Abstract
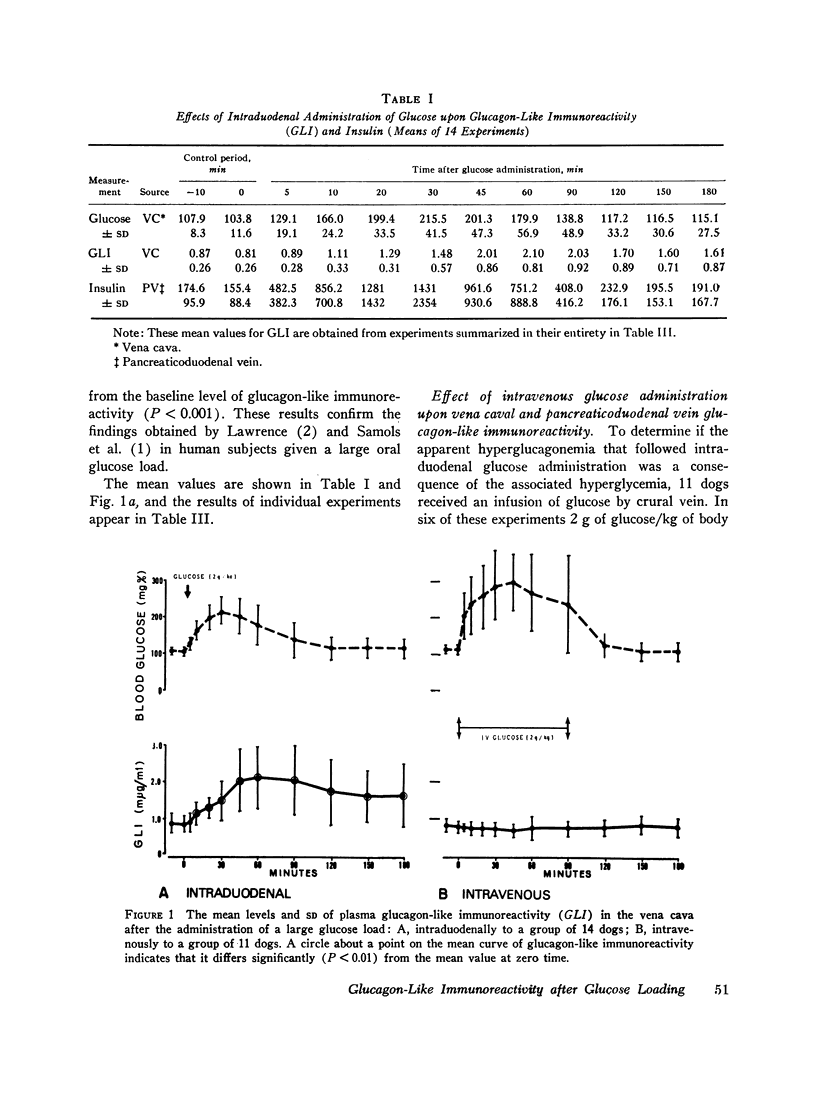
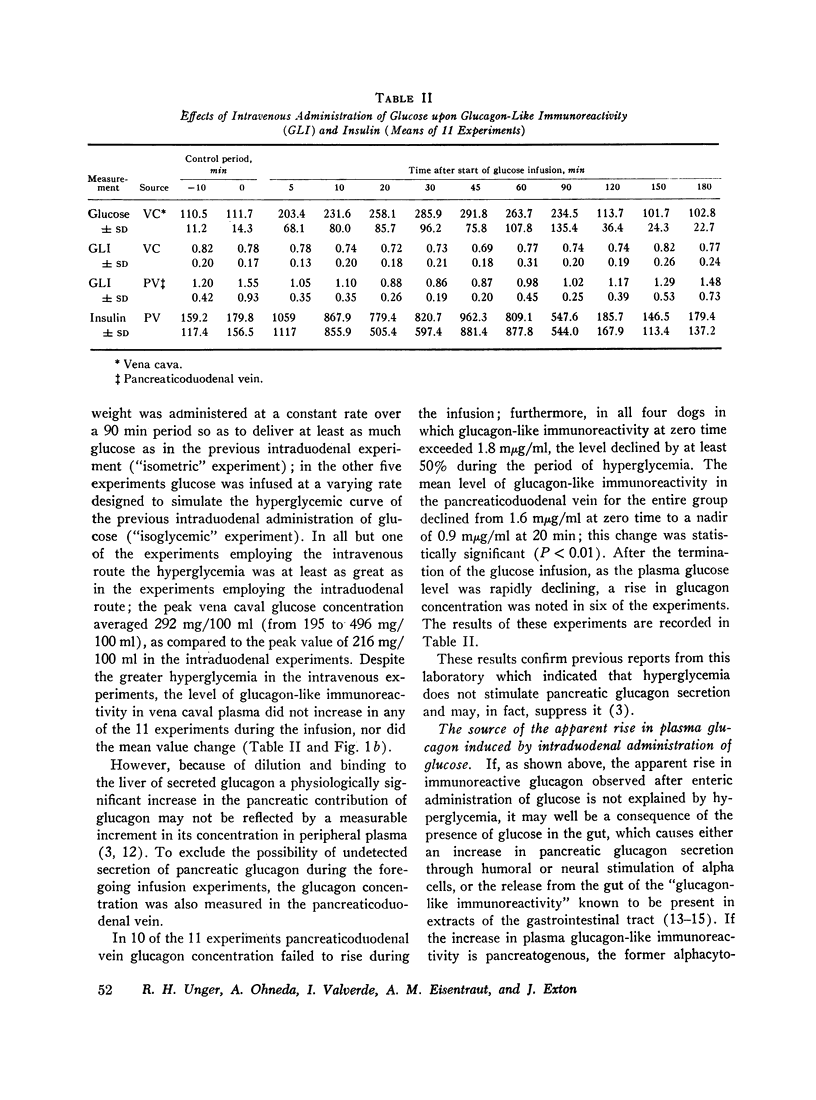
The effects of ingested and infused glucose upon circulating glucagon-like immunoreactivity (GLI) were compared in 14 triply catheterized conscious dogs. Within 60 min after the intraduodenal administration of 2 g/kg of glucose, the mean level of glucagon-like immunoreactivity in the vena caval plasma more than doubled, whereas