Abstract
Human immunodeficiency virus type 1 (HIV-1) and human T cell leukemia virus type II (HTLV-2) use a similar mechanism for –1 translational frameshifting to overcome the termination codon in viral RNA at the end of the gag gene. Previous studies have identified two important RNA signals for frameshifting, the slippery sequence and a downstream stem–loop structure. However, there have been somewhat conflicting reports concerning the individual contributions of these sequences. In this study we have performed a comprehensive mutational analysis of the cis-acting RNA sequences involved in HIV-1 gag–pol and HTLV-2 gag–pro frameshifting. Using an in vitro translation system we determined frameshifting efficiencies for shuffled HIV-1/HTLV-2 RNA elements in a background of HIV-1 or HTLV-2 sequences. We show that the ability of the slippery sequence and stem–loop to promote ribosomal frameshifting is influenced by the flanking upstream sequence and the nucleotides in the spacer element. A wide range of frameshift efficiency rates was observed for both viruses when shuffling single sequence elements. The results for HIV-1/HTLV-2 chimeric constructs represent strong evidence supporting the notion that the viral wild-type sequences are not designed for maximal frameshifting activity but are optimized to a level suited to efficient viral replication.
INTRODUCTION
Programmed ribosomal framshifting modulates the expression of two open reading frames (ORFs) in many retrovirus, plant virus, coronavirus and protozoan genes (reviewed in 1,2). In human immunodeficiency virus 1 (HIV-1) –1 translational frameshifting of its mRNA leads to synthesis of the Gag–Pol fusion protein which gives rise to the viral protease, reverse transcriptase and integrase (3). Without frameshifting only the precursor of structural proteins, Gag, is expressed. The ratio of Gag to Gag–Pol proteins is highly regulated and critical for viral propagation (4,5). Similarly, in human T cell leukemia virus type II (HTLV-2) two –1 ribosomal frameshift events result in synthesis of the fusion proteins Gag–Pro and Gag–Pro–Pol (6,7). It has been demonstrated by in vitro and in vivo studies that in both systems two cis-acting sequence elements located within the overlapping region of the gag and pol genes are critical for translational frameshifting to occur (reviewed in 1,2). One is a slippery heptamer sequence (U UUU UUA in HIV-1, A AAA AAC in HTLV-2) at which the frameshift takes place and the other is a structural RNA motif downstream that in retroviruses assumes either a stem–loop or pseudoknot structure. The general slippery sequence is X XXY YYZ, where spaces indicate the codons before shifting and X can equal Y. The simultaneous slippage model (8) proposes that the secondary structure of the second RNA signal stimulates the actual frameshift at the slippery site by representing a barrier to the mRNA translocation machinery inducing the two ribosome-bound tRNAs in the P and A sites to slip backwards in the 5′ direction simultaneously from their initial positions in the zero frame. This leaves two of the three codon–anticodon interactions unchanged if X ≠ Y. In the case of HIV-1 and HTLV-2 X equals Y and all three codon–anticodon interactions in the P site and two out of three in the A site are maintained.
For HIV-1 and HTLV-2 it has been demonstrated that a simple stem–loop structure promotes frameshifting at the slippery site in vitro and in vivo, however, the slippery site of HIV-1 alone is sufficient to mediate a basal level of frameshifting (9,10). When chimeric constructs of HIV-1 and HTLV-2 stem–loop sequences and slippery sequences were tested for frameshifting activity, conflicting results were obtained with regard to the individual contributions to frameshifting efficiency of the RNA elements (9,10). Kollmus et al. (9) came to the conclusion that the slippery sequence of HIV-1 combined with the stem–loop of either HIV-1 or HTLV-2 is more efficient in promoting –1 frameshifting than the HTLV-2 slippery site. However, Honda et al. (10) reported that the HTLV-2 slippery sequence is much more potent in inducing frameshifting when placed upstream of the HIV-1 stem–loop.
These discrepancies suggest that the slippery sequence and the stem–loop motif are not isolated components in determining frameshifting efficiency, but it is likely that the context in which they appear is also of importance. In order to address this question and to clarify the contradicting reports, we have performed a detailed and systematic analysis of RNA signals within the frameshifting regions of HIV-1 and HTLV-2. In addition to the slippery site and stem–loop motif we include in our study the region upstream of the slippery sequence as well as the spacer element that is located between the slippery site and the stem–loop. By shuffling individual or multiple elements of the HIV-1 and HTLV-2 wild-type sequences we were able to investigate the individual contributions of the RNA signals to frameshifting efficiency. Further, we show that the degree by which frameshifting efficiency is altered on exchange of the various RNA elements is strongly dependent on the nature of the particular stem–loop sequence.
