Abstract
Pro-inflammatory mediators can dramatically alter many responses of cultured endothelial cells in vitro, which are relevant to understanding the role played by the endothelium in inflammation in vivo. The aim of this study was to determine the ability of a comprehensive array of pro-inflammatory stimuli to modulate Cell Adhesion Molecule (CAM) expression in cultures of human microvascular cardiac endothelial cells (HMVEC.c). Cell ELISA, immunocy-tochemistry and flow cytometry were used to measure the CAM expressions in HMVEC.c in response to interleukins, TNF-α and LPS. Passage matched HMVEC.c from different donors showed different CAM expression profiles, confirming inherent variability in endothelial cells. Endothelial cells from different parts of the vasculature are exposed to different cytokines and thus different protein expression profiles. A thorough understanding of these innate differences in expression pattern of the microvasculatures of cardiac tissues might allow us the opportunity to target these tissues selectively.
Keywords: P-selectin, E-selectin, VCAM-1, ICAM-1, PECAM-1, Cell adhesion molecules, LPS, TNF-a, inflammation, Endothelium
Introduction
Adhesive interactions between cells and between cells and the extracellular matrix have been found to be crucial to multiple tissue functions including the inflammatory response. Early phases of inflammation involve the recruitment of inflammatory cells from the circulation and their transendothelial migration. This process is predominantly mediated by cellular adhesion molecules (CAMs), which are expressed on the vascular endothelium and on circulating leukocytes in response to several inflammatory stimuli. The CAMs mediate the sequential steps of leukocyte-endothelial cell interaction and together with inflammatory mediators, direct the influx of inflammatory cells and define the characteristics of inflammation [1]. Studies indicate that the expressions of these inducible leukocyte-endothelial adhesion molecules are important in the primary stages of recruitment and migration of leukocytes to the sites of inflammation [2]. The CAMs that mediate this cascade of events include selectins and members of the integrin and the immunoglobulin superfamilies [3]. The selectin family of adhesion molecules on the endothelium mediates transient interactions with their ligands on leukocytes, resulting in leukocyte rolling and tethering along the vascular endothelium [4]. Intercellular adhesion molecules (ICAMs) and vascular adhesion molecules (VCAMs) are involved in the adhesion of inflammatory cells to the endothelial surface [5], whereas the platelet endothelial cellular adhesion molecule-1 (PECAM-1 or CD31) is involved in extravasation of cells from the blood compartment into the vessel wall and underlying tissue [6]. The specificity of the leukocyte-endothelial interaction is the result of the differential utilization of distinct selectin, integrin, and immunoglobulin super family members by distinct leukocytes as they navigate through diverse beds of the vascular endothelium. In humans, aberrant expression of endothelial CAMs has been reported in various pathological conditions [7-11]. Thus, the details of these adhesive interactions at the cellular and molecular level are of great significant not only to understand the pathophysiology but also as an aid for therapeutic drug development.
Most of the previous studies of endothelial cells used human umbilical vein endothelial cells (HUVECs), however, HUVECs may not be an ideal model since they are close to senescence and are cultured from hypoxic and activated vessels [12]. Thus CAM expression in HUVECs may not represent the temporal and spatial pattern of expression of these molecules in microvascular endothelial cells derived from other tissues such as the heart. Thus, it is important to use the correct microvascular endothelial cells along with the pro-inflammatory mediators in vitro and in vivo models to understand the response of heart to reperfusion injury. In order to establish the optimal timing and dosage of cytoinflammatory mediators, a detailed understanding of the time and concentration dependence of CAM upregulation and cell surface expression is necessary. An earlier in vitro study [13], determined the changes in the expression of a limited set of CAMs, Esel, VCAM-1 and ICAM -1, in response to TNF-a and IL-ip in human cardiac microvasculature cells. Here we provide the concentration and time-dependences of expression of various proinflammatory mediators on the expression of five CAMs using a HMVEC.c culture model. The inflammatory mediators used in this study include tumor necrosis factor-α (TNF-α), lipopolysaccharides (LPS), interleukin l-β (IL-1β), IL-4, IL-13, histamine, thrombin, oncostatin-M (OSM) and substance P.
Materials and methods
Materials
The mouse anti-human Psel (clone AK-6) and mouse anti-human vWF (clone 2Q2134) were purchased from Abeam (Cambridge, MA). The mouse anti-human Esel (clone 68-5H11), Lsel (clone SK11), and mouse anti-human CD31-FITC were purchased from BD science (Franklin Lakes, NJ). Mouse anti-human CD31 antibody (Mab, clone JC7OA) purchased from Dako (Carpinteria, CA). The mouse anti-human VCAM-1 (clone 1G11) and ICAM-1 (clone 6.5B5) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The rabbit anti-human vWF was purchased from Millipore. The donkey anti-mouse Alexa Fluor 488, donkey anti-mouse Al-exa Fluor 555, goat anti-mouse Qdot 800 and goat anti-rabbit 655 were purchased from Invi-trogen (Carlsbad, CA). The human rlL-4 (hlL-4), human rhTNF-α (TNF-α), LPS (Ecoli 0111:B4), substance P, actinomycin D and cycloheximide were purchased from Sigma (Saint Louis, MO). The human Psel, Esel, Lsel, rOSM, rlL-6, rlL-11 and rIL-1β were purchased from R&D systems (Minneapolis, MN). Anti-mouse ImmPRESS and tetra-methylbenzidine-H2O2 substrate (TMB) from Kirkegaard and Perry laboratories, (Gaithesburg, MD). Polymyxin B (Sigma), anti-human TNF Receptor I (TNF-RI) and anti-human IL4 Receptor (IL-4R) antibodies (both from Abeam). Anti-human CD14 and anti-human TLR4 antibodies (R&D systems).
Endothelial cells and culture conditions
Human cardiac microvascular endothelial cells (HMVEC,c)s, (Lonza, Walkersville, MD) were cultured in EGM-2MV medium (Lonza). All the cells were maintained at 37°C in a humid 95%air/5% CO2 atmosphere. Details of the cells, their passage numbers and the donor are shown in Table 1. Unless otherwise stated all the studies with HMVEC.c were done using lot no.7F330 (12 y, ♂) and all the treatments were started at a post -confluent state (day 6). Unless otherwise stated all experiments were run in triplicate and each independent experiment was performed at least three times.
Table 1.
