Abstract
Background: There are currently no effective therapies for the treatment of ovarian cancer ascites fluid (OCAF). H19 is an RNA oncofetal gene that is present at high levels in human cancer tissues (ovarian cancer and OCAF among them), while existing at a nearly undetectable level in the surrounding normal tissue. There is evidence for a synergistic effect in cell cytotoxicity mediated by TNFα and diphtheria toxin in sensitive and resistant human ovarian tumor cell line. Thus, we tested the cytotoxic effect of TNF-α cytokine, together with the diphtheria toxin, in the therapy of ovarian cancer. Methods: The therapeutic potential of toxin vectors carrying the DT-A gene alone (pH19-DTA), or in combination with the TNF-α gene (pH19-TNF-DTA), driven by H19 regulatory sequences were tested in ovarian carcinoma cell lines and in a heterotopic ovarian cancer model. Results: The toxin vectors showed a high killing capacity when transfected into different ovarian cancer cell lines. In addition, intratumoral injection of the toxin vector into ectopically developed tumors caused 40% inhibition of tumor growth. The killing effect after injection of pH19-TNF-DTA plasmid into ectopically developed tumors was significantly higher than that showed by the pH19-DTA plasmid alone, particularly in diphtheria toxin and TNF resistant tumors. Conclusions: These observations may be the first step towards a major breakthrough in the treatment of human ovarian cancer. It should enable us to identify likely non-responders in advance, and to treat patients who are resistant to all known therapies, thereby avoiding treatment failure coupled with unnecessary suffering and cost.
Keywords: H19, TNF, DT-A, IRES, ascites, ovarian cancer
Introduction
Epithelial ovarian cancer (EOC) is the second most common gynecologic cancer, with an estimated 22,000 new cases and 15,000 deaths per year in the United States [http://www.cancer.gov/cancertopics/types/ovarian].
The median age of patients with ovarian cancer is 60 years old, and the average lifetime risk for the development of EOC is about 1 in 70 [1], with an overall five year survival rate not exceeding 35% [1].
EOC patients frequently have involvement of the pelvic and retroperitoneal lymph nodes as well [2,3]. The standard primary treatment of patients with advanced stage EOC is cytoreductive surgery followed by platinum and taxane doublet chemotherapy. Despite this aggressive approach, there is a high rate of recurrence. Although discovery of several other active non-platinum cytotoxic agents has improved outcome [4], long-term survival rates are still disappointing and most women will die as a result of their disease. Drug resistance and lack of specificity to mechanisms of disease formation and progression have limited success of traditional chemotherapy. Thus, novel targeted therapies are extensively explored in order to achieve improved long-term control with lower toxicity.
An attractive approach to human cancer gene therapy is to exploit the genetic and epigenetic alterations in a cancer for targeting the expression of toxic genes. Indeed, several attempts have been made in this direction, by employing promoters of the telomerase (hTERT) gene or promoters induced by hypoxia-inducible factors [4, 5].
We developed a novel therapy approach based on patient-specific gene expression profiles in each cancer, tailored to individual patients, by using selected transcriptional regulatory sequences for DNA-based therapy. This enables the directing of a tumor-selective expression of a toxin, delivered by a non-viral vector. Non-viral vectors appear promising due to their potential to overcome the main disadvantage of adenovi-ral vectors, causing immune responses directed against adenovirus proteins, and limit their ability to be administered iteratively.
Based on earlier studies from our group and others, transcriptional regulatory sequences of the H19 gene have emerged as candidates for cancer gene therapy. H19 is a paternally-imprinted, maternally expressed, oncofetal gene that encodes a RNA acting as “riboregulator” that has no protein product [6]. It is expressed at substantial levels in different human tumor types, but is only marginally or not at all expressed in normal adult tissues [7, 8]. Its precise function has been debated. Recent data suggested a role for H19 in promoting cancer progression, angiogenesis and metastasis [9, 10, 11]The list of cancers in which H19 gene expression is known to be elevated compared to normal tissue is still growing. Among those cancers are bladder cancer [7], hepatocellular carcinoma [8], choriocarcinoma [12], colorectal cancer [13], and lung carcinoma [14]. Detection of H19 expression in epithelial ovarian cancer using in-situ hybridization technique (ISH) revealed that H19 is expressed in the majority of serous epithelial tumors as compared to the normal tissue [15]. Moreover, H19 gene expression was detected in 90% of patients with ovarian cancer ascites fluid (OCAF) as determined by ISH [16] .As a toxic gene, we chose the diphtheria toxin A chain (DT-A), which has suitable properties for achieving efficacious cancer cell killing [17]. DT-A peptide catalyzes ADP-ribosylation at the dipthamide residue of the cellular translation elongation factor 2 (eEF-2), inhibiting protein synthesis and causing cell death [18].
It was previously demonstrated that the DTA-H19 construct was able to kill tumor cells both in vitro and in vivo in animal models for bladder cancer ,colorectal liver metastases[19]and ovarian cancer [20]. The safety, tolerability and preliminary efficacy of the therapuetic vector DTA-H19 was tested successfully in a phase 1/2a clinical trial for the treatment of superficial transitional cell carcinoma (TCC) of the bladder [21,22].
Because it is unlikely that gene transfer reaches every cancer cell, a ‘bystander’ effect might be used to induce the DNA based therapy approach. An interesting and novel approach for this purpose is cytokine DNA based therapy. In particular, use of the human tumor necrosis factor (TNF-α) seems an attractive strategy. Interestingly, some publications showed a synergistic effect in cell cytotoxicity mediated by TNF-α and diphtheria toxin in two types of human ovarian tumor cell lines: sensitive or resistant to both diphtheria toxin and TNF-α [23]. Previous reports showed that the combination of diphtheria toxin and the TNF proteins resulted in syner-gistic cytotoxic activity against human ovarian or renal cell carcinoma cell lines [24, 25].
However, systemic delivery of the TNF alpha protein had a clinically limited success due to severe dose limiting toxic effects [26]. This limitation can be overcome by the use of a gene delivery approach, combined with a tumor specific promoter to express TNF-α in the tumor tissue.
We investigated the therapeutic potential of a plasmid carrying in addition to DTA, the gene for human TNF alpha (h TNF-α). The expression of these genes is under control of the H19 promoter, with the construct carrying a viral IRES sequence 3′ of the TNF carrying sequence (pH19-TNF-IRES-DT-A). Here we propose a new approach in which the combined expression of cytotoxic and cytokine genes should enhance the cancer cell killing effect while preventing the potential toxic effect caused by systemic delivery of cytokines such as TNF-α.