after intravenous infusion of the same dose over a 90 min period no change in the mean vena caval level was observed; during glucose infusion mean glucagon-like immunoreactivity in the pancreatic venous effluent declined, suggesting that hyperglycemia suppresses rather than stimulates pancreatic glucagon secretion.
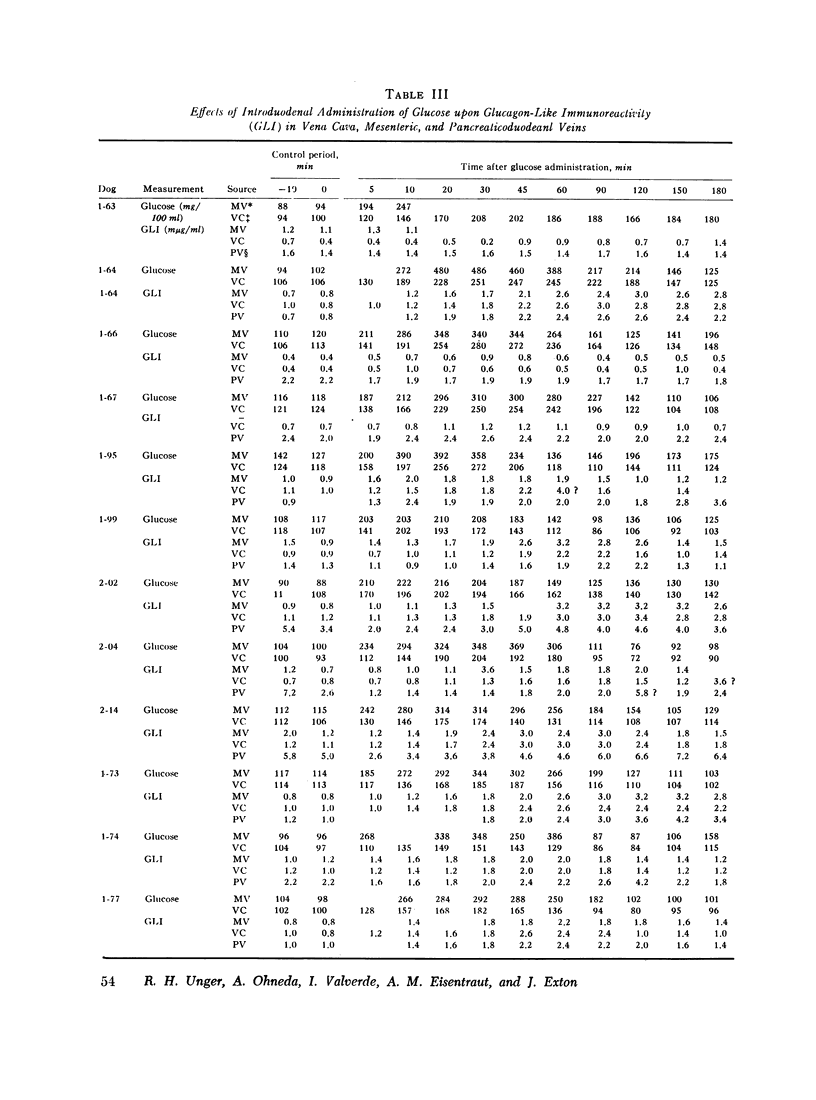
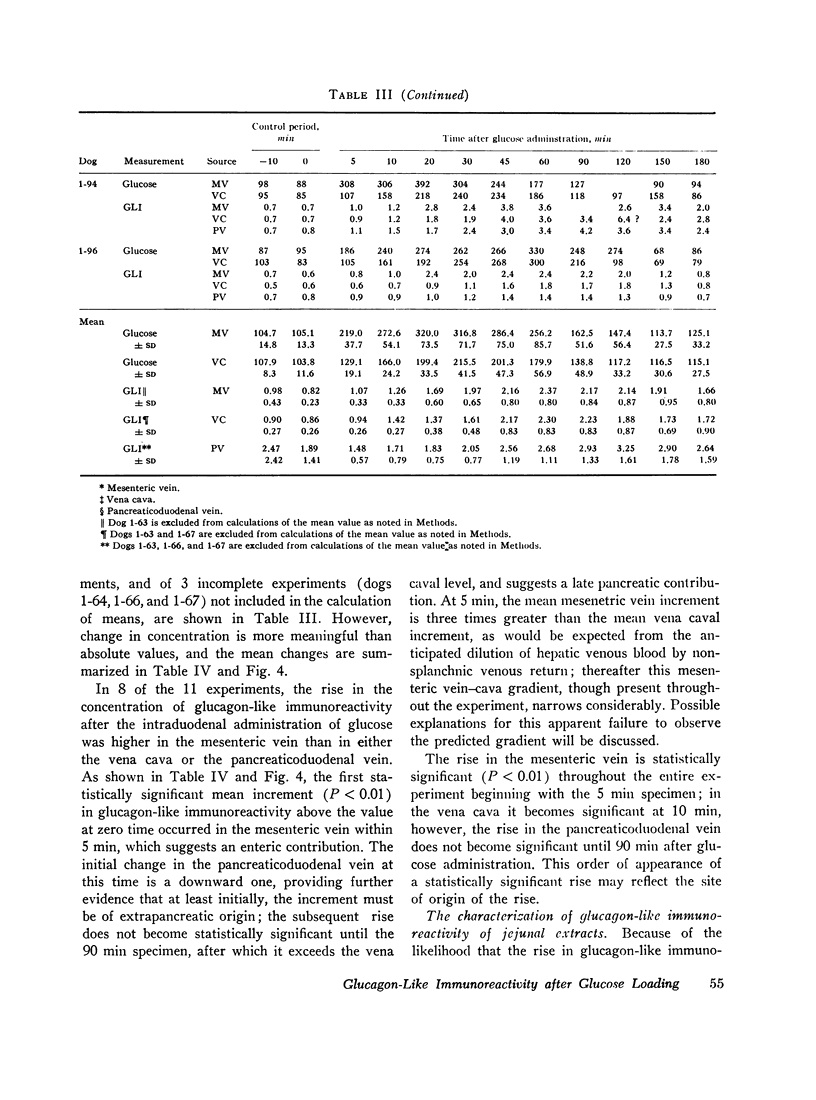
