Abstract
The main objectives of this study were to examine the acute effect of a single dose of smokeless tobacco (ST) on central aortic blood pressure and wave reflection characteristics. Fifteen apparently healthy male subjects (aged 30.6 ± 6.2 y) were given a 2.5 g oral dose of ST after baseline measurements were recorded. Pulse wave analysis using radial artery applanation tonometry was performed in triplicate at baseline (0 min) and at 10-min intervals during (10, 20 and 30 min) and after (40, 50 and 60 min) ST use. An acute dose of ST was associated with a significant increase in heart rate (HR), central aortic systolic and diastolic blood pressure, peripheral brachial systolic and diastolic blood pressure, and aortic augmentation index normalized to a fixed heart rate of 75 bpm (AIx@75). Furthermore, ejection duration and round trip travel time of the reflected pressure wave (Δtp) were significantly decreased as a result of one time ST use. As a result of changes in aortic pressure wave reflection characteristics, there was a significant increase in wasted left ventricular pressure energy (LVEw) and the tension–time index (TTI) as a result of ST use.
In conclusion, one time use of ST elicits significant transient increases in HR, central aortic pressures, AIx@75, the TTI and LVEw. Chronic users subjected to decades of elevated central pressures and left ventricular work may have an increased cardiovascular risk as central aortic pressures are even more strongly related to cardiovascular outcomes than peripheral blood pressures.
Keywords: smokeless tobacco, central aortic pressure, wave reflection, cardiovascular risk, pulse wave analysis
Introduction
The Centers for Disease Control estimates that the number of current male smokeless tobacco (ST) users in the USA is about 4.44 million, or 4.3% of the population.1 Furthermore, 19% of all ST users are between the ages of 18 and 24 y and could become habitual users for decades prior to reaching middle age. The most recognized health risk associated with any tobacco product is the carcinogenic side-effect. However, tobacco has other deleterious effects, including those on the cardiovascular system. The majority of tobacco studies have investigated the effects of cigarette smoking. It has been well established that cigarette smoking is strongly related to cardiovascular morbidity and mortality. In contrast, limited data are available concerning the effects of ST.
Previous studies have established the acute effects of ST on heart rate (HR) and peripheral blood pressure. A single bout of ST elicits a transient increase in HR and peripheral systolic (PSBP) and diastolic (PDBP) blood pressure in humans.2–5 However, no studies have investigated the acute changes in central blood pressure and central hemodynamics during and immediately after ST use in humans. Central aortic pressure is more strongly related to cardiovascular morbidity and mortality than peripheral blood pressure measurements.6–8 Moreover, it is now recognized that peripheral blood pressure measurements obtained by standard brachial artery sphygmomanometry are not reliable measures of ascending aortic pressure.9–11
Therefore, the purpose of the present study was to determine, for the first time, the effects of an acute bout of ST use on central aortic blood pressure and the amplitude and timing of the central aortic reflected pressure wave in humans. We hypothesized that a single bout of ST would transiently increase central blood pressures, central aortic pressure augmentation and indices of left ventricular work.
Materials and methods
Subjects
A total of 15 healthy men (aged 30 ± 6 y; body mass index [BMI] 30 ± 5 kg/m2) were recruited for participation in the study. All subjects had previous experience with ST, and all but two were habitual users. Additionally, all subjects were normotensive (<140/90 mmHg), free from overt cardiovascular disease, and were not receiving medication. The study was approved by the University of Florida Health Science Center Institutional Review Board and written informed consent was obtained from all subjects.
Brachial artery blood pressure measurements
After resting in an upright seated position for a period of 15 min, PSBP and PDBP measurements in the right arm were measured in triplicate by oscillometric brachial artery sphygmomanometry using an automated non-invasive device (BpTRU BPM-100, VSM MedTech Ltd, Vancouver, BC, Canada).
