0.006 (3) | −0.002 (3) |
| C7 | 0.024 (3) | 0.041 (3) | 0.036 (4) | −0.003 (2) | −0.003 (3) | 0.005 (3) |
| C8 | 0.030 (3) | 0.032 (3) | 0.049 (4) | 0.004 (2) | 0.006 (3) | −0.003 (3) |
| C9 | 0.024 (3) | 0.038 (3) | 0.043 (4) | 0.003 (2) | 0.007 (3) | −0.011 (3) |
| C10 | 0.035 (3) | 0.045 (4) | 0.040 (4) | 0.000 (3) | 0.013 (3) | −0.006 (3) |
| C11 | 0.049 (4) | 0.058 (5) | 0.045 (4) | −0.006 (3) | 0.011 (3) | 0.003 (4) |
| C12 | 0.070 (5) | 0.076 (6) | 0.074 (6) | −0.002 (4) | −0.003 (5) | 0.026 (5) |
| C13 | 0.051 (4) | 0.043 (4) | 0.069 (5) | −0.009 (3) | 0.005 (4) | −0.007 (4) |
| C14 | 0.054 (4) | 0.061 (5) | 0.077 (6) | −0.019 (4) | 0.008 (4) | 0.011 (4) |
| C15 | 0.055 (5) | 0.098 (7) | 0.071 (6) | −0.005 (5) | −0.002 (4) | 0.008 (5) |
Geometric parameters (Å, °)
| Zn1—O1 | 1.958 (4) | C7—H7 | 0.9300 |
| Zn1—N1 | 2.032 (5) | C8—C9 | 1.510 (9) |
| Zn1—I2' | 2.545 (6) | C8—H8A | 0.9700 |
| Zn1—I1 | 2.5627 (9) | C8—H8B | 0.9700 |
| Zn1—I2 | 2.5768 (18) | C9—C10 | 1.536 (8) |
| Br1—C5 | 1.902 (6) | C9—H9A | 0.9700 |
| O1—C2 | 1.315 (7) | C9—H9B | 0.9700 |
| O2—C15 | 1.353 (9) | C10—H10A | 0.9700 |
| O2—H2 | 0.8200 | C10—H10B | 0.9700 |
| N1—C7 | 1.278 (7) | C11—C12 | 1.516 (10) |
| N1—C8 | 1.478 (7) | C11—H11A | 0.9700 |
| N2—C10 | 1.499 (8) | C11—H11B | 0.9700 |
| N2—C13 | 1.503 (8) | C12—H12A | 0.9600 |
| N2—C11 | 1.506 (9) | C12—H12B | 0.9600 |
| N2—H2A | 0.91 (6) | C12—H12C | 0.9600 |
| C1—C6 | 1.412 (8) | C13—C14 | 1.519 (9) |
| C1—C2 | 1.421 (8) | C13—H13A | 0.9700 |
| C1—C7 | 1.447 (8) | C13—H13B | 0.9700 |
| C2—C3 | 1.411 (8) | C14—H14A | 0.9600 |
| C3—C4 | 1.368 (9) | C14—H14B | 0.9600 |
| C3—H3 | 0.9300 | C14—H14C | 0.9600 |
| C4—C5 | 1.383 (9) | C15—H15A | 0.9600 |
| C4—H4 | 0.9300 | C15—H15B | 0.9600 |
| C5—C6 | 1.370 (8) | C15—H15C | 0.9600 |
| C6—H6 | 0.9300 | ||
| O1—Zn1—N1 | 93.11 (18) | N1—C8—H8B | 109.4 |
| O1—Zn1—I2' | 111.2 (5) | C9—C8—H8B | 109.4 |
| N1—Zn1—I2' | 115.1 (3) | H8A—C8—H8B | 108.0 |
| O1—Zn1—I1 | 115.00 (13) | C8—C9—C10 | 112.7 (5) |
| N1—Zn1—I1 | 109.36 (14) | C8—C9—H9A | 109.1 |
| I2'—Zn1—I1 | 111.9 (5) | C10—C9—H9A | 109.1 |
| O1—Zn1—I2 | 104.79 (15) | C8—C9—H9B | 109.1 |
| N1—Zn1—I2 | 111.03 (15) | C10—C9—H9B | 109.1 |
| I2'—Zn1—I2 | 8.4 (5) | H9A—C9—H9B | 107.8 |
| I1—Zn1—I2 | 120.23 (7) | N2—C10—C9 | 112.5 (5) |
| C2—O1—Zn1 | 120.5 (4) | N2—C10—H10A | 109.1 |
| C15—O2—H2 | 109.5 | C9—C10—H10A | 109.1 |
| C7—N1—C8 | 118.9 (5) | N2—C10—H10B | 109.1 |
| C7—N1—Zn1 | 120.3 (4) | C9—C10—H10B | 109.1 |
| C8—N1—Zn1 | 120.7 (4) | H10A—C10—H10B | 107.8 |
| C10—N2—C13 | 110.2 (5) | N2—C11—C12 | 114.7 (6) |
| C10—N2—C11 | 113.6 (5) | N2—C11—H11A | 108.6 |
| C13—N2—C11 | 114.2 (5) | C12—C11—H11A | 108.6 |
| C10—N2—H2A | 103 (5) | N2—C11—H11B | 108.6 |
| C13—N2—H2A | 106 (5) | C12—C11—H11B | 108.6 |
| C11—N2—H2A | 109 (5) | H11A—C11—H11B | 107.6 |
| C6—C1—C2 | 119.4 (5) | C11—C12—H12A | 109.5 |
| C6—C1—C7 | 115.8 (5) | C11—C12—H12B | 109.5 |
| C2—C1—C7 | 124.9 (5) | H12A—C12—H12B | 109.5 |
| O1—C2—C3 | 119.9 (5) | C11—C12—H12C | 109.5 |
| O1—C2—C1 | 123.1 (5) | H12A—C12—H12C | 109.5 |
| C3—C2—C1 | 116.9 (6) | H12B—C12—H12C | 109.5 |
| C4—C3—C2 | 122.6 (6) | N2—C13—C14 | 112.2 (6) |