Details of the human cardiac microvascular endothehal cells (HMVEC,c) used in this study
| Passage number used for |
||||
|---|---|---|---|---|
| initiation | studies | |||
| HUVEC | 2F0515 | pooled | primary | 2-4 |
| HMVEC,c | 7F3303 | 12 y, ♂ | 6 | 7-9 |
| HMVEC,c | 3F1619 | 18 y, ♀ | 6 | 7-9 |
| HMVEC,c | 7F3276 | 45 y, ♂ | 6 | 7-9 |
Immunofluorescence (IF) microscopy
To study the cellular distribution of Psel, Esel, vWF and CD31, the HMVEC.c were plated (80×l03/cm2) on type I collagen coated 8-chamber slides (BD Biosciences). On day 6, cells were treated with hlL-4 for 24 h followed by histamine or medium for 10 min at 37°C. The monolayer was then fixed in 1% paraformalde-hyde (for cell surface staining) or acetone/methanol 3:1 volume (for intracellular staining) and rinsed in PBS. Psel was detected using mouse anti-human Psel (diluted 1:100), Esel was detected using mouse anti-human Esel (diluted 1:100). After washing, cells were stained with donkey anti-mouse Alexa Fluor 488 (diluted 1:500) and vWF was detected using goat anti-mouse Alexa Fluor 555 (1:500). PECAM-1 was detected using mouse anti-human CD31-FITC (diluted 1:5). For double IF, after the addition of the first primary and the corresponding secondary antibody, the sections were blocked with mouse IgG (Vector laboratories) before adding FITC-hCD31 antibody. Tissues were mounted in mounting media containing DAPI (Vectashield, Vector laboratories) and examined with a Nikon epifluorescence microscope equipped with a digital camera and digital capture device (Micron Optics, Cedar Knolls, NJ).
Cell enzyme-linked immunosorbent assay (cell-ELISA)
Cell ELISA for the detection of adhesion molecule expression was performed as follows. Briefly, cultured HMVEC.c were seeded onto a 96-well cell culture plate at a density of 20×103/well. Only monolayers of cultures that were post-confluent (day 6) were used for these studies. The monolayers were treated with various concentrations of inflammatory mediators before challenge with medium alone or medium containing histamine. After 10 min of challenge, cells were washed with phosphate buffered saline (PBS) and monolayers were fixed in 1% paraformaldehyde (for membrane protein). For intracellular protein the monolayer was perme-abilized (0.2% saponin) for 10 min at room temperature (RT). After washing the wells with PBS, 100 μl of primary antibody (2 μg/ml) was added to each well and incubated at RT for 2 h. The details of the primary antibodies used in cell-ELISA are given in Table 2. Binding was assessed using 100 μl of anti-mouse ImmPRESS (60 μl in 10 ml PBS) and TMB substrate. The plates were read at 650 nm using a VERSAmax microplate reader (Molecular Device, Sunnyvale, CA). All experiments were performed at least three times, and data are expressed as mean ± SD. The statistical significance of differences was determined by paired two-tailed Student's t-test using GraphPad Prism.
Table 2.
Details of the primary antibodies used in ELISA
| Target | Vendor | Clone | Immunogen | Dilutions used |
|---|---|---|---|---|
| Psel | Abeam | AK-6 | N/A | 1:500 |
| Esel | BD Bioscience | 5D11 | Activated endothelial cells | 1:250 |
| Lsel | BD Bioscience | SK11 | T-lymphocytes | 1:100 |
| ICAM-1 | Santa Cruz | 6.5B5 | Activated HUVEC | 1:100 |
| VCAM-1 | Santa Cruz | 1G11 | Recombinant VCAM-1 | 1:100 |
| PECAM-1 | Dako | JC7OA | Spleen cells | 1:250 |
Recombinant protein-based ELISA
Unless otherwise stated, all incubations were performed at room temperature. Ninety-six well plates (Maxi-Sorp) were coated in triplicate overnight with 100 ng/well of various recombinant proteins at 4°C. Plates were washed and blocked with 200 μl per well of 1% bovine serum albumin (BSA; Sigma)/PBS for 1 h. Plates were washed with PBS containing 1% tween (PBS-T) and serially diluted primary antibodies were added to the wells containing recombinant proteins and to wells lacking recombinant protein (as a negative control) and incubated for 2 h. Plates were washed in PBS-T and 100 μl of ImmPRESS reagent (60 μl in 10 ml PBS) was added. Following a final incubation for 30 min plates were washed with PBS-T and developed at room temperature with 100 μl of TMB substrate. Absorbance was measured at 650 nm at different time intervals starting at 2 min using a VERSAmax microplate reader (Molecular Dynamics).
Western blotting
SDS-PAGE electrophoresis was performed with recombinant selectin proteins under reducing and non-reducing conditions. Proteins (1.0μg each) were separated on 10% Tris-HCI poly-acrylamide gel (Bio-Rad) and transferred to PVDF membranes (0.2μ). Membranes were blocked with TTBS (10 mM Tris-HCI, pH8.0, 150 mM NaCI, 0.2% Tween-20) containing 5% nonfat dry milk for 1 h at room temperature. After three washing steps in TTBS, blots were incubated with 1:3000 diluted goat anti-mouse IgG-HRP conjugate (Bio-Rad). Following five washes with TTBS the peroxidase reaction was visualized by immune-star HRP substrate (Bio-Rad).
Flow cytometry
Flow cytometry was used to detect the changes in cell-surface adhesion molecule expression in response to different cytokines. After each treatment monolayers of HMVEC.c were detached with trypsin/EDTA and washed once with PBS. Detached cells were incubated with primary antibody [FITC-Psel (Abeam) diluted at 1:20 or PE-Esel (BD Biosciences) diluted at 1:5 or FITC-VCAM-1 (BD Biosciences) diluted at 1:5; PE-ICAM-1 (BD Biosciences) diluted at 1:5] for 45 min at 4°C. Cells were washed three times with 1% BSA (in PBS) to remove the unbound antibodies and fixed in 1% paraformaldehyde. Flow cytometry was carried out using a Guava PCA 96 base system (Millipore, Billerica, MA) at 532 nm excitation wavelength and using a 580 and 673 nm emission bandpass filter. Samples were analyzed with Gauva express software (Millipore) and 5,000 events per group were analyzed. Unstained cells and cells stained with isotype matched antibodies were included in all experiments. The positive cells were determined by linear regions that were set on double gated according to (1) forward scatter and side scatter parameters and (2) PECAM-1 staining. A positive event is defined in relation to the isotype matched control. All experiments were repeated at least three times.
Blocking studies on HMVEC,c using neutralizing agents or antibodies
To determine the specificity of LPS, TNF-α and IL-4 in the induction of CAMs, blocking studies were performed using polymyxin B, anti-human TNF Receptor I (TNF-RI) and anti-human IL4 Receptor (IL-4R) antibodies. To determine the role of downstream elements involved in the LPS recognition system, blocking studies were performed using anti-human CD14 and anti-human TLR4 antibodies. These experiments were performed by preincubating the cells with specific cytokine blocking agents or inhibitors for 1 h at 37°C before each experiment. A control goat anti-human IgG (Abeam) was used to determine the non-specific inhibition.
Results
Specificity of the antibodies
Western blot analyses were performed to determine the specificity and cross-reactivity of the antibodies based on reports of variability in commercial antibodies [14]. Results using recombinant selectin proteins showed all the three selectin antibodies used in this study, viz., anti-Psel (clone AK-6), anti-Esel (clone 5D11) and anti-Lsel (clone SKll) were specific to their corresponding protein and no cross-reactivity was seen with other members of the family (supplementary data 1). The confirmatory recombinant protein-ELISA also showed specificity and lack of cross reactivity of anti-selectin antibodies towards selectin proteins. The experiment was performed with other clones of anti-Psel (clone BBIG-E6) and anti-Esel (clones 5D11, CL2 and 68-5H11) antibodies. Among the clones tested, BBIG-E6 cross-reacted to both P-and E-selectins (data not shown).
