Abstract
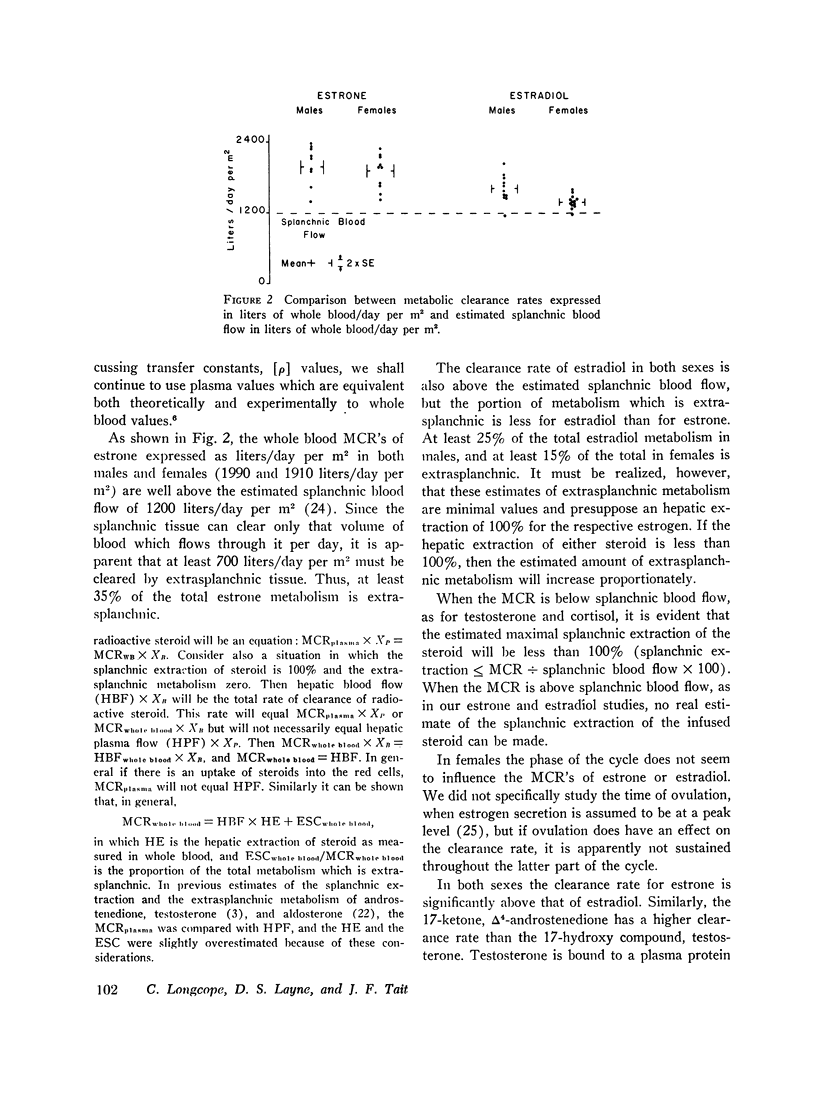
The continuous infusion of 3H-6,7-estrone and 3H-6,7-estradiol has been used to study the metabolic clearance rate (MCR), the interconversions, and the red cell uptake of these steroids in normal males and females. The whole blood MCR of estrone is 1,990 ± 120 liters per day/m2 (SE) in males and 1,910 ± 100 liters per day/m2 in females. The whole blood MCR of estradiol is 1,600 ± 80 liters per day/m2 in males and 1,360 ± 40 liters per day/m2 in females. The values in females do not vary significantly when studied in the follicular or luteal phase of the cycle. At least 35% of the total estrone metabolism in both sexes is extrasplanchnic and at least 25% of the total estradiol metabolism in males, and 15% in females is extrasplanchnic. The [ρ]BB2,1 [transfer constant of estradiol to estrone, which is equivalent to the fraction of the precursor (estradiol) converted to the product (estrone) when both the infusion of the precursor and the measurement of the product are in peripheral blood] is 15%; and the [ρ]BB1,2 [transfer constant of estrone to estradiol, which is equivalent to the fraction of the precursor (estrone) converted to product (estradiol) when both the infusion of the precusor and the measurement of the product are in peripheral blood] is 5% in both males and females. Our findings concerning the radioactivity in whole blood, as measured by our procedure, were the following: 15-20% of estrone in both sexes and 15% of estradiol in males is associated with red cells. Only 2% of the whole blood radioactivity of estradiol in females is associated with red cells. Changes in the distribution of radioactivity between plasma and red cells will influence the MCR as calculated from plasma, but not as calculated from whole blood.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEER C. T., GALLAGHER T. F. Excretion of estrogen metabolites by humans. I. The fate of small doses of estrone and estradiol-17beta. J Biol Chem. 1955 May;214(1):335–349. [PubMed] [Google Scholar]
- Bardin C. W., Lipsett M. B. Testosterone and androstenedione blood production rates in normal women and women with idiopathic hirsutism or polycystic ovaries. J Clin Invest. 1967 May;46(5):891–902. doi: 10.1172/JCI105588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow J. J., Logan C. M. Estrogen secretion, biosynthesis and metabolism: their relationship to the menstrual cycle. Steroids. 1966 Apr;7(4):309–320. doi: 10.1016/0039-128x(66)90102-4. [DOI] [PubMed] [Google Scholar]