MATERIALS AND METHODS
Template construct for frameshifting assay
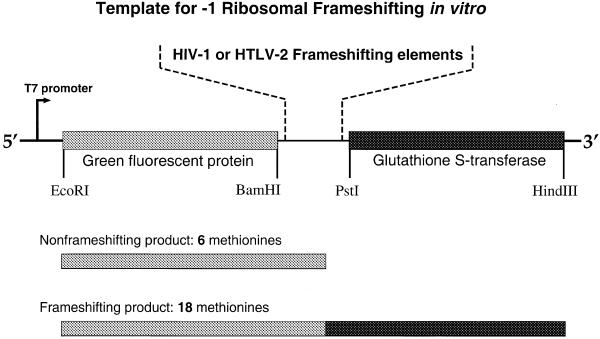
The green fluorescent protein (GFP) gene amplified from the pEGFP-c2 vector (Clontech) by PCR was ligated to an EcoRI/BamHI-digested pGEM-3Z vector (Promega) with a T7 promoter sequence upstream of the EcoRI site. Subsequently, the glutathione S-transferase (GST) gene, PCR-amplified from the pGEX-5X1 vector (Amersham Pharmacia), was inserted between the PstI and HindIII sites. For generation of the individual reporter constructs the BamHI and PstI sites of the resulting plasmid vector were used for cloning –1 frameshifting elements from either HIV-1 or HTLV-2. The respective sequences were obtained using annealed duplex DNA oligomers. To generate the stem–loop deletion mutants the internal BglII and PstI restriction sites were used. The accuracy of all wild-type and mutant constructs was confirmed by dideoxy DNA sequencing.
The UAG termination codon of the GFP ORF is located immediately after the inserted frameshifting region. If a –1 frameshift occurs at the slippery sequence, the termination codon is not read and translation proceeds through the GST gene, resulting in the production of a GFP–GST fusion protein.
Frameshifting assay
All plasmids were isolated and purified as described (11). The lyophilized DNA was dissolved in TE buffer (Tris–HCl, pH 8.0, and 1 mM EDTA). The TNT Quick coupled T7 transcription/translation system (Promega) was used according to the manufacturer’s protocol. We compared this system with the previously used TNT coupled T7 transcription/translation system (Promega) (11) and noticed no substantial difference in intra- and inter-assay variability except for a slightly lower product yield with the TNT Quick coupled T7 transcription/translation system. Aliquots of 400 ng template DNAs were used in a 20 µl reaction containing 10 µl reticulocyte lysate and 0.8 µl of 10 µCi/µl 35S-labeled methionine (NEN).
The GFP–GST fusion product yields a protein of 58 kDa that contains 18 methionine residues, whereas the non-frameshifting GFP protein product is 30 (HIV-1) or 28 kDa (HTLV-2) with six methionines. The high number of methionines in GST enhances the sensitivity for measuring frameshifting rates since the levels of frameshifting efficiency in HIV-1 and HTLV-2 are low compared to other viral systems such as BWYV and PLRV (11,12). In order to separate the GFP–GST fusion protein from the non-frameshifting product (GFP) the samples were separated through 12% SDS–polyacrylamide gels (Fig. 2B). After electrophoresis, gels were dried and exposed to a PhosphorImager screen (Molecular Dynamics). Quantitation of signal intensities was done using PhosphorImager software (Molecular Dynamics). Frameshifting efficiencies were calculated using the formula (IFS/18)/[(IFS/18) + (INFS/6)], where IFS is the signal intensity of the frameshifting product and INFS is the signal intensity of the non-frameshifting product. All individual in vitro assays were accompanied by HIV-1 wild-type controls and repeated three times or more to determine average frameshifting efficiencies. The mean ± standard deviation frameshifting efficiency of the HIV-1 wild-type reactions in this study was 5.6 ± 0.4%.
Figure 2.
Schematic representation of reporter constructs and resulting protein products used for in vitro ribosomal frameshifting measurements. Boxed regions indicate ORFs for GFP (shaded light gray) and GST (dark gray).
RESULTS
Experimental strategy
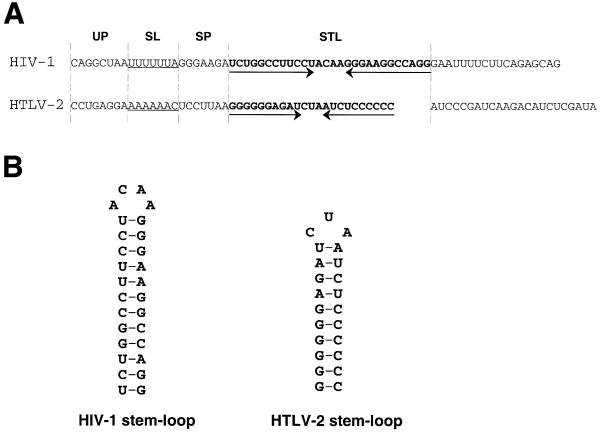
Figure 1A compares the sequence regions of HIV-1 and HTLV-2 involved in translational frameshifting of the viral gag–pol or gag–pro genes, respectively. The predicted secondary structure of the stem–loop motif is shown in Figure 1B. For quantitative in vitro analysis of frameshifting activity promoted by wild-type and mutant HIV-1 or HTLV-2 RNA elements we inserted them between the ORFs of GFP and GST (Fig. 2). The non-frameshifted product yields a protein of 30 (HIV-1 constructs) or 28 kDa (HTLV-2 constructs) with six internal methionines. Upon –1 frameshifting a fusion protein of 58 kDa containing 18 methionines is produced in reticulocyte extracts. To determine frameshifting efficiencies of HIV-1 and HTLV-2 constructs the ratios of frameshifting to non-frameshifting product were determined from at least three independent reactions each. Figure 3 shows an example of an autoradiogram obtained from electrophoretically separated in vitro translation products from incubations with HIV-1 and HTLV-2 wild-type reporter constructs and selected mutants. The HIV-1 wild-type construct yielded a frameshifting activity of ∼5.6%, whereas the HTLV-2 wild-type sequence yielded ∼9.3%. Deleting the stem–loop of the HIV-1 or HTLV-2 wild-types drastically reduced frameshifting (0.8% for HIV-1, 1.3% for HTLV-2) without eliminating it completely (Fig. 3), in accordance with earlier in vitro and in vivo results from other groups (10,13,14).