Pulse wave analysis
The assessment of arterial wave reflection characteristics were performed non-invasively using the SphygmoCor Pulse Wave Analysis Px system and SCOR-2000 Version 6.31 software (AtCor Medical, Sydney, Australia). High-fidelity radial artery pressure waveforms were recorded by applanation tonometry of the radial pulse in the left wrist using a ‘pencil-type’ micromanometer (Millar Instruments, Houston, TX, USA). The aortic pressure waveform was derived non-invasively from the radial pulse using applanation tonometry and synthesized using a generalized transfer function from high-fidelity radial pressure waveforms calibrated from standard brachial artery sphygmomanometry, which corrects for pressure wave amplification in the upper limb.12 The generalized transfer function is the average of many individual transfer functions as determined from simultaneous measures of radial and central aortic pressure waveforms. The generalized transfer function has been shown to estimate central arterial pressures to ≤0.2 ± 3.8 mmHg error13 and has been validated through comparison of both intra-arterially10,13 and non-invasively14 obtained radial pressure waves to intra-aortic micrometer pressure readings. The test–retest reproducibility of this procedure was previously established by others.15 In our laboratory, reproducibility has been established in young, healthy men with a mean coefficient of variation of 6.5%, 2.1%, 2.4% and 2.4% for aortic augmentation index (AIx), round trip travel time of the reflected pressure wave (Δtp), central systolic and diastolic blood pressure, respectively.16 At least three consecutive measurements were performed per time point, and the average of the three highest quality recordings, defined as an in-device quality index of over 90% (derived from an algorithm including average pulse height variation, diastolic variation and maximum rate of rise of the peripheral waveform) were used for analysis.
The central aortic pressure wave is composed of a forward traveling wave, generated by left ventricular ejection and a reflected wave that is returning to the ascending aorta from the periphery. A typical aortic pressure waveform synthesized from radial pulse pressure using applanation tonometry and the generalized transfer function is shown in Figure 1. The following pulse wave analysis parameters, related to the amplification and temporal characteristics of the reflecting wave, were used as dependent variables in the present study: central aortic SBP (CSBP), central aortic DBP (CDBP), mean arterial pressure (MAP), end systolic pressure (ESP), ejection duration (ED), AIx, AIx normalized to an HR of 75 bpm (AIx@75) and Δtp. ED is a measure of time, in milliseconds, of the duration of each cardiac systole. MAP was obtained from an integration of the waveform. ESP is defined as the pressure at the end of systole, which is the pressure at the end of ED.17 AIx, expressed as a percentage, characterizes augmentation of central pressures and is defined as reflected wave amplitude divided by pulse pressure.18,19 Δtp is the round trip travel time of the forward traveling wave from the ascending aorta to the major reflection site and back and is measured from the foot of the forward traveling pressure wave to the foot of the reflected wave.
Figure 1.
A typical central aortic pressure waveform synthesized from the radial artery pressure waveform using applanation tonometry, with superimposed waveform of aortic blood flow. The dotted line is representative of the theoretical aortic pressure waveform independent of wave reflection. The line labeled flow is a representative waveform of aortic blood flow. Augmentation index is the ratio of augmented pressure (CSBP − Pi) and central aortic pulse pressure (CSBP − CDBP). Wasted left ventricular pressure energy is directly related to augmented pressure (CSBP − Pi) and to the time duration of the reflected aortic pressure wave, (ED − Δtp). CSBP, central aortic systolic blood pressure; Pi, pressure at the first inflection point marking the onset of reflected aortic pressure wave return from the periphery; CDBP, central aortic diastolic blood pressure; Δtp, round trip travel time of the reflected pressure wave to the major peripheral reflecting site and back to the aorta; Δtr, systolic duration of the reflected aortic pressure wave; ED, ejection duration; ESP, end systolic pressure; LVEW, wasted left ventricular pressure energy; AIx, augmentation index
Additional calculations derived from pulse wave analysis included left ventricular wasted pressure energy (LVEW) and the tension–time index (TTI). LVEW is a component of extra myocardial oxygen requirement that is due to early systolic wave reflection.20 LVEW can be estimated in dynes s/cm2 as [(ED − Δtp)(CSBP − Pi)π/4]1.33322, where Pi is the first inflection point marking the onset of reflected aortic pressure wave return from the periphery (Figure 1). The TTI, a marker of left ventricular work and myocardial oxygen demand, was calculated as (ED × HR × mean aortic SBP)/1000, and is expressed in mmHg s/min. Assessment of central pressure waves is described in detail by Nichols and Singh.17
Laboratory procedure
All subjects arrived at the laboratory at least 2–3 h postprandial. All subjects were instructed to abstain from nicotine and caffeine for 12 h as well as exercise and alcohol for at least 24 h prior to testing. All studies were performed with the subjects in an upright seated position with their arms stabilized at the level of the fifth intercostal space. After baseline resting measurements were obtained (0 min), subjects placed 2.5 g of ST (Copenhagen long cut; U.S. Smokeless Tobacco Company, Richmond, VA, USA), containing approximately 10.5 mg/g nicotine content and 6.2 mg/g free nicotine,21 in the buccal sulcus under the bottom lip for 30 min. A 2.5 g dose of ST was used because it corresponded to a typical dose used in previous studies and was in accordance with the amount our subjects used. Brachial artery pressure and radial pressure waveforms were recorded in triplicate at 10-min intervals for a total duration of 60 min. Measurements were captured within the last two minutes of each 10-min interval. Time points 10, 20 and 30 min correspond to measurements made while using ST. Time points 40, 50 and 60 min correspond to measurements made after removal of ST. Subjects were instructed not to consume anything during data collection, but were allowed to rinse their mouth with bottled water following removal of the ST after 30 min. All measurements were performed by the same investigator in a quiet, temperature controlled room (21–23°C). To avoid potential diurnal variations, all measurements were conducted in the morning between 08:00 and 11:00 hours.
