Abstract
The ultra-structure of Mycobacterium tuberculosis (MTB) was examined by transmission electronic (TEM)) and atomic force microscopy (AFM). The study was performed to describe the morphology of susceptible, multidrug-resistant (MDR), extensively drug-resistant (XDR) and extremely drug-resistant tuberculosis isolates (XXDR-TB) during their exponential growth phase. Four types of cell division were observed and described. While three of them (symmetrical, asymmetrical and branching type) occurred in all isolates studied, the fourth one (adapted type) was seen only in XDR and XXDR-TB bacilli. In the fourth type of cell division, a rod shaped mother cell produced a small round shape bacillus (0.3-0.5 μm). These round cells were different from buds or polar division, but similar to terminal endospores without showing the typing heat resistance. Based on the present observation, we suggest that XDR-and XXDR-TB bacilli accommodate changes helping them to overcome the hostile environment. Viewed under AFM, the other frequently detected shapes in MTB isolates were oval, V, Y and multi-branching filaments. These shape variation confirmed pleomorphic phenomena in MTB populations and the specific features of pan-resistant strains.
Keywords: Extremely drug resistant tuberculosis, atomic force microscopy, cell division
Introduction
One hundred twenty eight years after Robert Koch's discovery of tubercle bacillus, this organism is still affecting mankind and will continue to do so in the near future [1]. Today, the appearance of extensively drug- resistant tuberculosis (XDR-TB) in addition to multidrug- resistant TB (MDR-TB) has further complicated TB control at the global level [2,3]. Equally alarming is the emergence of a new form of virtually incurable drug-resistant isolates, known as extremely drug resistant TB (XXDR-TB) [2,4]. XXDR-TB isolates showed in-vitro resistance to all first- and second-line drugs tested [2]. Such strains brought us back to the pre-antibiotic era and underlined the need to develop urgently new drugs and apply correctly the existing policies and strategies of TB control programmes [5]. In recent years, significant progress has been achieved in characterizing resistant isolates [6]. Unfortunately, our understanding of these bacilli at the ultra-structural level is still very limited for several reasons, including professional risk and available technology. We recently documented the morphological changes occurring in resistant and susceptible TB strains [7]. Furthermore, we showed cell shape alterations in exponential phase of XDR-TB strains [8]. These populations were clearly different: one showed ordinary pattern (70–80%), a second exhibited round or oval shape (15–20%) and the third showed extra-ordinary thick cell wall (21–26 nm) with features similar to stationary or anaerobic dormant bacilli (5–7%) [8]. Indeed, cell shape alteration in Mycobacterium tuberculosis has been reported by many researchers, e.g., in activated macrophages, tubercle bacilli elongate or enter in non-replicating persistence (NRP) state [9]. The literature described that when a patient is defined cured, he is cured of the proliferation cells only (and not necessarily of filterable or dormant bacilli) [10]. Therefore, the shape of bacilli can be seen as a marker of virulence, of biological defense against specific immune responses or of cell-division. Dahl demonstrated the existence of V and Y forms of cells at mid-log phase (OD 600 1.1) of Mycobacterium tuberculosis [11]. Later on Thanky et al [12], re-confirmed the unusual features of the cell cycle in Mycobacterium tuberculosis. The present study was aimed at demonstrating the cell-division morphological patterns (i.e., symmetrical, asymmetrical and branching) in resistant (MDR-, XDR- and XXDR-TB) and susceptible isolates.