This approach which is based on patient-specific gene expression profiles in ovarian cancer tailored to individual patients by using selected transcriptional regulatory sequences for DNA-based therapy, should enable us to identify likely non-responders in advance, and to treat patients who are resistant to all known therapies, thereby avoiding treatment failures with unnecessary suffering and costs.
Materials and methods
Cell culture
The human ovarian carcinoma cell lines (ES-2, SK-OV3, TOV-112D) used in this study were obtained from the American type culture collection (ATCC). Cells were maintained in DMEM-F12 (1:1) medium containing 10% fetal calf serum. For OV-CAR3, 0.01 mg/ml of human insulin was added to the culture medium.
Human ascites fluid
The Ascitic fluid samples from the peritoneum of patients suffering from ovarian cancer were submitted to this study following approval of the Israeli Ethics Committee. Samples were kindly given to us from the Division of Gynecologic Oncology, Wolfson Medical Center, and from the Department of Gynecology, Hadassah Medical Center. Cells were isolated by using centrifugation of a 15%, 30% and 60% percoll gradient. Cells from the 30% and 60% percoll gradient were collected and grown for further analysis (IHC,ISH PCRTransfection experiments etc).
Plasmids and constructs
All the luciferase gene reporter constructs were built from the pGL3 basic (Luc-1) vector (Promega, Madison, WI, USA) which lacks both promoter and enhancer sequences. The construct Luc-H19 contains the reporter gene under the control of the human H19 promoter region from nucleotide −818 to +14 was prepared as described [27].
The Luc-H19 plasmid was digested with Xba I and Nco I and the insert of the luciferase gene (luc) was replaced by the diphtheria toxin A-chain (DT-A) coding region to yield the DTA-H19 construct. Large-scale preparations of the plas-mids were performed using the EndoFree Plasmid Mega kit (Quiagen, Germany). All plasmids were modified by replacing the Amp res gene with the Kan res gene.
The reporter luciferases, the DT-A toxin or the human TNF-α genes were cloned by GENEART™into the expression vectors and their expression was regulated by the activity of the cloned hH19 promoter in different cell lines analyzed.
The pH19-TNF-IRES-DTA plasmid was prepared by GENEART™. The transcription of the DTA and the TNF genes were under the control of the H19 promoter, while the construct carries a viral IRES sequence (from the ECMV virus) 3′ of the TNF carrying sequence (pH19-TNF-IRES-DTA). This IRES construct is 619 bp long and responsible for the synthesis of DTA from the m-RNA transcript.
In vitro transfection and luciferase assay
A total of l*10^5 cells were plated in a twelve-well Nunc multidish (30 mm). Transient trans-fections were carried out using the JetPEI cation ic polymer transfection reagent (mean molecular weight of 22 kDa; Polyplus, Illkirsh, France). The transfection was carried out according to the manufacturer's instructions using 2 μg of DNA and 3 μl of JetPEI solution to obtain a N/P ratio of 5. Transfection experiments were stopped after 48 h and reporter gene activity was assessed. Luciferase activity was measured using the kit ‘Luciferase Assay System’ E-1500 (Promega, Madison, USA). Light output was detected using a Lumac Biocounter apparatus. Protein content was measured by the Bio-Rad (Hercules, CA, USA) protein assay reagent, and the results were expressed as light units/|jg protein. LucSV40 (Luc-4) was used as a reference for maximal luciferase activity, as it contains the SV40 promoter and enhancer, while Luc-1 lacking regulatory sequences was used as a negative control to determine the basal nonspecific luciferase expression, which was found to be negligible. All experiments were carried out in triplicate and the results represent the mean value and standard error was calculated.
In vitro activity and specificity of the regulatory sequences (cell killing assay)
Cells were cotransfected using 2 μg of the reporter vector Luc-4 and the indicated amounts of DTA-H19, TNF-H19 or pH19-TNF-IRES-DTA expression vectors using the transfection reagent JetPEI as described above. Cells were also transfected by 2 μg of Luc-4 alone. In vitro activity of the regulatory sequences was determined by calculating the percent decrease in the luc activity in the cotransfected cells compared to that obtained in the cells transfected with Luc-SV40only
RNA isolation and cDNA synthesis
Total RNA was extracted from cell lines or tissues, using the RNA STAT-60™ using total RNA/mRNA isolation reagent (Tel-Test, Inc., Friendswood, TX, USA), according to the manufacturer's instructions. The RNA was treated with RNase-free DNase I (Roche Diagnostics GmbH, Mannheim, Germany) to eliminate any contaminating DNA. The cDNA was synthesized from 2 μg total RNA in 20 μl reaction volume as described [28].
Determination of the level of RNA products of the H19, DT-A and luc genes
The PCR reactions were carried out in 25 μl volumes in the presence of 6 ng/μl of each of the forward and the reverse primers using 0.05 units/μl of Taq polymerase (TaKaRa Biomedi-cals, Japan) according to the manufacturer's instructions.
The primer sequences used to amplify the human H19 transcript was (5_-ACTGGAGACTAGG GAGGTCTCTAGCA) upstream and (5_-GCTGTGT GGGTCTGCTCTTTCAAGATG) downstream. The polymerase chain reaction (PCR) was carried out for 30 cycles (98 °C for 15 sec, 58 °C for 30 sec, and 72°C for 40 sec and finally 72°C for 5 min).
The PCR analysis for DT-A amplification was carried out as described [22]. The primer sequences used to amplify the luc transcript were (5-GAGGCGAACTGTGTGTGAGA) upstream and (5-TTTTCCGTCATCGTCTTTCC) downstream. The PCR was carried out for 34 cycles (94 °C for 5min, 94°C for 1 min, 56 °C for 30 sec, and 72°C for 30 sec and 72°C for 5 min). The primer sequences used to amplify the TNF transcript were (5- CGCCACCACGCTCTTCTG) upstream and (5- GCCATTGGCCAGGAGGGC) downstream. The PCR was carried out for 30 cycles (94 °C for 5 min, 94°C for 30 min, 53 °C for 30 sec, and 72°C for 30 sec and 72°C for 5 min). The integrity of the cDNA was assayed by RT-PCR analysis using the histone variant, H3.3 or GAPDH as positive control.