Statistical analysis
All data are reported as mean ± SEM. Changes in continuous dependent variables were analyzed using repeated-measures analysis of variance (ANOVA). When a significant effect of time was observed, post hoc analysis was performed to determine significant differences from baseline at each time point using Fisher’s least significant difference. The alpha level for statistical significance was set at 0.05 and data were analyzed using SPSS Statistics, Version 18.0 (IBM, Chicago, IL, USA).
Results
Repeated-measures ANOVA revealed that time had a significant effect for all dependent variables, except AIx. Age, weight and BMI were found to be non-significant covariates in this model. In addition, no significant interaction between habitual and recreational users was found.
Table 1 provides the mean ± SEM for blood pressure responses and pulse wave analysis parameters analyzed in the experiment. Post hoc analysis revealed that ST significantly increased HR, PSBP, CSBP, PDBP, CDBP and MAP at all time points respective to baseline while using ST (Table 1). HR peaked an average of 12 bpm above baseline at the 10-min time point, but was transiently elevated and returned to baseline values at the 60-min time point. PSBP, CSBP and MAP reached maximum values at the 20-min time point (an average of 17, 15 and 13 mmHg above baseline, respectively) and progressively declined through the 60-min time point, but remained significantly elevated respective to baseline. PDBP and CDBP peaked at the 30-min time point (an average of 11 and 12 mmHg above baseline, respectively) and declined thereafter, but also remained significantly elevated from baseline throughout the 60-min duration of the study. ED significantly decreased respective to baseline at the 10, 20 and 30 min time points while ESP was significantly elevated relative to baseline at all time points. AIx did not change throughout the duration of the study while AIx@75 was significantly elevated relative to baseline at the 10, 20 and 30 min time points (8.6 ± 2.1%, 8.5 ± 2.1% and 8.7 ± 1.9% versus 4.1 ± 2.3%, respectively). Δtp was significantly shortened at all time points compared with baseline.
Table 1.
Hemodynamic responses to smokeless tobacco
| Baseline | During ST | After ST | |||||
|---|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 40 | 50 | 60 | |
| HR (bpm) | 67 ± 3 | 79 ± 3* | 77 ± 4* | 77 ± 4* | 74 ± 4* | 73 ± 4** | 71 ± 4 |
| ED (ms) | 292 ± 6 | 281 ± 5* | 280 ± 7** | 281 ± 6* | 285 ± 8 | 288 ± 8 | 290 ± 7 |
| PSBP (mmHg) | 124 ± 3 | 140 ± 6* | 141 ± 5* | 139 ± 5* | 136 ± 4* | 134 ± 4* | 129 ± 4** |
| CSBP (mmHg) | 109 ± 3 | 123 ± 5* | 124 ± 4* | 122 ± 4* | 120 ± 3* | 118 ± 3* | 115 ± 3* |
| PDBP (mmHg) | 79 ± 2 | 90 ± 4* | 90 ± 3* | 90 ± 3* | 88 ± 2* | 86 ± 2* | 84 ± 3* |
