Abstract
Obesity is a metabolic disease characterized by chronic inflammation. Early studies indicated that adipose tissue from obese mice contains more saturated fatty acids and that the saturated fatty acids activate TLR4-mediated inflammatory signaling, which contributes to inflammation in adipose tissue. In this study, we determined fatty acid profile in non-adipose tissues from obese (db/db) mice and compared with that from lean mice. Unexpectedly, in contrast to a significant increase in saturated and decrease in unsaturated fatty acid in adipose tissue from obese mice, the non-adipose tissues from obese mice exhibited a significant decrease in saturated and increase in unsaturated fatty acid compared with that from lean mice. The liver from obese mice had a 15% and 32% decrease in palmitic acid and stearic acid, and a 20% increase in linoleic acid; the spleen had a 32% and 60% decrease in palmitic acid and stearic acid, and a 70% and 50% increase in oleic acid and linoleic acid; and the pancreas had a 50% and 75% decrease in palmitic acid and stearic acid, and a 130% and 113% increase in oleic acid and linoleic acid. These data suggest that, different from adipose tissue where elevated saturated fatty acids contributes to inflammation, fatty acids per se in non-adipose tissues such as liver, spleen and pancreas may not contribute to inflammatory responses in obese mice.
Keywords: Fatty acid, adipose tissue, non-adipose tissue, TLR4, NF-κB, inflammation, obesity
Introduction
Obesity is the abnormal accumulation of adipose tissues. While the classic function of adipose tissue is to store fatty acids after food intake, and to release them during fasting state to keep energy status balanced [1], accumulating evidence indicates that adipose tissue is also actively involved in a variety of metabolic regulations. Obesity is closely related to blood pressure elevation, HDL decrease in blood, impaired glucose metabolism, and atherogenic dyslipide-mia, which all together, are referred to as metabolic syndrome [2], leading to high susceptibility of cardiovascular disease and type 2 diabetes mellitus [3-4]. The rapidly increasing prevalence of metabolic syndrome was predicted to be one fourth of the populations in U.S. in the very near future [2].
Obesity is a chronic inflammatory disease [5], with macrophage infiltrating [6] and dysregulation of production of proinflammatory and anti-inflammatory cytokines [7] in adipose tissue. Toll like receptor 4 (TLR4) is a membrane spanning receptor which can induce the activation of nuclear factor-κB (NF-κB), a critical regulator of inflammatory responses [8], when binding to LPS ligand [9-10]. The NF-κB signaling pathway was found to be activated in insulin responsive tissues like liver, skeletal muscle and adipose tissue [11-14]. Recent studies demonstrated that saturated fatty acids activate TLR4-mediated NF-κB, and unsaturated fatty acids impedeTLR4-mediated NF- κB activation [15-16]. Interestingly, It has been shown that the ratio of palmitic acid (16:0) to linoleic acid (18:2) is significantly greater in epididymal adipose tissue of obese mice (ob/ob) than that of normal non-obese mice [17], suggesting that fatty acids in adipose tissue may contribute to inflammation observed in obesity. Nevertheless, whether the same mechanism can be applied to other tissues besides adipose tissue, contributing to inflammatory responses in obesity, still remains to be elucidated.
The objective of this study was to determine the fatty acid profile in non-adipose tissues from obese mice and compare it with the established role of fatty acids from adipose tissue. We report that the difference of saturated and un-saturated fatty acids composition in adipose tissue of obese mice compared with lean mice is opposite in non-adipose tissues, such as liver, spleen and pancreas, suggesting that saturated fatty acids per se may not contribute to inflammation in these non-adipose tissues.
Methods and methods
Animals
db/db obese mice [C57BLKS/J-leprdb/db) and lean mice [C57BLKS/J) were purchased from Jackson laboratory. The animals were fed with standard laboratory chow diet and used at 4-month old. Animal care and experiments were approved by the Institutional Animal Care and Use Committee of the University of Kentucky.
Quantification of fatty acids with gas-chromatography/mass spectrometer (GC/MS)
Quantification of fatty acids was conducted as we previously described[18]. Briefly, epididymal adipose tissues obtained from db/db obese and lean mice were homogenized in chloroform (containing 100 μg BHT/ml) with a glass rod. Liver, spleen and pancreas tissues from obese and lean mice were homogenized in MBST/OG buffer with a douncer pestle and the lipids were extracted with Folch/BHT reagent [19] and lower phase of the extract was collected and dried under N2. 50 μl Heptadecanoic acid (17:0) (5 mg/ml chloroform) was added to extract as an internal standard. Tissue total lipids were methyl esterified with BF3/Methanol (Supelco). The fatty acid methyl ester was analyzed using a gas chromatography system, Agilent 6890 GC G2579A system (Agilent) equipped with an OMEGAWAX 250 capillary column (Supelco) and a flame ionization detector. An Agilent 5973 mass selective detector was used to identify target peaks.