| C4—C3—H3 | 118.7 | N2—C13—H13A | 109.2 |
| C2—C3—H3 | 118.7 | C14—C13—H13A | 109.2 |
| C3—C4—C5 | 119.7 (6) | N2—C13—H13B | 109.2 |
| C3—C4—H4 | 120.2 | C14—C13—H13B | 109.2 |
| C5—C4—H4 | 120.2 | H13A—C13—H13B | 107.9 |
| C6—C5—C4 | 120.4 (6) | C13—C14—H14A | 109.5 |
| C6—C5—Br1 | 118.8 (5) | C13—C14—H14B | 109.5 |
| C4—C5—Br1 | 120.8 (5) | H14A—C14—H14B | 109.5 |
| C5—C6—C1 | 120.9 (6) | C13—C14—H14C | 109.5 |
| C5—C6—H6 | 119.5 | H14A—C14—H14C | 109.5 |
| C1—C6—H6 | 119.5 | H14B—C14—H14C | 109.5 |
| N1—C7—C1 | 126.3 (5) | O2—C15—H15A | 109.5 |
| N1—C7—H7 | 116.9 | O2—C15—H15B | 109.5 |
| C1—C7—H7 | 116.9 | H15A—C15—H15B | 109.5 |
| N1—C8—C9 | 111.1 (5) | O2—C15—H15C | 109.5 |
| N1—C8—H8A | 109.4 | H15A—C15—H15C | 109.5 |
| C9—C8—H8A | 109.4 | H15B—C15—H15C | 109.5 |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O2—H2···O1i | 0.82 | 1.86 | 2.640 (6) | 158 |
| N2—H2A···O2 | 0.91 (6) | 1.81 (6) | 2.716 (7) | 173 (8) |
Symmetry codes: (i) x+1, y, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BX2241).
References
- Ali, H. M., Mohamed Mustafa, M. I., Rizal, M. R. & Ng, S. W. (2008). Acta Cryst. E64, m718–m719. [DOI] [PMC free article] [PubMed]
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl.34, 1555–1573.
- Biswas, C., Drew, M. G. B. & Ghosh, A. (2008). Inorg. Chem.47, 4513–4519. [DOI] [PubMed]
- Bruker (2004). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Chen, Z., Morimoto, H., Matsunaga, S. & Shibasaki, M. (2008). J. Am. Chem. Soc.130, 2170–2171. [DOI] [PubMed]
- Darensbourg, D. J. & Frantz, E. B. (2007). Inorg. Chem.46, 5967–5978. [DOI] [PubMed]
- Habibi, M. H., Askari, E., Chantrapromma, S. & Fun, H.-K. (2007). Acta Cryst. E63, m2905–m2906.
- Kawamoto, T., Nishiwaki, M., Tsunekawa, Y., Nozaki, K. & Konno, T. (2008). Inorg. Chem.47, 3095–3104. [DOI] [PubMed]
- Lipscomb, W. N. & Sträter, N. (1996). Chem. Rev.96, 2375–2434. [DOI] [PubMed]
- Qiu, X.-Y. (2006a). Acta Cryst. E62, m717–m718.
- Qiu, X.-Y. (2006b). Acta Cryst. E62, m2173–m2174.
- Sheldrick, G. M. (2004). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Tomat, E., Cuesta, L., Lynch, V. M. & Sessler, J. L. (2007). Inorg. Chem.46, 6224–6226. [DOI] [PubMed]
- Wei, Y.-J., Wang, F.-W. & Zhu, Q.-Y. (2007). Acta Cryst. E63, m654–m655.
- Wu, J.-C., Liu, S.-X., Keene, T. D., Neels, A., Mereacre, V., Powell, A. K. & Decurtins, S. (2008). Inorg. Chem.47, 3452–3459. [DOI] [PubMed]
- Yuan, M., Zhao, F., Zhang, W., Wang, Z.-M. & Gao, S. (2007). Inorg. Chem.46, 11235–11242. [DOI] [PubMed]
- Zhu, X.-W. (2008). Acta Cryst. E64, m1456–m1457. [DOI] [PMC free article] [PubMed]
- Zhu, Q.-Y., Wei, Y.-J. & Wang, F.-W. (2007). Acta Cryst. E63, m1431–m1432.
- Zhu, X.-W. & Yang, X.-Z. (2008a). Acta Cryst. E64, m1090–m1091. [DOI] [PMC free article] [PubMed]
- Zhu, X.-W. & Yang, X.-Z. (2008b). Acta Cryst. E64, m1092–m1093. [DOI] [PMC free article] [PubMed]
- Zhu, X.-W. & Yang, X.-Z. (2008c). Acta Cryst. E64, m1094–m1095. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809038446/bx2241sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809038446/bx2241Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report