| CDBP (mmHg) | 80 ± 2 | 91 ± 4* | 92 ± 3* | 92 ± 3* | 89 ± 2* | 88 ± 3* | 85 ± 3* |
| MAP (mmHg) | 94 ± 3 | 106 ± 4* | 107 ± 3* | 106 ± 3* | 104 ± 3* | 102 ± 3* | 99 ± 3* |
| Pi (mmHg) | 106 ± 3 | 119 ± 5* | 119 ± 5* | 116 ± 5* | 116 ± 4* | 112 ± 4* | 109 ± 4 |
| ESP (mmHg) | 102 ± 3 | 114 ± 4* | 115 ± 4* | 114 ± 3* | 111 ± 3* | 110 ± 3* | 107 ± 3** |
| AIx (%) | 8.9 ± 2.2 | 7.1 ± 2.3 | 8.3 ± 2.6 | 8.7 ± 2.2 | 8.0 ± 2.4 | 9.2 ± 2.4 | 9.3 ± 2.5 |
| AIx@75 (%) | 4.1 ± 2.3 | 8.6 ± 2.1* | 8.5 ± 1.9** | 8.7 ± 1.9* | 6.8 ± 1.8 | 7.1 ± 2.3 | 7.0 ± 2.1 |
| Δtp (ms) | 160 ± 3 | 150 ± 2* | 152 ± 2* | 149 ± 2* | 151 ± 2* | 149 ± 2* | 152 ± 3* |
P < 0.01,
P < 0.05 versus respective baseline. Values reported as mean ± standard error of the mean. When significant within-subject variation was found, differences from baseline for each time point were determined using pairwise comparisons
HR, heart rate; ED, ejection duration; PSBP, peripheral systolic blood pressure; CSBP, central systolic blood pressure; PDBP, peripheral diastolic blood pressure; CDBP, central diastolic blood pressure; MAP, mean arterial pressure; Pi, central aortic pressure at inflection point
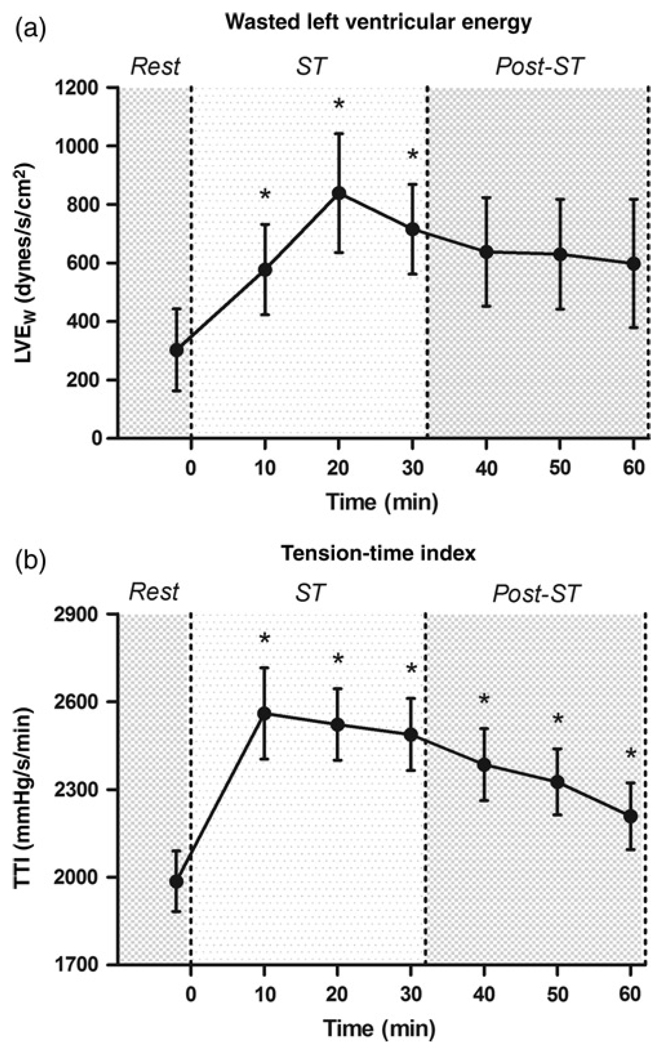
Figure 2 provides the mean ± SEM for indices of left ventricular work and myocardial oxygen consumption. LVEW was increased significantly from baseline at all time points during ST use, but returned to baseline values after ST. LVEW was elevated to 191%, 277% and 236% of baseline values at 10, 20 and 30 min, respectively. The TTI was significantly increased above baseline values at all time points. The TTI peaked at the 10-min time point at approximately 130% of baseline.
Figure 2.
Acute effects of smokeless tobacco on indices of left ventricular work. Values are mean ± SEM. *P < 0.05 versus baseline: (a) wasted left ventricular pressure energy (LVEW); (b) tension–time index (TTI). ST, smokeless tobacco
Discussion
The novel findings of this study are that central aortic blood pressure, LVEW and the TTI are transiently elevated due to an acute bout of ST use. Our results suggest that ST causes a significant transient alteration in cardiovascular hemodynamics both peripherally and centrally. HR, central aortic blood pressure and peripheral blood pressure values were all significantly elevated after one time ST use. LVEW, a measure of excess left ventricular work and oxygen demand, was elevated to twice baseline values during ST use. In addition, the TTI was significantly increased during ST use and remained elevated for 30 min post-ST use. These alterations in central aortic blood pressure and indices of left ventricular work are likely mediated by the systemic effects of nicotine and increasing sympathetic outflow.