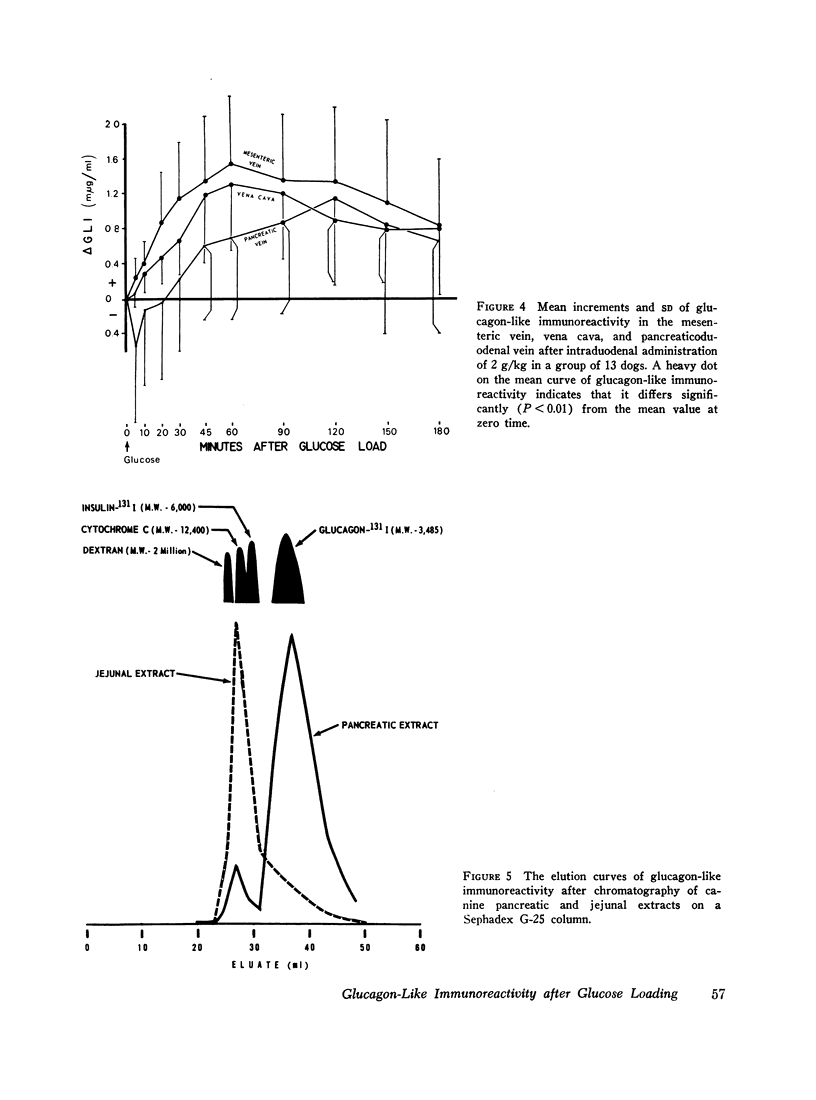
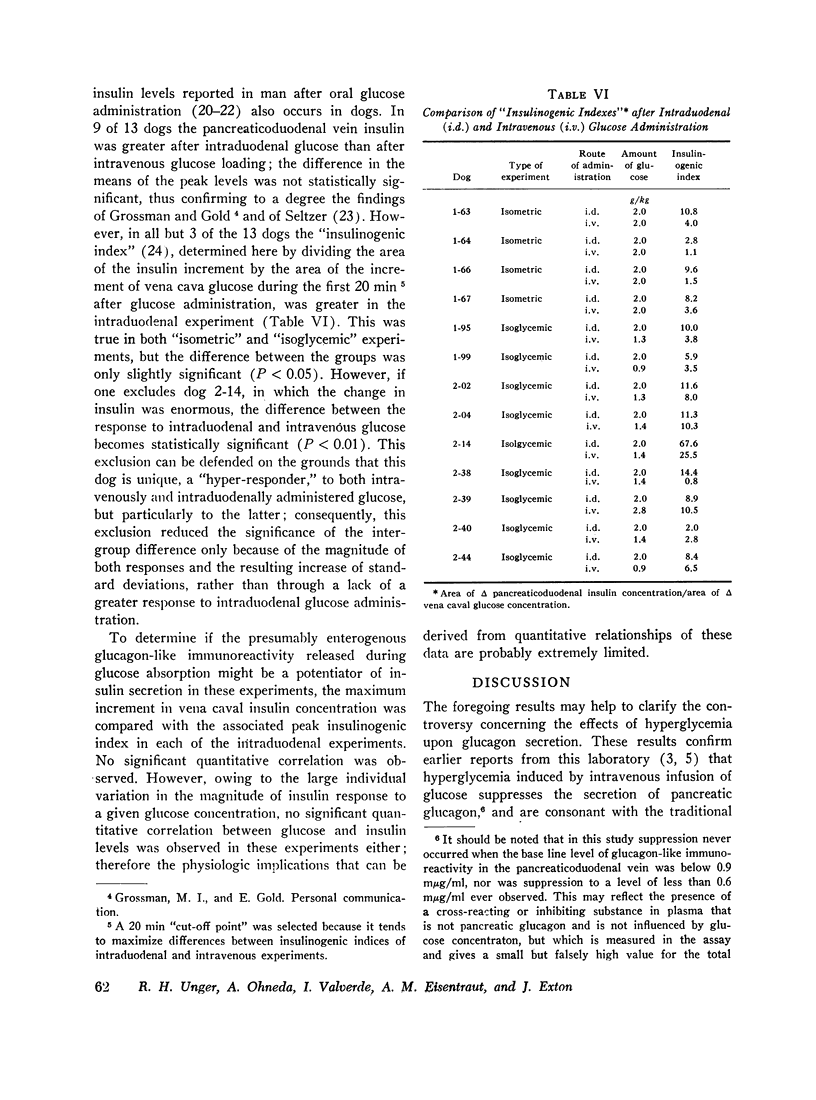
To determine if the rise in glucagon-like immunoreactivity that occurs during glucose absorption was of pancreatic origin, the effect of pancreatectomy performed 1 hr after the intraduodenal administration of glucose was determined. Although circulating insulin disappeared after resection of the pancreas, the level of glucagon-like immunoreactivity continued to rise, establishing its extrapancreatic origin. In other experiments, measurements of Glucagon-like immunoreactivity in plasma obtained simultaneously from pancreaticoduodenal and mesenteric veins and from the vena cava revealed the increment after intraduodenal glucose loading to be greatest in the mesenteric vein in 8 of 12 experiments, favoring the gut as the likely source of the rise.