HEK-Blue cell culture and analysis of TLR4-mediated NF-κB Activation
HEK-Blue cells (InvivoGen) stably expressing TLR4, CD14, MD2, and a NF-κB reporter were cultured in a 96-well plate in complete DMEM (Invitrogen) containing high glucose 4.5 g/L, 10% Endolow FBS, 1% P/S, 1% glutamine, Nor-mocin and Selection until 90% confluency and treated with 200 μl 100 μM d-BSA bound saturated or unsaturated fatty acid solution for 24 h. Cells treated with 200 μl 1% d-BSA in PBS solution for 24 h were used as a control. Then, 100 μl of the culture supernatant was mixed with 100 μl HEK-Blue detection medium and incubated at 37 °C for 2 h. Activated TLR4 induces NF-κB reporter expression, catalyzing the HEK-Blue detection medium to blue. Absorption at 650 nm was measured to quantify the blue color.
Statistical analysis
Data are expressed as means ± SD. Data analysis was performed using two-sided Student's t-test, and differences are considered significant at P < 0.05.
Results
Increased saturated fatty acid and reduced unsaturated fatty acid in adipose tissue of obese mice
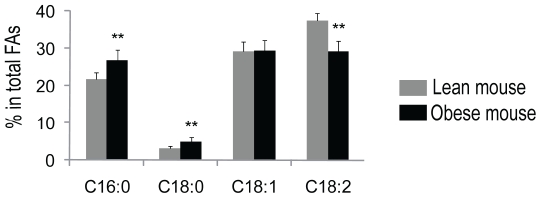
Early study showed that the ratio of palmitic acid (16:0) to linoleic acid (18:2) is significantly greater in epididymal adipose tissue of obese mice (ob/ob) than that of normal non-obese mice [17]. To confirm the finding, we determined fatty acid profile in the adipose tissue of obese mice (db/db) and lean mice. As shown in Table 1, myrstic acid (14:0), palmitic acid (16:0), palmitoleic acid (16:1), stearic acid (18:0), oleic acid (18:1), linoleic acid (18:2), and α-linoleic acid (18:3) were identified from adipose tissue. These fatty acids comprise > 95% of total fatty acids. Compared to lean mice, obese mice had significant increases in saturated fatty acids, a 22% increase in palmitic acid and 55% increase in stearic acid, and a significantly decrease in unsaturated fatty acids, a 20% reduction in linoleic acid (P < 0.01, Figure 1).
Table 1.
Fatty acid composition in adipose tissue of obese mice and lean mice
| Fatty Acid | C14:0 | C16:0 | C16:1 | C18:0 | C18:1 | C18:2 | C18:3 |
|---|---|---|---|---|---|---|---|
| Lean mice fatty acid composition % | 1.29 | 21.68 | 4.52 | 3.21 | 29.15 | 37.40 | 2.31 |
| Obese mice fatty acid composition % | 1.53 | 26.85 | 5.44 | 4.99 | 29.34 | 29.29 | 2.49 |
Figure 1.
Fatty acid composition in adipose tissue—obese mice versus lean mice. Mice epididymal adipose tissues were extracted with chloroform (containing 100 μg BHT/ml), followed by methylesterification of total fatty acids with BF3/Methanol. Analysis of fatty acids was performed using a gas chromatography system, and heptadecanoic acid (17:0) was used as an internal standard for data analysis. Adipose tissues of obese mice have increased saturated fatty acids and decreased unsaturated fatty acids. Dark bars are representative of obese mice, and light bars represent lean mice. n = 10. Data are expressed as means ± SD. **P < 0.01 versus lean mice.