An acute bout of ST use transiently elevates plasma concentrations of nicotine and its primary metabolite, cotinine. 3,5,22 These elevations in plasma levels of nicotine are associated with an increase in catecholamine secretion, particularly epinephrine from the adrenal medulla.5,23 – 26 When an exogenous sympathomimetic such as phenylephrine is administered to human subjects, there is a baroreflex-mediated decline in HR and muscle sympathetic nerve activity.5 However, in the present study, we observed a persistent parallel increase in HR, central aortic blood pressure and peripheral blood pressure despite activation of the baroreflex in response to ST. Our findings are consistent with Wolk et al.5 who previously reported that HR, peripheral blood pressure and muscle sympathetic nerve activity remain elevated during ST use. Therefore, the physiological response to ST is considerably different than that observed with sympathomimetics. The mechanism responsible for this discrepancy may be related to circulating plasma nicotine-mediated increases in sympathetic outflow prevailing over baroreflex-mediated effects.27 Nicotine-mediated increases in adrenal medulla catecholamine secretion as well as α-adrenergic-mediated vasoconstriction in the periphery likely contribute to the HR and pressor response observed in response to ST.23,25,27 Furthermore, animal studies have shown that there is an attenuation of endothelium-dependent vasodilation in muscular resistance arteries due to nicotine treatment.28 – 30 Similarly, ST has been shown to significantly impair flow mediated dilation of the brachial artery acutely following administration of one gram of ST in humans.31 Therefore, nicotine mediated sympathetic outflow and the acute attenuation of endothelium-dependent vasodilation may contribute to the persistently elevated HR and blood pressure response observed with a single dose of ST.
As a consequence of the changes in cardiovascular hemodynamics with an acute dose of ST, the timing and amplitude of aortic reflected pressure waves are altered. This is evidenced by our findings that AIx@75 was elevated to over 208% of baseline values at all measurement time points during ST use. Cardiac pacing studies have shown that AIx decreases approximately 5.6% for every 10 bpm increase in HR at supine rest.32 Rapid HRs decrease ED and shift the arrival of the aortic reflected pressure wave toward diastole, resulting in lower AIx values.19 In the present study, we observed a significant decrease in ED at all time points while using ST. However, in contrast to pacing32 and exercise studies,33 we observed no change in AIx values despite an average 10–13 bpm increase in HR. Therefore, Δtp must be decreasing due to an increase in pulse wave velocity and/or peripheral vasoconstriction, which may shift the primary reflecting site more proximal. Indeed, we observed a significant decline in Δtp while using ST, most likely due to sustained peripheral vascular resistance despite increasing HR.
As a result of the observed changes in the timing and amplitude of aortic pressure wave reflection, LVEW and the time–tension index were significantly elevated as a result of ST use (Figure 2). The TTI, another index of left ventricular work and myocardial oxygen demand,34 was persistently elevated for the 60- min duration of the study. Furthermore, LVEW was elevated at all time points during ST use. LVEW is the portion of the TTI curve attributed to earlier reflection of the pulse pressure wave (a shortening of Δtp in Figure 1) which increases central aortic pressure during systole and can be interpreted as the excess amount of energy expended by the left ventricle without a commensurate increase in flow.20 In the present study, LVEW was increased two-fold during ST use.
Although the effects of ST were transient, peripheral and central aortic blood pressures remained significantly elevated above baseline for the 60-min duration of the study. As these changes were observed in a cohort of chronic users, it is appropriate to conclude that a similar hypertensive stress would occur with each dose of ST. Participants in our study reported using ST, on average, 5–6 times per day. Therefore, these habitual users of ST would be subjected to a minimum of 5–6 h of elevated central and peripheral blood pressures per circadian cycle. Indeed, 24 h ambulatory brachial blood pressure values are significantly elevated in ST users compared with non-users.35 Over decades, repeated and/or persistent elevation of central aortic pressures secondary to ST use may confer increased cardiovascular risk.6 The Strong Heart Study has shown that non-invasively obtained central arterial pressure is more strongly related to cardiovascular outcomes and that a 10 mmHg increase in central aortic pressure is associated with a 15% increase in cardiovascular risk.6 In the present study, we observed an average increase in central aortic pressure of over 11 mmHg in the hour immediately following a single dose of ST.