Animal heterotopic model for In-vivo DNA based drug
Heterotopic model- Confluent ES-2 or SK-OV3 human ovarian carcinoma cells were trypsinized to a single cell suspension and resuspended in 2*106 cells /100 μl PBS, then subcutaneously injected into the back of 6-8 weeks old CD-1 or athymic female nude mice. 10 days after cell inoculation, the developed tumors were measured in two dimensions and subjected to different treatments. Intratumoral injections of 25 μg of one of the following toxin construct pH19-DTA - or the cytokine vector pH19-TNF-, or the double plasmid pH19-TNF-IRES-DTA or the reporter vector pH19-Luc- (control group) were performed at days 10, 12, 14 and 16 after cell inoculation. Tumor dimensions were measured, and the tumor volume was calculated according to the formula (width)2 × length × 0.5. The animals were sacrificed 3 days after the last injection, the tumors were excised and their ex-vivo weight and volume were measured. Samples of the tumors were fixed in 4% buffered formaldehyde and processed for histological examination for evidence of necrosis and persistent tumor. All of the surgical procedures and the animal care were approved by the local committee for animal welfare.
Statistical analysis
Transfections and Animal data analysis was conducted using the T test formula. Results of each treatment were compared to the control group (pH19-Luc) .P < 0.05 was considered statistically significant.
Results
We determined the cytotoxic effect of a vector carrying both the TNF and DT-A genes in ovarian cancer cell lines and in the nude mice heterotopic model by a plasmid construct in which the transcription of both the TNF and DTA genes is under control of the H19 promoter. This construct carries a viral IRES sequence 3′ to the TNF sequence followed by the DTA sequence (pH19-TNF-IRES-DTA). A number of different virus families and cellular RNAs can initiate translation via an internal ribosome entry segment (IRES), an RNA structure that directly recruits the 40S ribosomal subunits in a cap and 5’ end independent fashion [29].
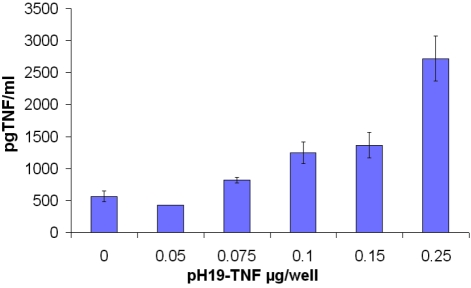
TNF is secreted from the cell and binds to its receptor. As a consequence of this binding, a broad range of cell activities are induced. The amount of TNF-α which was expressed and secreted from the cells after transfection with the pH19-TNF plasmid, was determined by ELISA assay, forty eight hours after transfection of TOV -122D cells with the pH19-TNF α construct, the supernatant was collected and assayed Figure 1
Figure 1.
The amount of TNF-α protein secreted after transfection of TOV-122D cells with pH19 -TNF plas-mid determined by ELISA. Medium from TOV-122D transfected cell was collected after 48h. TNF-α protein level was analyzed in the supernatant using a TNF-specific ELISA. The amount of TNF-α protein was normalized to a standard curve with increasing concentrations of TNF-α.
Increased levels of the TNF protein secreted from cells transfected with the pH19-TNF plas-mid are shown. This increase is correlated with the increased amount of plasmid used in the transfection of the TOV-122D cell lines. 2700 pg/ml of TNF-α was detected after transfection of TOV-122D cells with 0.25 μg plasmid /well. A small amount of TNF was detected in the cells transfected with the control plasmid pH19-Luc (indicated as 0 in Figure 1). The results described in Figure 1 indicate that the H19 promoter is able to drive the expression of the TNF-a gene and that the TNF-α protein is secreted to the culture medium.
We could not detect TNF in the supernatant from SK-OV3 cells after transfection under the same conditions (data not shown).
We determined the cytotoxic effect of a vector carrying both the TNF and DTA genes in ovarian cancer cell lines (ES-2, TOV-122D, SK-OV3) by using a plasmid in which the transcription of both the TNF and DTA genes is under the control of the H19 promoter.
This construct carries a viral IRES sequence 3′ to the TNF sequence followed by the DTA sequence (pH19-TNF-IRES-DTA). The transcription of both TNF and DTA genes is under the control of the H19 regulatory sequences while the synthesis mechanism of the TNF or DTA peptides is different: the TNF peptide is synthesized by a cap dependent mechanism and the synthesis of the DTA peptide is under the control of the IRES sequence.
The cytotoxic effect of pH19-TNF-IRES-DTA plasmid was determined by the luciferase assay and was compared to that of cells treated with a plasmid carrying either the DTA or TNF under the control of the H19 promoter.
Cells were cotransfected with 2 ug/well of LucSV40 and the indicated concentrations of pH19-DTA, pH19-TNF or pH19-TNF-IRES-DTA plasmids. Luciferase activity was determined and compared to that of cells transfected with Luc-SV40 alone (Figure 2).
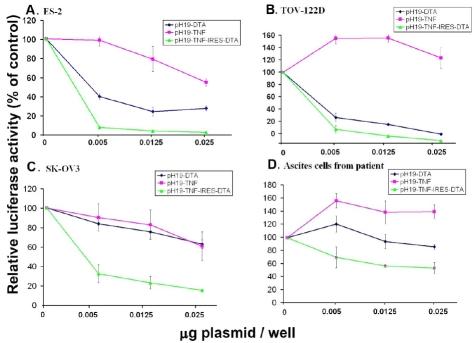
Figure 2.
The reduction of luciferase activity in ovarian cancer cell lines: ES-2, TOV-122D, SK-OV3 and in ascites cells of a patient due to co-transfection with the pH19-DTA, pH19-TNF or pH19-TNF-IRES-DTA vectors. The killing potential of each of the following vectors: pH19-DTA (blue), pH19-TNF (pink) and pH19-TNF-IRES-DTA (green) in (A) ES-2, (B) TOV-122D, (C) SK-OV3, (D) ascites cells isolated from a patient was measured as a reduction of luciferase activity induced by Luc-SV40. Cells were co-transfected with 2 ug/well of Luc-SV40 and the indicated concentrations of pH19 -DTA, pH19-TNFα, or pH19-TNF-IRES-DTA.
Figure 2A and B shows reduction of 96% in luciferase activity at a concentration of 0.025ug plasmid/well after transfection with the pH19-TNF-IRES-DTA plasmid in both ES-2 and TOV-122D ovarian carcinoma cells (IC50 values are 0.0025 and 0.003ug plasmid/well for ES-2 and TOV-122D respectively). This killing effect is higher than that showed by the pH19-DTA plasmid alone (75% and 85% respectively, with IC50 values of 0.004 ug plasmid/well for both cell lines,), at the same concentration (p<0.01). A very high killing effect in ES-2 cells was observed using only 0.005 ug plasmid/well of the combined plasmid as compared to each of the pH19-DTAor pH19-TNF plasmids (Figure 2A).