Reduced saturated fatty acids and increased unsaturated fatty acids in non-adipose tissues of obese mice
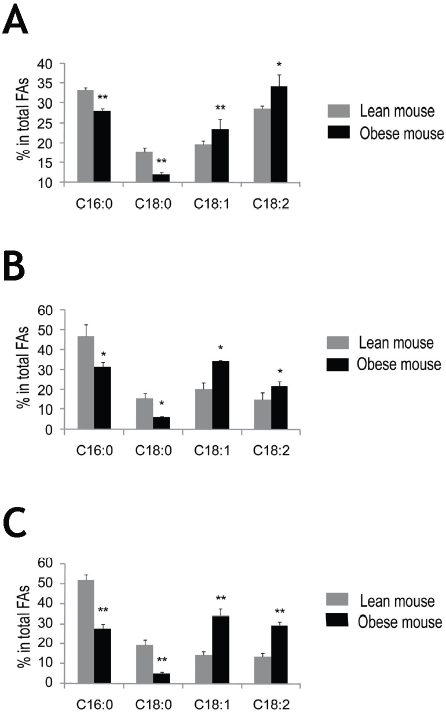
We then asked whether there are similar changes on fatty acid profile in non-adipose tissues in obese mice versus lean mice. We quantified fatty acids in liver, spleen and pancreas in obese and lean mice. Unexpectedly, in liver of obese mice, the unsaturated fatty acids distribution was significantly increased compared with lean mice, 20% increase in oleic acid and linoleic acid, (P < 0.05, Figure 2A). In contrast, the saturated fatty acids, palmitic acid and stearic acid were reduced by 15% and 32%, respectively, in obese mice (P < 0.01, Figure 2A). Similar to the liver, the increase in unsaturated fatty acid and decrease in saturated fatty acid distribution was observed in the spleen in obese mice, a 70% increase in oleic acid and 50% increase in linoleic acid, and 32% and 60% decline of palmitic acid and stearic acid, respectively (P < 0.05, Figure 2B). Similar data were also obtained in pancreas: a 50% and 75% decrease in palmitic acid and stearic acid, and a 130% increase in oleic acid and 113% increase in linoleic acid in obese mice (P < 0.01, Figure 2C).
Figure 2.
Fatty acid composition in liver, spleen and pancreas—obese mice versus lean mice. The liver, spleen and pancreas tissues were homogenized in MBST/OG buffer and extracted with Folch/BHT reagent, followed by methyl esterification of total fatty acids with BF3/Methanol. Analysis of fatty acids was performed using a gas chromatography system, and heptadecanoic acid (17:0) was used as an internal standard for data analysis. Obese mice exhibit reduced saturated fatty acids and increased unsatu-rated fatty acids distribution in the liver (A), spleen (B) and pancreas (C). Dark bars represent obese mice, and light bars represent lean mice. n = 4. Data are expressed as means ± SD. *P < 0.05, **P < 0.01 versus lean mice.
Saturated fatty acids promote TLR4-mediated NF-κB activation; in contrast, unsaturated fatty acids impede TLR4-mediated NF-κB activation
NF-κB induces the expression of proinflamma-tory cytokines in human adipose tissue [14]. Using a luciferase assay, early studies demonstrated that saturated fatty acids induce TLR4-mediated NF- κB activation, and unsaturated fatty acids impedeTLR4-mediated NF- κB activation [15-16]. Here we employed an alternative operating technique, HEK-Blue cell system, to elucidate the effect of fatty acid on TLR4-mediated NF-κB activation. The HEK-Blue cell stably expresses TLR4, CD14, MD2, and a NF-κB reporter. So it is a simple and convenient system to test TLR4-mediated inflammatory signaling. As shown in Figure 3, NF-κB activation was upregulated by stearic acid compared to BSA control (P < 0.01, Figure 3). In reverse, arachidonic acid attenuated TLR4-mediated NF-κB activation remarkably (P < 0.01, Figure 3).
Figure 3.
Effect of fatty acids on TLR4-mediated NF-κB activation. HEK-Blue cells were grew to 90% con-fluency and treated with 200 μl 100 μM fatty palmitic acid, stearic acid, oleic acid or arachidonic acid solution respectively, or 1% BSA in PBS as a control for 24 h. 100 μl of the culture supernatant was mixed with 100 μl HEK-Blue detection medium and incubated at 37°C for 2 h. Absorption at 650 nm was measured to quantify the blue color. Saturated fatty acids promote TLR4-mediated NF-κB activation while unsaturated fatty acids suppress TLR4-mediated NF-κB activation. Data are expressed as means ± SD of triplicate determinations. **P < 0.01 versus cells with no fatty acid solution or BSA treatment.
Palmitic acid seemed to enhance NF-kB activation as well but to a lesser extent compared to stearic acid. Oleic acid displayed inhibitory effect on TLR4-mediated NF-κB activation, but due to the relatively high value of standard deviations, the difference was not statistically significant (Figure 3).
Discussion
In this study, we compared the adipose tissue fatty acid profile between obese and lean mice. Our data confirm the early report that the ratio of palmitic acid (16:0) to linoleic acid (18:2) is significantly greater in epididymal adipose tissue of obese mice than that of normal non-obese mice [17]. As shown in Table 1 and Figure 1, there is a significant increase in stearic acid ratio in adipose tissue from obese mice compared with that from lean mice. Based on Figure 3, the stearic acid (18:0) is a more potent stimulator of TLR4 than palmitic acid (16:0). Thus, the stearic acid may be a major stimulator of inflammation among fatty acids in adipose tissue.