Materials and methods
Bacterial strains
Primary isolation and culturing of Mycobacterium tuberculosis isolates from sputum specimen were conducted in accordance to procedures manual [13]. Susceptible, MDR-, XDR-and XXDR-TB strains were isolated from patients diagnosed at National Research Institute of TB and Lung Diseases (NRITLD) in Tehran. Drug susceptibility test against isoniazid (INH), rifampicin (RF), streptomycin (SM) and etham-butol (ETB) were performed by the proportional method on Löwenstein-Jensen media at a concentration of 0.2, 40, 4.0 and 2.0μg/ml, respectively. Drug-susceptibility test against second-line drugs (kanamycin, amikacin, capreomycin, ciprofloxacin, cycloserine, ethionamide and para -aminosalicylic acid) was performed using two critical proportions of 1% and 10% [14]. Selected isolates were identified as M. tuberculosis by biochemical tests, including niacin production, catalase activity, nitrate reduction, pigment production and growth rate [14]. Thereafter, a loop of bacterial from Löwenstein-Jensen culture media was inoculated into Middle brook 7H9 broth supplemented with 0.2% glycerol and 10% Middle brook OAT enrichment. Ten batches of isolates from each class (drug susceptible, MDR-, XDR- and XXDR-TB) belonging to different patients were included in this study. Cells at an optical density (OD) of 0.6 at 600 nm were used for further experiments. These cells were first centrifuged at 800 rpm for 5 min, then the supernatant was adjusted to an OD of 580nm, corresponding to 6.3 × 107 colony-forming units of Mycobacterium tuberculosis per ml. Ten micro liter of this supernatant were stained according to the Z-N and Gram methods [13]. In another set up, 100 μL of the above suspension were exposed to 65°C heat for 35 minutes and plated on 7H10 agar plates, the growth was monitored in different intervals for 2 months.
Transmission electron microscopy (TEM)
Both negative staining and ultrathin section were used to study the general morphology of M. tuberculosis. For ultrathin section: bacterial suspension was prefixed in 4% glutaraldehyde in 0.1 M sodium cacodylate buffer. Five hundred micro litter of horse serum was then added to the decanted sample following centrifugation for 10 minutes at 3,000 rpm. Solidified samples were diced and thereafter processed according to standard protocol for TEM [15]. Briefly, the sample were washed in 0.1 M sodium cacodylate buffer (pH= 7.2), post fixed in 1% osmium tetraoxide for 1 h at 4°C and dehydrated in ascending grades of ethanol (35%,50%,75%,95% and 100%). Samples were embedded in Spur resin and following overnight polymerization, ultrathin sections were obtained using ultra microtome. Ultra thin sections on copper grids were stained with urinal acetate and lead citrate, washed in double distilled water and viewed under transmission electron microscope (Hitachi, H7100). Negative staining was performed using 0.5% uranyl acetate.
Atomic force microscopy (AFM)
AFM images were recorded in contact mode using an optical lever microscope equipped with a liquid cell (Nanoscope IV Multimode AFM; Veeco Metrology Group, Santa Barbara, CA [16]. To image mycobacteria, the bacteria were immobilized by mechanical trapping onto Isopore Polycarbonate membrane (Millipore), with pore size similar to the cell size. After filtering a concentrated cell suspension, the filter was gently rinsed with deionized water, carefully cut (1 × 1 cm), attached to a steel sample puck (Veeco Metrology Group LLC) using a small piece of adhesive tape and the mounted sample was transferred into the AFM liquid cell. Both height and deflection images were recorded, using oxide-sharpened micro fabricated Si3N4 cantilevers (Microlevers; Veeco Metrology LLC) with spring constant of 0.01 Nm−1 [17).
Statistical analysis
Expected and observed frequencies of cell division types in drug susceptible and MDR-versus XDR- and XXDR-TB cases were compared by Fisher Exact test. A P value ≥0.05 was considered statistically significant.
Reading methodology
Overall, for each strain 25 ultra-thin sections were observed under TEM, while AFM was performed on 20 steel sample packs.