One confounding factor in the literature regarding ST and cardiovascular risk is that two very different types of ST have been studied. A Swedish form of ST (i.e. snus) is prepared differently than the ST sold in the United States. Investigators examining the long-term effects of snus have found no evidence of increased risk for myocardial infarction or stroke with ST use.36 – 38 However, a large prospective study found that American ST use was significantly associated with mortality due to all-cause cardiovascular disease, coronary heart disease and stroke.39 American ST, but not snus, contains licorice, specifically glycyrrhetinic acid, which is an inhibitor of mineralocorticoid metabolism. Glycyrrhetinic acid may increase sodium retention and blood pressure in ST users in a dose-dependent manner and promote salt-sensitive hypertension.22,40 In addition to the effects of American ST on mineralocorticoid metabolism, the sodium content in all ST is of concern for development of hypertension and cardiovascular risk. In a cohort of ST users using several different brands of ST ad libidum for 3–4 d, 24-h urine sodium excretion increased significantly by as much as 41 mEq/L.41 Further studies that focus on the role of mineralocorticoid metabolism are necessary to characterize risks associated with long-term American ST use in the development of hypertension and cardiovascular disease.
Study limitations
A potential limitation of our study design was the lack of plasma catecholamine and nicotine concentration measurements. We can only speculate that humoral catecholamine responses and plasma nicotine levels would mimic previous studies. Indeed, the HR and peripheral blood pressure responses observed in this study are in agreement with previous ST studies.2 – 5,22 We also did not measure glycyrrhetinic acid or sodium levels in our subjects. However, alteration of blood pressure via sodium retention and the renin–angiotensin–aldosterone system is outside the timeframe examined in this study.
Conclusion
Central aortic pressures are transiently elevated in response to one time use of ST. As a result of central aortic pressure augmentation, LVEW and the TTI are significantly increased. These alterations in central pressure dynamics indicate an acute increase in left ventricular work and myocardial oxygen demand due to a dose of ST. The change in wave reflection characteristics, and ultimately central aortic pressure, is likely mediated by the systemic effects of nicotine in ST increasing sympathetic outflow while maintaining peripheral vascular resistance. Chronic users subjected to decades of elevated central pressures and left ventricular work may be exposed to increased risk of cardiovascular disease, as central aortic pressures are even more strongly related to cardiovascular outcomes than peripheral blood pressures.
ACKNOWLEDGEMENTS
No grant, contract or industrial support was provided for this paper.
Footnotes
Author contributions: All authors participated in the design, interpretation of the studies, analysis of the data and review of the manuscript. JSM, DTB and RWB all contributed to the formulation and design of the current study. JSM collected all measurements. JSM and ANG were responsible for all aspects of the data analysis. JSM, DTB, ANG and RWB collectively interpreted the results of the experiment as well as participated in the review of manuscript drafts. JSM was solely responsible for writing the manuscript. All persons who contributed to this study in any way are listed as authors.
REFERENCES
- 1.Rodu B, Cole P. Smokeless tobacco use among men in the United States 2000 and 2005. J Oral Pathol Med. 2009;38:545–550. doi: 10.1111/j.1600-0714.2009.00780.x. [DOI] [PubMed] [Google Scholar]
- 2.Benowitz NL, Porchet H, Sheiner L, Jacob P., III Nicotine absorption and cardiovascular effects with smokeless tobacco use: comparison with cigarettes and nicotine gum. Clin Pharmacol Ther. 1988;44:23–28. doi: 10.1038/clpt.1988.107. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch JM, Hedner J, Wernstedt L, Lundberg J, Hedner T. Hemodynamic effects of the use of oral snuff. Clin Pharmacol Ther. 1992;52:394–401. doi: 10.1038/clpt.1992.161. [DOI] [PubMed] [Google Scholar]