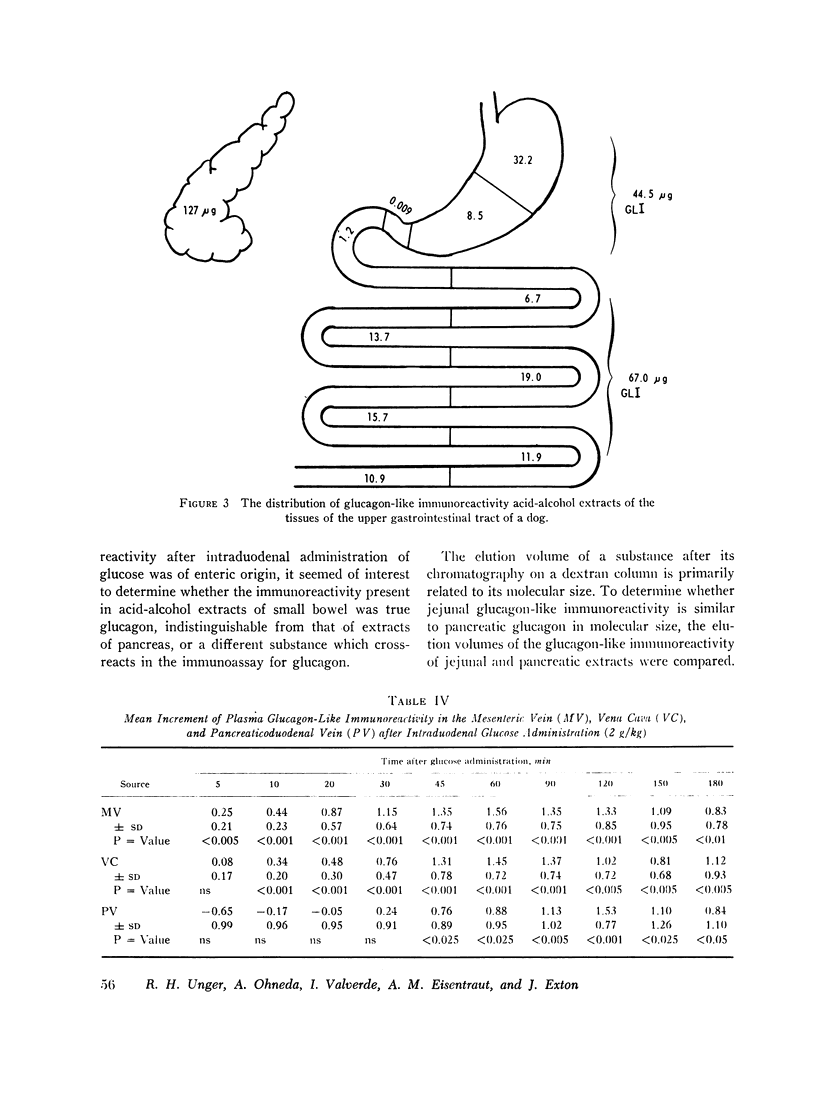
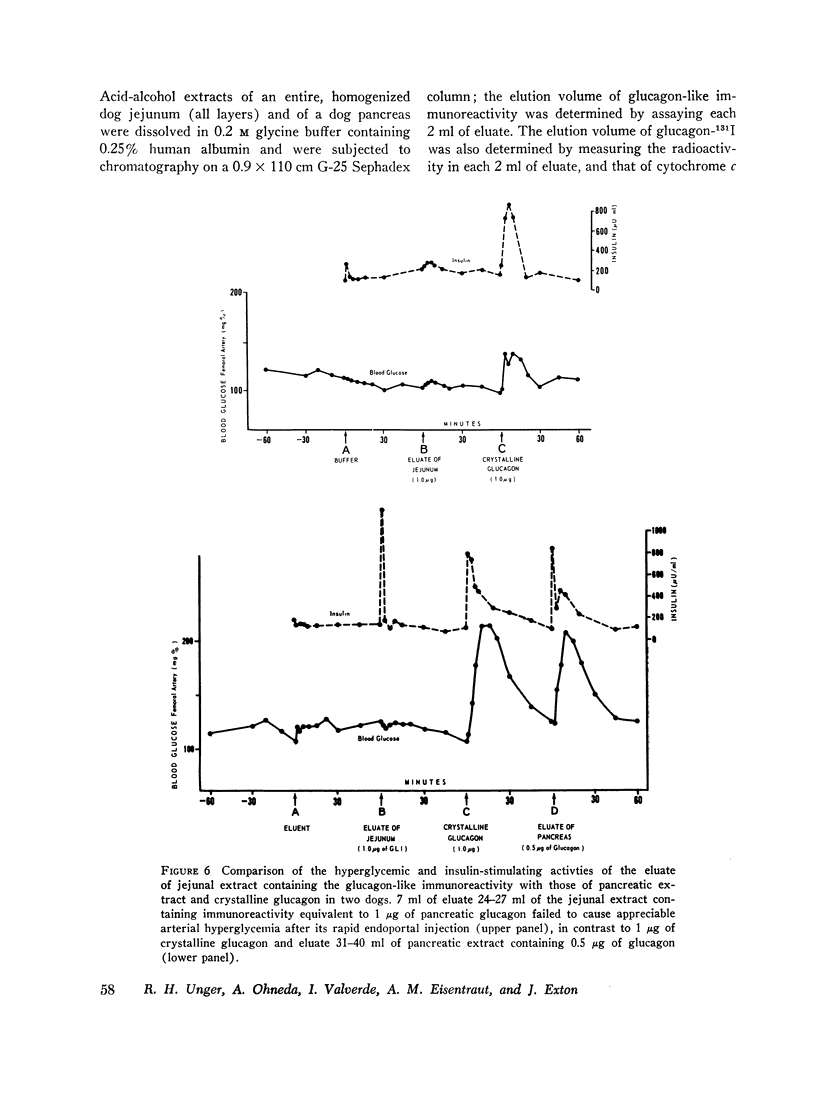
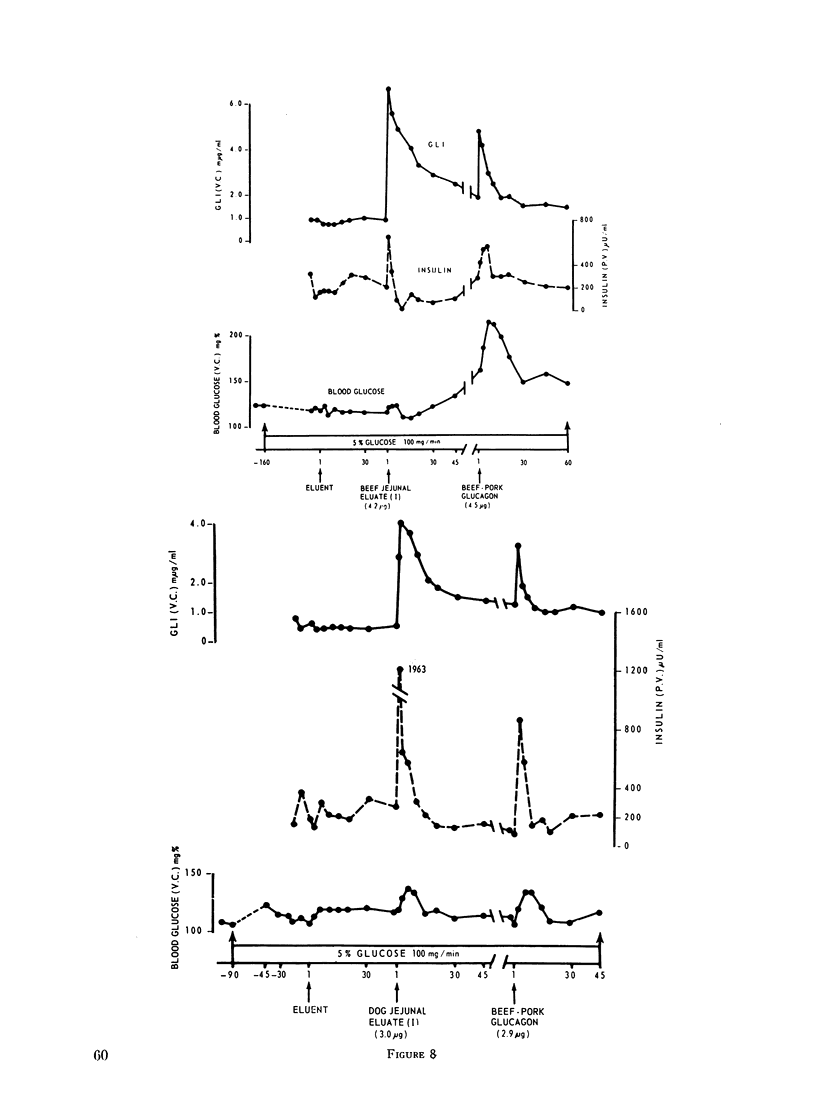
To characterize gut glucagon-like immunoreactivity, acid-alcohol extracts of canine jejunum were compared with similar glucagon-containing extracts of canine pancreas with respect to certain physical and biological properties. On a G-25 Sephadex column the elution volume of the jejunal immunoreactivity was found to be smaller than that of glucagon, which suggested a molecular size at least twice that of pancreatic glucagon. Furthermore, the in vivo and in vitro biological activities of the eluates containing jejunal glucagon-like immunoreactivity appeared to differ from those of eluates containing pancreatic glucagon. The jejunal material lacked hyperglycemic activity when injected endoportally into dogs, was devoid of glycogenolytic activity in the isolated perfused rat liver, and did not increase hepatic 3′,5′ cyclic adenylate in the perfused liver; however, like glucagon it appeared to stimulate insulin release. It seems quite clear the material in intestinal extracts either is a different substance or a different form from that of true pancreatic glucagon, although it crossreacts in the radioimmunoassay with antibodies to glucagon.
It is concluded, (a) that hyperglycemia does not stimulate and probably suppresses the secretion of pancreatic glucagon; (b) that during intestinal absorption of glucose, a rise in glucagon-like immunoreactivity occurs; (c) this immunoreactivity is derived from an extrapancreatic site, probably the gut; (d) that the glucagon-like immunoreactivity extractable from jejunum