- Bradley S. E., Ingelfinger F. J., Bradley G. P., Curry J. J. THE ESTIMATION OF HEPATIC BLOOD FLOW IN MAN. J Clin Invest. 1945 Nov;24(6):890–897. doi: 10.1172/JCI101676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHMAN J., BRADLOW H. L., GALLAGHER T. F. Oxidative metabolism of estradiol. J Biol Chem. 1960 Nov;235:3104–3107. [PubMed] [Google Scholar]
- FLOOD C., LAYNE D. S., RAMCHARAN S., ROSSIPAL E., TAIT J. F., TAIT S. A. An investigation of the urinary metabolites and secretion rates of aldosterone and cortisol in man and a description of methods for their measurement. Acta Endocrinol (Copenh) 1961 Feb;36:237–264. doi: 10.1530/acta.0.0360237. [DOI] [PubMed] [Google Scholar]
- GOLD N. I. Partial characterization of the metabolites of cortisol-4-C-14 in the dog. II. The totally hepatectomized dog. J Biol Chem. 1961 Jul;236:1930–1933. [PubMed] [Google Scholar]
- GURPIDE E., ANGERS M., VANDE WIELE R. L., LIEBERMAN S. Determination of secretory rates of estrogens in pregnant and nonpregnant women from the specific activities of urinary metabolites. J Clin Endocrinol Metab. 1962 Sep;22:935–945. doi: 10.1210/jcem-22-9-935. [DOI] [PubMed] [Google Scholar]
- Horton R., Tait J. F. Androstenedione production and interconversion rates measured in peripheral blood and studies on the possible site of its conversion to testosterone. J Clin Invest. 1966 Mar;45(3):301–313. doi: 10.1172/JCI105344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton R., Tait J. F. In vivo conversion of dehydroisoandrosterone to plasma androstenedione and testosterone in man. J Clin Endocrinol Metab. 1967 Jan;27(1):79–88. doi: 10.1210/jcem-27-1-79. [DOI] [PubMed] [Google Scholar]
- Lipsett M. B., Wilson H., Kirschner M. A., Korenman S. G., Fishman L. M., Sarfaty G. A., Bardin C. W. Studies on Leydig cell physiology and pathology: secretion and metabolism of testosterone. Recent Prog Horm Res. 1966;22:245–281. doi: 10.1016/b978-1-4831-9825-5.50009-9. [DOI] [PubMed] [Google Scholar]
- Little B., Tait J. F., Tait S. A., Erlenmeyer F. The metabolic clearance rate of progesterone in males and ovariectomized females. J Clin Invest. 1966 Jun;45(6):901–912. doi: 10.1172/JCI105405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIGEON C. J., WALL P. E., BERTRAND J. Some aspects of the metabolism of 16-C14-estrone in normal individuals. J Clin Invest. 1959 Apr;38(4):619–629. doi: 10.1172/JCI103840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migeon C. J., Lescure O. L., Zinkham W. H., Sidbury J. B. IN VITRO INTERCONVERSION OF 16-C-ESTRONE AND 16-C-ESTRADIOL-17beta BY ERYTHROCYTES FROM NORMAL SUBJECTS AND FROM SUBJECTS WITH A DEFICIENCY OF RED CELL GLUCOSE-6-PHOSPHATE DEHYDROGENASE ACTIVITY. J Clin Invest. 1962 Nov;41(11):2025–2035. doi: 10.1172/JCI104661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman W. H., Crépy O. Steroid-protein interaction with particular reference to testosterone binding by human serum. J Biol Chem. 1967 Jan 25;242(2):182–189. [PubMed] [Google Scholar]