- 4.Squires WG, Jr, Brandon TA, Zinkgraf S, Bonds D, Hartung GH, Murray T, Jackson AS, Miller RR. Hemodynamic effects of oral smokeless tobacco in dogs and young adults. Prev Med. 1984;13:195–206. doi: 10.1016/0091-7435(84)90051-3. [DOI] [PubMed] [Google Scholar]
- 5.Wolk R, Shamsuzzaman AS, Svatikova A, Huyber CM, Huck C, Narkiewicz K, Somers VK. Hemodynamic and autonomic effects of smokeless tobacco in healthy young men. J Am Coll Cardiol. 2005;45:910–914. doi: 10.1016/j.jacc.2004.11.056. [DOI] [PubMed] [Google Scholar]
- 6.Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, Ali T, Umans JG, Howard BV. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension. 2007;50:197–203. doi: 10.1161/HYPERTENSIONAHA.107.089078. [DOI] [PubMed] [Google Scholar]
- 7.Wang KL, Cheng HM, Chuang SY, Spurgeon HA, Ting CT, Lakatta EG, Yin FC, Chou P, Chen CH. Central or peripheral systolic or pulse pressure: which best relates to target organs and future mortality? J Hypertens. 2009;27:461–467. doi: 10.1097/hjh.0b013e3283220ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams B, Lacy PS. Central aortic pressure and clinical outcomes. J Hypertens. 2009;27:1123–1125. doi: 10.1097/HJH.0b013e32832b6566. [DOI] [PubMed] [Google Scholar]
- 9.Nichols WW. Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. Am J Hypertens. 2005;18:3S–10S. doi: 10.1016/j.amjhyper.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Pauca AL, O’Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension. 2001;38:932–937. doi: 10.1161/hy1001.096106. [DOI] [PubMed] [Google Scholar]
- 11.Kroeker EJ, Wood EH. Comparison of simultaneously recorded central and peripheral arterial pressure pulses during rest, exercise and tilted position in man. Circ Res. 1955;3:623–632. doi: 10.1161/01.res.3.6.623. [DOI] [PubMed] [Google Scholar]
- 12.Nichols WW, O’Rourke MF. McDonald’s Blood Flow in Arteries: Theoretical Experimental and Clinical Principles. London: Hodder Arnold Publishing; 2005. [Google Scholar]
- 13.Chen CH, Nevo E, Fetics B, Pak PH, Yin FC, Maughan WL, Kass DA. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation. 1997;95:1827–1836. doi: 10.1161/01.cir.95.7.1827. [DOI] [PubMed] [Google Scholar]
- 14.Gallagher D, Adji A, O’Rourke MF. Validation of the transfer function technique for generating central from peripheral upper limb pressure waveform. Am J Hypertens. 2004;17:1059–1067. doi: 10.1016/j.amjhyper.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 15.Wilkinson IB, Fuchs SA, Jansen IM, Spratt JC, Murray GD, Cockcroft JR, Webb DJ. Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens. 1998;16:2079–2084. doi: 10.1097/00004872-199816121-00033. [DOI] [PubMed] [Google Scholar]
- 16.Casey DP, Pierce GL, Nichols WW, Braith RW. Measurement of pulse wave velocity and augmentation index is reproducible in young, healthy men (abstract) Med Sci Sport Exerc. 2006;38 [Google Scholar]
- 17.Nichols WW, Singh BM. Augmentation index as a measure of peripheral vascular disease state. Curr Opin Cardiol. 2002;17:543–551. doi: 10.1097/00001573-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Murgo JP, Westerhof N, Giolma JP, Altobelli SA. Aortic input impedance in normal man: relationship to pressure wave forms. Circulation. 1980;62:105–116. doi: 10.1161/01.cir.62.1.105. [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol. 2000;525(Part 1):263–270. doi: 10.1111/j.1469-7793.2000.t01-1-00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashimoto J, Nichols WW, O’Rourke MF, Imai Y. Association between wasted pressure effort and left ventricular hypertrophy in hypertension: influence of arterial wave reflection. Am J Hypertens. 2008;21:329–333. doi: 10.1038/ajh.2007.49. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention (CDC) Determination of nicotine, pH, and moisture content of six U.S. commercial moist snuff products –Florida, January–February 1999. MMWR Morb Mortal Wkly Rep. 1999;48:398–401. [PubMed] [Google Scholar]
- 22.Benowitz NL. Systemic absorption and effects of nicotine from smokeless tobacco. Adv Dent Res. 1997;11:336–341. doi: 10.1177/08959374970110030501. [DOI] [PubMed] [Google Scholar]