is not the same as pancreatic glucagon but is a larger molecule devoid of hyperglycemic and glycogenolytic activity, a cross-reactant in radioimmunoassay for glucagon; and (e) that the eluate in which jejunal immunoreactivity is contained can stimulate insulin release in conscious dogs.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butcher R. W., Ho R. J., Meng H. C., Sutherland E. W. Adenosine 3',5'-monophosphate in biological materials. II. The measurement of adenosine 3',5'-monophosphate in tissues and the role of the cyclic nucleotide in the lipolytic response of fat to epinephrine. J Biol Chem. 1965 Nov;240(11):4515–4523. [PubMed] [Google Scholar]
- Crockford P. M., Porte D., Jr, Wood F. C., Jr, Williams R. H. Effect of glucagon on serum insulin, plasma glucose and free fatty acids in man. Metabolism. 1966 Feb;15(2):114–122. doi: 10.1016/0026-0495(66)90032-1. [DOI] [PubMed] [Google Scholar]
- ELRICK H., STIMMLER L., HLAD C. J., Jr, ARAI Y. PLASMA INSULIN RESPONSE TO ORAL AND INTRAVENOUS GLUCOSE ADMINISTRATION. J Clin Endocrinol Metab. 1964 Oct;24:1076–1082. doi: 10.1210/jcem-24-10-1076. [DOI] [PubMed] [Google Scholar]
- KENNY A. J. Extractable glucagon of the human pancreas. J Clin Endocrinol Metab. 1955 Sep;15(9):1089–1105. doi: 10.1210/jcem-15-9-1089. [DOI] [PubMed] [Google Scholar]
- Ketterer H., Eisentraut A. M., Unger R. H. Effect upon insulin secretion of physiologic doses of glucagon administered via the portal vein. Diabetes. 1967 May;16(5):283–288. doi: 10.2337/diab.16.5.283. [DOI] [PubMed] [Google Scholar]
