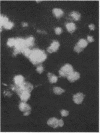
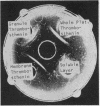
Abstract
Thrombosthenin, an immunologically distinct contractile protein was isolated in relatively pure form from human platelets. The protein, which was of high molecular weight appeared to be composed of multiple polypeptide subunits, probably polymeric in nature.
Thrombosthenin had magnesium-dependent ATPase activisty, releasing an average of 3 μg phosphorus per mg protein in 30 min. After the addition of ATP, there was a reversible alteration in viscosity with calculated ATP sensitivity ranging from 64 to 90%. These biochemical properties of thrombosthenin resemble those of smooth muscle.
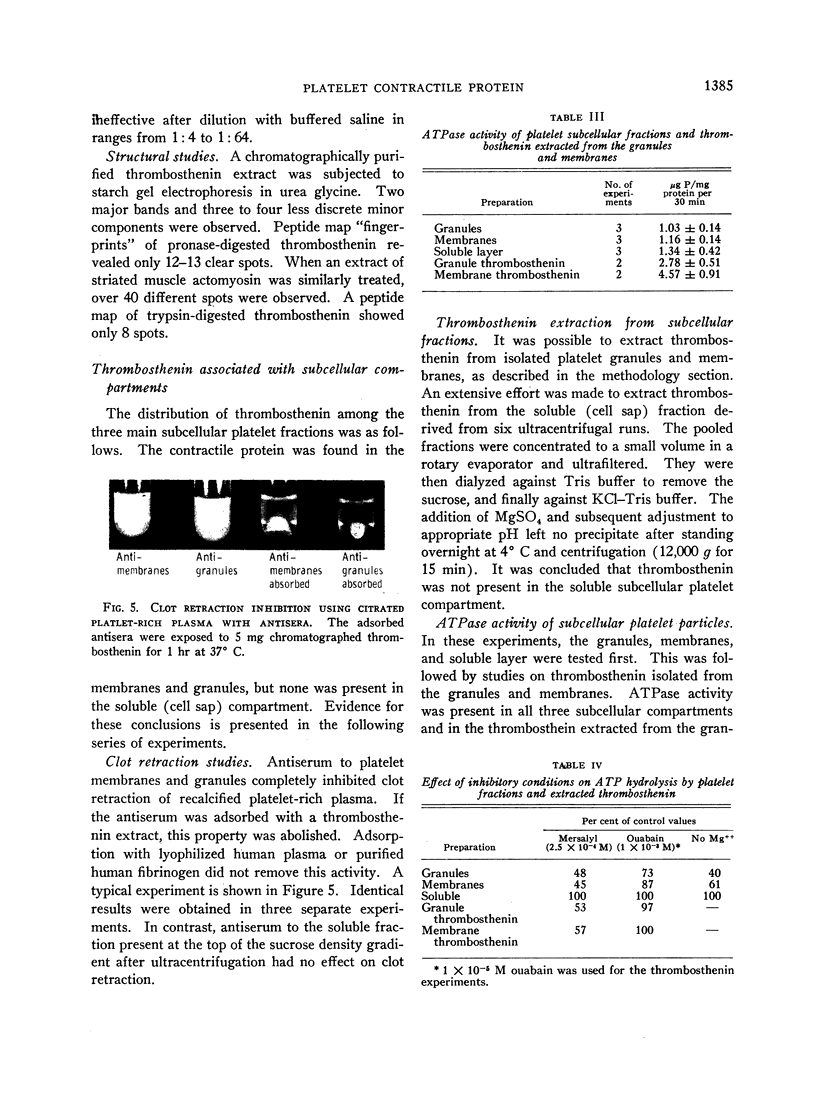
Specific antisera to thrombosthenin significantly inhibited the ATPase activity of the protein. Clot retraction of recalcified platelet-rich plasma and clot retraction of clotted fibrinogen-platelet mixtures were also inhibited by the antisera. The findings suggest that thrombosthenin is an important component of the clot retraction system.