- RIONDEL A., TAIT J. F., GUT M., TAIT S. A., JOACHIM E., LITTLE B. Estimation of testosterone in human peripheral blood using S35-thiosemicarbazide. J Clin Endocrinol Metab. 1963 Jul;23:620–628. doi: 10.1210/jcem-23-7-620. [DOI] [PubMed] [Google Scholar]
- Rivarola M. A., Saez J. M., Meyer W. J., Jenkins M. E., Migeon C. J. Metabolic clearance rate and blood production rate of testosterone and androst-4-ene-3,17-dione under basal conditions, ACTH and HCG stimulation. Comparison with urinary production rate of testosterone. J Clin Endocrinol Metab. 1966 Nov;26(11):1208–1218. doi: 10.1210/jcem-26-11-1208. [DOI] [PubMed] [Google Scholar]
- Rosenbaum W., Christy N. P., Kelly W. G. Electrophoretic evidence for the presence of an estrogen-binding beta-globulin in human plasma. J Clin Endocrinol Metab. 1966 Dec;26(12):1399–1403. doi: 10.1210/jcem-26-12-1399. [DOI] [PubMed] [Google Scholar]
- SANDBERG A. A., SLAUNWHITE W. R., Jr Studies on phenolic steroids in human subjects. II. The metabolic fate and hepato-biliary-enteric circulation of C14-estrone and C14-estradiol in women. J Clin Invest. 1957 Aug;36(8):1266–1278. doi: 10.1172/JCI103524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segre E. J., Friedrich E. H., Dodek O. I., Jr, Lloyd C. W., Lobotsky J., Levin J., Klaiber E. L. Effects of epinephrine on the production and metabolic clearance of cortisol in normal men and women and in women with idiopathic hirsutism. Acta Endocrinol (Copenh) 1966 Dec;53(4):561–570. doi: 10.1530/acta.0.0530561. [DOI] [PubMed] [Google Scholar]
- TAIT J. F., TAIT S. A., LITTLE B., LAUMAS K. R. The disappearance of 7-H-3-d-aldosterone in the plasma of normal subjects. J Clin Invest. 1961 Jan;40:72–80. doi: 10.1172/JCI104239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavernetti R. R., Rosenbaum W., Kelly W. G., Christy N. P., Roginsky M. S. Evidence for the presence in human plasma of an estrogen-binding factor other than albumin: abnormal binding of estradiol in men with hepatic cirrhosis. J Clin Endocrinol Metab. 1967 Jul;27(7):920–926. doi: 10.1210/jcem-27-7-920. [DOI] [PubMed] [Google Scholar]
- WALL P. E., MIGEON C. J. In vitro studies with 16-C14-estrone: distribution between plasma and red blood cells of man. J Clin Invest. 1959 Apr;38(4):611–618. doi: 10.1172/JCI103839. [DOI] [PMC free article] [PubMed] [Google Scholar]