Figure 1.
(A) Alignment of the HIV-1 and HTLV-2 frameshifting regions. Individual sequence elements are separated by dotted lines and the stem–loop regions (STL) are in bold. The base pairing residues of the stem–loop are indicated by arrows. UP, upstream sequence; SL, slippery sequence; SP, spacer element. (B) Predicted secondary structure of the stem–loop element from the frameshifting regions of the HIV-1 and HTLV-2 mRNAs.
Figure 3.
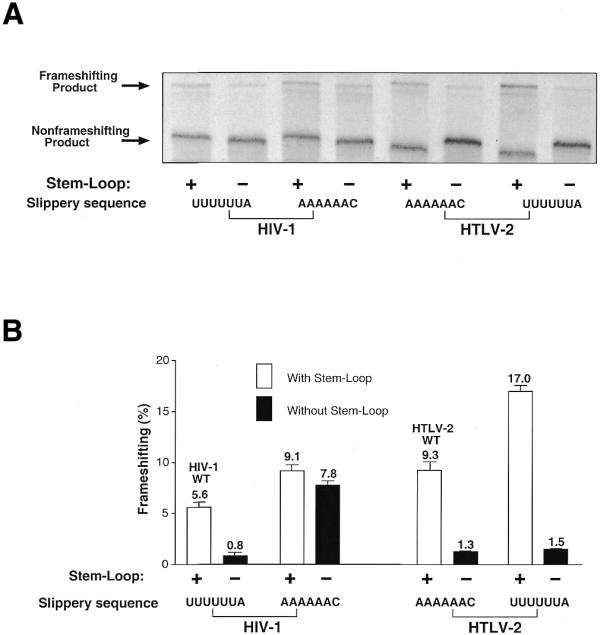
(A) SDS–PAGE analysis of [35S]methionine-labeled translation products from the in vitro ribosomal frameshifting assay of wild-type HIV-1 and HTLV-2 constructs as well as selected mutants. The positions of the frameshifting and non-frameshifting products are indicated by arrows. The slight shift in positions of the bands is due to small size differences between the wild-type and mutant translation products. The constructs for HIV-1 and HTLV-2 harbor the wild-type sequences with (+) or without (–) the stem–loop and with either the HIV-1 slippery site (UUUUUUA) or the HTLV-2 slippery sequence (AAAAAAC). (B) Quantitative analysis of frameshifting results from the autoradiogram in (A). The average percentages of frameshifting from at least three independent experiments are depicted, including error bars.
Contribution of slippery sites to frameshifting in HIV-1 and HTLV-2 is context dependent
The slippery sequences in HIV-1 and HTLV-2 have been shown to be essential for frameshifting (9,10), with an optimal repetition of A6 or U6 within the consensus X6Y. If the HIV-1 slippery site U6A is replaced by G3A3C, frameshifting is almost eliminated (Table 1). In the case of HTLV-2 the same slippery sequence (G3A3C) leaves a residual frameshifting activity of 2.7%. However, when in addition the stem–loop is replaced by the HIV-1 stem–loop frameshifting is nearly abolished.
Table 1. Frameshifting efficiencies of HIV-1 (I) and HTLV-2 (T) wild-type constructs and chimeras.
