Abstract
The cerebral cortex is tightly and reciprocally linked to the cerebellum and the ascending dentato-thalalmo-cortical pathway influences widespread cortical regions. Using a rodent model of middle cerebral artery stroke, we showed previously that chronic, 20 Hz stimulation of the contralateral lateral cerebellar nucleus (LCN) improved motor recovery, while 50 Hz stimulation did not. Using motor evoked potentials (MEP) elicited by intracortical microstimulation, we now show the effect of LCN stimulation on motor cortex excitability as a function of pulse frequency in propofol-anesthetized rats. MEPs were recorded serially, at 15-second intervals, with cerebellar stimulation delivered in 10-minute blocks at rates of 20, 30, 40, 50 or 100 Hz. Stimulation at 20, 30, 40 or 50 Hz enhanced the average MEP response across the block, with the maximal overall increase observed during 30 Hz stimulation. However, the effect varied as a function of both repeated trials within the block and LCN stimulation frequency, such that 40 Hz and 50 Hz stimulation showed a reduced effect over time. Stimulation at 100 Hz produced a transient increase in MEP amplitude in some animals; however the overall effect across the block was a trend towards reduced cortical excitability. These results suggest that direct stimulation of the LCN can yield frequency-dependent changes in cortical excitability and may provide a therapeutic approach to modulating cortical activity for the treatment of strokes or other focal cortical lesions, movement disorders and epilepsy.
Keywords: electrical stimulation, lateral cerebellar nucleus, dentate nucleus, evoked potentials, motor, cortical excitability, stroke
INTRODUCTION
Functional reorganization of perilesional cortex is known to play a role in spontaneous and therapy-related recovery of motor function following stroke or other focal injuries (Carmichael, 2003; Liepert et al., 2000; Rossini et al., 2003). While the mechanisms underlying this reorganization remain unclear, evidence suggests that intrinsic changes in the excitability of spared, perilesional cortex may be involved (Manto et al., 2006; Murphy et al., 2004). In humans, physical therapy-related motor recovery following stroke is associated with changes in both cortical excitability and perilesional organization (Boake et al., 2007; Kim et al., 2006; Liepert, 2006). In light of this potential link, techniques capable of artificially enhancing cortical excitability are being explored as a means of promoting functional reorganization, including direct cortical stimulation (Huang et al., 2008; Plautz et al., 2003), repetitive transcranial stimulation (Di Lazzaro et al., 2006; Fregni et al., 2006; Hummel et al., 2005; Kim et al., 2006) and sub-threshold sensorimotor stimulation (Deletis et al., 1987; Hamdy et al., 1998). Each approach has been shown to enhance plasticity-related recovery in animal models of stroke and in preliminary human trials (Adkins-Muir and Jones, 2003; Fregni et al., 2006; Huang et al., 2008; Hummel et al., 2005; Levy et al., 2008). Unfortunately, clinical success has been limited to date, with a recent pivotal trial evaluating the use of direct cortical stimulation via chronically implanted epidural electrodes failing to achieve its intended endpoints (Plow et al., 2009).
We have proposed previously that direct, electrical activation of the ascending cerebellar projections represents an alternative approach to enhance excitability chronically across perilesional cortical areas (Machado et al., 2009). Previous work has shown that damage to the dentato-thalamo-cortical (DTC) pathway at its origin results in reduced cortical excitability (Di Lazzaro et al., 1994), while paradigms consistent with activation of DTC output enhance cortical excitability (Rispal-Padel et al., 1981) and facilitate motor behavior in normal individuals (Iwata et al., 2004). Through our approach, chronic stimulation is applied directly to the cerebellar output nuclei, driving the naturally excitatory projections in order to facilitate activity within spared thalamo-cortical pathways. Given the widespread projections of this circuit (Asanuma et al., 1983; Dum and Strick, 2003), augmentation of excitability is expected across premotor, supplementary motor as well as post-central associative cortical areas that are typically spared along the margins of a middle cerebral artery infarct (Eisner-Janowicz et al., 2008; Frost et al., 2003). Our early results in the rodent stroke model have been promising, with enhanced post-stroke motor recovery observed after six weeks of stimulation of the lateral cerebellar nucleus (LCN) (Machado et al., 2009). In that study, therapeutic benefit was observed in animals that received 20 Hz LCN stimulation but not among those that received 50 Hz stimulation. The objective of the current work was to determine the relationship between continuous LCN stimulation frequency and changes in cortical excitability as indexed using intracortical microstimulation-derived motor evoked potentials (MEPs) in the rat.
METHODS
Animals
All experiments were performed using male Wistar rats (250–350 grams, Charles River, Wilmington, MA, USA). The animals were housed in an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-approved animal facility in a climate controlled environment that included a 12-hour light/dark cycle and free access to water. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the Cleveland Clinic.
Surgery
Cerebellar Electrode Implantation
Anesthesia was initiated in a chamber saturated with isoflurane at 5% and maintained under mechanical ventilation with continuous isoflurane (1.5% – 3.0%) and oxygen. The rat was positioned on a stereotactic frame (David Kopf, Tujunga, CA), fixed at the external auditory canals and maxilla. An incision was opened over the calvaria, exposing the bregma, lambda and occipital region. A small (~1.5 mm) bur hole was created over the posterior calvaria, through which a 0.28 mm diameter concentric bipolar stimulating electrode (Model MS306, Plastics One Inc., Roanoke, VA, USA) with 0.3 mm of exposed tip was inserted to the pre-calculated target of the right LCN: −11.0 mm (posterior) to Bregma, 3.7 mm lateral and 6.0 mm ventral. The electrode was fixed to the skull using dental acrylic, with small stainless steel screws (MX-0090-2; Small part Inc., Miami Lakes, FL) placed across the exposed skull for reinforcement. The plastic connector at the proximal end of the electrode remained exposed above the level of the dental acrylic to allow for its attachment to an external stimulator during the experiment. Once the electrode was implanted and secured, the skin edges were approximated around the acrylic with absorbable suture. The animals were monitored during recovery from anesthesia, with food and water provided ad-libitum. A period of one week was allowed for recovery between implantation of the cerebellar electrode and the acute MEP experiment.