Results and discussion
Cell division of various strains of M. tuberculosis (Susceptible, MDR-, XDR- and XXDR-TB Bacilli)
As shown in Figures 1-4, four types of cell division (symmetrical, asymmetrical, branching and adapted) were observed in Mycobacterium tuberculosis. While in drug susceptible (73.3%) and MDR-TB strains (71%) symmetrical division was observed more frequently, in XDR- and XXDR-TB cases, asymmetrical (42.2%; 44%) branching (20%; 24.4%) and adapted cell - division (11.1%; 13.3%) were the commonest findings (Table 1). In fact, symmetrical division was lower to average of 20% in highly resistant strains (P=0.0005). Symmetrical division occurred in both rod and round shape bacilli (Figures 1 and 5). To our knowledge this is the first report showing round shape bacilli are dividing into two equal daughter cells (Figure 5). Round shape bacilli are frequently seen among XDR and XXDR-TB cell populations. They are very small with average size of 0.3μm to 0.5μm. These round XDR- or XXDR-TB bacilli are different from morphological transition that occurred in the stationary phase of M. tuberculosis [18]. In this phase the bacilli will return to rod shape in a high-nutrient medium, but in these resistant strains, the round shape cell remained round even in next generations. Generally, when the bacterial cells acquire the cocci form, they attach firmly to the surface; since environmental challenges are then distributed over more individuals, they have more possibility to survive [19]. In addition, round form bacilli may spread and transmit faster than rod shape bacilli (1-3μm). In asymmetrical division (as shown in Figure 2), unlike the symmetrical division the inner layer is not dividing the cells into equal parts. Therefore, after rupturing the outer cell wall, one of the daughter cells is bigger than the other one [11,20]. Sometimes during last stage of cell-division the outer layers are still inter-twined together whereas, the inner layer has been ruptured. This results in V-shape bacteria and is referred as a snapping post-fission movement (Figure 2). In susceptible and MDR-TB isolates, The average of cells showing asymmetrical type of divisions is 18% in susceptible and MDR-TB isolates, and 39% in XDR- and XXDR-TB isolates (P=0.0012). Another type of division is the “branching” type of cell division is also more common in XDR- and XXDR-TB isolates (Figure 3); sometimes branching resulting in Y-shape, oval and multi-branching bacilli (Figures 3 and 6). Previous study has shown that Mycobacterium tuberculosis grows from the ends of cell and not along the length of the cylinder as seen in other well-characterized rod-shape bacteria [12]. This might be true for susceptible isolates but in highly resistant strains we observed branches along the cylinder (Figure 3c). Recently Dahl has observed V and Y forms of cells at mid-log phase (OD600 1.1) of Mycobacterium tuberculosis. He described two features of cell division for these shapes; snapping and transient branching. Later on Thanky et al [12], suggested that the deposition of newly synthesized peptidoglycon in mycobacteria is restricted to the poles of cell. In our study, the susceptible and MDR-TB isolates follow the same rule (Figure 1), but this may not be fully applicable in XDR-and XXDR-TB bacilli. Generally, Mycobacterium tuberculosis faces two major challenges when dividing; they must maintain their normal rod-shape and they need to regulate cell division in response to environmental stimuli. In XDR- and XXDR-TB bacilli, about 11% has a new form of division which we named “adapted type of division” (Figure 4). During what appear to be an early stage of division, the enlargement of electron transparent zone (outer layer) towards head region occurred (Figure 4a). Then elongated layer derived from outer electron dense (peptidoglycon) and outer plasma membrane extended towards moderately dense substances. This extension folds into tubular shape in one side (Figure 4b). Finally, there was formation of round bodies inside the XDR- and XXDR-TB bacilli (Figure 4c) that were detached detached from mother cells (Figure 4d). These structures are different from buds or polar division. Recently, a group of researcher in Sweden suggested sporulation as a lifecycle adapted by MTB under dormant condition [21]. But other studies cast doubt on spore formation by MTB [22]. The round shape bacilli very much resembled the spore, although these cells could not withstand the temperature above 65°C, and they also fail to form heat resistant forming colonies. Therefore, they were not spore. In addition, these bacilli increased their number by symmetrical type of cell-division. At present we have no explanation for these types of cell-division, and suggest further molecular studies to investigate their life-cycle.
Figure 1.
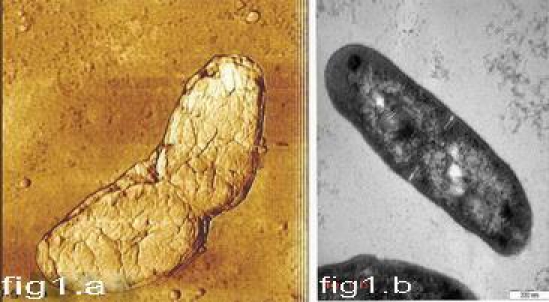
Symmetrical type of cell division using AFM(1.a)andTEM (1.b).
Figure 4.
Different stages (4a- d) of adapted type of cell division in XDR and XXDR-TB cells. The round cells formed inside the rod–like mother cells(4.d).