The DTA and TNF resistant ovarian carcinoma cells (SK-OV3) were efficiently killed (80% reduction in luciferase activity and IC50 value of 0.004 ug plasmid/well), using the pH19-TNF-IRES-DTA plasmid (Figure 2C), while very low cytotoxic effect (35% in luciferase activity) is detected using the pH19-DTA plasmid (p<0.03) at a concentration of 0.025 ug plasmid/well.
The reduction in luciferase activity using the pH19-TNF plasmid in the ES-2 and SK-OV3 cells was 45% & 36% respectively (Figure 2 A, C), however an increase in luciferase activity was noted in both the TOV-122D cells and the as-cites cells from patient . This is in accordance with previous data on MDAH 2774 and TOV-112D ovarian cancer cell line [30, 31].
It is interesting to note that the ovarian cancer cells which were isolated from patient's ascites fluid were also DTA and TNF resistant (Figure 2D). Although very little cytotoxic effect was me-sured after transfection with pH19-DTA (16%) plasmid alone, a significant reduction of 52% (IC50 value of 0.0125ug plasmid/well) was noted in luciferease activity due to transfection with 0.025 ug of the pH19-TNF-IRES-DTA plasmid (p<0.01).
We determined whether the anti cell proliferation effect shown in Figure 2 was due to the activity of both TNF and DTA. SK-OV3 cells were transfected with Luc-SV40 (2 μg/well) and the indicated concentrations of pH19-DTA and pH19-TNF plasm ids. Luciferase activity was determined and compared to that of cells transfected with Luc-SV40 alone (Figure 3).
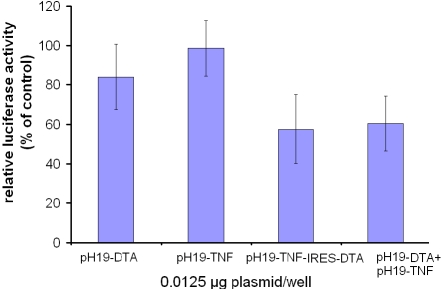
Figure 3.
The reduction of luciferase activity in SK-OV3 cell line due to co-transfection with the pH19-DTA and pH19-TNF vectors, compared with transfection of the pH19-TNF-IRES-DTA vector. The killing potential of the pH19-DTA and pH19-TNF vectors or pH19-TNF-IRES-DTA was measured as a reduction of Luc-SV40 activity. Cells were transfected with Luc-SV40 (2 μg/well) and 0.0125 μg/well of each of the following vectors:pH19-DTA, pH19-TNF, orpH19-TNF -IRES-DTA, co-transfected with pH19-DTA and pH19-TNF 0.0125 μg/well of each plasmid). Cells were also transfected with LucSV40 alone. Transfection experiments were stopped after 48 hours and reporter gene activity was assessed.
Figure 3 shows 43% reduction in luciferase activity in cells transfected with 0.0125 μg/well of the pH19-TNF-IRES-DTA plasmid (p<0.005). Luciferase activity in cells that were transfected with both 0.0125 μg/well of the pH19-DTAand 0.0125 μg/well of the pH19-TNF, showed a similar reduction of luciferase activity (40%), (p<0.03). Thus, the reduction in luciferase activity is the result of the activity of both TNF and DTA.
It is important to rule out a possible false positive result by using the combined plasmid. We reversed the TNF sequence in the pH19-TNF-IRES-DTA plasmid to eliminate the expression of TNF (pH19-TNFrev-IRES-DTA). This plasmid was then co-transfected into SK-OV3 and ES-2 cells with Luc-SV40 and luciferase activity was measured, and compared to that determined following transfectio with pH19-DTAor pH19-TNF-IRES -DTA plasmids respectively (Figure 4).
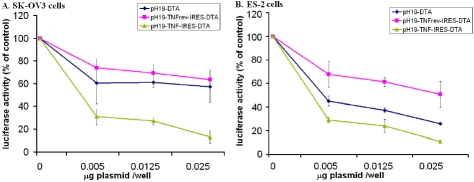
Figure 4.
The reduction of luciferase activity in SK-OV3 and ES-2 cell lines transfected with LucSV40 due to transfection with pH19-DTA, pH19-TNF-IRES-DTA or pH19-TNFrev-IRES-DTA vectors. The killing potential of the pH19-DTA and pH19-TNF-IRES-DTA or pH19-TNFrev-IRES-DTA was measured as a reduction of luciferase activity in SK-OV3 cell line (A) and in ES-2 cell line (B). Cells were transfected with 2 μg/well of Luc-SV40 and the indicated concentration of pH19-DTA, pH19-TNF-IRES-DTA, pH19-TNFrevDTA respectively. Cell lines were also transfected with Luc-SV40 alone. Transfection experiments were stopped after 48 hours and reporter gene activity was assessed. The luciferase activity in the co-transfected cells was compared to the luciferase activity of the cells transfected with the Luc-SV40 plas-mid alone.
Figure 4A and B shows that using 0.025 μg plasmid/well, the reduction of luciferase activity after transfection with the pH19-TNFrev-IRES-DTA plasmid was lower than that measured after transfection with pH19-DTA (33% & 39% respectively in SK-OV3 cells and 75% & 25% respectively in ES-2 cells) while much more efficient reduction (79% & 89% in SK-OV3 cells and in ES-2 cells, respectively ) was noticed after transfection with the pH19-TNF-IRES-DTA plasmid. These results indicate that the synergistic effect showed after transfection with the pH19-TNF-IRES-DTA plasmid is due to the activity of both TNF and DTA and not to an artifact resulting from the plasmid structure.
From the results in Figures 2, 3 and 4 it can also be concluded that the ECMVIRES construct inserted into the pH19-TNF-IRES-DTA plasmid was active and enabled the synthesis of the DTA peptide.
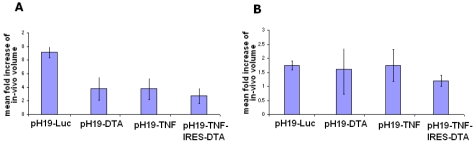
The ability of the pH19-TNF-IRES-DTA, pH19 - TNFα or pH19-DTA, to cause cancer cell killing and inhibit tumor growth in-vivo was analyzed. ES-2 or SK-OV-3 cells were subcutaneously injected into the dorsa of 6-7 weeks old athymic female mice. Measurable heterotopic tumors developed 10 days after cell inoculation. Mice were divided into 4 groups. Mice of each group were injected with one of the following plasmids: pH19-TNF-IRES-DTA, pH19 -TNFα, pH19-DTA or pH19-Luc (control) vectors. Tumors from each group of mice were directly injected with 25 μg plasmid/tumor in a two day interval for a total number of 4 injections. The size of the tumors was determined at the end of each treatment. The in-vivo fold increase of the tumor size is indicated in Figure 5.