Lateral Cerebellar and Cortical Motor Thresholds
Cerebellar stimulation was delivered as biphasic, square wave pulses using a stimulus isolation unit (Model SIU-102, Warner Instruments, Hamden, CT USA) with pulse timing characteristics controlled by a Grass stimulator (Model S88). In order to identify the motor threshold for LCN stimulation (Asanuma and Hunsperger, 1975), the freely moving and awake animal was placed in a clear acrylic chamber (open at the top) and with the LCN electrode connected to the stimulator by means of a tethering system. Thresholds for stimulation of the LCN were determined by visual inspection for stimulation delivered at frequency values of 20, 30, 40, 50 and 100 Hz with the pulse width maintained at 400 µs.
Once LCN stimulation thresholds were determined, anesthesia was induced with intravenous propofol (10 mg/kg bolus i.v., followed by continuous infusion at 45–60 micrograms/kg/min). The depth of anesthesia was verified by toe pinch and corneal reflexes. A median incision was created and the skin overlying the region of the skull anterior to the LCN electrode implant was retracted, exposing the skull. A 5 mm by 3 mm craniotomy was opened over the left motor cortical area followed by opening and retraction of the underlying dura. Intracortical microstimulation was used to map the motor area of the cerebral cortex (Liddell and Phillips, 1950) and to identify the cortical site with the lowest threshold for activation of the contralateral hamstrings. Intracortical stimuli were delivered in brief bursts (Asanuma and Ward, 1971), consisting of a series of six, charge-balanced square-wave pulses with an intra-burst frequency of 330 pulses per second, with each pulse phase 400 µs in duration. Motor representation of the cerebral cortex was mapped along an x/y grid in 0.5 mm increments. For each penetration the electrode was advanced ventrally in 0.1 mm steps from 0.5 mm to 1.0 mm below the pial surface.
Electromyography
EMG data were recorded using paired, coiled-wire electrodes placed subcutaneously in a belly-tendon configuration through a small (1–2 cm) skin incision made using sterile technique. A ring electrode placed around the ankle of the animal served as the ground. Electromyographic activity was amplified and filtered (100 – 1,500 Hz, Model SM2000, Nicolet,Madison, WI), with the 60 Hz notch filter active. Gain was adjusted to take advantage of the full range of the analog-to-digital (A/D) converter without clipping and held constant for the duration of each study. The raw data were digitized (Model 6711, National Instruments, Austin, TX) at a rate of 25,000 samples per second and stored to a PC computer.
Experimental Procedure
Motor evoked potentials were generated in the hamstring musculature by time-locking EMG activity to the onset of intracortical stimulation. Intracortical stimulation was delivered at 125% of motor threshold for hamstring activation, with each sample epoch comprised of a 250 ms baseline followed by a 1,000 ms response window. MEPs were elicited serially, at approximately 15-second intervals using a block design, consisting of 40 trials per 10-minute block. A random interval of +/− 500 ms was included in the interval between MEP elicitation in order to minimize the possibility of the cortical stimulus pulse being time-locked to the onset of any single stimulus pulse from the continuous cerebellar stimulation. Following an initial 10-minute MEP baseline “OFF” period, LCN stimulation was alternately turned “ON” for 10-minute blocks separated by a 10-minute “OFF” block. The intervening “OFF” blocks were included to examine the reversibility of the LCN stimulation effect and to provide a washout period between “ON” blocks. During the “ON” blocks, LCN stimulation was delivered at 80% of the previously determined threshold for motor activation from LCN stimulation for the particular frequency being tested.
Data Processing and Analysis
Individual MEP responses were reviewed by an investigator blinded to the status of the LCN stimulator, with each response windowed using a pair of vertical cursors placed at its onset and offset. A second set of cursors, separated by the same number of data points (i.e., same ΔT) as the MEP window was positioned immediately prior to the onset of intracortical microstimulation. Once the windows were in place, the root mean square (RMS) of each segment was calculated and the ratio of the MEP window to the pre-stimulus baseline sample was determined. This approach was selected to minimize the effect of any changes in the baseline activity between the “OFF” and “ON” stimulation conditions. If a clear MEP could not be identified, and in the absence of marked artifact, a value of one was recorded. In order to correct for baseline signal variability between animals, the RMS ratio values were normalized to the baseline data for each animal by dividing each RMS ratio data point by the mean of the “OFF” block data for that animal.
A preliminary analysis of the data revealed no difference in the response pattern within blocks of the same stimulus frequency. As such, data from blocks of the same frequency were averaged within a given animal. A mixed model approach was conducted to account for multiple measurements taken from a single animal over time, with frequency, trial, and their interaction used as independent, fixed variables. Additionally, the effect of a given frequency on the magnitude of the MEP response across the 10-minute “ON” block was determined by examining model effects. Trial was identified as the repeated measurement and animal within frequency as the subject identifier. A compound symmetry variance-covariance structure was used. In order to maintain model assumptions, a transformation (square root) of the normalized RMS was used as the dependent variable. A Tukey adjustment was applied to control for multiple comparisons.