Effect of Interleukins (ILs) on CAM expression by cell ELISA
The effect of IL family members (IL-1β, IL-4, IL-13 and OSM) on the cell surface expression of CAMs was examined in cultures of HMVEC,c using Cell ELISA. Very modest increases in surface expression of Psel, and VCAM-1 expression were observed with various concentrations of IL-1β, IL-13 and 0SM (not shown). Significant induction of Psel was seen with IL-4 in combination with histamine. This induction occurred in a concentration-dependent manner and the optimum concentration for IL-4 and histamine was 20 ng/ml and 0.1 mM respectively (supplementary data 2 A.B and D). The time course of histamine treatment indicated the maximum cell surface expression of Psel occurred 10’ post treatment (supplementary data 2 C). VCAM-1 was induced by IL-4 treatment. Cell surface expression of Esel (supplementary data 2 E). PECAM-l (supplementary data 2G) or ICAM-1 (not shown) did not change in response to various concentrations of IL-4. Similarly, no change in the cell surface expression of Lsel was seen with any of the treatments with ILs (data not shown).
Kinetics of cell surface Psel expression
To determine the maximum cell surface expression of Psel in HMVEC.c experiments were performed with different doses, frequency and duration of hlL-4 and /histamine treatment. The results showed maximum Psel expression was dependent on dose, frequency and duration of IL-4/histamine treatment (supplementary data 2 H). A 3-day treatment of 20ng/ml twice per day IL-4 followed by O.lmM histamine induced the maximum Psel over any other type of treatment or combination.
Effect of TNF-a and LPS on CAM expression by cell ELISA
To determine whether TNF-a or LPS affected the cell surface expression of selectins, ICAM-1 and VCAM-1 in HMVEC.c, we treated post-confluent monolayers with different concentration of LPS and TNF-α. The amount of cell surface selectins along with VCAM-1, ICAM-1 and PECAM-1 were measured using cell-ELISA. The basal level expression of Psel, Esel, Lsel, ICAM-1 and VCAM-1 was negligible while LPS and TNF-a up-regulated Esel expression (supplementary data 3 A-C) as well as VCAM-1 and ICAM-1 (not shown). The up-regulation of Esel by LPS and TNF-a was concentration dependent with maximum expression observed with 10 μg/ml of LPS and 12.5 ng/ml of rhTNF-a for 24 h. In contrast, no changes were seen with cell surface expression of Psel or Lsel.
Differential expression of CAMs by LPS and IL-4/histamine
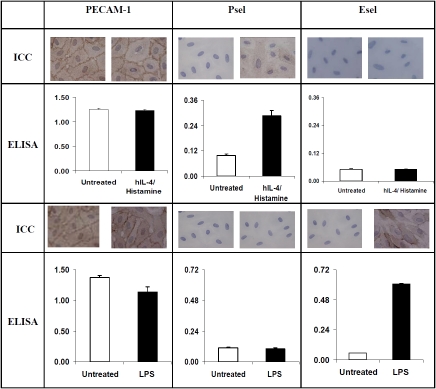
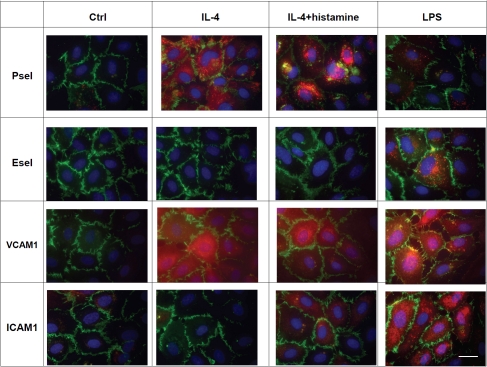
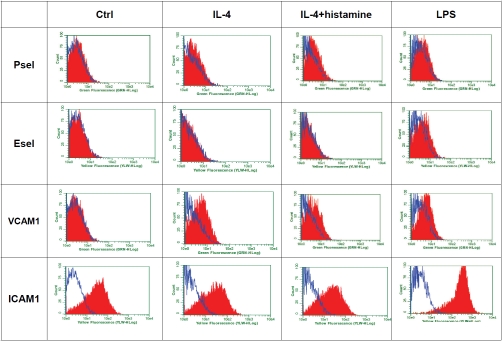
To explore the differential effect of inflammatory mediators on selectin expression, we treated HMVEC.c with IL-4, IL-4/histamine or LPS. The Immunocytochemistry (ICC) and cell-ELISA showed hlL-4/histamine significantly increased cell surface expression of Psel but not Esel. In contrast, LPS specifically up-regulated cell surface expression of Esel but not Psel (Figure 1). Immunofluorescence studies confirmed the selective up-regulation of Psel by IL-4 and Esel by LPS and showed that subsequent histamine treatment prompted expression of Psel on the cell surface (Figure 2). Constitutive levels of PECAM-1 expression were unchanged during the treatment. Both ICAM-land VCAM-1 were up -regulated by LPS treatment, while VCAM-1 was up-regulated by both LPS and IL-4 treatment. The effect of IL-4 and LPS on cell surface expression of Psel, Esel, VCAM-1 and ICAM-1 on HMVEC.c was also analyzed by flow cytometry. Data shown in Figure 3 demonstrate that unstimulated HMVEC,c expressed undetectable amounts of Psel, Esel and VCAM-1 but did express ICAM-1. An increase in the fluorescence staining for Psel and VCAM-1 was observed with IL-4 and IL-4/histamine treatment. Similarly an increase in fluorescence staining for Esel, VCAM −1 and ICAM-1 was seen with the treatment of LPS.
Figure 1.
Differential expression of Psel and Esel by inflammatory mediators: Post-confluent HMVEC,c were treated with IL-4 (20 ng/ml) or LPS (10 ug/ml) for 24 h. The concentration of proinfiammatory mediators that induced maximal response was determined in preliminary experiments. At the end of the incubation period, IL-4 treated cells were exposed to histamine (0.1 mM). Cell surface expression of PECAM-l, Psel and Esel were measured by cell ELISA as described in materials and methods. All experiments were repeated at least three times and each experiment was done in triplicate wells. Each data point is mean ± SD (n=3). Also shown are representative figures (ICC) corresponding to each treatment.
Figure 2.
Inflammatory mediators up-regulates Psel, Esel, VCAM-1 and ICAM-1. HMVEC,c were cultured and treated with of IL-4, IL4/histamine or LPS. At the end of each treatment cells were fixed and cells were triple stained for nuclear stain DAPI (blue), PECAM-1 (green) and Psel or Esel or VCAM-1 or ICAM-1 (red) as described in materials and methods. Ctrl=indicate no treatment, bar=20μm. These experiments were repeated three times with nearly identical results.
Figure 3.