Figure 5.
The effect of intratumoral injection of the pH19-TNF-IRES-DTA, pH19 -TNFα, pH19-DTA or pH19-Luc plasmid on the growth of subcutaneous ovarian tumors in nude mice. After tumors developed, mice were divided into 4 groups of 6 mice. Mice received 4 injections of 25 μg of pH19-TNF-IRES-DTA, pH19 -TNFα, pH19-DTA or pH19-Luc plasmids complexed with PEI within two day intervals. One day after the last treatment animals were sacrificed. The tumor dimensions were measured in vivo prior to the first treatment and immediately before sacrifice. The mean fold increase of the final volume compared to the initial volume in the treated tumors was calculated. A and B represents tumors developed by ES-2 or by SK-OV3 cells respectively.
Figure 5A shows that the mean fold increase after 4 injections of the pH19-TNF-IRES-DTA, pH19 -TNFα or pH19-DTA plasmids into the ES-2 subcutaneous tumors were much lower (70%, 59% and 59% of tumor growth inhibition, respectively) compared to the control group treated with 4 injections of the pH19-Luc plasmid (P<0.095).
No significant difference in the tumor growth was noticed between the pH19 -TNFα and pH19-DTA treated tumors, while a larger increase in tumor growth inhibition was noted in the pH19-TNF-IRES-DTA treated tumors.
Although no difference in the mean fold increase was detected after 4 injections of pH19-TNF or pH19-DTA into the SK-OV-3 subcutaneous tumors (Figure 5B), as compared to pH19-Luc treated tumors, 4 injections of the pH19-TNF-IRES-DTA plasmid were able to reduce the mean fold increase by 31% (P<0.0004).
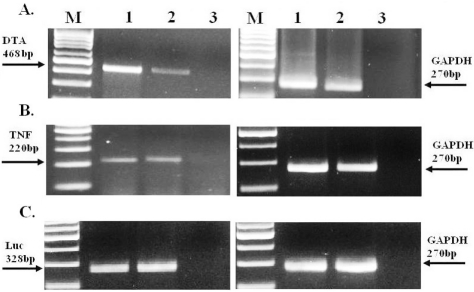
We analyzed the RNA level of DTA, TNF and Luciferase in the SK-OV3 tumors after intratumoral injection of 25 μg of pH19-DTA, pH19-TNF, pH19-TNF- IRES -DTA or the reporter vector pH19-Luc (as described above). Animals were sacrificed 48 h after plasmid injection; the tumors were excised and snap frozen. The levels of the DTA, TNF and Luc mRNAs in the total RNA extracted from the ovarian tumors were determined by RT-PCR (Figure 6).
Figure 6.
The relative RNA level of DTA, TNF and Luciferase after treatment of SK-OV3 subcutaneous tumors with pH19-DTA, pH19 -TNF-α, pH19-TNF-IRES-DTA or pH19-Luc plasmids determined by RT-PCR. 48h after the animals were treated with the plasmid; samples from the ovarian cancer subcutaneous tumors were excised and frozen immediately. 400 ng RNA from the tumors were used for the determination of DTA, TNF and Luc by RT-PCR reactions. A. Lane 1: DTA transcripts 48h after pH19-DTA plasmid injection, Lane 2: DTA transcripts 48h after pH19-TNF- IRES -DTA plasmid injection. B. Lane 1: TNF transcripts 48h after pH19-TNF plasmid injection, Lane 2: TNF transcripts 48h after pH19-TNF- IRES-DTA plasmid injection. C. Lane1-2: Luc transcripts 48h after pH19-Luc plasmid injection. The sizes of the PCR products are 468, 220, 328 and 270 bp for DTA, TNF, Luc and GAPDH internal control respectively. M-100 bp molecular weight marker.
Discussion
The present work shows the use of the regulatory sequences of the H19 gene for the development of DNA-based therapy for human ovarian cancer. The successful development of antitumor gene therapy depends on the use of a combinatorial approach aimed at targeted delivery and specific expression of effective antitumor agents. Various gene therapy strategies for the treatment of ovarian cancer are currently under development and aim towards maximal treatment efficacy and minimal adverse effects. In this study, a tumor-selective promoter was used in conjunction with both cytotoxic and cytokine genes to achieve targeted tumor cell destruction.
The choice of the Diphtheria toxin A-fragment (DTA) not only insures high killing activity but also has great advantage against any unintended toxicity to normal cells. Since only the cDNA coding for the A-fragment is expressed, the released DTA toxin from the lysed cells cannot enter neighboring cells in the absence of the DTB fragment, which was proven to promote specific binding of the toxin to a host cell receptor leading to the entrance of the toxin into the cell [32]. However, several malignant cell lines such as renal cell carcinoma and ovarian carcinoma cell lines are resistant to diphtheria toxin and therefore will not be affected by the pH19-DTA vector (refer to your published paper). Some interesting evidence for a synergistic effect in cell cytotoxicity mediated by TNFα and diphtheria toxin in sensitive and resistant human ovarian tumor cell lines, led us to test the combined use of TNF and DTA for selective killing of tumor cells [23-24].
Systemic delivery of the TNF-α protein has had limited success clinically because of severe dose limiting toxic effects. We overcame this limitation by constructing a plasmid in which TNF-α and DTA genes are expressed under the control of the H19 tumor specific promoter.
We introduced the regulatory sequences of the H19 gene which are active in cells from ovarian cancer ascites fluid (OCAF), to control DTA and/ or TNF expression. This significantly favors the specificity of the treatment with no or minimum cytotoxic activity against the surrounding normal tissue. Moreover, since H19 is highly expressed during cancer development, angiogenesis and metastases (Matouk et al, 2005), the killing effect of the pH19-DTA plasmid in H19 expressing tumor cells may also limit cancer progression and metastases [11].
The development of resistance of tumor cells to anti cancer drugs is one of the complex challenges facing cancer chemotherapy treatments. Ovarian carcinomas are particularly known in their ability to develop multi drug resistance (Modesitt SC et al, 2007). During this research we noticed that ovarian carcinoma cells such as SK-OV3 and ascites cells isolated from a patient can develop resistance to DTA and TNF (Figure 2C and D). To overcome this problem we designed the pH19-TNF-IRES-DTA construct in which the transcription of the plasmid is controlled by H19 regulatory sequences but the translation mechanism of the human TNF alpha cytokine and the cytotoxic DTA is cap dependent and IRES dependent respectively.
Significant killing effect of the pH19-TNF-IRES-DTA plasmid after transfection into all ovarian cancer cell lines tested was detected (Figure 2).