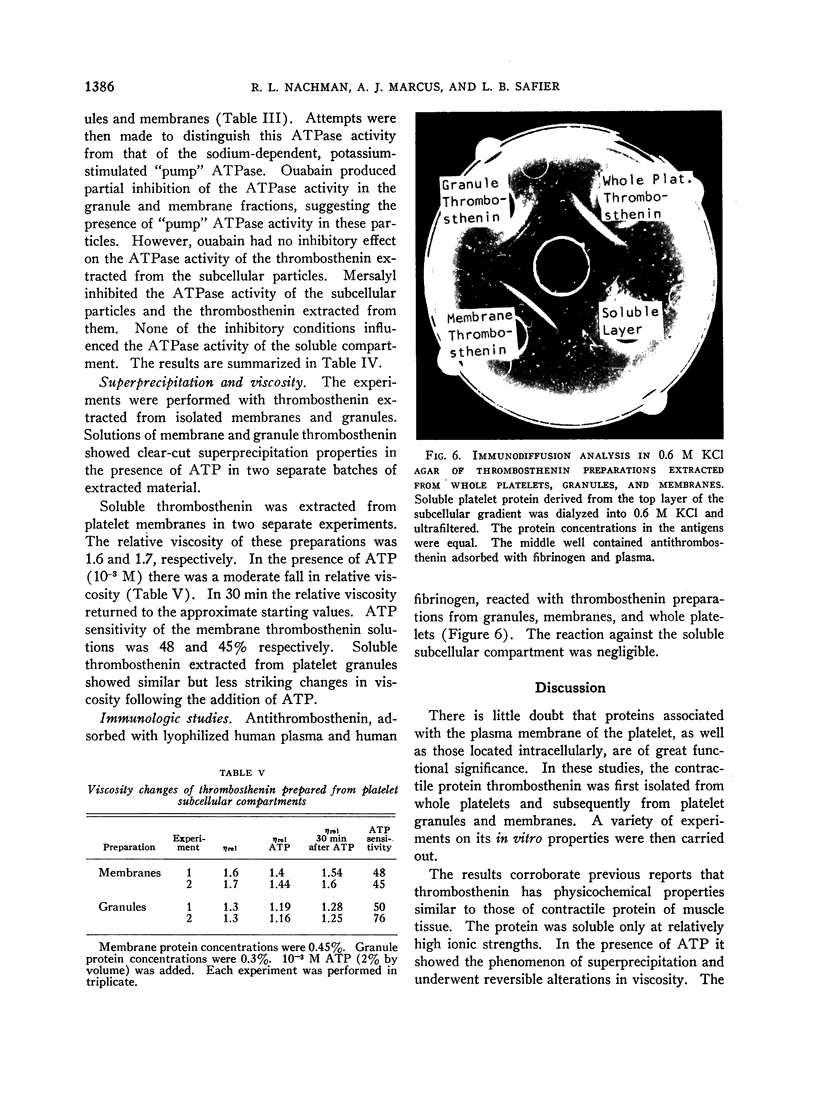
Thrombosthenin was extracted from isolated platelet granule and membrane fractions. The contractile protein derived from the membrane compartment was more active as an ATPase and appeared to be more homogeneous on immunologic analysis.
Full text
PDF


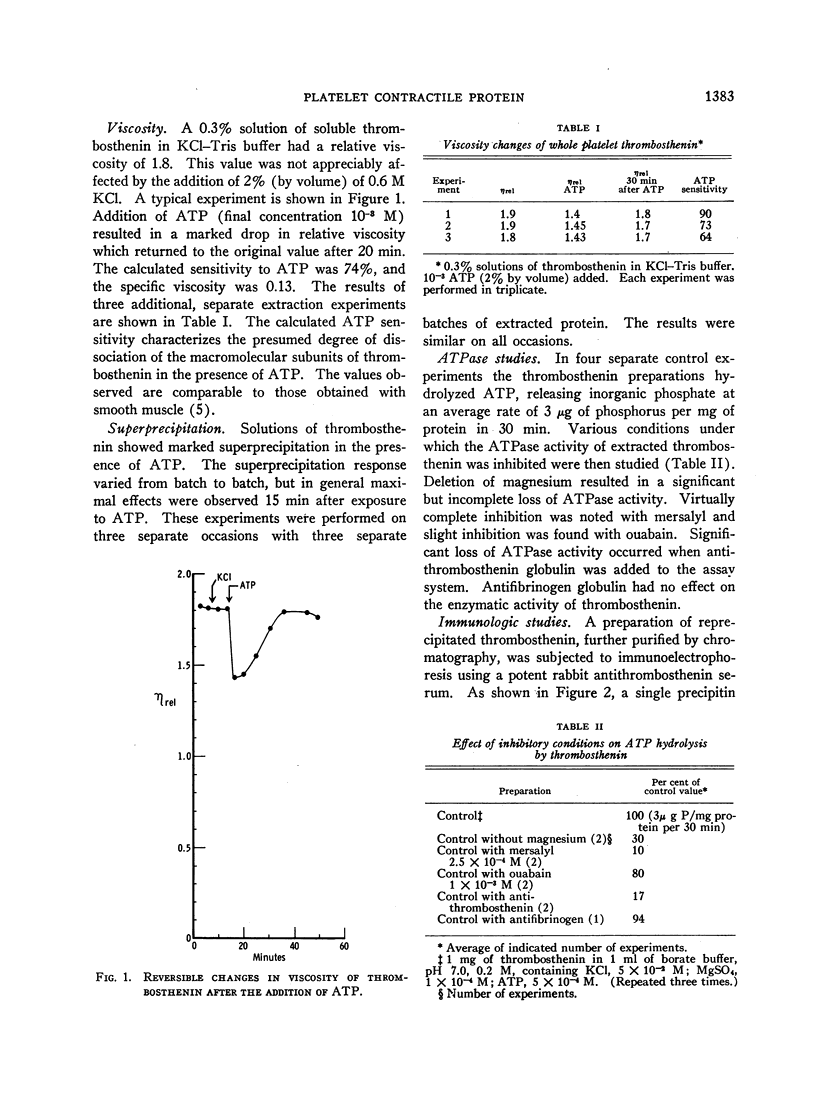
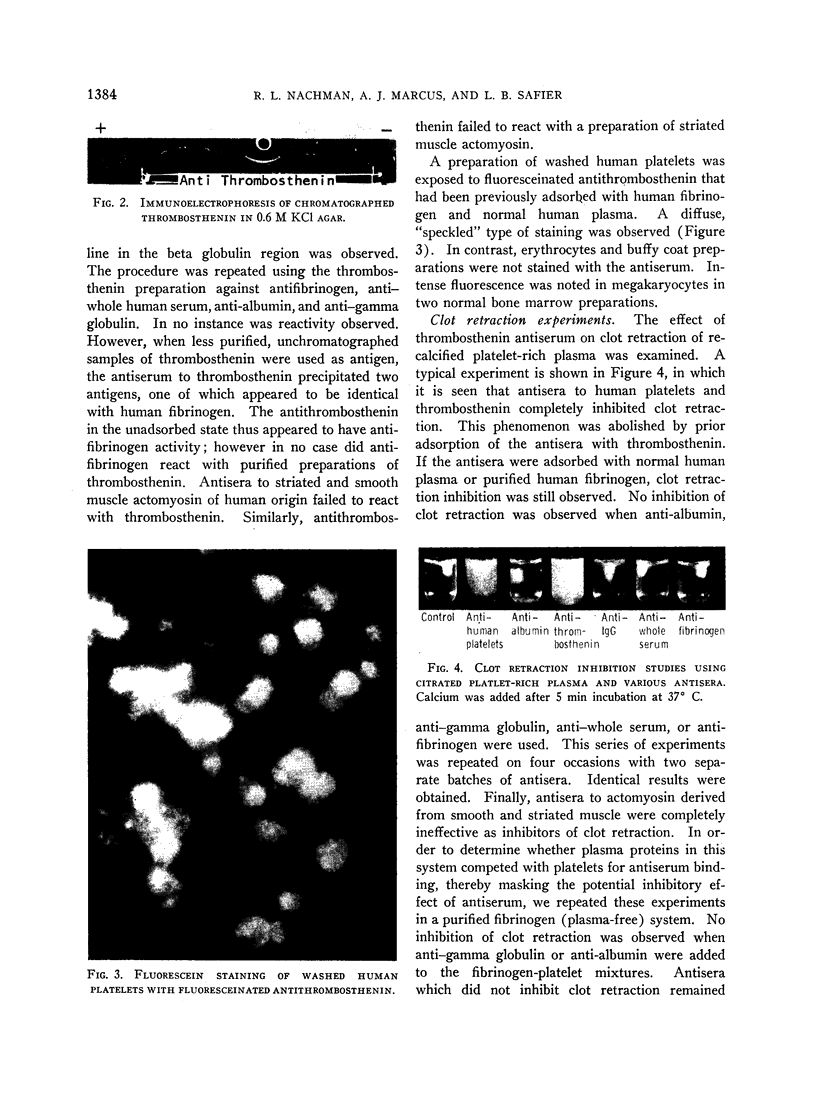



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chambers D. A., Salzman E. W., Neri L. L. Characterization of "ecto-ATPase" of human blood platelets. Arch Biochem Biophys. 1967 Mar;119(1):173–178. doi: 10.1016/0003-9861(67)90444-4. [DOI] [PubMed] [Google Scholar]
- Cohen S., Porter R. R. Heterogeneity of the peptide chains of gamma-globulin. Biochem J. 1964 Feb;90(2):278–284. doi: 10.1042/bj0900278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRYER R. L., TAMMES A. R., ROUTH J. I. The determination of phosphorus and phosphatase with N-phenyl-p-phenylenediamine. J Biol Chem. 1957 Mar;225(1):177–183. [PubMed] [Google Scholar]
- KORNGOLD L. The distribution and immunochemical properties of human tissue and tumor antigens. Ann N Y Acad Sci. 1957 Dec 16;69(4):681–697. doi: 10.1111/j.1749-6632.1957.tb49709.x. [DOI] [PubMed] [Google Scholar]