Differential expression of cell surface Psel, Esel, VCAM-1 and ICAM-lby HMVEC, c in response to distinct inflammatory mediators. Monolayers of HMVEC,c were incubated with LPS (10 μg/ml) or IL-4 (20 ng/ml) for 24 h and/or followed by 0.1 mM histamine. After each treatment cells were detached with EDTA and used for staining and flow cytometric analyses as described in materials and methods. Flow cytometric histograms of HMVEC,c stained for Psel, Esel, VCAM-1 and ICAM-1 (red). Isotype matched control are shown (open blue). Unstimulated control cells were similar to isotype control (data not shown). Results are from a single representative of three separate experiments.
Effect of Passage number on IL-4 and LPS induced expression of Psel and Esel
Using the cell ELISA, we found that the cell surface expression of Psel and Esel protein stimulated with IL-4/histamine and LPS respectively varies significantly in HMVEC.c with passage number. There was a significant reduction (P<0.01) in the induction of both Psel and Esel expression in cells from passage 12 compared to 7 (supplementary data 3 D-F). The experiments were repeated using other passage numbers and the results showed there is a gradual reduction in the inducibility/cell surface expression by IL-4 and LPS with higher passage numbers. Consequently, further experiments were performed on cells from passage 7 to 9.
Regulation of Psel in HMVEC,c in vitro
To further characterize the up regulated Psel expression in HMVEC,c we measured the surface as well as total Psel by cell ELISA. As shown in supplementary data 3 G-H, treatment of cells with IL-4 alone significantly increased the total Psel but no increase was seen in surface Psel. In contrast, no increase in total Psel was seen with histamine alone but there was a significant increase in cell surface Psel. As expected, significant increases in total and cell surface Psel were seen in cells treated with IL-4 and histamine. To reveal the localization of Psel, monolayers of permeabilized HMVEC,c were immunostained using Psel antibody. Immunofluroscence studies (supplementary data 4) showed baseline Psel expression seen in untreated cells was distributed mostly in the cytoplasm. This pattern of Psel staining was similar to the staining pattern for vWF, a marker for Weibel-Palade (WP) bodies. The histamine treatment alone mobilized most of the Psel towards the cell surface (data not shown). Treatment with IL-4 for 24 h increased the cytoplasmic Psel, while IL-4 treatment along with histamine treatment results in mobilization of upregulated Psel towards the cell surface. In resting cells, Psel was co-localized with vWF, while IL-4 plus histamine treatment results in increased cell surface expression of Psel.
Effect of donor on the induced expression of CAMs
To determine the inherent endothelial diversity relative to the donor source of HMVEC.c, passage matched cells from three different donors were compared for their inducible cell surface expression of CAMs. As shown in supplementary data 5, constitutive expression of Psel, Esel, Lsel and VCAM-1 and ICAM-1 was negligible for all donors. A combination of IL-4/histamine treatment induces Psel and VCAM-1 cell surface expression by all three donors. However, the induction of VCAM-1 varied among the three donors with the highest amount seen in cells from a 12-year-old male (P<0.05). Upon activation of all three HMVEC.c with LPS, two of them (12- and 45-year -old males) consistently showed a 4-fold induction of Esel while the cells from the 18-year-old female showed less than a 2 fold induction. This disparity was also seen in the expression of ICAM-1, where the cells from both 12- and 45-year-old males showed more than 4 fold inductions, while the cells from the 18-year-old female showed less than a 2 fold. None of the cells showed any significant level of expression of Lsel.
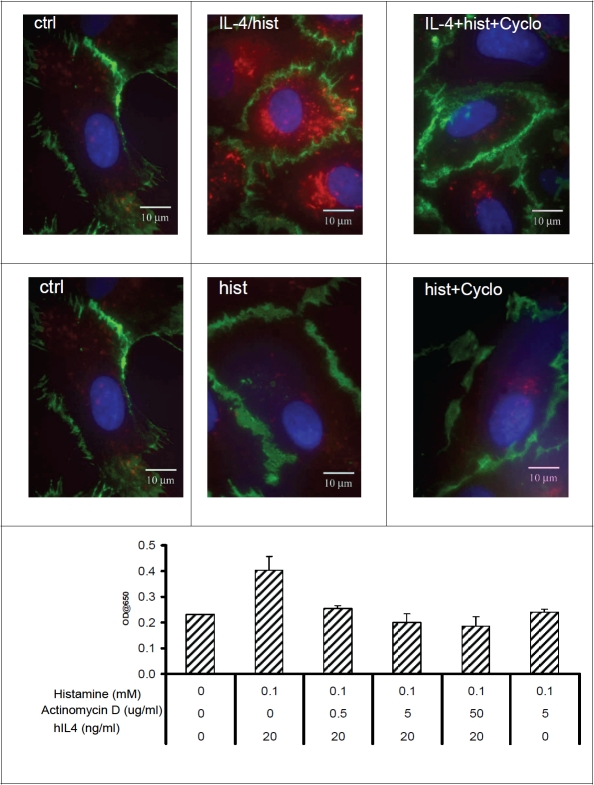
IL-4 induced expression of Psel requires de novo protein synthesis
In order to determine whether IL-4 induced expression of Psel required protein synthesis, we treated HMVEC.c with IL-4 in the presence or absence of cycloheximide. The cycloheximide treatment inhibits the IL-4/histamine induced expression of Psel in HMVEC,c (Figure 4, upper panel). Correspondingly, treatment of actinomy-cin D also inhibits the IL-4/histamine induced expression of Psel, indicating de novo protein synthesis is involved in the induction (Figure 4, lower panel). In contrast, cycloheximide or actinomycin D treatment had no effect on the basal level expression of Psel.
Figure 4.
hlL-4 induced expression of Psel requires protein synthesis: Representative figures from localization studies of Psel (red) and PECAM-1 (green) in HMVEC,c, treated with IL-4/histamine and histamine alone in presence or absence of cycloheximide. After treatment, the cells were permeabilized and double IF were done as described in material and methods. PECAM-1 is constitutively expressed on the lateral borders of the cell. Cell nuclei are stained with DAPI (blue). Lower panel showing dose-dependent inhibition of cell surface Psel induction by actinomycin D. Monolayer of HMVEC,c were stimulated with indicated IL-4 and/or histamine along with actinomycin D for 24 h. Cell surface Psel was measured by cell-ELISA as described in materials and methods. Data are from one experiment representative of at least three independent experiments per condition.
Specificity of IL-4 in the induction of CAMs
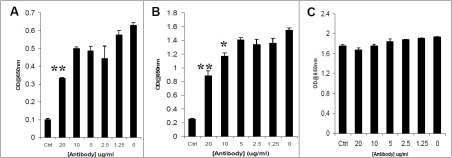
To explore off receptor effects of IL-4 on the induction of Psel in HMVEC,c, blocking experiments were performed using a specific IL4-R antibody. A goat anti-human IL4-R antibody directed against human IL-4Ra reduced the IL-4 induced expression of Psel and VCAM-1 in a dose dependent manner. Conversely, anti-IL-4R antibody treatment had no effect on PECAM-1 expression (Figure 5).
Figure 5.