| |
UP |
SL |
SP |
STL |
|
|
| HIV-1 | CAGGCUAA | UUUUUUA | GGGAAGA | UCUGGCCUUCCUACAAGGGAAGGCCAGGGAAUUUUCUUCAGAGCAG | ||
| HTLV-2 |
CCUGAGGA |
AAAAAAC |
UCCUUAA |
GGGGGGAGAUCUAAUCUCCCCCCAUCCCGAUCAAGACAUCUCGAUA |
||
| |
UP |
SL |
SP* |
STL |
Composition |
Efficiency (%) |
| 1 | CAGGCUAA | UUUUUUA | GGGAA | I-STL | IIII | 5.6 ± 0.4 (WT) |
| 2 | CCUGAGGA | UUUUUUA | GGGAA | I-STL | TIII | 4.5 ± 0.3 |
| 3 | CAGGCUAA | AAAAAAC | GGGAA | I-STL | ITII | 9.1 ± 0.6 |
| 4 | CAGGCUAA | UUUUUUA | UCCUU | I-STL | IITI | 3.2 ± 0.2 |
| 5 | CCUGAGGA | AAAAAAC | GGGAA | I-STL | TTII | 2.3 ± 0.7 |
| 6 | CCUGAGGA | UUUUUUA | UCCUU | I-STL | TITI | 2.2 ± 0.1 |
| 7 | CAGGCUAA | AAAAAAC | UCCUU | I-STL | ITTI | 10.9 ± 0.4 |
| 8 | CCUGAGGA | AAAAAAC | UCCUU | I-STL | TTTI | 1.9 ± 0.3 |
| 9 | CCUGAGGA | AAAAAAC | UCCUU | T-STL | TTTT | 9.3 ± 0.9 (WT) |
| 10 | CAGGCUAA | AAAAAAC | UCCUU | T-STL | ITTT | 14.3 ± 0.5 |
| 11 | CCUGAGGA | UUUUUUA | UCCUU | T-STL | TITT | 17.0 ± 0.6 |
| 12 | CCUGAGGA | AAAAAAC | GGGAA | T-STL | TTIT | 11.0 ± 1.0 |
| 13 | CAGGCUAA | UUUUUUA | UCCUU | T-STL | IITT | 17.9 ± 1.1 |
| 14 | CAGGCUAA | AAAAAAC | GGGAA | T-STL | ITIT | 18.9 ± 0.8 |
| 15 | CCUGAGGA | UUUUUUA | GGGAA | T-STL | TIIT | 15.5 ± 0.5 |
| 16 | CAGGCUAA | UUUUUUA | GGGAA | T-STL | IIIT | 12.8 ± 0.7 |
| 17 | CAGGCUAA | GGGAAAC | GGGAA | I-STL | IGII | 0.5 ± 0.1 |
| 18 | CCUGAGGA | GGGAAAC | UCCUU | I-STL | TGTI | 0.2 ± 0.1 |
| 19 | CCUGAGGA | GGGAAAC | UCCUU | T-STL | TGTT | 2.7 ± 0.1 |
UP, upstream sequence; SL, slippery site; SP, spacer element; STL, stem–loop (underlined).
aFirst 5 bases of spacer sequence.
The seemingly conflicting results of Kollmus et al. (9) and Honda et al. (10) concerning the influence of the slippery sequence on frameshifting efficiencies of HIV-1 and HLTV-2 prompted us to investigate the contribution of the slippery sequence on frameshifting within the different sequence backgrounds of the HIV-1 and HTLV-2 mRNAs. As shown in Figure 3, frameshifting increases by 63% (from 5.6 to 9.1%) when the wild-type slippery sequence UUUUUUA is replaced by the slippery site AAAAAAC of HTLV-2. Furthermore, an HIV-1 construct lacking the stem–loop but with the HTLV-2 slippery site still had higher frameshifting activity than the complete HIV-1 wild-type sequence (7.8% compared to 5.6%; Fig. 3).
Intriguingly, changing the wild-type slippery sequence of HTLV-2 from A6C to the U6A sequence of HIV-1 also greatly increased frameshifting efficiency, from 9.3 to 15.3%. Taken together, these results strongly argue for a modulatory role of the upstream sequences and/or the spacer element on frameshifting function, since these represent the only non-constant components within the described reporter constructs.
The upstream sequences and spacer elements in HIV-1 and HTLV-2 modulate frameshifting efficiency
In order to analyze the influence of the sequence residing immediately upstream of the slippery site we tested the frameshifting activities of HIV-1 and HTLV-2 constructs with switched upstream regions (Table 1). Exchanging the upstream sequence CAGGCUAA of HIV-1 for the HTLV-2 sequence CCUGAGGA slightly reduced frameshifting activity (5.6 versus 4.5%), whereas in HTLV-2 insertion of the HIV-1-derived upstream sequence led to an ∼50% increase in frameshifting rate (9.3 versus 14.3%). Interestingly, in HIV-1 frameshifting activity was further reduced when in addition to the HTLV-2 upstream sequence either the slippery sequence or the spacer was replaced by the corresponding sequence of HTLV-2 (see Table 1). Exchanging all these elements with HTLV-2 sequences, leaving only the stem–loop of HIV-1, further decreased frameshifting. Equally, transferring the HTLV-2 upstream sequence and spacer elements individually compromised frameshifting efficiency when placed in the HIV-1 background. The exception was the HTLV-2 slippery sequence, which enhanced the frameshifting described above. In this case the HTLV-2 A6C slippery sequence was extended to an A8C motif because of the HIV-1 upstream sequence. A stimulatory effect on frameshifting through an increasing number of adenines in the slippery sequence is consistent with the earlier results of Honda et al. (10).
In HTLV-2 double replacement of the upstream and slippery sequences or the upstream sequence and the spacer both increased frameshifting rates (17.9 and 18.9%, respectively; Table 1). Individual exchange of the slippery sites boosted frameshifting to 17%, while spacer replacement yielded 11% (Table 1). However, exchanging all three elements resulted in 12.8% frameshifting.
Frameshiftings mediated by the HIV-1 or HTLV-2 stem–loops show different sensitivities to changes in surrounding sequences
Of the several RNA elements examined, the stem–loop sequence, in concert with a slippery site, is certainly the most important component of the frameshifting region, as has been demonstrated by several groups (9,10,13). This was confirmed in our in vitro experiments (see Fig. 3). Further, it has been shown for HIV-1 that frameshifting rate correlates with the thermodynamic stability of the stem–loop (15).