Histology
Following the experiments, histological analysis was performed to verify the location of the cerebellar electrode. Prior to sacrifice, a lesion was generated at the distal tip of the deep cerebellar electrode by electrocoagulation (1.0 mA, DC anodal current, 15 seconds duration). The animal was placed under deep anesthesia with pentobarbital (45mg/kg) and transcardially perfused with saline followed by 4% formaldehyde. The brains were then removed and immersed in 4% paraformaldehyde. All brains were blocked in paraffin and the cerebellum was sectioned at 40 µm, with every other slice mounted. The Pearls / DAB stain was used to facilitate recognition of the iron deposits in the trajectory of the electrode.
RESULTS
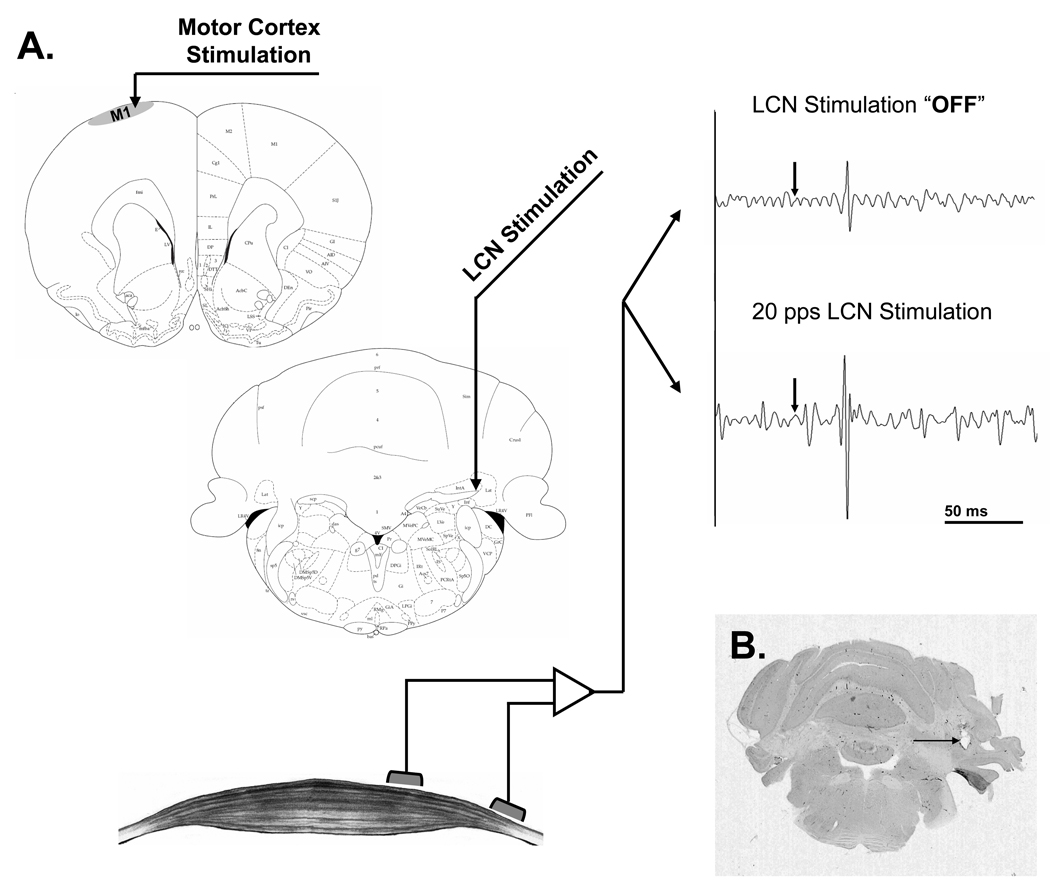
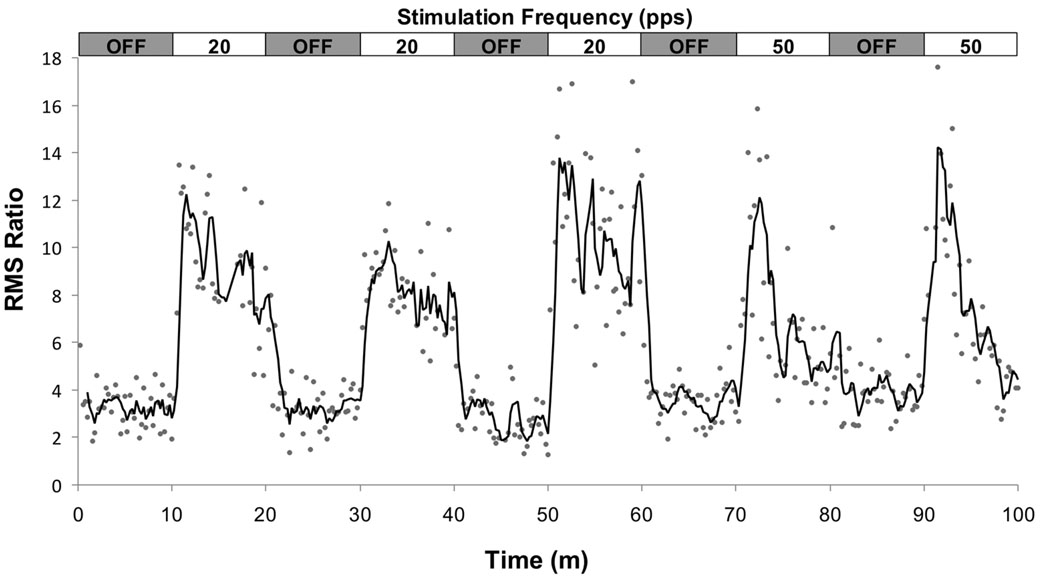
Twenty-six rats underwent MEP testing: 18 at a single frequency each with the remaining 8 tested at two LCN stimulation frequencies. An overview of the stimulation and recording protocol is shown in Figure 1. The raw data shown were elicited from the same preparation and include one sample recorded with the cerebellar stimulation turned “OFF” (upper right) and the second with cerebellar stimulation turned “ON” (lower right) at a rate of 20 Hz. An example of the time series data, which displays the RMS ratio of each individual MEP response as a function of time for a single animal, is shown in Figure 2. The data depicted were taken from an animal that underwent cerebellar stimulation at both 20 Hz (3 blocks) and 50 Hz (2 blocks). A three-point moving average (dark line) is overlaid on the individual data points to highlight the observed changes in MEP magnitude.