Effect of anti-human IL-4Rα on IL-4 induced expression of Psel, VCAM-1 and PECAM-1 in HMVEC,c . Cells were preincubated for 1 hour with increasing concentrations of neutralizing antibody to IL-4Rα and were then incubated for 24 hours in the presence of IL-4 (20 ng/ml) followed by histamine (0.1 mM). The amount of cell surface Psel (A), VCAM-1 (B) and PECAM-1 (C) was measured by Cell-ELISA as described in materials and methods. Ctrl, indicates unstimulated cells. **P<0.01 compared with IL-4 stimulated in the absence of antibody. The data presented are representative of three experiments
Effects of polymyxin B on LPS induced expression of Esel, VCAM-1 and ICAM-1
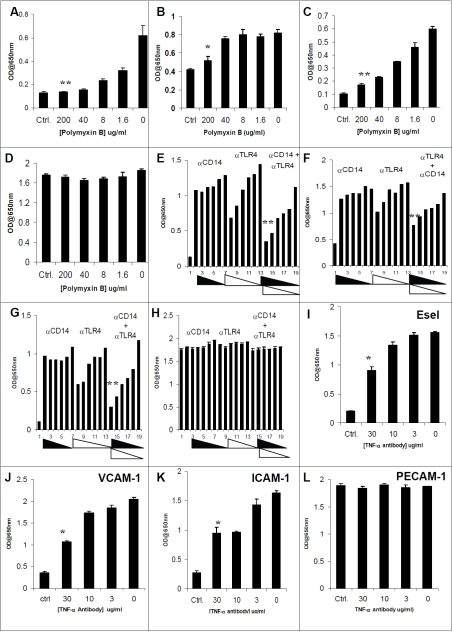
As an inhibitor of LPS, polymyxin B blocked or significantly decreased LPS-induced cell surface expression of Esel, VCAM-1 and ICAM-1 proteins in HMVEC,c (Figure 6, A-D). Cell ELISA showed the 200μg/mL of polymyxin B completely abrogated LPS induced expression of Esel and ICAM-1, while only a modest but statistically significant reduction is seen with VCAM-1 expression. As expected, polymyxin B treatment had no effect on PECAM-1 expression.
Figure 6.
Effect of polymyxin B, anti-CD14, anti-TLR4 and anti-TNF-α on the LPS and TNF-α induced expression of cell adhesion molecules in HMVEC,c. Dose response of polymyxin B presence in the induction of Esel (A), VCAM-1 (B), ICAM-1 (C) and PECAM-1 (D). Dose response of anti-CD14 or anti-TLR4 alone or in combination (lane 1, unstimulated; lanes 2-7, 20-1.25 ug/ml of anti-CD 14 antibody, lanes 8-13, 9.0-0.5 ug/ml of anti-TLR4 antibody, lanes 14-19, combination of anti-CD14 and anti-TLR4 antibodies) on LPS induced expression of Esel (E), VCAM-1 (F), ICAM-1 (G) and PECAM-1 (H). Effect of anti-TNF-α antibody on TNF-α induced expression of Esel (l), VCAM-1 (J), ICAM-1 (K) and PECAM-1 (L). Statistical difference with respect to the group treated with LPS or TNF-α alone, **p<0.01 and *P<0.05. The experiment was repeated three times and yielded similar results.
LPS mediated induction requires LPS recognition system
To investigate whether the LPS-mediated up-regulation of CAMs depends on an LPS recognition system, we employed antibody inhibitors to interrupt this pathway. Pretreatment of HMVEC,c with anti-CD14 and anti-TLR4 alone or in combination suppressed the LPS-induced Esel, VCAM-1 and ICAM-1 expression. Cell ELISA showed pretreatment of HMVEC,c with anti-CD14 and anti-TLR4 resulted in a modest decrease in LPS induced Esel, VCAM-1 and ICAM-1. However in combination, the pretreatment resulted in a statistically significant reduction in LPS induced cell surface expression of Esel, VCAM-1 and ICAM-1 (Figure 6 E-H).
Specificity of TNF-a in the induction of CAMs
To determine the specificity of TNF-a induced expression of cell adhesion molecules, blocking studies were performed using an antibody directed against the human TNF-Receptor 1 (TNF-R1). Pre-treatment of HMVEC,c with anti-TNF-R1 antibody resulted in a dose dependent reduction in TNF-α induced Esel, VCAM-1 and ICAM-1 (Figure 6 I-L).
Discussion
During the inflammation process, the activation of endothelium by proinflammatory cytokines is a critical step, as it is directly responsible for the recruitment and extravasation of the circulating leukocytes at the site of inflammation. This process is facilitated by the coordinated expression and functioning of adhesion molecules on endothelial cells as well as on the leukocytes. To initiate the inflammatory process circulating leukocytes, while withstanding the shear force of the blood stream have to establish contact (tethering) with the endothelial cells. The tethering and rolling of leukocytes over the endothelium is largely mediated by selectins and their ligands [15]. Endothelial Psel is stored in Weibel-Palade (WP) bodies and after activation with inflammatory mediators such as histamine, thrombin, WP bodies fuse with the plasma membrane and Psel is rapidly mobilized to the endothelial apical surface [16]. The tethering slows the speed of leukocytes and permits them to roll over the endothelial surface, allowing further interactions mediated by integrin and their ligands [17]. The members of the immu-noglobulin superfamily, VCAM-1 (CD54) and ICAM-1 (CD106) are the main endothelial adhesion molecules implicated in binding to integrins which are expressed on leukocytes [18, 19]. VCAM-1 binds to integrins ouPi or Very Late Anti-gen-4 (VLA-4) while ICAM-1 binds to integrins αLβ2 or lymphocyte Associate Antigen-1 (LFA-1). Previous studies indicate that ICAM-1 is scarcely expressed on the quiescent endothelium surface, however expression of both ICAM-1 and VCAM-1 was induced after cell activation by proinflammatory mediators such as interleukin (IL)-1 and TNF-α [20-22]. PECAM-1, another member of the immunoglobulin superfamily is a 130-kD type I transmembrane adhesion glyco-protein and its expression is limited to cells of the vascular system [23]. In endothelial cells, PECAM-1 is localized to cell-cell borders and is known to be involved in adhesion [24] and tran-sendothelial migration of leukocytes [25].