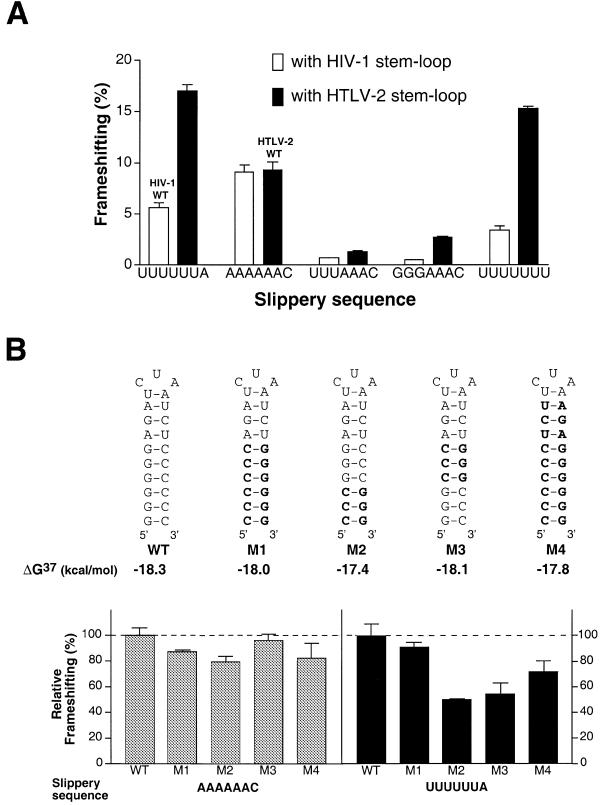
We asked whether frameshifting mediated by the HIV-1 and HTLV-2 stem–loops would be similarly influenced by changes in the other cis-acting RNA elements, a behavior that would indicate a purely additive contribution from each element to frameshifting function. As shown in Figure 4A, the changes in the extent of frameshifting brought about by changes in the slippery sequence were different for the HIV-1 and HTLV-2 stem–loops. The range of frameshifting rates in HIV-1 was much smaller (1–9%) than the range for HTLV-2 stem–loop constructs (2–18%). This behavior might be a direct consequence of the inherently different thermodynamic stabilities of the two stem–loop structures at 37°C, which for HTLV-2 was calculated to be ΔG = –18.3 kJ/mol, while for HIV-1 ΔG = –20.9 kJ/mol, as estimated using the Mufold program (16). In order to examine this property more directly we introduced mutations into the HTLV-2 stem–loop that only changed the orientation of base interactions but did not substantially influence the calculated thermodynamic stability of the stem–loop structures. As documented in Figure 4B, only minor changes in frameshifting efficiency were observed with mutants M1–M4 in combination with the HTLV-2 wild-type slippery sequence. However, when introducing the HIV-1 slippery site, 2-fold differences in frameshifting rates were measured between mutants (Fig. 4B). The most drastic change was apparent after inversion of the terminal three G-C base pairs of the stem, which might alter base stacking within the helix. Similarly, inverting the medial three G-C base pairs decreased frameshifting substantially, whereas inversion of all six G-C base pairs yielded frameshifting activities close to wild-type levels (Fig. 4B). In line with the results described above, an analogous mutational analysis with the HIV-1 stem–loop resulted in only minor differences in frameshifting efficiencies (data not shown).
Figure 4.
(A) Influence of mutated slippery sequences on –1 frameshifting in HIV-1 and HTLV-2. The percentage of frameshifting obtained from constructs with altered slippery sites is analyzed when placed in the HIV-1 compared to HTLV-2 sequence background. (B) Sensitivity of frameshifting rates to changes in the slippery sequence is altered in HTLV-2 stem–loop mutants. The wild-type (WT) HTLV-2 stem–loop secondary structure is depicted, with base changes in mutants M1–M4 in bold. The results from in vitro translation assays are shown as columns with error bars. The relative extent of frameshifting is determined as the ratio between each mutant and the wild-type stem–loop. All mutants were tested in combination with the HTLV-2 slippery sequence (AAAAAAC, left) and the HIV-1 slippery site (UUUUUUA, right).
DISCUSSION
The frameshifting regions of HIV-1 and HTLV-2 mRNA contain several RNA elements, of which the slippery sequence and the stem–loop have been studied extensively with respect to their importance for frameshifting function. Here we have extended the analysis of cis-acting RNA signals to the neighboring upstream region and the spacer sequence. We have examined their functional interrelationships by shuffling HIV-1- and HTLV-2-derived elements. Our results from interchanging the slippery sites provide an explanation for the apparently contradictory results obtained by other groups (9,10). As reported by Honda et al. (10) and confirmed in our experiments, the combination of the HIV-1 stem–loop and the HTLV-2 slippery sequence results in higher frameshifting activity than the HIV-1 stem–loop combined with the HIV-1 slippery site. However, if the surrounding RNA elements, the upstream sequence and the spacer, are also exchanged for the respective HTLV-2 sequences, then we obtain the opposite result. In fact, an HTLV-2-based construct with the HIV-1 stem–loop was used in the study by Kollmus et al. (9) and this explains how they came to the conclusion that the HIV-1 slippery sequence is more efficient in mediating –1 frameshifting.