ES-2 and TOV-122D cells (Figure 2A and B, respectively), were efficiently killed by very low concentrations of the pH19-DTA plasmid (0.005 μg/well). Therefore, no significant increase in the killing effect of these cells by using the pH19-TNF-IRES-DTA was determined as compared to that obtained with the pH19-DTA plasmid. On the other hand, in the SK-OV3 cells and in the ascites cells isolated from a patient (Figure 2C and D), the killing effect caused by the pH19-TNF-IRES-DTA plasmid was much higher than that caused by the pH19-DTA plasmid alone at the same concentrations.
It is also important to note that even though an increase in luciferase activity was detected in TOV-122D cells and in the ascites cells isolated from a patient after co-transfection with the pH19-TNF plasmid (Figure 2B and D, respectively), a very significant reduction in luciferase activity was noticed after co-transfection with the pH19-TNF-IRES-DTA plasmid.
Increased killing effect of TNF in combination with other cytotoxic reagents such as cisplatin leading to enhanced cytotoxic effect in which supra-additive cytotoxicity and apoptosis via a strong bystander effect was reported [33].
Figure 3 shows that co-transfection in SK-OV3 cells of both pH19-TNF and pH19-DTA plasmids caused 40% reduction in luciferase activity which was similar to that caused by transfection of the pH19-TNF-IRES-DTA plasmid (43%). This indicates that the killing effect caused by the pH19-TNF-IRES-DTA plasmid is the result of the activity of both DTA and TNF and not an artifact caused by the plasmid backbone.
The co-transfection of pH19-TNF and pH19-DTA plasmids into the cell population (as shown in Figure 3) can result in one of the three following scenarios at the level of the single cell: first, the transfection of the pH19-DTA plasmid alone, which can cause low killing efficiency in the case of diphtheria toxin resistance cells (such as SK-OV3). Second, transfection of the pH19-TNF plasmid alone may lead to cell proliferation [34] as was shown in Figure 2B and D. Third; the co-transfection of both pH19-TNF and pH19-DTA may result in a more efficient cell killing. Hence, transfection of cancer cells by the pH19-TNF-IRES-DTA plasmid can assure the expression of both TNF and DTA in a transfected cell, leading to an increase in cell death indicating a significant advantage over simultaneous transfection of both pH19-TNF and pH19-DTA plasmids. The use of pH19-TNF-IRES-DTA plasmid ensures the effect of TNF and DTA in the same cell, avoiding the possibility of transfection of only one of the two plasmids into the target cell.
In addition, the results in Figure 4A shows that the reduction in luciferase activity caused following co-transfection of SK-OV3 cells with Luc-SV40 and the pH19-TNFrev-IRES-DTA construct was similar to that caused by the pH19-DTA plasmid while the reduction in luciferase activity was much higher after transfection with the pH19-TNF-IRES-DTA construct. The reduction in luciferase activity after transfection of ES-2 cells with the pH19-DTA plasmid was higher as compared to the reduction in luciferease activity after transfection with the pH19-TNFrev-IRES-DTA construct (Figure 4B). This may imply that the translation of DTA transcript under the control of the IRES sequence is less efficient than translation by the cap dependent mechanism.
Therefore, the results in Figure 2 strengthen our conclusion that TNF is expressed from the pH19 -TNF-IRES-DTA plasmid by cap dependent mechanism and that DTA is expressed by IRES dependent mechanism from the same plasmid.
We used the heterotopic animal model to evaluate the tumor growth inhibition of pH19-TNF-IRES-DTA plasmid treated tumors as compared to that of pH19-DTA or pH19- TNF plasmids treated tumors. The ES-2 cell line and the SK-OV3 (DTA and TNF resistance cell line) were used in the tumor formation. The results obtained in the in vivo experiments are shown in Figure 5. Growth inhibition of tumors induced by ES-2 cells was noticed after treatment with each one of the pH19-DTA, pH19-TNF or pH19-TNF-IRES-DTA plasmids as compared to the control group. The SK-OV3 tumors treated with the pH19-DTA or pH19- TNF plasmids showed no growth inhibition as compared to the control group, which is an expected result considering the resistance of these cells to either TNF or DTA. On the other hand, growth inhibition of SK-OV3 treated tumors was only noticed using the pH19-TNF-IRES-DTA plasmid demonstrating the advantage of using this plasmid as compared to the treatment with pH19-DTA or pH19- TNF plasmids alone. Recently, some studies have shown that the combined treatment of cell killing agents such as Rexin-G which is a retrovirus carrying the cycline G gene to block the cell cycle and Reximmune-C which incorporates the immune system stimulator GM-CSF gene may prevent recurrence of the tumor by activation of the patient's immune cells in the area of the residual tumor [35]. Ongoing research by Gordon and Hall showed that this combination of gene therapy kills cancer cells, thereby exposing new tumor antigens, while promoting a local immune response [36]. Similarly, TNF-α is known in its ability to stimulate the immune system. Hence, treatment of tumors with the pH19-TNF-IRES-DTA plasmid leading to the expression of both DTA and TNF genes, followed by the secretion of TNF protein from the cells to the area of residual tumor may lead to locally stimulation of the immune system.
In these experiments, we used nude/athymic mice, which have a genetic mutation that causes a deteriorated or absence of the thymus gland. Therefore, mature T lymphocytes cells cannot be generated. As a result, these mice have only partial immune response; they are unable to mount some types of immune responses. Thus, local stimulation of the immune response should be further investigated using immunocompetent mice. Development of mouse models representing human spontaneous ovarian cancer has been hampered by the lack of understanding of the etiology of this very complex disease. Until now, all the available models reported have shown limitations of each model in establishing a reproducible and inheritable line to study this disease [37].
In addition, it is important to note that systemic TNF application has severe toxic effects [38]. Figure 1 showed that following transfection with pH19-TNF plasmid the TNF peptide was secreted from the cells to the culture medium which may result in unwanted toxicity following injection of the plasmid into cancer tissue. Thus, appropriate toxicology studies should be conducted before applying this plasmid in human therapy.
What could be the mechanism of the increased cell killing effect using the pH19-TNF-IRES-DTA plasmid? Polunovsky VA et. al. showed that inhibition of protein synthesis by agents such as cyclohexamide significantly increases TNF-mediated apoptotic rates in malignant cells. Apoptosis is the result of the caspase cascade which can be initiated by TNFR1 and TNF engagement, while pro-survival signals require de-novo expression of anti-apoptotic proteins [39].