Figure 1.
A. Stimulation and recording set-up. MEPs recorded from the hamstring muscle in response to intracortical microstimulation of the contralateral motor cortex before (upper right) and during (lower) stimulation of the LCN. Each raw EMG tracing presents a 200ms segment, comprised of a 50ms baseline followed by a 150ms response window, with intracortical stimulation denoted by the arrow. B. Coronal cut of the rat's cerebellum stained for H&E. The arrow points to the artifact of the location of the tip of the electrode at the topography of the LCN.
Figure 2.
Time series plot for a single experimental animal depicting changes in the RMS ratio of the individual MEP responses (dots) across the 100 minute experimental window. The animal underwent cerebellar stimulation at both 20 (3 blocks) and 50 Hz (2 blocks) as shown in the horizontal box at the top of the figure. A three-point moving average is overlaid on the data to highlight changes in the MEP as a function of stimulation status and frequency.
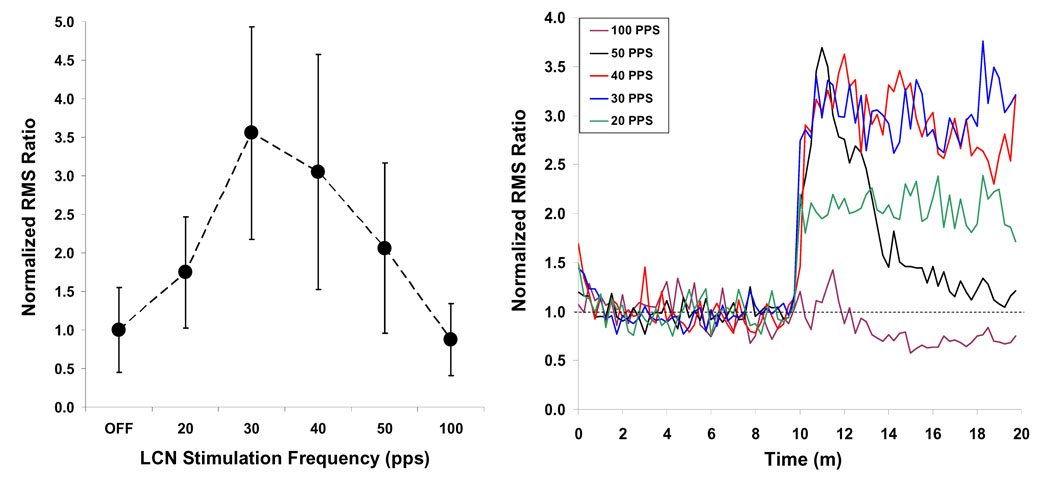
The analysis model revealed a significant effect of LCN stimulation on MEP magnitude (p < 0.01). Subsequent analysis revealed that the MEP response was enhanced, relative to the “OFF” condition, when LCN stimulation was delivered at 20 (p < 0.01), 30 (p < 0.001), 40 (p < 0.001) and 50 Hz (p < 0.01). In contrast, stimulation at 100 Hz was associated with an overall reduction in the magnitude of the MEP response; however this change failed to achieve statistical significance for the combined data set. The summary data are shown in Figure 3 (left) for each of the six stimulation conditions.
Figure 3.
LEFT: The effect of LCN stimulation on mean MEP amplitude across the 10-minute recording window is frequency dependent, with maximal increase observed at 30 Hz. RIGHT: The persistence of the LCN stimulation effect across the 10-minute recording window is frequency dependent. The mean response is shown as a function of time for each of the five frequency levels, with the initial 10 minutes of data representing the “off” condition, followed by the 10 minute “on” block. With the exception of 100 Hz, all frequency groups show an initial increase in response magnitude at the start of LCN stimulation. At 50 Hz however, the effect is transient, with the response approximating baseline levels by the end of the 10 minute block. A similar pattern is seen for stimulation at 40 Hz, though the decrement is less dramatic. The enhancement is sustained at both 20 and 30 Hz, while there appears to be a negative effect of 100 Hz stimulation on cortical excitability over time.