If we compare all possible combinations of HIV-1/HTLV-2 chimeras that differ only in the slippery sequence (mutant nos 1/3, 2/5, 4/7, 6/8 and 11/9), then three out of five combinations give higher frameshifting with the HIV-1 slippery site. Clearly, the frameshifting activity induced by a given combination of RNA elements is not predictable from the individual contributions of single components, but rather is the result of a complex interplay between each sequence region within the mRNA and probably their interaction with the translational machinery.
Interestingly, the construct with the highest frameshifting activity (Table 1, mutant no. 14) was a mosaic of alternating RNA elements from HIV-1 and HTLV-2. Also, the second best frameshifting chimera (mutant no. 13) contained two RNA elements from each virus. The question arises, why did the frameshifting region evolve to harbor at least four cis-acting RNA signals even though one or two of these elements, optimally combined, can yield the same level of frameshifting? It is widely known that the efficiency of translational frameshifting is regulated during the viral life cycle (for reviews see 17–19). Such mechanisms require additional sequences that interact with viral and/or host factors. A number of trans-acting factors that positively or negatively influence the efficiency of frameshifting have already been genetically identified (20–23). Within viral RNA the candidate region most likely to be subject to regulatory control is the stem–loop. It is well known that during translation of the frameshift region the secondary structure has to be unfolded for the ribosome to proceed along the mRNA. The stem–loop is believed to collide with the moving ribosomal machinery causing it to stall (24,25). This pausing is a prerequisite for efficient frameshifting and it has recently been shown that the average ribosomal pause time is greater for that fraction of ribosomes that proceed in the –1 frame (26). Cellular or viral trans-acting factors that interact with the stem–loop region could thus positively or negatively influence frameshifting by stabilizing or destabilizing the secondary structure. In fact, Kollmus et al. (27) have demonstrated that when replacing the HIV-1 stem–loop by the iron-responsive element, frameshifting rates increase under conditions that allow binding of iron regulatory proteins.
When comparing HIV-1 and HTLV-2 constructs, mutants of the frameshifting region had a more profound effect on frameshifting in the context of the HTLV-2 stem–loop than with the HIV-1 sequence, indicating a higher sensitivity of this element to changes in the surrounding sequences. These results are in line with earlier work demonstrating that the HTLV-2 stem–loop is much more sensitive to changes in the length of the spacer element than the HIV-1 stem–loop (9,28). The differences might be solely dependent on the thermodynamic stability of the stem–loop structure. However, when we tested mutants of the HTLV-2 stem–loop that do not change the overall thermodynamic stability of the secondary structure but only alter base pair orientation, the frameshifting efficiencies of individual mutants still varied depending on the nature of the slippery sequence. The HIV-1 slippery site proved much more sensitive to mutations in the stem–loop than that derived from HTLV-2. Taken together, these findings also argue for the involvement of additional cis- and/or trans-acting factors.
It has recently been shown that the introduction of an upstream or downstream termination codon relative to the slippery site also influences frameshifting efficiency (29,30). An upstream termination codon in the –1 frame located at various positions impairs frameshifting via an unknown mechanism (29). However, a downstream termination codon in the –1 frame (30) enhances frameshifting, probably because it represents another pausing element for the translating ribosomes acting in concert with the RNA secondary structure. The translational termination signal probably leads to sequestration of protein factors to the frameshifting region either positively or negatively interfering with the frameshifting process.
Although the mechanics of ribosomal frameshifting are largely unknown, we should not be surprised to find that frameshifting efficiency is affected by the upstream sequences and spacer sequences, as well as the more thoroughly studied slippery sequence and downstream structural motif, in this case a stem–loop. The spacer element usually has 6 or 7 nt. It is likely that they are normally in a stacked configuration with ∼3.4 Å per base. A number of nuclease digestion experiments have been done and they reveal that the number of nucleotides found between the coding site and the outside of the ribosome where the nuclease acts is 12–15 nt in prokaryotes (31,32) and 20 nt in eukaryotes (33). This clearly suggests that the spacer segment undergoes considerable elongation before ribosomal frameshifting occurs. The power behind this extension is the translocational mechanism of the ribosome, which moves the mRNA–tRNA complex one codon (probably ∼10 Å) associated with tRNA translocation from the A site to the P site. This translocation moves in the upstream direction and presumably elongation of the spacer segment is due to the fact that the downstream secondary structure, in this case the stem–loop, cannot enter the ribosomal mRNA channel. We do not as yet know the mechanism behind the translocation process and mRNA movement, but it is likely that a significant component consists of pressures to move the tRNA itself. It is this movement in the upstream direction which, when faced with an extended mRNA and a secondary structural element that does not unravel, leads to sliding of the tRNA by –1 nt in the upstream direction. The detailed sequence of bases in the spacer segment will determine both its initial stacking energy and its gradual loss through unstacking and extension, as well as the extent to which it interacts with other ribosomal components forming the mRNA channel. In the same way, the upstream sequence of the message must continue to move through the ribosome during the translocational process. Thus, it too may have an opportunity to be influenced by contacts with those elements that make the ribosomal mRNA channel. However, it has recently been shown that specific inhibition of EF-2-mediated translocation by pokeweed antiviral protein does not change the efficiency of –1 ribosomal frameshifting. Therefore, the frameshift must occur before completion of the peptidyltransferase reaction (34).