- Lawrence A. M. Radioimmunoassayable glucagon levels in man: effects of starvation, hypoglycemia, and glucose administration. Proc Natl Acad Sci U S A. 1966 Feb;55(2):316–320. doi: 10.1073/pnas.55.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAKMAN M. H., SUTHERLAND E. W., Jr USE OF LIVER ADENYL CYCLASE FOR ASSAY OF GLUCAGON IN HUMAN GASTRO-INTESTINAL TRACT AND PANCREAS. Endocrinology. 1964 Jul;75:127–134. doi: 10.1210/endo-75-1-127. [DOI] [PubMed] [Google Scholar]
- MCINTYRE N., HOLDSWORTH C. D., TURNER D. S. NEW INTERPRETATION OF ORAL GLUCOSE TOLERANCE. Lancet. 1964 Jul 4;2(7349):20–21. doi: 10.1016/s0140-6736(64)90011-x. [DOI] [PubMed] [Google Scholar]
- SAMOLS E., MARRI G., MARKS V. PROMOTION OF INSULIN SECRETION BY GLUCAGON. Lancet. 1965 Aug 28;2(7409):415–416. doi: 10.1016/s0140-6736(65)90761-0. [DOI] [PubMed] [Google Scholar]
- SCHENK W. G., Jr, MCDONALD K. E., CAMP F. A., POLLOCK L. THE MEASUREMENT OF REGIONAL BLOOD FLOW. J Thorac Cardiovasc Surg. 1963 Jul;46:50–56. [PubMed] [Google Scholar]
- SELTZER H. S., SMITH W. L. Plasma insulin activity after glucose: an index of insulogenic reserve in normal and diabetic man. Diabetes. 1959 Nov-Dec;8:417–424. doi: 10.2337/diab.8.6.417. [DOI] [PubMed] [Google Scholar]
- Samols E., Tyler J., Marri G., Marks V. Stimulation of glucagon secretion by oral glucose. Lancet. 1965 Dec 18;2(7425):1257–1259. doi: 10.1016/s0140-6736(65)92278-6. [DOI] [PubMed] [Google Scholar]
- Samols E., Tyler J., Megyesi C., Marks V. Immunochemical glucagon in human pancreas, gut, and plasma. Lancet. 1966 Oct 1;2(7466):727–729. doi: 10.1016/s0140-6736(66)92982-5. [DOI] [PubMed] [Google Scholar]
- Sokal J. E. Glucagon--an essential hormone. Am J Med. 1966 Sep;41(3):331–341. doi: 10.1016/0002-9343(66)90079-9. [DOI] [PubMed] [Google Scholar]