Endothelial cells are remarkably heterogeneous in terms of morphology, protein expression and function. The evidence emerging from the analyses of microvascular endothelial cells from different organs, as well as endothelial cells obtained from different vessels from the same organ show the heterogeneity is at multiple levels [13, 26-32]. Hence it has become an important issue to study the response to specific inflammatory mediators of the relevant endothelial cells in vitro and in vivo. An earlier in vitro study [13], determined the changes in the expression of Esel, VCAM-1 and ICAM-1 in response to TNF-α and IL-1β in human cardiac microvasculature. The finding of the present study was that human microvascular endothelial cells derived from cardiac tissues although largely alike in overall CAM expression profiles, still exhibit heterogeneous patterns for some proteins. These cells in culture respond differentially to different inflammatory markers. Among the inflammatory mediators used in this study, IL-4/histamine treatment differentially up-regulated Psel expression, while LPS induced Esel expression. IL-4 is a pleiotropic T cell-derived cytokine that is known to invoke B cell proliferation and to induce Ig class switching in activated B cells, thus playing a major role in the inflammatory responses. By activating endothelial cells to express Psel and VCAM-1, the IL-4 is also responsible for the adhesion of PSGL and VLA-4-expressing leukocytes, viz., neutro-phils, monocytes and eosinophils. The fact that neither Esel nor ICAM-1 is enhanced in response to IL-4 may indicate selective adhesion [33] and may facilitate selective entrance of different leukocyte subsets into the vessel walls and underlying tissues. Previous studies in HU-VEC showed both IL-4 and IL-13 equally effective in inducing Psel and VCAM-1 expression [34, 35]. There is experimental evidence suggesting that these similar biological activities are due to a shared receptor component [36-38]. However, in our experiments with HMVEC,c IL-13 treatment up-regulated Psel and VCAM-1 at only marginal levels above non-treated controls. Similarly, unlike many other cell types that respond to both cytokines equally, T cells only respond to IL-4 not to IL-13 [39]. LPS and TNF-α differentially up-regulated expression of Esel, VCAM-1 and ICAM-1, not Psel or Lsel. At various concentrations, LPS significantly induced higher amounts of cell surface Esel than TNF-a. As suggested by earlier studies, this is probably due to the synergistic effect of LPS and the endogenous TNF-α, which is produced in response to LPS [40, 41]. LPS stimulates endothelial cells through the LPS recognition systems, binding with CD14 and transfer to Toll Like Receptor (TLR)-4 which in turn result in the production of downstream adhesion molecules by the activation of NF-kB- and AP-1-dependent transcriptional pathways. Antibody blocking studies indicate that LPS mediates these effects on HMVEC,c through CD14 and TLR4.
Thus, using comparable concentrations of inflammatory mediators used with other celllines [13, 26, 30], we were able to selectively upregulates specific CAMs. However, by increasing the treatment time and frequency we were able to exacerbate the cell surface expression of specific CAMs. This was essential for achieving maximum expression of CAMs in subsequent in vivo studies using HMVEC,c to better reflect the chronic exposure seen in vivo. The present study also demonstrated passage- and organmatched cardiac endothelial cells from different donors have different CAM expression profiles, although the number of individual donors used in this study was low (n=3). The different expression patterns between male and female donors is interesting and worthy of follow up. These cell and organ-specific differences should be considered when interpreting findings derived from studies using endothelial cells derived from different donors and organs.
A thorough understanding of these innate differences in expression pattern of the microvascu-latures of cardiac tissues might allow us the opportunity to target these tissues selectively. Understanding the molecular mechanisms that underlie leukocyte adhesion and the extravasation cascade has allowed molecular targets to be identified for antiadhesion therapy. The emerging possible therapeutic targets in human include the blockade of selectins using selectin antagonists to prevent damage caused during ischemic-reperfusion processes such as transplantation, myocardial infarction and thrombosis [42]. Similarly, immunoblockade of ICAM-1 reduces ischemic brain injury and neutrophil accumulation in rat [43] and rabbit [44] models of cerebral ischemia. Monoclonal antibodies against α4 and αL integrin chains have shown a clear beneficial effect in animal models as well as in human diseases [1]. Humanized anti-VLA-4 [45] and anti-LFA-1 [46] antibodies have shown a significant therapeutic effect in inflammatory and autoimmune diseases in human. Although the synthesis of new drugs able to targets CAMs and/or their receptors is challenging, convincing results obtained in animals models of diseases together with the fact that some approaches targeting CAMs led to promising results in humans, suggests that more efforts are needed to definitely validate this potentially effective therapeutic approach.
Supplemental material
Supplementary data 1 Specificity and cross reactivity of selectin antibodies against members of selectin family of proteins: All the selectin antibodies used in this study were tested for their specificity and cross-reactivity to the members of the selectin protein family using recombinant Psel, Esel and Lsel. After electrophoresis of selectin proteins, western blots were performed using specific antibodies as described in material and methods. Representative coomassie blue stained gels and western blots are shown (upper panel). Specificity and cross reactivity of the antibodies were also determined in cell ELISA using recombinant selectin proteins (P=Psel; E=Esel, L=Lsel and N = no protein) as described in materials and methods. Representative figures of the ELISA plate and OD650 readings are shown (lower panel). Each experiment was repeated at least two times and each data point represents means ± SD
Supplementary data 2. Kinetics of IL-4/histamine induced Psel expression in HMVEC,c by Cell ELISA (A-C): (A) A representative figure showing the dose-dependent Psel expression induced by hlL-4 for 24 h followed by histamine. HMVECc were incubated with various concentrations of hIL-4 for 24 h followed by histamine (0.1mM) for 10 min. (B), A representative figure showing the effect of histamine on the mobilization to the membrane of hPsel induced IL-4. HMVECc were incubated with hIL-4 (20 ng/ml) for 24 h followed by different concentrations of histamine for 10 min. (C) Figure showing the time course of histamine treatment on the expression of Psel in HMVEC,c. Post confluent HMVEC,c were treated with IL-4 for 24 h followed by histamine (0.1 mM). Figure showing dose response of hIL-4 and histamine on the surface expression of cell adhesion molecules in HMVEC,c. Post-confluent HMVECc were treated for 24 h with increasing concentrations of hIL-4. At the end of the incubation period, cells were treated with different concentrations of histamine (0, 0.1 and 1 mM) or medium alone and cell surface expression of Psel (D), Esel (E), VCAM-1 (F), and PECAM-1 (G) was measured by ELISA. In the negative control the primary antibody was replaced with isotype matched mouse IgG (not shown). Note no differences were seen in the cell surface expression of Esel, PECAM-1 and ICAM-1 (not shown). Each point is the mean of triplicate well ± SD and experiment was repeated at least three times with identical results. (H) Figure showing a robust induction of Psel can be achieved by changing the dose and frequency of IL-4 in HMVEC,c. A representative figure showing Psel expression in HMVEC,c treated twice a day with IL-4 alone [hlL-4 (bid)] or with histamine [hlL-4 (bid)+hist] or once a day with IL-4 [hIL-4 (qd)+hist] over a 3-day period. These experiments were repeated at least three times and each data point is mean ± SD (n=3).
Supplementary data 3. Differential Expression of Psel and Esel in response to TNF-α, LPS and IL-4: Representative figures showing the dose–dependent up-regulation of cell surface cell adhesion molecules in HMVEC,c. Monolayers of HMVEC,c were stimulated with various concentrations of LPS (A), TNF-α (B) and cell surface expression of Psel, Esel, and PECAM-1 were measured using Cell ELISA. (C) Time dependent cell surface expression of Esel by LPS and TNF-α. Representative figures showing the effect of passage number on the induction of CAMs in HMVEC,c. Cell surface expression of Psel (D), Esel (E) and PECAM-1 (F) in HMVEC,c from passage 7 (closed) and 12 (hatched) in response to hIL-4/histamine and LPS. In negative controls (ctr), the primary antibody was replaced with isotype matched mouse IgG. Representative figures showing the subcellular distribution of Psel in stimulated HMVEC,c. Monolayer of HMVEC,c were stimulated with IL-4, histamine or IL-4/histamine combinations. Cell surface (G) and total (H) Psel expression was measured by Cell ELISA. In negative controls (ctr), the primary antibody was replaced with isotype matched mouse IgG. These experiments were repeated three times with nearly identical results.