An analysis of the interaction between LCN stimulation frequency and the location of a trial within the “ON” block revealed frequency-specific differences (p < 0.001) in the sustainability of the augmentative effect of LCN stimulation on MEP magnitude (Figure 3, right). This variation in response over time was significant for stimulation at 40 Hz (p < 0.001), 50 Hz p < 0.001) and 100 Hz (p < 0.01). At 40 Hz and 50 Hz LCN stimulation, the response was characterized by an initial increase, comparable in amplitude to that observed for 30 Hz, followed by marked reduction in amplitude as a function of repeated stimulation trials. The decay in response magnitude was more pronounced at 50 Hz and corroborated by a strong negative correlation (r = −0.93) between trial number within the block and RMS ratio. Negative correlations were similarly identified for the 100 Hz (r = −0.75) and the 40 Hz (r = −0.42) groups, though the strength of the correlation was smaller. The overall effect is illustrated in Figure 3 (right), which provides the mean response as a function of time relative to the onset of the 10 minute stimulation block. For each average, twenty minutes of data are shown representing the average response across the ten minute “OFF” period followed by the ten minute “ON” period. Correlations for 0 (OFF), 20, and 30 Hz were mild-to-moderate and were not found to be significant; indicating that stimulation at these frequencies was associated with a sustained increase in MEP magnitude across the ten minute epoch. In a subset of animals, stimulation at 100 Hz produced a sharp (i.e., 2 – 3 trials), transient increase in the MEP response; however this effect was minimized by data averaging leaving only the trend towards reduction over time.
DISCUSSION
Changes in cortical excitability can be achieved through a variety of interventions, including physical activity (Liepert et al., 2001), pharmacological agents (Li et al., 2009; Paulus et al., 2008) and magnetic or electrical stimulation of specific regions of the nervous system (Deletis et al., 1987; Di Lazzaro et al., 2006; Kim et al., 2006). In the current study, continuous electrical stimulation of the LCN was found to modulate excitability of the contralateral primary motor cortex in normal rodent. Moreover, the pulse frequency of LCN stimulation influenced the overall magnitude and persistence of this effect. These findings are consistent with our recent work showing frequency-specific improvements in motor recovery following chronic LCN stimulation in a rodent model of middle cerebral artery stroke (Machado et al., 2009), where animals treated chronically with 20 Hz LCN stimulation showed significant improvements in motor performance over a period of six weeks post-infarct while those that received 50 Hz stimulation did not. Together, the data support a possible link between changes in cortical excitability and post-stroke motor recovery, in which lower stimulation frequencies provide sustained augmentation of cortical excitability and facilitate reorganization of spared, peri-lesional cortex. Additional studies are needed to characterize further any relationship between the effects of chronic LCN stimulation-related changes on cortical excitability and perilesional plasticity.
Cerebellar control over cerebral cortex excitability
The cerebellum is uniquely positioned to modulate cortical excitability and thought by some to be a key player in regulating cerebral plasticity (Molinari et al., 2002). Extensive and reciprocal connections exist between the cerebral and cerebellar hemispheres (Allen and Tsukahara, 1974), with the ascending component of this link a di-synaptic, net excitatory pathway that has been well characterized across a variety of mammalian species, including the rat (Dum and Strick, 2003; Faull and Carman, 1978; Haroian et al., 1981). Cerebellar efferents terminate broadly across multiple thalamic subnuclei, including the intralaminar nuclei, the medial dorsal nucleus as well as the rostral portion of the ventral tier nuclei (Asanuma et al., 1983; Dum and Strick, 2003; Haroian et al., 1981). Single pulse electrical stimulation of the deep cerebellar nuclei yields a short latency increase in neuronal activity in primary motor cortex of both cats and non-human primates (Holdefer et al., 2000; Sasaki et al., 1976). In awake animals, stimulation can elicit both simple and complex movements of the contralateral hemibody (Schultz et al., 1979), with evidence to suggest the involvement of a trans-cortical loop via the ascending pathway (Rispal-Padel et al., 1982). At sub-threshold levels, pairing single-pulse stimulation of the contralateral motor cortex with electrical or magnetic conditioning cerebellar pulse modulates motor evoked responses (McCaffrey and Erickson, 1987; Rispal-Padel et al., 1981). The paired-pulse paradigms used in those studies depict acute effects that are transient in nature and only occur when the test pulse is delivered within a specific time window following the conditioning stimulus. Our findings extend those results to reveal that low frequency, but not high frequency, chronic stimulation of the cerebellar output pathways modulates cortical excitability independent of temporal synchrony between the cerebellar and cerebral stimulus pulses.
Significance of LCN pulse frequency
Chronic electrical stimulation forms the basis of deep brain stimulation (DBS) therapy; with most therapeutic applications relying upon high (> 100 Hz) pulse frequencies to achieve their effect (Ashby et al., 1999). Typically the therapeutic effect of high-frequency DBS mirrors that derived from surgical destruction of the targeted region, a parallelism that has generated considerable debate as to the underlying mechanism. At low pulse frequency levels (< 50Hz) the effects of stimulation tend to be more consistent in suggesting activation of the output of the stimulated subcortical region (Alesch et al., 1995). Though our data are consistent with activation of cerebellar output, they suggest further that the persistence of the facilitative effect is frequency dependent, with a decline in activation observed over time for frequencies of 40 Hz and higher. Indeed, when present, the effect faded rapidly at 100 Hz, with evidence of an overall reduction from baseline in cortical excitability over time, a finding that is perhaps consistent with studies suggesting that high frequency stimulation of the dentate reduces seizure frequency (Chkhenkeli et al., 2004). At the low end of the frequency range, it was noted that while 20 Hz also provided a significant and sustained enhancement of excitability, the effect was less robust than that observed with 30 Hz stimulation. Meanwhile, the magnitude of the early response within each stimulation block was actually similar for pulse rates of 30, 40 and 50 Hz, perhaps suggestive of a ceiling effect in the response. A possible explanation for peak and persistent efficacy at 30 Hz is that this frequency maximizes temporal summation of the cerebellar output pathways without overdriving the system (e.g., the synapse) to failure. Indeed, the mean spontaneous activity of neurons within the dentate / LCN in awake animals is reported to fall within the beta frequency range (Harvey et al., 1979; Thach, 1975), with modulations occurring around the beta band at the time of movement onset (Aumann et al., 1998). Aumann and collaborators interpret their data as signifying that one role of the deep cerebellar nuclei is to generate and propagate beta oscillations throughout the central and peripheral motor systems (Aumann and Fetz, 2004). Although higher frequency stimulation can generate robust, albeit short-lived increments in cortical excitability, stimulation at frequencies within the beta band – the natural frequency band of coherence in the cerebellothalamocortical motor system – allows for a robust yet sustainable facilitation of motor activity and excitability. Thus, while further data are needed to determine the persistence of the effect beyond the 10-minute window examined, our goal of identifying a frequency for chronic activation of cerebellar output appears to be best fulfilled by a pulse frequency of 30 Hz.