- Knight V. A., Jones B. M., Jones P. C. Inhibition of the aggregation of dissociated embryo-chick fibroblast cells by adenosine triphosphate. Nature. 1966 Jun 4;210(5040):1008–1010. doi: 10.1038/2101008a0. [DOI] [PubMed] [Google Scholar]
- LEVY H. M., FLEISHER M. STUDIES ON THE SUPERPRECIPITATION OF ACTOMYOSIN SUSPENSIONS AS MEASURED BY THE CHANGE IN TURBIDITY. I. EFFECTS OF ADENOSINE TRIPHOSPHATE CONCENTRATION AND TEMPERATURE. Biochim Biophys Acta. 1965 May 4;100:479–502. doi: 10.1016/0304-4165(65)90018-8. [DOI] [PubMed] [Google Scholar]
- MARSH B. B. The estimation of inorganic phosphate in the presence of adenosine triphosphate. Biochim Biophys Acta. 1959 Apr;32:357–361. doi: 10.1016/0006-3002(59)90607-9. [DOI] [PubMed] [Google Scholar]
- MORSE E. E., JACKSON D. P., CONLEY C. L. ROLE OF PLATELET FIBRINOGEN IN THE REACTIONS OF PLATELETS TO THROMBIN. J Clin Invest. 1965 May;44:809–816. doi: 10.1172/JCI105193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus A. J., Zucker-Franklin D., Safier L. B., Ullman H. L. Studies on human platelet granules and membranes. J Clin Invest. 1966 Jan;45(1):14–28. doi: 10.1172/JCI105318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NACHMAN R. L. IMMUNOLOGIC STUDIES OF PLATELET PROTEIN. Blood. 1965 May;25:703–711. [PubMed] [Google Scholar]