Supplementary data 4. Cellular distribution of Psel on HMVEC,c by immunofluroscence. Representative figures from co-localization studies of Psel (red) and vWF (green) in naive HMVEC,c and cells treated with IL-4/histamine. After treatment the cells were permeabilized and incubated with mouse anti-human Psel antibody and rabbit anti-human vWF antibody. Bound antibodies were detected using goat anti-mouse Qdot 800 (red) and goat anti-rabbit Qdot 655 (green). In naive cells Psel co-localized with vWF, while IL-4/histamine treatment significantly increased the membrane Psel expression which was not co-localized with vWF. Cell nuclei were stained with DAPI (blue), (bar=10μm).
Supplementary data 5. Effect of donor on the induced expression CAMs: Passage-matched HMVEC,c from three different donors, 12-year-old male (12y,M), 18-year-old female (18y,F) and 45-year-old male (45y,M) were treated with IL-4/histamine and LPS. Cell surface expression of Psel, Esel, Lsel, VCAM-1 and ICAM-1 were measured as described in materials and methods. Left panel shows the representative figures of ELISA plate corresponding to each treatment. Right panel shows the OD650 reading from each treatment. Each data point is mean ± SD from three independent experiments and each experiment was done in triplicate (**=P<0.01).
References
- 1.Barreiro O, Sanchez-Madris F. Molecular basis of leukocyte-endothelium interactions during the inflammatory response. Rev Esp Cardiol. 2009;62:552–556. doi: 10.1016/s1885-5857(09)71837-7. [DOI] [PubMed] [Google Scholar]
- 2.Montefort S, Holgate ST. Adhesion molecules and their role in inflammation. Respir Med. 1991;59:91–99. doi: 10.1016/s0954-6111(06)80284-2. [DOI] [PubMed] [Google Scholar]
- 3.Bevilacqua MP, Nelson RM. Endothelial-leukocyte adhesion molecules in human disease. Annu Rev Med. 1994;45:361–78. doi: 10.1146/annurev.med.45.1.361. [DOI] [PubMed] [Google Scholar]
- 4.Lasky LA. Selectins: interpreters of cell-specific carbohydrates information during inflammation. Science. 1992;258:964–969. doi: 10.1126/science.1439808. [DOI] [PubMed] [Google Scholar]
- 5.Sawa Y, Sugimoto Y, Ueki T, Ishikawa H, Sato A, Ngato T, Yoshida S. Effects of TNF-alpha on leukocyte adhesion molecule expressions in cultured human lymphatic endothelium. J Histochemistry and Cytochemistry. 55:721–733. doi: 10.1369/jhc.6A7171.2007. [DOI] [PubMed] [Google Scholar]
- 6.Woodfin A, Reichel CA, Khandoga A, Corada, Voisin M, Scheiermann, Haskard, Dejana E, Krombach F, Nourshargh S. JAM-A mediates neutrophil transmigration in a stimulus-specific manner in vivo: evidence for sequential roles for JAMA-A and PECAM-1 in neutrophil transmigration. Blood. 2007;110:1848–1856. doi: 10.1182/blood-2006-09-047431. [DOI] [PubMed] [Google Scholar]
- 7.Barker J. Adhesion molecules in cutaneous inflammation. Ciba Found Symp. 1995;189:91–106. [PubMed] [Google Scholar]
- 8.Bennion SD, Middleton MH, David-Bajar KM, Brice S Norris D. In three types of interface dermatitis, different patterns of expression of intercellular adhesion molecule (ICAM-1) indicates different triggers of diseases. J Invest Dermatol. 1995;105:71S–79S. doi: 10.1111/1523-1747.ep12316107. [DOI] [PubMed] [Google Scholar]
- 9.De Vries IJM, Langeveld-Wildshut EG, van Reijsen FC, Dubois GR, van den Hoek JA, Bihari IC, van Wichen D, de Weger RA, Knol EF. Adhesion molecule expression on skin endothelia in atopic dermatitis: Effects of TNF-α and IL-4. J Allergy Clin Immunol. 1998;102:461–468. doi: 10.1016/s0091-6749(98)70136-8. [DOI] [PubMed] [Google Scholar]
- 10.Groves RW, Allen MH, Barker J, MacDonald DM. Endothelial leukocyte adhesion molecule (ELAM-1) expression in cutaneous inflammation. Br J Dermatol. 1991;124:117–123. doi: 10.1111/j.1365-2133.1991.tb00419.x. [DOI] [PubMed] [Google Scholar]
- 11.Groves RW, Ross EL, Barker J, MacDonald DM. Vascular cell adhesion molecule-1: Expression in normal and diseased skin and regulation in vivo by interferon gamma. J Am Acad Dermatol. 1993;29:67–71. doi: 10.1016/0190-9622(93)70154-l. [DOI] [PubMed] [Google Scholar]
- 12.Garlanda C, Dejana E. Heterogeneity of Endothelial cells: specific markers. Arterioscler Thromb Vase biol. 1997;17:1193–1202. doi: 10.1161/01.atv.17.7.1193. [DOI] [PubMed] [Google Scholar]
- 13.McDouall RM, Farrar MW, Khan A, Yacoub MH, Allen AP. Unique sensitivities to cytokine regulated expression of adhesion molecules in human heart derived endothelial cells. Endothelium. 2001;8:25–40. doi: 10.3109/10623320109063155. [DOI] [PubMed] [Google Scholar]
- 14.Couchman JR. Commercial Antibodies: The Good, Bad, and Really Ugly. J Histochem & Cytochem. 2009;57:7–8. doi: 10.1369/jhc.2008.952820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alon R Ley K. Cells on the run: shear–regulated integrin activation in leukocyte rolling and arrest on endothelial cells. Curr opin Cell biol. 2008;20:525–535. doi: 10.1016/j.ceb.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonfanti R, Furie B, Wagner D. PADGEM (GMP140) is a component of Weibel-Palade bodies of human endothelial cells. Blood. 1989;73:1109–1112. [PubMed] [Google Scholar]
- 17.Barreiro O, Vicente-Manzanares M, Urzainqui A, YanezMo M, Sancez-Madrid F. Interactive protrusive structures during leukocytes adhesion and transendothelial migration. Fron Biosci. 2004;9:1849–1863. doi: 10.2741/1285. [DOI] [PubMed] [Google Scholar]
- 18.Elices MJ, Osborn L, Takeda Y, Crouse C, Luhowskyj S. VACM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-/fibronectin binding site. Cell. 1990;10:577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- 19.Marlin SD, Springer TA. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocytes function-associated antigen (LFA-1) Cell. 1987;51:813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- 20.Haraldsen G, Kvale D, Lien B, Farstad IN, Brandtzaeg P. Cytokine-regulated expression of E-selectin, intercellular adhesion molecule-1 (ICAM-1), and Vascular cell adhesion molecule-1 (VCAM-1) in human microvasuclar endothelial cells. J Immunol. 1996;156:2558–2565. [PubMed] [Google Scholar]