Potential therapeutic application
In the 1970s and 1980s, Wright (Wright et al., 1984), Davis (Davis et al., 1983) and Cooper (Cooper et al., 1973) attempted to modulate cerebellar output to treat epilepsy, reporting modest improvements in seizure frequency. Those studies did not target the deep cerebellar nuclei directly, instead using electrodes placed “upstream” over the cerebellar cortex. One methodological factor that may have contributed to the failure of this approach is that the surface electrodes were too small relative to the cortical surface of the cerebellum. As such, the area modulated by stimulation was not large enough to generate a spatially effective inhibition of cerebellar output. The recent failed trial of epidural stimulation for the treatment of stroke (Plow et al., 2009) may represent a modern corollary of this dilemma. One potential factor for why the human trial failed despite strong evidence from non-human primate (Plautz et al., 2003) and rodent (Adkins-Muir and Jones, 2003) experiments may relate to profound differences in the complexity of gyral folding patterns between humans and sub-human models (Van Essen and Dierker, 2007). Direct, electrical stimulation of the more compact dentate nucleus may represent a more efficient means of driving the output of the DTC pathway, resulting in a more widespread increase in cortical excitability.
Implications
Continuous stimulation of the LCN evokes increments in cortical excitability in the contralateral hemisphere, indexed by increased MEP amplitudes. Stimulation in the beta band results in sustained augmentation in excitability, with the most robust results identified with stimulation at 30 Hz. These results are likely related to the natural propagation properties of motor-related signaling in the DTC pathway. While the magnitude of these effects in cortical areas beyond the motor representation remains to be characterized, this approach for modulating cortical excitability may offer therapeutic possibilities for movement disorders, epilepsy and for the modulation of perilesional plasticity following strokes or other focal cortical lesions.
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health Grant R21HD056515. We thank Erik Novak for statistical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adkins-Muir DL, Jones TA. Cortical electrical stimulation combined with rehabilitative training: enhanced functional recovery and dendritic plasticity following focal cortical ischemia in rats. Neurol Res. 2003;25:780–788. doi: 10.1179/016164103771953853. [DOI] [PubMed] [Google Scholar]
- Alesch F, Pinter MM, Helscher RJ, Fertl L, Benabid AL, Koos WT. Stimulation of the ventral intermediate thalamic nucleus in tremor dominated Parkinson's disease and essential tremor. Acta Neurochir (Wien) 1995;136:75–81. doi: 10.1007/BF01411439. [DOI] [PubMed] [Google Scholar]
- Allen GI, Tsukahara N. Cerebrocerebellar communication systems. Physiol Rev. 1974;54:957–1006. doi: 10.1152/physrev.1974.54.4.957. [DOI] [PubMed] [Google Scholar]
- Asanuma C, Thach WR, Jones EG. Anatomical evidence for segregated focal groupings of efferent cells and their terminal ramifications in the cerebellothalamic pathway of the monkey. Brain Res. 1983;286:267–297. doi: 10.1016/0165-0173(83)90016-4. [DOI] [PubMed] [Google Scholar]
- Asanuma H, Hunsperger RW. Functional significance of projection from the cerebellar nuclei to the motor cortex in the cat. Brain Res. 1975;98:73–92. doi: 10.1016/0006-8993(75)90510-7. [DOI] [PubMed] [Google Scholar]
- Asanuma H, Ward JE. Patterns of contraction of distal forelimb muscles produced by intracortical stimulation in cats. Brain Res. 1971;27:97–109. doi: 10.1016/0006-8993(71)90374-x. [DOI] [PubMed] [Google Scholar]
- Ashby P, Kim YJ, Kumar R, Lang AE, Lozano AM. Neurophysiological effects of stimulation through electrodes in the human subthalamic nucleus. Brain. 1999;122(Pt 10):1919–1931. doi: 10.1093/brain/122.10.1919. [DOI] [PubMed] [Google Scholar]
- Aumann TD, Fetz EE. Oscillatory activity in forelimb muscles of behaving monkeys evoked by microstimulation in the cerebellar nuclei. Neurosci Lett. 2004;361:106–110. doi: 10.1016/j.neulet.2003.12.091. [DOI] [PubMed] [Google Scholar]
- Aumann TD, Rawson JA, Horne MK. The relationship between monkey dentate cerebellar nucleus activity and kinematic parameters of wrist movement. Exp Brain Res. 1998;119:179–190. doi: 10.1007/s002210050332. [DOI] [PubMed] [Google Scholar]
- Boake C, Noser EA, Ro T, Baraniuk S, Gaber M, Johnson R, et al. Constraint-induced movement therapy during early stroke rehabilitation. Neurorehabil Neural Repair. 2007;21:14–24. doi: 10.1177/1545968306291858. [DOI] [PubMed] [Google Scholar]
- Carmichael ST. Plasticity of cortical projections after stroke. Neuroscientist. 2003;9:64–75. doi: 10.1177/1073858402239592. [DOI] [PubMed] [Google Scholar]