- NEIFAKH S. A., AVRAMOV J. A., GAITSKHOKI V. S., KAZAKOVA T. B., MONAKHOV N. K., REPIN V. S., TUROVSKI V. S., VASSILETZ I. M. MECHANISM OF THE CONTROLLING FUNCTION OF MITOCHONDRIA. Biochim Biophys Acta. 1965 May 4;100:329–343. doi: 10.1016/0304-4165(65)90002-4. [DOI] [PubMed] [Google Scholar]
- Nachman R. L., Engle R. L., Jr, Copeland L. Correlation of immunologic and structural heterogeneity of Bence Jones proteins. J Immunol. 1966 Sep;97(3):356–362. [PubMed] [Google Scholar]
- Nachman R. L., Marcus A. J., Zucker-Franklin D. Immunologic studies of proteins associated with subcellular fractions of normal human platelets. J Lab Clin Med. 1967 Apr;69(4):651–658. [PubMed] [Google Scholar]
- SCHMID H. J., JACKSON D. P., CONLEY C. L. Mechanism of action of thrombin on platelets. J Clin Invest. 1962 Mar;41:543–553. doi: 10.1172/JCI104508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHULMAN N. R. Immunoreactions involving platelets. III. Quantitative aspects of platelet agglutination, inhibition of clot retraction, and other reactions caused by the antibody of quinidine purpura. J Exp Med. 1958 May 1;107(5):697–710. doi: 10.1084/jem.107.5.697. [DOI] [PMC free article] [PubMed] [Google Scholar]