TNF is at most weakly cytotoxic or cytostatic to malignant cells. It is only in combination with metabolic inhibitors that its cytotoxic potential is unmasked; the default cell survival and inflammatory pathways downstream of TNF signaling are inactivated by the metabolic inhibitors, allowing apoptosis to proceed.
DTA, similarly to cyclohexamide inhibits protein synthesis through a different mechanism; by expressing both TNF and DTA from the pH19-TNF-IRES-DTA plasmid, no de-novo protein synthesis, which is necessary for the cell proliferation pathway, takes place due to the activity of DTA. Instead, the TNF mediated pathway is shifted to the apoptosis pathway resulting in massive cell death.
The results described in this study may be the first step towards a major breakthrough in the treatment of human ovarian cancer. It should enable us to identify likely non-responders in advance, and to treat patients who are resistant to all known therapies, thereby avoiding treatment failure coupled with unnecessary suffering and cost.
References
- 1.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–252. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 2.Louis MH, Dutoit S, Denoux Y, Erbacher P, De-slandes E, Behr JP, P Gauduchon1, L Poulain. Intraperitoneal linear polyethylenimine (L-PEI)-mediated gene delivery to ovarian carcinoma nodes in mice. Cancer Gene Ther. 2006;13:367–74. doi: 10.1038/sj.cgt.7700893. [DOI] [PubMed] [Google Scholar]
- 3.Jandu N, Richardson M, Singh G, Hirte H, Hatton MW. Human ovarian cancer ascites fluid contains a mixture of incompletely degraded soluble products of fibrin that collectively possess an antiangiogenic property. Int J Gynecol Cancer. 2006;16:1536–44. doi: 10.1111/j.1525-1438.2006.00624.x. [DOI] [PubMed] [Google Scholar]
- 4.Ruan H, Su H, Hu L, Lamborn KR, Kan YW, Deen DF. A hypoxia-regulated adeno-associated virus vector for cancer-specific gene therapy. Neoplasia. 2001;3:255–263. doi: 10.1038/sj.neo.7900157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu J, Fang B. Telomerase promoter-driven cancer gene therapy. Cancer Biol Ther. 2003;2:S64–S70. [PubMed] [Google Scholar]
- 6.Erdmann VA, Barciszewska MZ, Szymanski M, Hochberg A, de Groot N, Barciszewski J. The non-coding RNAs as riboregulators. Nucl Acids Res. 2001;29:189–193. doi: 10.1093/nar/29.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ariel I, Lustig O, Schneider T, Pizov G, Sappir M, de Groot N, Hochberg A. The imprinted H19 gene as a tumor marker in bladder carcinoma. Urology. 1995;45:335–338. doi: 10.1016/0090-4295(95)80030-1. [DOI] [PubMed] [Google Scholar]
- 8.Ariel I, Miao HQ, Ji XR, Schneider T, Roll D, de Groot N, Hochberg A, Ayesh S. Imprinted H19 oncofetal RNA is a candidate tumour marker for hepatocellular carcinoma. Mol Pathol. 1998;51:21–25. doi: 10.1136/mp.51.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayesh S, Matouk I, Schneider T, Ohana P, Laster M, Al-Sharef W, de Groot N, Hochberg A. The possible physiological role of H19 RNA. Mol Carcinog. 2002;35:63–74. doi: 10.1002/mc.10075. [DOI] [PubMed] [Google Scholar]
- 10.Matouk IJ, DeGroot N, Mezan S, Ayesh S, Abu-lail R, Hochberg A, Galun E. The H19 non-coding RNA is essential for human tumor growth. PLoS ONE. 2007;2:e845. doi: 10.1371/journal.pone.0000845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ariel I, Sughayer M, Fellig Y, Pizov G, Ayesh S, Podeh D, Li bdeh BA, Levy C, Birman T, Tykocinski ML, de Groot N, Hochberg A. The imprinted H19 gene is a marker of early recurrence in human bladder carcinoma. Mol Pathol. 2000;53:320–3. doi: 10.1136/mp.53.6.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lustig-Yariv O, Schulze E, Komitowski D, Erdmann V, Schneider T, de Groot N, Hochberg A. The expression of the imprinted genes H19 and IGF-2 in choriocarcinoma cell lines. Is H19 a tumor suppressor gene? Oncogene. 1997;5:169–77. doi: 10.1038/sj.onc.1201175. [DOI] [PubMed] [Google Scholar]
- 13.Cui H, Onyango P, Brandenburg S, Wu Y, Hsieh CL, Feinberg AP. Loss of imprinting in colorectal cancer linked to hypomethylation of H19 and IGF2. Cancer Res. 2002;62:6442–6. [PubMed] [Google Scholar]
- 14.Kaplan R, Luettich K, Heguy A, Hackett NR, Harvey BG, Crystal RG. Monoallelic up-regulation of the imprinted H19 gene in airway epithelium of phenotypically normal cigarette smokers. Cancer Res. 2003;63:1475–82. [PubMed] [Google Scholar]
- 15.Tanos V, Prus D, Ayash S, Weinstein D, Tykocinski ML, De-Groot N, Hochberg A. Expression of the imprinted H19 oncofetal RNA in epithelial ovarian cancer. Eru J Obstet Gynecol Reprod Biol. 1999;85:7–11. doi: 10.1016/s0301-2115(98)00275-9. [DOI] [PubMed] [Google Scholar]
- 16.Mizrahi A, Czerniak A, Levy T, Amiur S, Gallula J, Matouk I, Abu-lail R, Sorin V, Birman T, de Groot N, Hochberg A, Ohana P. Development of targeted therapy for ovarian cancer mediated by a plasmid expressing diphtheria toxin under the control of H19 regulatory sequences. J Transl Med. 2009;7:69. doi: 10.1186/1479-5876-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breitman ML, Clapoff S, Rossant J, Tsui LC, Glode LM, Maxwell IH, Bernstein A. Genetic ablation: targeted expression of a toxin gene causes microphthalmia in transgenic mice. Science. 1987;238:1563–1565. doi: 10.1126/science.3685993. [DOI] [PubMed] [Google Scholar]
- 18.Pastan I, Chaudhary V, FitzGerald DJ. Recombinant toxins as novel therapeutic agents. Annu Rev Biochem. 1992;61:331–354. doi: 10.1146/annurev.bi.61.070192.001555. [DOI] [PubMed] [Google Scholar]