An important element is, of course, the interaction of the stem–loop structure with the ribosome, which helps to determine whether it will unravel, and therefore not frameshift, or maintain its structure, leading to frameshifting. Here it is not a matter of the stabilizing energy of the isolated stem–loop. Instead, it is the energy of the stem–loop as it abuts the ribosome. As seen by the variation in frameshifting associated with changes in the stem–loop structure (Fig. 4B), it seems clear that a string of CG base pairs are important for stability near the end of the stem–loop structure. However, there is a curious destabilization associated with mutants M2 and M3 in which the six CG base pairs have been changed to blocks of three with the adjacent block inverted. These lose considerable frameshifting ability with the HIV-1 slippery sequence, but much less so with HTLV-2. This may reflect the fact that interaction of the stem–loop with the ribosome is a much more important component of frameshifting in HIV-1 than in HTLV-2.
Discussions of the mechanics of ribosomal frameshifting will shortly undergo an abrupt change due to the recent publication of high resolution X-ray crystallographic studies of both the large and small ribosomal subunits (35–37). At present it is possible to locate the position of the two tRNAs in the A and T sites and give a precise description of that environment. In the near future, with further developments in this area, we will be able to transform the discussion of ribosomal frameshifting from generalities to highly specific suggestions about which structural elements and interactions may be important in understanding this process.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Analeah O’Neill and Stephanie C. Wang for excellent technical assistance. This work was supported by an Anna Fuller fellowship for molecular oncology to S.M. and by grants to A.R. from the National Institutes of Health, the National Science Foundation and the National Cancer Research Foundation.
References
- 1.Farabaugh P.J. (1996) Programmed translational frameshifting. Microbiol. Rev., 60, 103–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gesteland R.F. and Atkins,J.F. (1996) Recoding: dynamic reprogramming of translation. Annu. Rev. Biochem., 65, 741–768. [DOI] [PubMed] [Google Scholar]
- 3.Jacks T., Power,M.D., Masiarz,F.R., Luciw,P.A., Barr,P.J. and Varmus,H.E. (1988) Characterization of ribosomal frameshifting in HIV-1 gag-pol expression. Nature, 331, 280–283. [DOI] [PubMed] [Google Scholar]
- 4.Dinman J.D. and Wickner,R.B. (1992) Ribosomal frameshifting efficiency and gag/gag-pol ratio are critical for yeast M1 double-stranded RNA virus propagation. J. Virol., 66, 3669–3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karacostas V., Wolffe,E.J., Nagashima,K., Gonda,M.A. and Moss,B. (1993) Overexpression of the HIV-1 gag-pol polyprotein results in intracellular activation of HIV-1 protease and inhibition of assembly and budding of virus-like particles. Virology, 193, 661–671. [DOI] [PubMed] [Google Scholar]
- 6.Falk H., Mador,N., Udi,R., Panet,A. and Honigman,A. (1993) Two cis-acting signals control ribosomal frameshift between human T-cell leukemia virus type II gag and pro genes. J. Virol., 67, 6273–6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mador N., Panet,A. and Honigman,A. (1989) Translation of gag, pro and pol gene products of human T-cell leukemia virus type 2. J. Virol., 63, 2400–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacks T., Madhani,H.D., Masiarz,F.R. and Varmus,H.E. (1988) Signals for ribosomal frameshifting in the Rous sarcoma virus gag-pol region. Cell, 55, 447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kollmus H., Honigman,A., Panet,A. and Hauser,H. (1994) The sequences of and distance between two cis-acting signals determine the efficiency of ribosomal frameshifting in human immunodeficiency virus type 1 and human T-cell leukemia virus type II in vivo. J. Virol., 68, 6087–6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Honda A., Nakamura,T. and Nishimura,S. (1995) RNA signals for translation frameshift: influence of stem size and slippery sequence. Biochem. Biophys. Res. Commun., 213, 575–582. [DOI] [PubMed] [Google Scholar]
- 11.Kim Y.G., Maas,S., Wang,S.C. and Rich,A. (2000) Mutational study reveals that tertiary interactions are conserved in ribosomal frameshifting pseudoknots of two luteoviruses. RNA, 6, 1157–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim Y.G., Su,L., Maas,S., O’Neill,A. and Rich,A. (1999) Specific mutations in a viral RNA pseudoknot drastically change ribosomal frameshifting efficiency. Proc. Natl Acad. Sci. USA, 96, 14234–14239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parkin N.T., Chamorro,M. and Varmus,H.E. (1992) Human immunodeficiency virus type 1 gag-pol frameshifting is dependent on downstream mRNA secondary structure: demonstration by expression in vivo. J. Virol., 66, 5147–5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reil H., Kollmus,H., Weidle,U.H. and Hauser,H. (1993) A heptanucleotide sequence mediates ribosomal frameshifting in mammalian cells. J. Virol., 67, 5579–5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bidou L., Stahl,G., Grima,B., Liu,H., Cassan,M. and Rousset,J.P. (1997) In vivo HIV-1 frameshifting efficiency is directly related to the stability of the stem–loop stimulatory signal. RNA, 3, 1153–1158. [PMC free article] [PubMed] [Google Scholar]