- Chkhenkeli SA, Sramka M, Lortkipanidze GS, Rakviashvili TN, Bregvadze E, Magalashvili GE, et al. Electrophysiological effects and clinical results of direct brain stimulation for intractable epilepsy. Clin Neurol Neurosurg. 2004;106:318–329. doi: 10.1016/j.clineuro.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Cooper IS, Amin I, Gilman S. The effect of chronic cerebellar stimulation upon epilepsy in man. Trans Am Neurol Assoc. 1973;98:192–196. [PubMed] [Google Scholar]
- Davis R, Gray E, Engle H, Dusnak A. Reduction of intractable seizures using cerebellar stimulation. Appl Neurophysiol. 1983;46:57–61. doi: 10.1159/000101243. [DOI] [PubMed] [Google Scholar]
- Deletis V, Dimitrijevic MR, Sherwood AM. Effects of electrically induced afferent input from limb nerves on the excitability of the human motor cortex. Neurosurgery. 1987;20:195–197. doi: 10.1097/00006123-198701000-00038. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Dileone M, Profice P, Pilato F, Cioni B, Meglio M, et al. Direct demonstration that repetitive transcranial magnetic stimulation can enhance corticospinal excitability in stroke. Stroke. 2006;37:2850–2853. doi: 10.1161/01.STR.0000244824.53873.2c. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Molinari M, Leggio MG, Nardone R, Fogli D, et al. Excitability of the motor cortex to magnetic stimulation in patients with cerebellar lesions. J Neurol Neurosurg Psychiatry. 1994;57:108–110. doi: 10.1136/jnnp.57.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. J Neurophysiol. 2003;89:634–639. doi: 10.1152/jn.00626.2002. [DOI] [PubMed] [Google Scholar]
- Eisner-Janowicz I, Barbay S, Hoover E, Stowe AM, Frost SB, Plautz EJ, et al. Early and late changes in the distal forelimb representation of the supplementary motor area after injury to frontal motor areas in the squirrel monkey. J Neurophysiol. 2008;100:1498–1512. doi: 10.1152/jn.90447.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faull RL, Carman JB. The cerebellofugal projections in the brachium conjunctivum of the rat I. The contralateral ascending pathway. J Comp Neurol. 1978;178:495–517. [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Valle AC, Rocha RR, Duarte J, Ferreira MJ, et al. A sham-controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke. 2006;37:2115–2122. doi: 10.1161/01.STR.0000231390.58967.6b. [DOI] [PubMed] [Google Scholar]
- Frost SB, Barbay S, Friel KM, Plautz EJ, Nudo RJ. Reorganization of remote cortical regions after ischemic brain injury: a potential substrate for stroke recovery. J Neurophysiol. 2003;89:3205–3214. doi: 10.1152/jn.01143.2002. [DOI] [PubMed] [Google Scholar]
- Hamdy S, Rothwell JC, Aziz Q, Singh KD, Thompson DG. Long-term reorganization of human motor cortex driven by short-term sensory stimulation. Nat Neurosci. 1998;1:64–68. doi: 10.1038/264. [DOI] [PubMed] [Google Scholar]
- Haroian AJ, Massopust LC, Young PA. Cerebellothalamic projections in the rat: an autoradiographic and degeneration study. J Comp Neurol. 1981;197:217–236. doi: 10.1002/cne.901970205. [DOI] [PubMed] [Google Scholar]
- Harvey RJ, Porter R, Rawson JA. Discharges of intracerebellar nuclear cells in monkeys. J Physiol. 1979;297:559–580. doi: 10.1113/jphysiol.1979.sp013057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdefer RN, Miller LE, Chen LL, Houk JC. Functional connectivity between cerebellum and primary motor cortex in the awake monkey. J Neurophysiol. 2000;84:585–590. doi: 10.1152/jn.2000.84.1.585. [DOI] [PubMed] [Google Scholar]
- Huang M, Harvey RL, Stoykov ME, Ruland S, Weinand M, Lowry D, et al. Cortical stimulation for upper limb recovery following ischemic stroke: a small phase II pilot study of a fully implanted stimulator. Top Stroke Rehabil. 2008;15:160–172. doi: 10.1310/tsr1502-160. [DOI] [PubMed] [Google Scholar]
- Hummel F, Celnik P, Giraux P, Floel A, Wu WH, Gerloff C, et al. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128:490–499. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- Iwata NK, Hanajima R, Furubayashi T, Terao Y, Uesugi H, Shiio Y, et al. Facilitatory effect on the motor cortex by electrical stimulation over the cerebellum in humans. Exp Brain Res. 2004;159:418–424. doi: 10.1007/s00221-004-1979-x. [DOI] [PubMed] [Google Scholar]
- Kim YH, You SH, Ko MH, Park JW, Lee KH, Jang SH, et al. Repetitive transcranial magnetic stimulation-induced corticomotor excitability and associated motor skill acquisition in chronic stroke. Stroke. 2006;37:1471–1476. doi: 10.1161/01.STR.0000221233.55497.51. [DOI] [PubMed] [Google Scholar]
- Levy R, Ruland S, Weinand M, Lowry D, Dafer R, Bakay R. Cortical stimulation for the rehabilitation of patients with hemiparetic stroke: a multicenter feasibility study of safety and efficacy. J Neurosurg. 2008;108:707–714. doi: 10.3171/JNS/2008/108/4/0707. [DOI] [PubMed] [Google Scholar]