- 19.Ohana P, Schachter P, Ayesh B, Mizrahi A, Birman T, Schneider T, Matouk I, Ayesh S, Kuppen PJK, DeGroot N, Czerniak A, Hochberg A. Regulatory sequences of H19 and IGF2 genes in DNA-based therapy of colorectal rat liver metastases. J Gene Med. 2005;7:366–74. doi: 10.1002/jgm.670. [DOI] [PubMed] [Google Scholar]
- 20.Mizrahi A, Czerniak A, Levy T, Amiur S, Gallula J, Matouk I, Abu-lail R, Sorin V, Birman T, de Groot N, Hochberg A, Ohana P. Development of targeted therapy for ovarian cancer mediated by a plasmid expressing diphtheria toxin under the control of H19 regulatory sequences. J Transl Med. 2009;7:69. doi: 10.1186/1479-5876-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohana P, Gofrit O, Ayesh S, Al-Sharef W, Mizrahi A, Birman T, Schneider T, Matouk I, de Groot N, Tavdy E, Sidi A, Hochberg A. Regulatory sequences of the H19 gene in DNA based therapy of bladder cancer. Gene Ther and Mol Biol. 2004;8:181–191. [Google Scholar]
- 22.Sidi A, Ohana P, Shalva B, Shalev M, Ransom JH, Lamm D, Hochberg A, Leibovitch I. Phase I/II Marker Lesion Study of Intravesical BC-819 DNA Plasmid in H19 Overexpressing Superficial Bladder Cancer Refractory to Bacillus Cal-mette Guerin. Am J of Urol. 2008;180:2379–2383. doi: 10.1016/j.juro.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Morimoto H, Bonavida B. Diphtheria toxin- and Pseudomonas A toxin-mediated apoptosis. ADP ribosylation of elongation factor-2 is required for DNA fragmentation and cell lysis and synergy with tumor necrosis factor-alpha. J Immunol. 1992:2089–94. [PubMed] [Google Scholar]
- 24.Morimoto H, Safrit JT, Bonavida B. Synergistic effect of tumor necrosis factor-alpha- and diphtheria toxin-mediated cytotoxicity in sensitive and resistant human ovarian tumor cell lines. J Immunol. 1991;147:2609–16. [PubMed] [Google Scholar]
- 25.Mizutani Y, Bonavida B, Yoshida O. Cytotoxic effect of diphtheria toxin used alone or in combination with other agents on human renal cell carcinoma cell lines. Urol Res. 1994;22:261–6. doi: 10.1007/BF00541904. [DOI] [PubMed] [Google Scholar]
- 26.Pilati P, Rossi CR, Mocellin S. Strategies to enhance the anticancer potential of TNF. Front Biosci. 2008;13:3181–93. doi: 10.2741/2919. [DOI] [PubMed] [Google Scholar]
- 27.Ohana P, Kopf E, Bibi O, Ayesh S, Schneider T, Laster M, Tykocinski ML, de-Groot N, Hochberg A. The expression of the H19 gene and its function in human bladder carcinoma cell lines. FEBS Lett. 1999;454:81–84. doi: 10.1016/s0014-5793(99)00780-2. [DOI] [PubMed] [Google Scholar]
- 28.Ohana P, Bibi O, Matouk I, Levy C, Birman T, Ariel I, Schneider T, Ayesh S, Giladi H, Laster M, de Groot N, Hochberg A. The use of H19 regulatory sequences for targeted gene therapy in cancer. Int. J. Cancer. 2001;98:645–650. doi: 10.1002/ijc.10243. [DOI] [PubMed] [Google Scholar]
- 29.López-Lastra M, Rivas A, Barría MI. Protein synthesis in eukaryotes: the growing biological relevance of cap-independent translation initiation. Biol Res. :121–46. doi: 10.4067/s0716-97602005000200003. 200538. [DOI] [PubMed] [Google Scholar]
- 30.Kim DS, Jang YJ, Jeon OH, Kim DS. Saxatilin inhibits TNF-alpha-induced proliferation by suppressing AP-1-dependent IL-8 expression in the ovarian cancer cell line MDAH 2774. Mol Immunol. 2007;44:1409–16. doi: 10.1016/j.molimm.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Kulbe H, Thompson R, Wilson JL, Robinson S, Hagemann T, Fatah R, Gould D, Ayhan A, Balkwill F. The inflammatory cytokine tumor necrosis factor-alpha generates an autocrine tumor-promoting network in epithelial ovarian cancer cells. Cancer Res. 2007;67:585–92. doi: 10.1158/0008-5472.CAN-06-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maxwell IH, Glode LM, Maxwell F. Expression of diphtheria toxin A-chain in mature B-cells: a potential approach to therapy of B-lymphoid malignancy. Leuk Lymphoma. 1992;7:457–462. doi: 10.3109/10428199209049802. [DOI] [PubMed] [Google Scholar]
- 33.Shamimi-Noori S, Yeow WS, Ziauddin MF, Xin H, Tran TL, Xie J, Loehfelm A, Patel P, Yang J, Schrump DS, Fang BL, Nguyen DM. Cisplatin enhances the antitumor effect of tumor necrosis factor-related apoptosis-inducing ligand gene therapy via recruitment of the mitochondria-dependent death-signaling pathway. Cancer Gene Ther. 2008;15:356–70. doi: 10.1038/sj.cgt.7701120. [DOI] [PubMed] [Google Scholar]
- 34.Hagemann T, Robinson SC, Schulz M, Trumper L, Balkwill FR, Binder C. Enhanced invasiveness of breast cancer cell lines upon co-cultivation with macrophages is due to TNF-(R) dependent upregulation of matrix metalloproteases. Carcinogenesis. 2004;25:1543–1549. doi: 10.1093/carcin/bgh146. [DOI] [PubMed] [Google Scholar]
- 35.Brower V. Cancer gene therapy steadily advances. J Natl Cancer Inst. 2008;100:1276–1278. doi: 10.1093/jnci/djn335. [DOI] [PubMed] [Google Scholar]
- 36.Gordon EM, Levy JP, Reed RA, Petchpud WN, Liu L, Wendler CB, Hall FL. Targeting metastatic cancer from the inside: a new generation of targeted gene delivery vectors enables personalized cancer vaccination in situ. Int J Oncol. 2008;33:665–75. [PubMed] [Google Scholar]
- 37.Fong MY, Kakar SS. Ovarian cancer mouse models: a summary of current models and their limitations. J Ovarian Res. 2009;2(12) doi: 10.1186/1757-2215-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balkwill F. Tumor necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–71. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- 39.Polunovsky VA, Wendt CH, Ingbar DH, Peterson MS, Bitterman PB. Induction of endothelial cell apoptosis by TNF alpha: modulation by inhibitors of protein synthesis. Am J Physiol. 1994;267(4 Pt 1):C893–900. doi: 10.1152/ajpcell.1994.267.4.C893. [DOI] [PubMed] [Google Scholar]