- 16.Jaeger J.A., Turner,D.H. and Zuker,M. (1989) Improved predictions of secondary structures for RNA. Proc. Natl Acad. Sci. USA, 86, 7706–7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson G.M. and Brewer,G. (1999) Slip-sliding the frame: programmed –1 frameshifting on eukaryotic transcripts. Genome Res., 9, 393–394. [PubMed] [Google Scholar]
- 18.Brierley I. (1995) Ribosomal frameshifting viral RNAs. J. Gen. Virol., 76, 1885–1892. [DOI] [PubMed] [Google Scholar]
- 19.Farabaugh P.J. (2000) Translational frameshifting: implications for the mechanism of translational frame maintenance. Prog. Nucleic Acid Res. Mol. Biol., 64, 131–170. [DOI] [PubMed] [Google Scholar]
- 20.Cui Y., Dinman,J.D. and Peltz,S.W. (1996) Mof4-1 is an allele of the UPF1/IFS2 gene which affects both mRNA turnover and –1 ribosomal frameshifting efficiency. EMBO J., 15, 5726–5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dinman J.D. and Wickner,R.B. (1994) Translational maintenance of frame: mutants of Saccharomyces cerevisiae with altered –1 ribosomal frameshifting efficiencies. Genetics, 136, 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dinman J.D. and Wickner,R.B. (1995) 5 S rRNA is involved in fidelity of translational reading frame. Genetics, 141, 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruiz-Echevarria M.J., Yasenchak,J.M., Han,X., Dinman,J.D. and Peltz,S.W. (1998) The upf3 protein is a component of the surveillance complex that monitors both translation and mRNA turnover and affects viral propagation. Proc. Natl Acad. Sci. USA, 95, 8721–8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tu C., Tzeng,T.H. and Bruenn,J.A. (1992) Ribosomal movement impeded at a pseudoknot required for frameshifting. Proc. Natl Acad. Sci. USA, 89, 8636–8640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Somogyi P., Jenner,A.J., Brierley,I. and Inglis,S.C. (1993) Ribosomal pausing during translation of an RNA pseudoknot. Mol. Cell. Biol., 13, 6931–6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopinski J.D., Dinman,J.D. and Bruenn,J.A. (2000) Kinetics of ribosomal pausing during programmed –1 translational frameshifting. Mol. Cell. Biol., 20, 1095–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kollmus H., Hentze,M.W. and Hauser,H. (1996) Regulated ribosomal frameshifting by an RNA-protein interaction. RNA, 2, 316–323. [PMC free article] [PubMed] [Google Scholar]
- 28.Hatfield D.L., Levin,J.G., Rein,A. and Oroszlan,S. (1992) Translational suppression in retroviral gene expression. Adv. Virus Res., 41, 193–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Honda A. and Nishimura,S. (1996) Suppression of translation frameshift by upstream termination codon. Biochem. Biophys. Res. Commun., 221, 602–608. [DOI] [PubMed] [Google Scholar]
- 30.Lucchesi J., Makelainen,K., Merits,A., Tamm,T. and Makinen,K. (2000) Regulation of –1 ribosomal frameshifting directed by cocksfoot mottle sobemovirus genome. Eur. J. Biochem., 267, 3523–3529. [DOI] [PubMed] [Google Scholar]
- 31.Steitz J.A. (1969) Polypeptide chain initiation: nucleotide sequences of the three ribosomal binding sites in bacteriophage R17 RNA. Nature, 224, 957–964. [DOI] [PubMed] [Google Scholar]
- 32.Kuechler E. and Rich,A. (1970) Position of the initiator and peptidyl sites in the E. coli ribosome. Nature, 225, 920–924. [DOI] [PubMed] [Google Scholar]
- 33.Kang C.W. and Cantor,C.R. (1985) Structure of ribosome-bound messenger RNA as revealed by enzymatic accessibility studies. J. Mol. Biol., 181, 241–251. [DOI] [PubMed] [Google Scholar]
- 34.Tumer N.E., Parikh,B.A., Li,P. and Dinman,J.D. (1998) The pokeweed antiviral protein specifically inhibits Ty1-directed +1 ribosomal frameshifting and retrotransposition in Saccharomyces cerevisiae. J. Virol., 72, 1036–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schluenzen F., Tocilj,A., Zarivach,R., Harms,J., Gluehmann,M., Janell,D., Bashan,A., Bartels,H., Agmon,I., Franceschi,F. and Yonath,A. (2000) Structure of functionally activated small ribosomal subunit at 3.3 angstroms resolution. Cell, 102, 615–623. [DOI] [PubMed] [Google Scholar]
- 36.Wimberly B.T., Brodersen,D.E., Clemons,W.M.,Jr, Morgan-Warren,R.J., Carter,A.P., Vonrhein,C., Hartsch,T. and Ramakrishnan,V. (2000) Structure of the 30S ribosomal subunit. Nature, 407, 327–339. [DOI] [PubMed] [Google Scholar]
- 37.Ban N., Nissen,P., Hansen,J., Moore,P.B. and Steitz,T.A. (2000) The complete atomic structure of the large ribosomal subunit at 2.4 Å resolution. Science, 289, 905–920. [DOI] [PubMed] [Google Scholar]