- Li X, Ricci R, Large CH, Anderson B, Nahas Z, George MS. Lamotrigine and valproic acid have different effects on motorcortical neuronal excitability. J Neural Transm. 2009;116:423–429. doi: 10.1007/s00702-009-0195-z. [DOI] [PubMed] [Google Scholar]
- Liddell EG, Phillips CG. Thresholds of cortical representation. Brain. 1950;73:125–140. doi: 10.1093/brain/73.2.125. [DOI] [PubMed] [Google Scholar]
- Liepert J. Motor cortex excitability in stroke before and after constraint-induced movement therapy. Cogn Behav Neurol. 2006;19:41–47. doi: 10.1097/00146965-200603000-00005. [DOI] [PubMed] [Google Scholar]
- Liepert J, Bauder H, Wolfgang HR, Miltner WH, Taub E, Weiller C. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000;31:1210–1216. doi: 10.1161/01.str.31.6.1210. [DOI] [PubMed] [Google Scholar]
- Liepert J, Uhde I, Graf S, Leidner O, Weiller C. Motor cortex plasticity during forced-use therapy in stroke patients: a preliminary study. J Neurol. 2001;248:315–321. doi: 10.1007/s004150170207. [DOI] [PubMed] [Google Scholar]
- Machado AG, Baker KB, Schuster D, Butler RS, Rezai A. Chronic electrical stimulation of the contralesional lateral cerebellar nucleus enhances recovery of motor function after cerebral ischemia in rats. Brain Res. 2009;1280:107–116. doi: 10.1016/j.brainres.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manto M, Oulad ben Taib N, Luft AR. Modulation of excitability as an early change leading to structural adaptation in the motor cortex. J Neurosci Res. 2006;83:177–180. doi: 10.1002/jnr.20733. [DOI] [PubMed] [Google Scholar]
- McCaffrey M, Erickson JP. Modulation of cat motor evoked potential by prior cerebellar or somatosensory stimulation. Neurosurgery. 1987;20:193–195. doi: 10.1097/00006123-198701000-00037. [DOI] [PubMed] [Google Scholar]
- Molinari M, Filippini V, Leggio MG. Neuronal plasticity of interrelated cerebellar and cortical networks. Neuroscience. 2002;111:863–870. doi: 10.1016/s0306-4522(02)00024-6. [DOI] [PubMed] [Google Scholar]
- Murphy GG, Fedorov NB, Giese KP, Ohno M, Friedman E, Chen R, et al. Increased neuronal excitability, synaptic plasticity, and learning in aged Kvbeta1.1 knockout mice. Curr Biol. 2004;14:1907–1915. doi: 10.1016/j.cub.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Paulus W, Classen J, Cohen LG, Large C, Di Lazzaro V, Nitsche MA, et al. State of the art: Pharmacologic effects on cortical excitability measures tested by transcranial magnetic stimulation. Brain Stimulat. 2008;1:151–163. doi: 10.1016/j.brs.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Plautz EJ, Barbay S, Frost SB, Friel KM, Dancause N, Zoubina EV, et al. Post-infarct cortical plasticity and behavioral recovery using concurrent cortical stimulation and rehabilitative training: a feasibility study in primates. Neurol Res. 2003;25:801–810. doi: 10.1179/016164103771953880. [DOI] [PubMed] [Google Scholar]
- Plow EB, Carey JR, Nudo RJ, Pascual-Leone A. Invasive cortical stimulation to promote recovery of function after stroke: a critical appraisal. Stroke. 2009;40:1926–1931. doi: 10.1161/STROKEAHA.108.540823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rispal-Padel L, Cicirata F, Pons C. Contribution of the dentato-thalamo-cortical system to control of motor synergy. Neurosci Lett. 1981;22:137–144. doi: 10.1016/0304-3940(81)90077-x. [DOI] [PubMed] [Google Scholar]
- Rispal-Padel L, Cicirata F, Pons C. Cerebellar nuclear topography of simple and synergistic movements in the alert baboon (Papio papio) Exp Brain Res. 1982;47:365–380. doi: 10.1007/BF00239355. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Calautti C, Pauri F, Baron JC. Post-stroke plastic reorganisation in the adult brain. Lancet Neurol. 2003;2:493–502. doi: 10.1016/s1474-4422(03)00485-x. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Kawaguchi S, Oka H, Sakai M, Mizuno N. Electrophysiological studies on the cerebellocerebral projections in monkeys. Exp Brain Res. 1976;24:495–507. doi: 10.1007/BF00234966. [DOI] [PubMed] [Google Scholar]
- Schultz W, Montgomery EB, Jr, Marini R. Proximal limb movements in response to microstimulation of primate dentate and interpositus nuclei mediated by brain-stem structures. Brain. 1979;102:127–146. doi: 10.1093/brain/102.1.127. [DOI] [PubMed] [Google Scholar]
- Thach WT. Timing of activity in cerebellar dentate nucleus and cerebral motor cortex during prompt volitional movement. Brain Res. 1975;88:233–241. doi: 10.1016/0006-8993(75)90387-x. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Dierker DL. Surface-based and probabilistic atlases of primate cerebral cortex. Neuron. 2007;56:209–225. doi: 10.1016/j.neuron.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Wright GD, McLellan DL, Brice JG. A double-blind trial of chronic cerebellar stimulation in twelve patients with severe epilepsy. J Neurol Neurosurg Psychiatry. 1984;47:769–774. doi: 10.1136/jnnp.47.8.769. [DOI] [PMC free article] [PubMed] [Google Scholar]