- 23.Van Slyke CB, Larson PS. Observations on the role of the adrenal medulla in the blood pressure response to nicotine. J Pharmacol Exp Ther. 1950;98:400–404. [PubMed] [Google Scholar]
- 24.Narkiewicz K, van de Borne PJ, Hausberg M, Cooley RL, Winniford MD, Davison DE, Somers VK. Cigarette smoking increases sympathetic outflow in humans. Circulation. 1998;98:528–534. doi: 10.1161/01.cir.98.6.528. [DOI] [PubMed] [Google Scholar]
- 25.Cryer PE, Haymond MW, Santiago JV, Shah SD. Norepinephrine and epinephrine release and adrenergic mediation of smoking-associated hemodynamic and metabolic events. N Engl J Med. 1976;295:573–577. doi: 10.1056/NEJM197609092951101. [DOI] [PubMed] [Google Scholar]
- 26.Benowitz NL, Jacob P., III Intravenous nicotine replacement suppresses nicotine intake from cigarette smoking. J Pharmacol Exp Ther. 1990;254:1000–1005. [PubMed] [Google Scholar]
- 27.Marano G, Ramirez A, Mori I, Ferrari AU. Sympathectomy inhibits the vasoactive effects of nicotine in conscious rats. Cardiovasc Res. 1999;42:201–205. doi: 10.1016/s0008-6363(98)00326-5. [DOI] [PubMed] [Google Scholar]
- 28.Mayhan WG, Patel KP. Effect of nicotine on endothelium-dependent arteriolar dilatation in vivo. Am J Physiol. 1997;272:H2337–H2342. doi: 10.1152/ajpheart.1997.272.5.H2337. [DOI] [PubMed] [Google Scholar]
- 29.Mayhan WG, Sharpe GM. Superoxide dismutase restores endothelium-dependent arteriolar dilatation during acute infusion of nicotine. J Appl Physiol. 1998;85:1292–1298. doi: 10.1152/jappl.1998.85.4.1292. [DOI] [PubMed] [Google Scholar]
- 30.Mayhan WG, Sharpe GM. Chronic exposure to nicotine alters endothelium-dependent arteriolar dilatation: effect of superoxide dismutase. J Appl Physiol. 1999;86:1126–1134. doi: 10.1152/jappl.1999.86.4.1126. [DOI] [PubMed] [Google Scholar]
- 31.Rohani M, Agewall S. Oral snuff impairs endothelial function in healthy snuff users. J Intern Med. 2004;255:379–383. doi: 10.1046/j.1365-2796.2003.01279.x. [DOI] [PubMed] [Google Scholar]
- 32.Wilkinson IB, Mohammad NH, Tyrrell S, Hall IR, Webb DJ, Paul VE, Levy T, Cockcroft JR. Heart rate dependency of pulse pressure amplification and arterial stiffness. Am J Hypertens. 2002;15:24–30. doi: 10.1016/s0895-7061(01)02252-x. [DOI] [PubMed] [Google Scholar]
- 33.Casey DP, Nichols WW, Braith RW. Impact of aging on central pressure wave reflection characteristics during exercise. Am J Hypertens. 2008;21:419–424. doi: 10.1038/ajh.2007.74. [DOI] [PubMed] [Google Scholar]
- 34.Sarnoff SJ, Braunwald E, Welch GH, Jr, Case RB, Stainsby WN, Macruz R. Hemodynamic determinants of oxygen consumption of the heart with special reference to the tension–time index. Am J Physiol. 1958;192:148–156. doi: 10.1152/ajplegacy.1957.192.1.148. [DOI] [PubMed] [Google Scholar]
- 35.Bolinder G, de Faire U. Ambulatory 24-h blood pressure monitoring in healthy, middle-aged smokeless tobacco users, smokers, and nontobacco users. Am J Hypertens. 1998;11:1153–1163. doi: 10.1016/s0895-7061(98)00137-x. [DOI] [PubMed] [Google Scholar]
- 36.Hergens MP, Ahlbom A, Andersson T, Pershagen G. Swedish moist snuff and myocardial infarction among men. Epidemiology. 2005;16:12–16. doi: 10.1097/01.ede.0000147108.92895.ba. [DOI] [PubMed] [Google Scholar]
- 37.Hergens MP, Alfredsson L, Bolinder G, Lambe M, Pershagen G, Ye W. Long-term use of Swedish moist snuff and the risk of myocardial infarction amongst men. J Intern Med. 2007;262:351–359. doi: 10.1111/j.1365-2796.2007.01816.x. [DOI] [PubMed] [Google Scholar]
- 38.Hergens MP, Lambe M, Pershagen G, Terent A, Ye W. Smokeless tobacco and the risk of stroke. Epidemiology. 2008;19:794–799. doi: 10.1097/EDE.0b013e3181878b33. [DOI] [PubMed] [Google Scholar]
- 39.Henley SJ, Thun MJ, Connell C, Calle EE. Two large prospective studies of mortality among men who use snuff or chewing tobacco (United States) Cancer Causes Control. 2005;16:347–358. doi: 10.1007/s10552-004-5519-6. [DOI] [PubMed] [Google Scholar]
- 40.Schambelan M. Licorice ingestion and blood pressure regulating hormones. Steroids. 1994;59:127–130. doi: 10.1016/0039-128x(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 41.Benowitz NL, Jacob P, III, Yu L. Daily use of smokeless tobacco: systemic effects. Ann Intern Med. 1989;111:112–116. doi: 10.7326/0003-4819-111-2-112. [DOI] [PubMed] [Google Scholar]