Table 1.
Summary of the TEM and AFM findings on the number and ratio of each cell type division in various strains of M. Tubuculosis. (* P is significant)
| Type of Cell division | Type of strains | ||||
|---|---|---|---|---|---|
| A a)Drug-susceptible | B b)MDR-TB | c)XDR-TB | d)XXDR-TB | P value (a + b) vs (c+d) | |
| Symmetrical | 33/45 section(73.3%) | 32/45 section (71 %) | 12/45 section (26.6%) | 8/45(17.7%) | 65/20 P=0.0005* |
| Asymmetrical | 9/45section(20%) | 9/45 section (20%) | 19/45 section(42.2%) | 20/45(44.4%) | 18/39 P=0.0012* |
| Branching | 4/45 section(6.6%) | 4/45 section (8.8. %) | 9/45 section( 20%) | 11/45(24.4%) | 8/20 P=0.022* |
| Adapted | 0/45 section(0%) | 0/45 section(0%) | 5/45(11.1%) | 6/45 (13.3%) | 0/11 p=0.0007* |
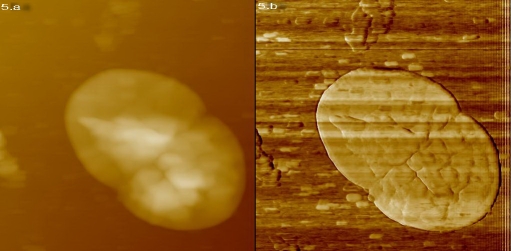
Figure 5.
The round XXDR-TB cells under AFM in height (5.a) and deflection mode (5.b): they are dividing into two equal daughter cells . The size of the cells is about 0.3μm.
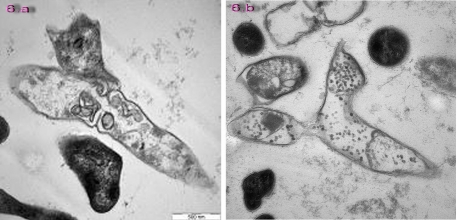
Figure 2.

Asymmertical type of cell division under AFM (2.a) and TEM(2.b).
Figure 3.
Different types of branching in Mycobacterium tuberculosis. Multi-branching (3.a, 3.b and 3.d) was frequent in XDR and XXDR-TB cells.
Figure 6.
The Y-shape XDR-TB bacilli under TEM ( 6.a & 6.b both are Y-shape TB bacilli).
Shape and size of various strains of M. tuberculosis (Susceptible, MDR and XDR and XXDR-TB Bacilli)
Under AFM and TEM, five different types of cell shapes were observed. They were rod, round, oval, V and Y-shape (Figures 1, 2, 6 and 7). They are present in exponential phase of all studied isolates. However, their ratio in a population is different among strains with various drug susceptibility. In susceptible and MDR-TB strains, rod shapes bacilli were more frequent (85%), whereas in XDR- and XXDR-TB, the round (35%), oval (15%) or sometimes even multiple branching (15%) could be seen. These shape variations may confirm plemorphism phenomena in Mycobacterium tuberculosis. Plemorphism has been described in other rod-like actinobacteria such as Rhodococcus, Arthobacter, Cornebacterium and Nocardia species [23]. Generally, pleomorphic bacteria can and do change shape and some display a morphological progression during life cycles. These morphological changes might accommodate changes in uptake and metabolic flux in response to altered environments. The pleomorphic phenomenon has been reported in Mycobacterium smegmatis [24]. Even in Mycobacterium tuberculosis it has been shown their morphological changes during persistence in lungs as well as starvation in-vitro [10]. Recently, the importance of morphology in determining the host immune responses was highlighted in experiments by Champion and Mitragotri [25]. These authors found that shapes and not size was the dominant factor in determining whether particles were phagocytized. In this context Chauhan et al [26], showed that TB bacilli elongatesafter being phagocytized. Here, we found that the majority of XDR- and XXDR-TB is round and small in size. These changes may enhance the ability of bacilli to escape specific immune responses and to better accommodate changes in response to altered environment. Taken together, the XDR-and XXDR-TB bacilli appear to be morphologically different from drug-susceptible and MDR-TB ones. These morphological changes may help them escape the immune response and / or overcome the inhibitory effect of most antibiotics. The work presented here may help to improve our understanding of the adaptation and life-cycle of resistant bacilli, and help scientists to identify targets for novel therapies.