- 21.May JM, Caroline PD, Wheeler-Jones Pearson JD. Effects of protein kinase inhibitors on cytokine induced adhesion molecule expression by human umbilical vein endothelial cells. Bri J Pharm. 1996;118:1761–1771. doi: 10.1111/j.1476-5381.1996.tb15602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dustin ML, Rothlein R, Bhan AK, Dinarello CA, Springer TA. Induction by IL-1 and interferon-gamma: tissue distribution, biochemistry and function of natural adherence molecule (ICAM-1) J Immunol. 1986;137:245–254. [PubMed] [Google Scholar]
- 23.Man N, Madri JA. PECAM-1: old friend, new partners. Curr Opin Cell Biol. 2003;15:515–524. doi: 10.1016/s0955-0674(03)00100-5. [DOI] [PubMed] [Google Scholar]
- 24.Piali L, Hammel P, Uhrek C, Bachmann F. CD31/PECAM-1 is al ligand for αvβ3 integrin involved in adhesion of leukocytes to endothelium. J Pharmacol Sci. 1996;92:7–12. [Google Scholar]
- 25.Cepinskas G, Savickiene J, Ionescu CV, Kvietys PR. PMN transendothelial migration decreases nuclear NFkB in IL-1b-activated endothelial cells: role of PECAM-1. J Cell Biol. 2003;161:641–651. doi: 10.1083/jcb.200212048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Auerbach R, Alby L, Morrissey W, Tu M, Joseph J. Expression of organ specific antigens on capillary endothelial cells. Microvasc. Res. 1985;29:401–411. doi: 10.1016/0026-2862(85)90028-7. [DOI] [PubMed] [Google Scholar]
- 27.Kumar S, West DC, Ager A. Heterogeneity in endothelial cells from large vessels and microvessels. Differentiation. 1987;36:57–70. doi: 10.1111/j.1432-0436.1987.tb00181.x. [DOI] [PubMed] [Google Scholar]
- 28.Lodge PA, Haisch GE, Huber SA, Martin B, Craigghead GC. Biological differences in endothelial cells depending upon organ differentiation. Trans Proc. 1991;23:216–218. [PubMed] [Google Scholar]
- 29.Page C, Rose M, Yacoub M, Pigott R. Antigenic heterogeneity of vascular endothelium. Am J Pathol. 1992;141:673–683. [PMC free article] [PubMed] [Google Scholar]
- 30.Petzelbauer P Bender JR, Wilson J, Pober JS. Heterogeneity of dermal microvasular endothelial cell antigen expression and cytokine responsiveness in situ and in cell culture. J Immunol. 1993;148:78–83. [PubMed] [Google Scholar]
- 31.Swerlick RA, Lee KH, Wick TM, Lawley TI. Human dermal microvascular endothelial cells but not human umbilical vein cells express CD36 in vivo and in vitro. J Immunol. 1992;148:78–83. [PubMed] [Google Scholar]
- 32.Aird W. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ. Res. 2007;100:174–190. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- 33.Huo Y, Xia L. PSGL-1 plays a crucial role in the selective recruitment of leukocytes into the atherosclerotic arterial wall. Trends Cardiovascular Med. 2009;19:140–145. doi: 10.1016/j.tcm.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Etter H, Althaus R, Eugster HP, Santamaria-Babi LF, Weber FL. IL-4 and IL-13 downregulates rolling adhesion of leukocytes to IL-1 or TNF-α activated endothelial cells by limiting the interval of E-selectin expression. Cytokine. 1998;10:395–403. doi: 10.1006/cyto.1997.0308. [DOI] [PubMed] [Google Scholar]
- 35.Fukushi J, Ono M, Morikawa W, Iwamoto Y, Kuwano M. The activity of soluble VACM-1 in angiogenesis stimulated by IL-14 and IL-13. J Immunol. 2000;165:2818–2823. doi: 10.4049/jimmunol.165.5.2818. [DOI] [PubMed] [Google Scholar]
- 36.Zurawski A, Chomarat P, Djossou O, Bidaud C, Mckenzie AN, Miossec A. The primary biding subunit of the human interleukin-4 receptor is also a component of the interleukin-13 receptor. J Bio Chem. 1995;270:13869–8. doi: 10.1074/jbc.270.23.13869. [DOI] [PubMed] [Google Scholar]
- 37.Zurawski SM, Vega F, Huyghe B, Zurawski G. Receptors for IL-13 and IL-4 are complex and share a novel component that functions in signal transduction. EMBO J. 1993;12:2663–67. doi: 10.1002/j.1460-2075.1993.tb05927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Avesa G, Punnonen J, Cocks B, de Waal Malefyt R, Vega J, Zurawski S, Zuraski G, deVries JE. An IL-4 mutant protein inhibits both IL-4 or IL-13 induced human immunoglobulin G4 (IgG4) and IgE synthesis and B cell proliferation: support for a common component shared by IL-4 and IL-13 receptors. J Expt Med. 1993;178:2213–17. doi: 10.1084/jem.178.6.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zurawski G, deVries JE. Interleukin 13, an interleukin 4-like cytokine that acts on monocytes and B cells, but not on T cells. Immunol Today. 1994;15:19–24. doi: 10.1016/0167-5699(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 40.Cardoso L, Araujo MI, Goes AM, Pacifico LG, Oliveira RR, Oliveira SC. Polymyxin B as inhibitor of LPS contamination of Schistosoma mansoni recombinant proteins in human cytokine analysis. Microb Cell Fact. 2007;6:1–6. doi: 10.1186/1475-2859-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wanidworanum C, Strober W. Predominant role of tumor necrosis factor-alpha in human monocytes IL-10 synthesis. J Immunol. 1993;151:6853–6861. [PubMed] [Google Scholar]
- 42.Chamoun F, Burne M, O'Donnell M, Rabb H. Pathophysiologic role of selectins and their ligands in ischemia reperfusion injury. Front BioSci. 2000;5:E103–109. doi: 10.2741/chamoun. [DOI] [PubMed] [Google Scholar]
- 43.Zhang RL, Chopp M, LiY, Zaloga C. Anti-ICAM-1 antibody reduces ischemic cell damage after transient middle cerebral artery occlusion in the rat. Neurology. 1994;44:1747–1751. doi: 10.1212/wnl.44.9.1747. [DOI] [PubMed] [Google Scholar]
- 44.Bowes MP, Zivin JA, Rothlein R. Monoclonal antibody to the ICAM-1 adhesion site reduces neurological damage in a rabbit cerebral embolism stroke model. Exp Neurol. 1993;119:215–219. doi: 10.1006/exnr.1993.1023. [DOI] [PubMed] [Google Scholar]
- 45.Noseworthy JH, Kirkpatrick P. Natalizumab. Nat Rev Drug Discov. 2005;4:101–102. doi: 10.1038/nrd1637. [DOI] [PubMed] [Google Scholar]
- 46.Marecki S, Kirkpatrik P. Efalizumab. Nat Rev Drug Discov. 2004;3:473–474. doi: 10.1038/nrd1420. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.