- UNGER R. H., EISENTRAUT A. M., MADISON L. L. The effects of total starvation upon the levels of circulating glucagon and insulin in man. J Clin Invest. 1963 Jul;42:1031–1039. doi: 10.1172/JCI104788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNGER R. H., EISENTRAUT A. M., McCALL M. S., MADISON L. L. Glucagon antibodies and an immunoassay for glucagon. J Clin Invest. 1961 Jul;40:1280–1289. doi: 10.1172/JCI104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNGER R. H., EISENTRAUT A. M., McCALL M. S., MADISON L. L. Measurements of endogenous glucagon in plasma and the influence of blood glucose concentration upon its secretion. J Clin Invest. 1962 Apr;41:682–689. doi: 10.1172/JCI104525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNGER R. H., EISENTRAUT A. M. STUDIES OF THE PHYSIOLOGIC ROLE OF GLUCAGON. Diabetes. 1964 Nov-Dec;13:563–568. doi: 10.2337/diab.13.6.563. [DOI] [PubMed] [Google Scholar]
- Unger R. H., Ketterer H., Dupré J., Eisentraut A. M. The effects of secretin, pancreozymin, and gastrin on insulin and glucagon secretion in anesthetized dogs. J Clin Invest. 1967 Apr;46(4):630–645. doi: 10.1172/JCI105565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. H., Ketterer H., Eisentraut A. M. Distribution of immunoassayable glucagon in gastrointestinal tissues. Metabolism. 1966 Oct;15(10):865–867. doi: 10.1016/0026-0495(66)90156-9. [DOI] [PubMed] [Google Scholar]
- YALOW R. S., BERSON S. A. Immunoassay of endogenous plasma insulin in man. J Clin Invest. 1960 Jul;39:1157–1175. doi: 10.1172/JCI104130. [DOI] [PMC free article] [PubMed] [Google Scholar]