We then compared the non-adipose tissue fatty acid profile between obese and lean mice to determine whether the increase in saturated and decrease in unsaturated fatty acid presents in non-adipose tissue. Oppositely, we found that the non-adipose tissues, such as liver, spleen and pancreases, have significantly lower saturated and higher unsaturated fatty acid distribution in obese mice compared with that of lean mice. Based on Figure 3, the fatty acid profile of non-adipose tissue implies that the fatty acids from non-adipose tissue per se may play an inhibitory role in modulating TLR4/NF-κB signaling in obese mice. We should point out that other factors in non-adipose tissues also contribute to inflammatory response in non-adipose tissues.
Acknowledgments
This work was supported by grants to XAL from American Heart Association (0530241N), NIH (R01GM085231 and 3R01GM085231-02S1) and Children's Miracle Network.
Glossary
Abbreviations
- TLR4
Toll Like Receptor 4
- NF-κB
Nuclear Factor-κB
- BF3
Boron Trifluoride
- DMEM
Dulbecco's Modified Eagle's Minimal Essential Medium
- FBS
Fetal Bovine Serum
- PBS
Phosphate Buffered Saline
- d-BSA
Defatted Bovine Serum Albumin
References
- 1.Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008;29(24):2959–71. doi: 10.1093/eurheartj/ehn387. Dec. [DOI] [PubMed] [Google Scholar]
- 2.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) Jama. 2001;285(19):2486–97. doi: 10.1001/jama.285.19.2486. May 16. [DOI] [PubMed] [Google Scholar]
- 3.Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care. 2005;28(11):2745–9. doi: 10.2337/diacare.28.11.2745. Nov. [DOI] [PubMed] [Google Scholar]
- 4.Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366(9497):1640–9. doi: 10.1016/S0140-6736(05)67663-5. Nov 5. [DOI] [PubMed] [Google Scholar]
- 5.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340(2):115–26. doi: 10.1056/NEJM199901143400207. Jan 14. [DOI] [PubMed] [Google Scholar]
- 6.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–808. doi: 10.1172/JCI19246. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96(9):939–49. doi: 10.1161/01.RES.0000163635.62927.34. May 13. [DOI] [PubMed] [Google Scholar]
- 8.Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336(15):1066–71. doi: 10.1056/NEJM199704103361506. Apr 10. [DOI] [PubMed] [Google Scholar]
- 9.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, et al. Defective LPS signaling in C3H/ HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282(5396):2085–8. doi: 10.1126/science.282.5396.2085. Dec 11. [DOI] [PubMed] [Google Scholar]
- 10.1Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274(16):10689–92. doi: 10.1074/jbc.274.16.10689. Apr 16. [DOI] [PubMed] [Google Scholar]
- 11.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11(2):183–90. doi: 10.1038/nm1166. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11(2):191–8. doi: 10.1038/nm1185. Feb. [DOI] [PubMed] [Google Scholar]
- 13.Kim JK, Kim YJ, Fillmore JJ, Chen Y, Moore I, Lee J, et al. Prevention of fat-induced insulin resistance by salicylate. J Clin Invest. 2001;108(3):437–46. doi: 10.1172/JCI11559. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lappas M, Yee K, Permezel M, Rice GE. Sulfasalazine and BAY 11-7082 interfere with the nuclear factor-kappa B and I kappa B kinase pathway to regulate the release of proinflammatory cytokines from human adipose tissue and skeletal muscle in vitro. Endocrinology. 2005;146(3):1491–7. doi: 10.1210/en.2004-0809. Mar. [DOI] [PubMed] [Google Scholar]
- 15.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem. 2001;276(20):16683–9. doi: 10.1074/jbc.M011695200. May 18. [DOI] [PubMed] [Google Scholar]
- 16.Suganami T, Tanimoto-Koyama K, Nishida J, Itoh M, Yuan X, Mizuarai S, et al. Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macro-phages. Arterioscler Thromb Vasc Biol. 2007;27(1):84–91. doi: 10.1161/01.ATV.0000251608.09329.9a. Jan. [DOI] [PubMed] [Google Scholar]
- 17.Haessler HA, Crawford JD. Alterations in the fatty acid composition of depot fat associated with obesity. Ann N Y Acad Sci. 1965;131(1):476–84. doi: 10.1111/j.1749-6632.1965.tb34813.x. Oct 8. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Sabeva NS, Bhatnagar S, Li X, Pujol A, Graf GA. ABCD2 is abundant in adipose tissue and opposes the accumulation of dietary erucic acid (C22:1) in fat. J Lipid Res. 2009 doi: 10.1194/jlr.M900237-JLR200. Jun 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. May. [PubMed] [Google Scholar]