Figure 7.
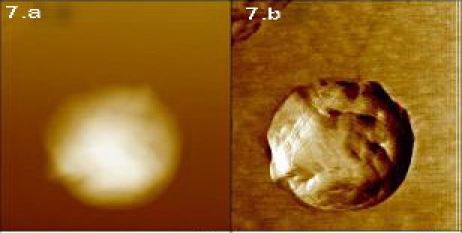
The round shape XXDR-TB bacilli under AFM in height (7.a) and deflection mode (7.b).
Acknowledgments
The authors wish to thank the staff of Microbiology unit at the Republican Research and Practical Centre for Epidemiology and Microbiology (Minsk, Belarus). Also we sincerely thank the TB laboratory staff at the Mycobacteriology Research Centre/NRITLD (Tehran, Iran).
This study was sponsored by Ministry of Health, Medical Education, Deputy of Research (2009-2010) and National Research Institute of TB & Lung Diseases (NRITLD), Tehran, Iran.
References
- 1.Migliori GB, Loddenkemper R, Blasi F, Raviglione MC. 125 years after Robert Koch's discovery of the tubercle bacillus: the new XDRTB threat. Is “science” enough to tackle the epidemic? Eur Respir J. 2007;29:423–427. doi: 10.1183/09031936.00001307. [DOI] [PubMed] [Google Scholar]
- 2.Migliori GB, De Iaco G, Besozzi G, Centis R, Cirillo DM. First tuberculosis cases in Italy resistant to all tested drugs. Euro surveill. 2007;12:20–21. doi: 10.2807/esw.12.20.03194-en. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs-worldwide. MMWR Morb Mortal Wkly Rep. 2006;55:301–305. [PubMed] [Google Scholar]
- 4.Velayati AA, Masjedi MR, Farnia P, Tabarsi P, Ghanavei J, ZiaZarifi AH, Hoffner S. Emergence of new forms of totally drug-resistant tuberculosis bacilli:super extensively drug – resistant tuberculosis or totally drug resistant strains in Iran. Chest. 2009;136:420–425. doi: 10.1378/chest.08-2427. [DOI] [PubMed] [Google Scholar]
- 5.Lenaerts AJ, De Groote MA, Orme IM. Pre-clinical testing of new drugs for tuberculosis: current challenges. Trends Microbiol. 2008;16:48–54. doi: 10.1016/j.tim.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Comas I, Gagneux S. The past and future of tuberculosis Research. PLOS Path. 2009;5:1–7. doi: 10.1371/journal.ppat.1000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Velayati AA, Farnia P, Masjedi MR, Tengku AI, Rafiuz ZH, Ho OK, Ghanavei J, Farnia P, Kabarei AN, Tabarsei P, Omar AR, Varahram M. Differences in cell wall thickness between resistant and non resistant of Mycobacterium tuberculosis ; using transmission electron microscopy. J chemo. 2009;55:303–307. doi: 10.1159/000226425. [DOI] [PubMed] [Google Scholar]
- 8.Velayati AA, Farnia P, Masjedi MR, Ibrahim TA, Tabarsei P, Haroun RZ, Kuan HO, Ghanavi J, Farnia P, Varahram M. Totally drug–resistant tuberculosis strains: evidence of adaptation at the cellular level. Eur Respir J. 2009;34:1–3. doi: 10.1183/09031936.00081909. [DOI] [PubMed] [Google Scholar]
- 9.Chauhan A, Madiraju MV, Fol M, Lofton H, Maloney E, Reynolds R, Rajagopalan M. Mycobacterium tuberculosis cells growing in macrophages are filamentous and deficient in FtsZ rings. J Bacteriol. 2006;188:1856–1865. doi: 10.1128/JB.188.5.1856-1865.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khomenko AG. The variability of Mycobacterium tuberculosis in patients with cavitary pulmonary tuberculosis in the course of chemotherapy. Tubercle. 1987;68:243–253. doi: 10.1016/0041-3879(87)90064-x. [DOI] [PubMed] [Google Scholar]
- 11.Dahl JL. Electron microscopy analysis of Mycobacterium tuberculosis cell division. FEMS Microbiol Lett. 2004;240:15–20. doi: 10.1016/j.femsle.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Thanky NR, Young DB, Robertson BD. Unusual features of the cell cycle in mycobacteria: polar –restricted growth and the snapping – model of cell division. Tubercle. 2007;87:231–236. doi: 10.1016/j.tube.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Kent PT, Kubica GP. Atlanta, AG: 1985. public Health Mycobacteriology : a guide for a level III laboratory.. Edited by Public Health Services, U.S. Department of Health and Human Services. [Google Scholar]
- 14.World Health Organization (WHO), editor. Geneva, Switzerland: Guidelines for drug susceptibility testing for second line anti tuberculosis drugs for DOTs-plus WHO/CDC/TB/2001.288. [Google Scholar]
- 15.Toda T, Takeya K, Koike M, Mori R. Electron microscopy of ultrathin sections of Mycobacterium. Fine structure of the cells grown in- vitro and in -vivo. Proc Japan Acad. 1960;36:372–375. [Google Scholar]
- 16.Alsteen D, Verbelen C, Dague E, Raze D, Baulard AR, and Dufrene YF. Organization of the mycobacterial cell: a nanoscale view. Pflugers Arch Eur J phys. 2008;456:117–125. doi: 10.1007/s00424-007-0386-0. [DOI] [PubMed] [Google Scholar]
- 17.Verbelen C, Dupres V, Menozzi FD, Raze D, Baulard AR, Hols P, Dufrene YF. Ethambutolinduced alterations in Mycobacterium bovis BCG imaged by atomic force microscopy. FEMS Microbiol let. 2006;264:192–194. doi: 10.1111/j.1574-6968.2006.00443.x. [DOI] [PubMed] [Google Scholar]
- 18.Cunningham AF, Spreadbury CL. Mycobacterial stationary phase Induced by Low Oxygen Tension: Cell Wall Thickening and Localization of the 16-Kilodalton α-Crystallin Homolog. J Bacteriol. 1998;180:801–808. doi: 10.1128/jb.180.4.801-808.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young KD. The selective value of bacterial shape. Microbiol Mol Rev. 2006;70:660–703. doi: 10.1128/MMBR.00001-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Letek M, Fiuza M, Ordonez E, Villadangos AF, Ramos A, Mateos LM, Gil JA. Cell growth and cell division in the rod-shaped actinomycete Corynebacterium glutamicum. Anto Van Leewen. 2008;94:99–109. doi: 10.1007/s10482-008-9224-4. (2008) [DOI] [PubMed] [Google Scholar]
- 21.Ghosh J, Larsoon P, Singh B, Pettersson BMF, Islam NM, Sarkar SN, Dasgupta S, Kirsebom LA. Sporulation in mycobacteria. Proc Natl Acad Sci USA. 2009;106:10781–10786. doi: 10.1073/pnas.0904104106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Traag BA, Driks A, Stragier P, Bitter W, Broussard G, Hatfull G, Chu F, Adams KN, Ramakrihnan L, Losick R. Do mycobacterium produce endospore? Proc Natl Acad Sci USA. 2009;107:878–881. doi: 10.1073/pnas.0911299107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young KD. Bacterial shape. Mol Microbiol. 2003;49:571–580. doi: 10.1046/j.1365-2958.2003.03607.x. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen L, Scherr N, Gatfield J, Walburger A, Pieters J, Thompson CJ. Antigen 84, an effector of pleiomorphism in Mycobacterium smegmatis. J Bacteriol. 2007;189:7896–7910. doi: 10.1128/JB.00726-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Champion J A, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci USA. 2006;103:4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chauhan A, Madiraju MVVS, Fol M, Lofton H, Maloney E, Reynolds R, Rajagopalan M. Mycobacterium tuberculosis cells growing in macrophages are filamentous and deficient in FtsZ rings. J Bacteriol. 2006;188:1856–1865. doi: 10.1128/JB.188.5.1856-1865.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]